Abstract
Methods to increase temperature stability of vaccines and adjuvants are needed to reduce dependence on cold chain storage. We report herein creation and application of pVEX expression vectors to improve vaccine and adjuvant manufacture and thermostability. Defined media fermentation yields of 6 g/L thermostable toll-like receptor 5 agonist flagellin were obtained using an IPTG inducible pVEX-flagellin expression vector. Alternative pVEX vectors encoding Pyrococcus furiosus maltodextrin-binding protein (pfMBP) as a fusion partner improved Influenza hemagglutinin antigen vaccine solubility and thermostability. A pfMBP hemagglutinin HA2 domain fusion protein was a potent immunogen. Manufacturing processes that combined up to 5 g/L defined media fermentation yields with rapid, selective, thermostable pfMBP fusion protein purification were developed. The pVEX pfMBP-based thermostable tag (TST) platform is a generic protein engineering approach to enable high yield manufacture of thermostable recombinant protein vaccine components.
Keywords: Vaccine, Maltodextrin-binding protein, Thermostable, Flagellin, Hemagglutinin, Affinity tag
1. Introduction
Vaccines lose potency in a temperature-dependent fashion over time, necessitating cold-chain storage until point of use. To eliminate cold storage, various formulation strategies to improve temperature stability of vaccines and adjuvants have been developed (Chen and Kristensen, 2009). However, thermotolerant product-specific formulations are not a generic approach to stabilize vaccines.
Many protein-based vaccine antigens are manufactured in Escherichia coli. Protein based adjuvants, such as the toll-like receptor 5 (TLR5) agonist flagellin, could also be produced in E. coli. Activation of TLRs induces immediate antiviral or antibacterial responses and secretion of stimulatory signals (such as interleukin-12 and Type I interferon production) that activate and differentially regulate the adaptive immune response. TLR agonists have potential application as new generation adjuvants (Lahira et al., 2008).
A variety of fusion protein carriers have been developed to enable soluble production of passenger proteins in E. coli (Xu and Foong, 2008; Zou et al., 2008; reviewed in Sørensen and Mortensen, 2005). Of these, maltose binding protein (MBP) fusions have been used to improve protein solubility (Fox and Waugh, 2003), and as carrier proteins for vaccination (Kink and Williams, 1998). However, most recombinant proteins produced in E. coli are not inherently thermostable, and these carriers do not improve passenger protein thermostability.
Fusion to a thermostable carrier protein can increase passenger protein thermostability. For example, thermostable Methanopyrus kandleri Ftr fusion proteins retained thermostability when fused to small target proteins of <20 kDa (de Marco et al., 2004). Thermostable Archaea maltodextrin-binding proteins (MBPs), such as Pyrococcus furiosus maltodextrin-binding protein (pfMBP), confer solubilization properties onto fusion proteins (Fox et al., 2003). The pfMBP (43 kDa) carrier protein improved thermostabilization of a small (27 kDa) target green fluorescent protein (Huang et al., 2006). The fusion:target protein size ratio of 1.6:1 was consistent with the approximately 2:1 thermostable carrier partner to target protein size ratio determined by de Marco et al. (2004) to be required for target protein thermostabilization.
We report herein application of pfMBP fusion proteins for vaccination. We determined that the pfMBP fusion partner [thermostable tag (TST)] generically improved target protein thermostability. Potent thermostable Influenza hemagglutinin (HA) antigen and flagellin adjuvant were created, and manufacturing processes for rapid purification of high purity fusion protein were developed. The TST platform is thus a generic method to improve thermostability of recombinant protein vaccine components.
2. Materials and methods
2.1. Molecular biology
Plasmid cloning was in Escherichia coli (E. coli) strain DH5α [F-Φ80dlacZΔM15 Δ(lacZYA -argF) U169 recA1 endA1 hsdR17(rK−, mK+) phoA supE44 λ-thi-1 gyrA96 relA1; Invitrogen, Carlsbad, CA] using standard methodologies. All clones were sequence verified.
Protein expression was performed in E. coli BL21 [E. coli B F− dcm ompT hsdS(rB− mB−) gal] codon plus (Novagen, Madison, WI). For immunization, low endotoxin (<100 EU/mg) plasmid DNA was purified from DH5α using Nucleobond AX 2000 columns (Macherey Nagel, Düren, Germany).
2.2. pVEX-MBP vectors
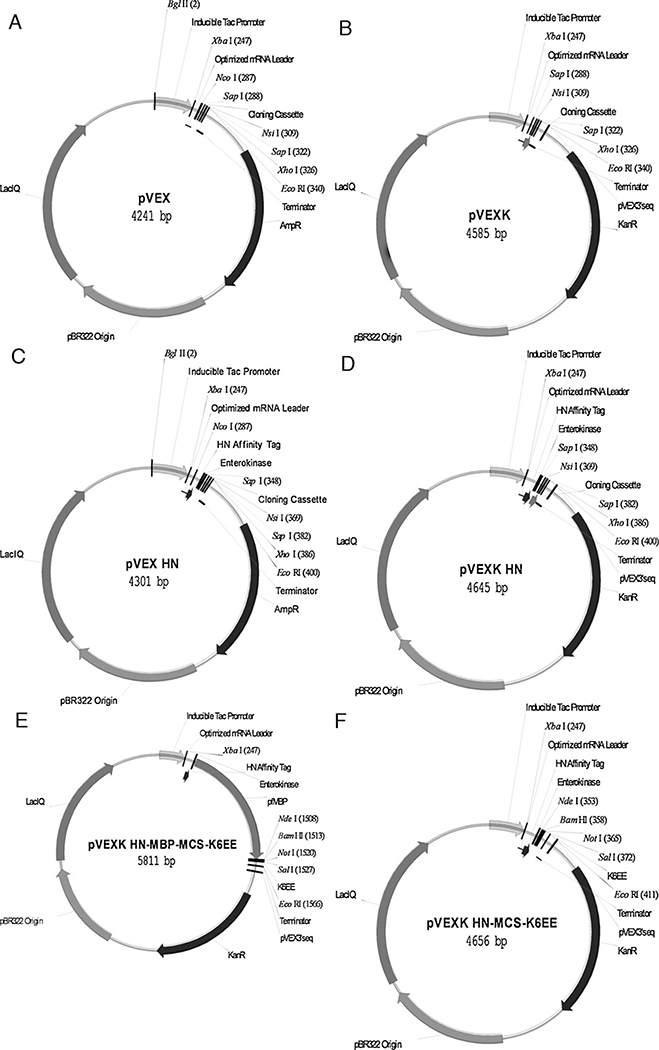
The pVEX vectors (Fig. 1) are moderate copy number ROP-pBR322 replication origin (without the pUC plasmid G to A high copy mutation) E. coli recombinant protein expression vectors.
Fig. 1.
pVEX vectors. Maps of the IPTG resistant ampicillin resistant pVEX (A) and kanamycin resistant pVEXK (B) E. coli expression vectors are shown, along with HN-tagged derivatives (C and D, respectively). Target genes are precisely cloned into these vectors using the SapI type IIS enzyme. Briefly, genes are PCR amplified with primers incorporating SapI sites into termini to generate 5′ ATG (pVEX and pVEXK) or 5′ AAG (pVEX HN, pVEXK HN) and 3′ TAA (all four vectors) 3 bp sticky ends upon digestion with SapI. The pVEXK HN-MBP-MCS-K6EE vector for cloning genes with an N-terminus HN-pfMBP and optional C-terminal K6EE stabilization tag (E) and pfMBP-deleted control vector pVEXK HN-MCS-K6EE (F) are shown. Target genes are cloned into these vectors using an NdeI (CATATG, ATG is in frame with the upstream HN- or HN-pfMBP reading frame) BamHI, NotI, SalI polylinker. Target genes designed to also encode the C-terminus K6EE tag are precisely cloned into these vectors by PCR amplification to introduce an in frame 5′ NdeI site and a 3′ SalI site (in frame and deleting the target gene stop codon). Heat inducible bacteriophage lambda pR pL promoter versions of HN-MBP-MCS-K6EE and HN-MCS-K6EE are also available (pVEXK pR pL HN-MBP-MCS-K6EE; pVEXK pR pL HN-MCS-K6EE).
pVEXB-MBP (MKIEE-pfMBP-GIEGR): The pfMBP gene (PF1938) from P. furiosus DSM 3638 (ATCC 43587D-5) was PCR amplified from genomic DNA using the following primer pair to make a cytoplasmic expression construct.
PF1938F: 5′-tcccagcacctgcacccATGAAAATCGAAGAAGGAAAAGTTGTTATTTGGCATGCAATG-3′
Underlined in the primer above are substitutions at the 5′ end to delete the pfMBP secretion leader and replace the 5′ end with MKIEE amino acids (residues 1–5).
PF1938R: 5′-tcggagcacctgcactaCCTTccctcgatTCCTTGCATGTTGTTAAGGATTTCTTG-3′
Double underlined in the primer above is a glycine amino acid, single underlined is the Factor Xa cleavage linker (GIEGR). This facilitates removal of pfMBP from downstream fusions if desired. The cloning site is pfMBP-gga atc gag gga agg cat atg (CATATG is the NdeI cloning site for the start of the downstream fusion protein).
The 1.2 kb product was digested with type IIS restriction enzyme AarI (cacctgc sites underlined in primers; cleaves at adjacent +4 to +8 site to create 4 bp sticky end) and cloned into the pVEXB arabinose inducible expression vector backbone (Williams, J.A., Hodgson, C.P., 2006. WO/2006/026125).
pVEXB-MBP-gly ser (MKIEE-pfMBP-GGGGGS): A pVEXB-MBP derivative, with the pfMBP linker modified to replace the Factor Xa cleavage site with a five glycine one serine residue linker immediately before the CATATG NdeI target protein cloning site was constructed by PCR mediated mutagenesis of the pVEXB-MBP clone.
pVEXB HN-MBP (M HN-MKIEE-pfMBP-GIEGR): This derivative of pVEXB-MBP was constructed by modification of the N-terminus of pfMBP to incorporate a 6xHN histidine tag (Clontech, Mountain View, CA) -enterokinase (DDDDK) cleavage linker (MG-HNHNHNHNHNHN-GGDDDDK, herein referred to as HN).
pVEXK HN-MBP-MCS-K6EE (M HN-MKIEE-pfMBP-GIEGR-MCS-K6EE): The HN-pfMBP insert was transferred into the pVEXK vector. A polylinker multiple cloning site (MCS) was inserted after the Factor Xa linker (downstream of the NdeI site) followed by a six lysine, two glutamic acid residue C-terminal tag (K6EE). Open reading frames (ORFs) cloned as NdeI (CAT ATG; ATG is ORF start codon)/SalI (GTC GAC; reading frame as shown) fragments are expressed with N-terminal HN-pfMBP and C-terminal K6EE tags. The map of pVEXK HN-MBP-MCS-K6EE, and the control vector without pfMBP (pVEXK HN-MCS-K6EE), are shown in Fig. 1E and F, respectively. Heat inducible versions (pVEXK pR pL HN-MBP-MCS-K6EE; pVEXK pR pL HN-MCS-K6EE) were made by precise replacement of the IPTG inducible ptac promoter and lacIQ repressor with the bacteriophage lambda pR pL promoters and C1857ts lambda repressor.
2.3. pfMBP-HA fusion proteins
The arabinose-inducible pVEXB-MBP vector was utilized to construct a series of four fusions (B–E in Table 1) with the Influenza A H5N1 A/Vietnam/1203/2004 HA gene using the primer pairs and PCR fragments as described in Table 1 and shown in Fig. 2A.
Table 1.
pfMBP fusion proteins.
| Target proteind | Primers | Fusion protein size (kDa) | pfMBP:target protein size ratioa |
|---|---|---|---|
| B = HA1–HA2 (1 to 23) | HAABEF01 + HABR01 | 85 | 1:1 |
| C = HA2 | HACF01 + HAACDR01 | 66b | 1.9:1 |
| D = HA2 (−1 to 23) | HADF01 HAACDR01 | 63 | 2.2:1 |
| E = HA1 | HAABEF01 + HAER01 | 82 | 1.1:1 |
| Flagellin | fliCF01 + fliCR01 | 95c | 0.8:1 |
pfMBP carrier protein = 43kDa.
Fermentation yield (RF269) of 4.5 g/L soluble protein in defined media fermentation (pVEXK HN-pfMBP-HA2-K6EE).
Fermentation yield (RF268) of 4.9g/L soluble protein in defined media fermentation (pVEXK HN-pfMBP-flagellin).
See Fig. 2A for HA regions expressed in each construct.
Fig. 2.
pfMBP-HA fusions. (A) Influenza serotype H5 HA protein, with the locations of the HA regions expressed as pfMBP-HA fusion proteins shown. The proprietary H5 HA DNA construct utilized to make these clones contained a deletion of the high pathogenicity RRRRKK H5 HA cleavage loop. (B) High level expression of soluble, thermostable HN-pfMBP-HA2 fusion protein. SDS-PAGE gel loaded with total (lane 1), soluble (lane 2) and thermostable (75 °C, 40 min; lane 3) extracts from E. coli lysates after IPTG induction of the pVEXK HN-pfMBP-HA2-K6EE vector. M = BioRad Broad Range Marker.
All clones deleted the N-terminal HA signal peptide and C-terminal transmembrane and cytoplasmic domains. 1–23 of HA2 is the hydrophobic membrane insertion region that is conserved across multiple isolates.
The NdeI site on the forward PCR primers (below) was compatible and in frame with the NdeI cloning site in the pVEXB-MBP vector.
HAABEF01: 5′-tcccagCATATGagtgatcagatttgcattggttac-3′
HACF01: 5′-tcccagCATATGagaggattatttggagctatagcaggttttatag-3′
HADF01: 5′-tcccaaCATATGcaccatagcaatgagcaggggagtgggtac-3′
The reverse PCR primers (below) encoded a seven amino acid glycine-K6EE C-terminal extension to each pfMBP-HA fusion protein. The stop codon is double underlined. Digestion of the PCR product with AarI (cacctgc site underlined in primers) created an EcoRI compatible end (bolded AATT), at the 3′ end of the gene (in the reverse primers, below).
HABR01: 5′-cgtgagcacctgcaactAATTCTTATTCTTCTTTCTTTTTCTTTTTTTTGCCCCCATACCAACCATCTACCATTCCCTG-3′
HAACDR01: 5′-cgtgagcacctgcaactAATTCTTATTCTTCTTTCTTTTTCTTTTTTTTGCCTCCTATTGATTCCAATTTTACTCCAC-3′
HAER01: 5′-cgtgagcacctgcaactAATTCTTATTCTTCTTTCTTTTTCTTTTTTTTGCCCTCTCTTTGAGGGCTATTTCTGAGC-3′
2.4. pfMBP-flagellin fusions
The fliC (flagellin) gene from Salmonella enterica serovar Typhimurium (ATCC genomic DNA 700720D) was PCR amplified with the following primers:
fliCF01: 5′-ggaaggcatatggcacaagtcattaatacaaacagc-3′
fliCR01: 5′-ggaagggaattcttaacgcagtaaagagaggacgttttgc-3′
The 1.5 kb PCR product was digested with NdeI/EcoRI (sites are underlined in the primers) and cloned into the pVEXK MBP and the pVEXK MBP-glyser vectors. This fusion of pfMBP (43 kDa) and flagellin (52 kDa) was also made in arabinose inducible (pVEXB) versions of the pfMBP (Factor Xa linker) and the pfMBP-glyser encoding vectors.
A HN-tagged version of pfMBP-flagellin was made by transfer of the pfMBP-flagellin (Factor Xa linker) insert to the pVEXK HN-MBP vector (pVEXK HN-MBP-flagellin). PCR mediated deletion of pfMBP was used to create the HN-flagellin control plasmid (pVEXK HN-flagellin).
2.5. IMAC protein purification
Fusion protein was purified from 1 LIPTG-induced LB shake flask cultures. Cultures were grown at 30–37 °C in LB media containing 0.2% glucose and induced (pVEX using 1mM IPTG, pVEXB using 0.2% arabinose) at 0.5–1.0 OD600 for 4–8 h. Cells were harvested by centrifugation, resuspended in 1/25th culture volume of binding buffer (50 mM NaPO4, pH 7, 300 mM NaCl containing 2 mg/L lysozyme), lysed by freeze-thawing and sonication and centrifuged. Where indicated for thermostable protein purification, E. coli proteins were then precipitated by heat treatment (75 °C for 40 min). HN-tagged protein in the clarified lysate was purified on a Talon resin (Clontech Laboratories, Mountain View, CA) immobilized metal affinity chromatography (IMAC) column, including a wash in binding buffer containing 0.1% Triton X-114 at 4 °C to reduce endotoxin (Zimmerman et al., 2006). Endotoxin was quantified using the EndoSafe PTS system (Charles River Laboratories, Wilmington, MA). Reduced SDS-PAGE analysis using 4–20% Tris–HCl precast gels (Biorad, Hercules, CA) was as described (Williams et al., 1994). Recombinant protein was quantified by A280 using the theoretical extinction coefficient.
2.6. Fermentation
Seed stocks for fermentation of the pVEXK HN-flagellin, pVEXK HN-MBP-flagellin and pVEXK HN-MBP HA2-K6EE vectors were manufactured in BL21 codon plus and the resultant cell lines fermented using 7 L working volumes of a proprietary defined media in New Brunswick BioFlo 110 bioreactors in a fed-batch fermentation process with glucose carbon source. Samples were removed and assayed for biomass (A600) and glucose (One Touch Blood Glucose Monitor, Lifescan, Milptas CA). Glucose feeding was adjusted as necessary to prevent growth restriction without accumulating excessive glucose (>20 g/L). Protein production was induced by IPTG addition to 1mM at 50–60 OD600 (T = 0). Cultures were grown for 8 h post induction and samples removed for biomass, glucose and protein analysis at T = 0, 2, 4, 6 and 8 h post induction. Protein expression was assessed by SDS-PAGE analysis of total and soluble protein samples prepared from frozen 5 OD600 pellets from each timepoint. The cell pellets were resuspended in TE buffer (10mM Tris, 1mM EDTA, pH 8.0) to 20 OD600/mL, lysed by sonication (total protein), and clarified by centrifugation (soluble protein) prior to mixing 1:1 with 2× SDS-PAGE sample buffer containing β-mercaptoethanol, heating to 95 °C for 5 min and resolving on 4–20% Tris–HCl precast gels. Harvest yields were quantified by comparison of the fusion protein band to a standard curve of purified, quantified, fusion protein.
2.7. Cell culture
Adherent human embryonic kidney cell line HEK293 (American Type Culture Collection, Manassas, VA) was propagated in DMEM/F12 containing 10% fetal bovine serum and split (0.25% trypsin–EDTA) using Invitrogen (Carlsbad, CA) reagents and conventional methodologies. For transfections, cells were plated on 24-well tissue culture dishes. Supercoiled plasmids were transfected into cell lines using Lipofectamine 2000 following the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Total cellular lysates for luciferase determination were prepared by resuspending cells in cell lysis buffer (Promega, Madison WI). Lysed cells were clarified by centrifugation and luciferase assayed in the supernatants in opaque 96 well plates using a FLX800 microplate fluorescence reader after activation with the Promega luciferase assay system. The supernatants were assayed for EGFP (transfection control) was assayed with the fluorescence reader using black 96 well plates.
TLR5 activation assay: HEK293 cells were cotransfected with NFκB -luc (luciferase reporter plasmid responsive to NFκB activation), pDNAVACCUltra5 EGFP (EGFP transfection control plasmid), and either (1) pUNO-TLR9 (unmethylated CpG DNA responsive TLR) or (2) pUNO-TLR5 (flagellin responsive TLR; pUNO vectors were from Invivogen San Diego, CA). TLR9 and TLR5 transfected cells were stimulated 20 h post transfection with 0.2 or 2μg of test protein and luciferase activity (and control EGFP) determined 6 h later.
TLR4 activation assay: HEK293 cells were cotransfected with NFκB-luc and pUNO-hTLR4A +pDUO2-hMD2/CD14 (Invivogen; hMD2/CD14 is a cofactor for TLR4A activation) and pDNAVACCUltra5 EGFP (EGFP transfection control plasmid). Control groups included transfections with pUNO-hTLR4A or pDUO2-hMD2/CD14 plasmids separately, or without either receptor plasmid. Transfected cells were stimulated 24 h post transfection with 0.05, 0.2 or 1.0 μg low endotoxin HN-pfMBP, 1.0 μg HN-Taq (recombinant HN-tagged taq DNA polymerase, negative control) or 1 μL saturated E. coli culture (endotoxin positive control TLR4 agonist) and luciferase activity (and control EGFP) determined 6.5 h later.
2.8. Murine study
10 μg of either HA plasmid DNA or HN-pfMBP-HA2 protein (low endotoxin DNA and protein preparations), mixed in 50 μL PBS, pH7.4, was injected bilaterally (25 μL per site), intradermally into BALB/c mice (6–8 weeks old, 5 per group) in a prime dose (day 0). Boost doses were performed on days 21 and 49. Terminal bleed was on day 56.
Serum samples were taken on days 0, 21, 49 and 56 and tested for anti-HA2 IgG response. Anti-HA2 specific total IgG, IgG2a, and IgG1 levels were determined in a standard ELISA assay using plates coated with HN-HA2-K6EE protein (HA2 antigen without the pfMBP carrier) that was purified under denaturing conditions (binding, wash, and elution buffers contained 4 M urea) on a Talon resin column. Sigma (St. Louis, MO) detection antibodies and the BD Biosciences OptEIA kit (BD Biosciences Pharmingen, San Diego, CA) reagents were used to develop ELISA plates. Briefly, coated wells were blocked and incubated 2 h with diluted serum samples. The wells were washed and the signal was detected by subsequent, sequential incubations and washing with either: (1) goat anti-mouse IgG (Fc)-biotin, then Streptavidin-horse radish peroxidase conjugate (total IgG); or (2) goat anti-mouse IgG1, then anti-goat IgG-biotin, then Streptavidin-horse radish peroxidase conjugate (IgG1); or (3) goat anti-mouse IgG2a, then anti-goat IgG-biotin, then Streptavidin-horse radish peroxidase conjugate (IgG1).
2.9. Circular dichroism (CD) spectroscopy
CD spectra were measured on an Aviv Model 202 spectrometer at 25 °C in a 1mm cell in PBS buffer, pH 7.4, at protein concentrations of 0.53 mg/mL, 0.30 mg/mL, for HN-flagellin, and HN-pfMBP-flagellin, respectively. The heat-treated samples were prepared by a 40 min heat treatment at 75 °C. The thermodynamic stability was recorded at 220 nm by monitoring the CD signal at 25–75 °C with a scan rate of 1 °C/min for increasing temperature assay. Additional CD spectra were measured at 75 °C when samples reached 75 °C after the thermodynamic stability assay mentioned above. The spectra were corrected against those of the buffer reference.
3. Results
3.1. pVEX expression vectors
The 6xHN histidine tag (HNHNHNHNHNHN; Clontech) is a metal binding tag alternative to the His-tag (HHHHHH) that is used for purification by immobilized metal chelate chromatography (IMAC). E. coli recombinant protein expression vectors (pVEX vectors) with ampicillin (pVEX; Fig. 1A) or kanamycin (pVEXK; Fig. 1B) resistance markers with or without an N-terminal HN-tag (pVEX HN, pVEXK HN; Fig. 1C and D) were created. The pVEXK HN-MCS-K6EE expression vector (Fig. 1F) was then constructed and is designed to express a target protein as a C-terminal fusion to HN with an optional octapeptide K6EE C-terminus. Dipeptide glutamic acid (EE) or aspartic acid (DD) acidic residues at the C-terminus increased E. coli protein half-life (Parsell et al., 1990; Trepod and Mott, 2000). Consistent with this, the composite K6EE tag increased E. coli produced recombinant protein stability and yield (Williams, unpublished observations). In these pVEX vectors recombinant protein expression is induced from the strong ptac promoter by IPTG induction. A heat inducible version of this vector was also constructed (pVEXK pR pL HN-MCS-K6EE).
3.2. pVEX pfMBP fusion protein expression vectors
An IPTG inducible pVEX expression vector containing a HN-tagged derivative of pfMBP was constructed (pVEXK HN-MBP-MCS-K6EE; Fig. 1E) along with a heat inducible version (pVEXK pR pL HN-MBP-MCS-K6EE). These vectors express a target protein as a C-terminal fusion to HN-pfMBP optionally with the octapeptide K6EE C-terminus stabilization tag. High levels of soluble pfMBP accumulate in the cell after IPTG induction of the pVEXK ptac promoter. Purification from a 1 L shake flask culture of HN-pfMBP (without the K6EE tag) using heat treatment combined with IMAC chromatography incorporating an endotoxin reduction step yielded 92 mg highly pure, low endotoxin (20 EU/mg), thermostable (resistant to 85 °C for 40 min treatment) protein.
E. coli MBP is a potential toll-like receptor 4 (TLR4) agonist (Fernandez et al., 2007). HN-pfMBP was tested for TLR4 agonist activity to determine if this activity was conserved. While positive control endotoxin activated TLR4-dependent NFκB expression, 0.05, 0.2 or 1.0 μg low endotoxin HN-pfMBP protein did not activate TLR4 (data not shown). This demonstrates HN-pfMBP is not a strong TLR4 activator.
3.3. pfMBP HA fusions
The Influenza H5N1 HA2 domain (Fig. 2A) expressed in E. coli as a HN-HA2-K6EE fusion protein was insoluble (data not shown). A previous study reported that the H5N1 HA1 domain was also insoluble when expressed in E. coli (Chiu et al., 2009). Thus Influenza H5N1 HA1 and HA2 domain antigens expressed in E. coli are insoluble.
The application of the pfMBP carrier protein to improve solubility and thermostability of HA1 and HA2 HA antigens was evaluated. An arabinose-inducible pfMBP expression vector (pVEXBMBP) was utilized to construct a series of four fusions (B–E in Table 1) with HA1 or HA2 domains of the Influenza A H5N1 A/Vietnam/1203/2004 HA gene using the fragments described in Table 1 and shown in Fig. 2A. All clones expressed fusion proteins (pfMBP-HA-K6EE) of the predicted size in bacterial cells after arabinose induction. All four pfMBP fusions, HA1–HA2 (1–23), HA2, HA2 (−1–23) and HA1, were stable; soluble in TE buffer when lysed in small scale cultures; and thermostable after heat treatment (data not shown). Therefore the pfMBP carrier has general utility to express HA antigens as soluble thermostable fusion proteins.
The HA2 region (target protein ‘C’, Table 1, Fig. 2A) was selected for further evaluation because it: (1) contains the 1–23 hydrophobic membrane insertion region of HA2 that is conserved across multiple viral isolates and (2) HA2 is more highly conserved between viral isolates than HA1. HA2-C was cloned into the IPTG inducible pVEXK HN-MBP-MCS-K6EE vector (Fig. 1E) and the pVEXK HN-MCS-K6EE control vector (Fig. 1F). High level production of thermostable fusion protein (HN-pfMBP-HA2-K6EE) was observed after IPTG induction of pVEXK HN-MBP-HA2-K6EE (Fig. 2B, lane 3). This demonstrated that addition of the 2.3 kDa N-terminal HN-tag to pfMBP did not affect expression or thermostability of pfMBP fusion proteins. In contrast, the HN-HA2-K6EE protein without the pfMBP gene was insoluble (data not shown). This demonstrated that the pVEXK HN-MBP-HA2 expression system was necessary to obtain high yields of high quality, soluble HA2 protein.
Thermostable HN-pfMBP-HA2-K6EE fusion protein was purified from a 1 L IPTG-induced shake flask culture using heat treatment combined with IMAC chromatography incorporating an endotoxin reduction step. The final yield was 12 mg of high purity, intact, low endotoxin fusion protein (247 EU/mg). Production of HN-pfMBP-HA2-K6EE was then assessed in defined media fermentation. A production yield of 4.5 g/L soluble thermostable pfMBP-HA2 fusion protein was obtained (Table 1) demonstrating this system is an efficient production platform for thermostable Influenza HA antigen production.
3.4. pfMBP-HA2 fusion is an effective immunogen
The immunogenicity of: (1) HN-pfMBP-HA2 protein and (2) a CMV promoter DNA vaccine plasmid expressing the entire Influenza A H5N1 A/Vietnam/1203/2004 HA gene (HA plasmid), was determined. The results, summarized in Table 2, demonstrated that the HN-pfMBP-HA2 recombinant protein was an effective immunogen, generating significantly more anti-HA2 total IgG (day 56, prime and two boosts) and IgG1 (day 49, prime-boost) antibodies than the comparator DNA immunogen. The plasmid immunogen induced more anti-HA2 IgG2a antibodies than HN-pfMBP-HA2 (day 49, prime-boost) indicating the HN-pfMBP-HA2 protein immunogen generated a Th2 biased response compared to the DNA immunogen. Significantly, these results demonstrated that HN-pfMBP fusion proteins can generate strong immune responses against a target antigen (HA2) at low dose, and in physiological buffers, without requiring adjuvant.
Table 2.
pfMBP-HA2 is an effective immunogen.
| Immunogen | 21 day anti-HA2 IgG (A450)b | 49 day anti-HA2 IgG1 (A450)c | 49 day anti-HA2 IgG2a (A450)d | 49 day anti-HA2 IgG (A450)b | 56 day anti-HA2 IgG (A450)b |
|---|---|---|---|---|---|
| HN-pfMBP-HA2 protein | 0.073 ± 0.015 | 1.377 ± 0.08a | 0.395 ± 0.410 | 0.301 ± 0.102 | 0.615 ± 0.245a |
| HA plasmid | 0.073 ± 0.015 | 0.485 ± 0.468a | 0.904 ± 0.472 | 0.210 ± 0.154 | 0.190 ± 0.138a |
Titers between protein and DNA were significantly different at a P value = 0.05 [Wilcoxon (Mann–Whitney) rank-sum test and two sided Student t-test].
Total A450 average ± SD at 1/62,500 serum dilution. T = 0 preimmune anti-HA2 IgG (total) (A450 at 1/62,500) was 0.056 ± 0.002 (pfMBP-HA2) or 0.057 ± 0.002 (HA plasmid).
Buffer blanked A450 average ± SD at 1/500 serum dilution. T = 0 preimmune anti-HA2 IgG1(A450 at 1/500) was 0.03 ± 0.03 (pfMBP-HA2) or 0.03 ± 0.03 (HA plasmid).
Buffer blanked A450 average ± SD at 1/500 serum dilution. T = 0 preimmune anti-HA2 IgG2a (A450 1/500) was 0.003 ± 0.002 (pfMBP-HA2) or 0.001 ± 0.002 (HA plasmid).
3.5. Thermostable flagellin adjuvants
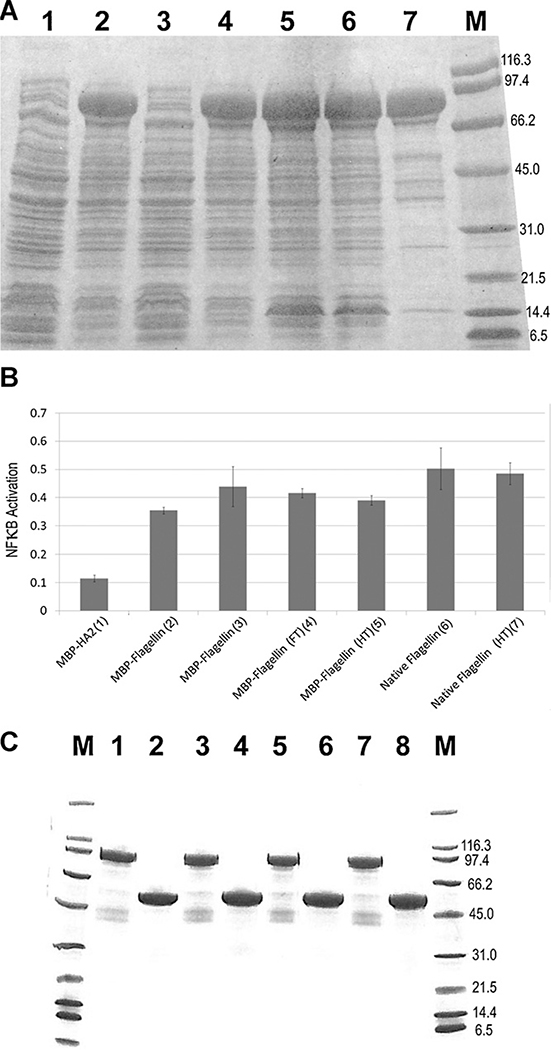
S. enterica serovar Dublin flagellin is heat stable (90 °C for 20 min) when produced in E. coli (Burdelya et al., 2008). A pVEX HN-flagellin (N-terminal HN-tagged S. enterica serovar Typhimurium flagellin) vector also expressed a soluble fusion protein that was tolerant to heat treatment. pVEX HN-flagellin was expressed to high levels in E. coli. Purification from a 1 L shake flask culture of HN-flagellin by IMAC chromatography incorporating an endotoxin reduction step yielded 67 mg highly pure, low endotoxin (<11 EU/mg), thermostable flagellin (resistant to 85 °C for 40 min treatment; Fig. 3C, lane 8). In a defined media fermentation high production yields of 6.2 g/L soluble, thermostable HN-flagellin were obtained.
Fig. 3.
HN-flagellin and HN-pfMBP-flagellin. (A) HN-pfMBP-flagellin heat treatment. SDS-PAGE of harvest samples from a pVEXK HN-MBP-flagellin culture. Lane 1= uninduced total protein, lane 2 = induced total protein, lane 3 = uninduced soluble protein, lane 4 = induced soluble protein, lane 5 = induced total protein (1 L scale purification), lane 6 = induced soluble protein (1 L scale purification), lane 7 = induced soluble protein after 75 °C heat treatment (1 L scale purification), M = BioRad Broad Range Marker (B) TLR5 mediated induction of NFκB by HN-pfMBP-flagellin. (1) HN-pfMBP-HA2 negative control; (2 and 3) two lots HN-pfMBP-flagellin; (4 and 5) HN-pfMBP-flagellin stressed by 3× freeze–thaw (4) or heat treatment (71 °C for 1 h) (5); (6 and 7) isolated flagellum (Invivogen) without (6) or with (7) heat treatment. All samples were centrifuged at 12,000 × g to remove precipitates prior to analysis and assayed in duplicate in 24 well plates (0.2 μg protein/well) as described in Section 2. Ratio of Luciferase (NFκB promoter activation) to EGFP (transfection control) is shown. (C) Purified HN-flagellin and HN-pfMBP-flagellin thermostability. The proteins were buffer exchanged into phosphate buffered saline and subjected to a freeze–thaw cycle prior to analysis. SDS-PAGE of 5 μg samples of Talon purified HN-pfMBP-flagellin (lanes 1, 3, 5 and 7) or HN-flagellin (lanes 2, 4, 6 and 8) pretreated by a 40 min heat treatment at 75 °C (lanes 3 and 4), 80 °C (lanes 5 and 6), or 85 °C (lanes 7 and 8). M = BioRad Broad Range Marker.
Production of flagellin with the pfMBP tag was then assessed. pVEX vectors (ptac or pBAD promoter) encoding two different pfMBP-flagellin fusion proteins [i.e. two different linkers, GIEGR (factor Xa) or GGGGGS, between pfMBP and flagellin] were also made. These vectors expressed pfMBP as an N-terminal fusion partner with the entire S. enterica serovar Typhimurium flagellin gene. To retain full biologic activity, the fusions (as with HN-flagellin) did not include the C-terminal K6EE tag since the C-terminal domain of flagellin is required for inflammasome activation (Lightfield et al., 2008).
Thermostable fusion protein (not denatured by 75 °C for 20 min heat treatment) of the correct size was produced after arabinose induction of the pBAD promoter (pVEXB) vectors. Native E. coli proteins were precipitated with a 75 °C for 20 min heat treatment. Fusions of pfMBP and flagellin using the Factor Xa and GGGGGS linkers were proteolytically and thermally stable.
The flagellin gene was also cloned into the pVEXK HN-MBP vector (Factor Xa linker). High levels of thermostable fusion protein were induced after IPTG induction of the ptac promoter. Thermostable fusion protein was purified by IMAC incorporating an endotoxin reduction step from a 1 L IPTG induced pVEXK HN-MBP-flagellin shake flask culture using heat treatment (75 °C for 40 min) to remove E. coli proteins (Fig. 3A, lane 7). The final yield was 66 mg of high purity, intact, low endotoxin (<26 EU/mg) HN-pfMBP-flagellin fusion protein that was stable in standard buffer formulations such as phosphate buffered saline (PBS).
Production of HN-pfMBP-flagellin was then assessed in defined media fermentation. A production yield of 4.9 g/L soluble thermostable HN-pfMBP-flagellin was obtained (Table 1).
The immunostimulatory activity of a low endotoxin preparation of fusion proteins HN-pfMBP-flagellin, HN-pfMBP (negative control) and native Salmonella flagellum (Positive control; Invivogen, San Diego, CA) was determined in a cell culture bioassay. Strong NFκB promoter activation was observed with TLR5, but not the TLR9 negative control, with both doses of native flagellum and HN-pfMBP-flagellin but not with HN-pfMBP. TLR5 activation activity was not reduced with HN-pfMBP-flagellin preparations that were further stressed by 3× freeze–thaw or additional heat treatment (71 °C for 1 h; Fig. 3B, columns 4 and 5). This demonstrated that the pfMBP-flagellin fusion combines thermostability with immunostimulatory activation of TLR5.
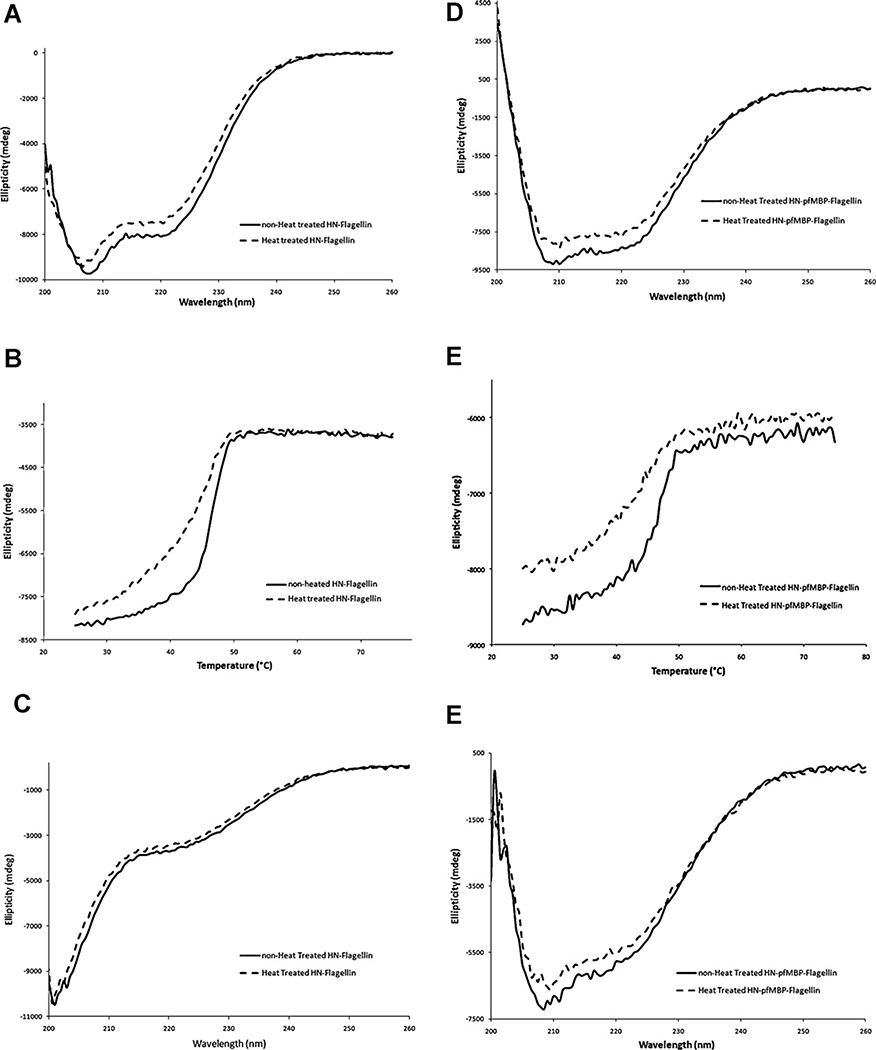
Both HN-flagellin and HN-pfMBP-flagellin were resistant to freeze–thaw and highly thermostable (Fig. 3C, lanes 7 and 8). We compared the far-UV circular dichroism (CD) spectra of the non-heat treated and heat treated HN-flagellin and HN-pfMBP-flagellin (Fig. 4A and D). The two spectra for each sample were very similar, indicating the 40 min heat treatment at 75 °C did not affect the secondary composition of each sample. Second, we compared the stability of the two species (non-heat treated and heat treated) of those two samples by conducting thermal melting experiments monitored by CD (Fig. 4B and E). Both species for each sample exhibited very similar melting transition temperatures: 46.6 °C for non-heat treated HN-flagellin, 46.3 °C for heat treated HN-flagellin. The non-heat treated HN-pfMBP-flagellin (Tm = 46.9 °C) was slightly more stable than the heat treated one (Tm = 44.7 °C). Finally, we also compared the far-UV CD spectra of the non-heat treated and heat treated HN-flagellin and HN-pfMBP-flagellin after the extended 3 h heat treatment from 25 °C to 75 °C when performing the thermal melting experiments. Interestingly, both non-heat treated and heat treated HN-flagellin lost most of their secondary structures (Fig. 4C) while both species of HN-pfMBP-flagellin maintained the majority of their secondary structures (Fig. 4F). This indicates that thermo-stability was improved with the pfMBP fusion partner.
Fig. 4.
Circular dichroism (CD) characterization of HN-flagellin and HN-pfMBP-flagellin. (A) CD spectra of non-heat treated and heat-treated (40 min in a 75 °C water bath) HN-flagellin. (B) Melting curves of non-heat treated and heat treated HN-flagellin recorded at 220 nm. (C) CD spectra of non-heat treated and heat-treated (40 min in a 75 °C water bath) HN-flagellin after 3 h temperature scan. (D) CD spectra of non-heat treated and heat-treated (40 min in a 75 °C water bath) HN-pfMBP-flagellin. (E) Melting curves of non-heat treated and heat treated HN-pfMBP-flagellin recorded at 220 nm. (F) CD spectra of non-heat treated and heat-treated (40 min in a 75 °C water bath) HN-pfMBP-flagellin after 3 h temperature scan.
Collectively these results demonstrated that both the pVEXK HN-flagellin and the pVEXK HN-MBP-flagellin expression systems can be utilized in shake flask or industrial fermentation to produce large amounts of high quality thermostable flagellin protein that is compatible with standard formulations and freeze–thaw.
4. Discussion
Application of the pfMBP tag as a thermo-stabilization fusion partner for vaccine antigen production was demonstrated with Influenza H5N1 HA-based antigens. High level expression of intact thermostable soluble fusion protein was obtained, and pfMBP fusion protein was purified by heat treatment. This flocculated and removed host E.coli proteins and thermolabile components, simplifying the purification process. A pfMBP fusion protein would also be compatible with the thermolysis-mediated cell disruption process for efficient extractive purification of thermostable fusion protein (Ren et al., 2007).
Fusion to HN-pfMBP simplified use of the HA2 antigen as an immunogen since the native HA2 antigen without pfMBP (HN-HA2) was insoluble. HN-pfMBP-HA2 was soluble, produced to high yield in defined media fermentation, easily purified to high purity, and was a potent immunogen. While E. coli derived MBP was demonstrated to be an innate immune TLR4 agonist (Fernandez et al., 2007), this activity was not observed with pfMBP. Perhaps pfMBP-mediated binding to cell surface maltodextrin motifs facilitates antigen presentation by improving cellular uptake of the fusion protein.
Many bacteria contain a flagellum composed of polymers of the protein flagellin arranged in helical chains so as to form a hollow core. Conserved regions of both the N- and C-terminus of flagellin (e.g. Salmonella species fliC) are critical for TLR5 activation, perhaps through formation of a tertiary structure (Murthy et al., 2004). TLR5 recognizes these regions when present in polymeric flagellum or recombinant purified flagellin. The C-terminal 35 amino acids of flagellin is also a ligand for inflammasome receptor Naip5 (and perhaps Ipaf), inducing release of inflammatory cytokines interleukin 1β (IL-1β) and IL-18 (Lightfield et al., 2008). TLR5 is expressed on a spectrum of cell types, notably epithelial cells of the gut, kidneys and airways mucosal surfaces (reviewed in Ramos et al., 2004) and flagellin-mediated TLR5 signaling is conserved in the vertebrate lineage, including bony fish (Rebl et al., 2010). Salmonella flagellin has been demonstrated to induce innate immune responses in the lungs after intratracheal delivery (Honko and Mizel, 2004) and is an adjuvant that improved immune responses to: Influenza vaccine after intranasal (IN) administration in mice (Skountzou et al., 2010); and Yersinia pestis F1 antigen after IN or intratracheal administration in mice, or after IN or intramuscular administration in cynomolgus monkeys. Importantly, flagellin is an effective adjuvant even in the presence of preexisting flagellin antibodies (Honko et al., 2006). These results collectively demonstrate the potential for flagellin as a mucosal and systemic adjuvant, especially when delivered to epithelial cells.
Assembled native polymeric flagellum in crude culture media filtrates were thermostable (Fig. 3B, lane 7) as was recombinant E. coli produced HN-tagged flagellin (Fig. 3C, lane 8). The results from dynamic light scattering measurements suggest HN-flagellin is a monomer–dimer mixture (PS and DSW, unpublished observations). To meet manufacturing and safety requirements for vaccine adjuvant applications, this highly purified recombinant flagellin would be preferable to crude flagellum polymers. The HN-pfMBP-flagellin fusion protein described herein also retained flagellin thermostability and flagellin-mediated TLR5 activation. Both HN-flagellin and HN-pfMBP-flagellin fusion proteins were produced in high yield in defined media fermentation, and were easily purified to high purity (Fig. 3C, lanes 1 and 2). These thermostable flagellin compositions are viable TLR5 agonist for adjuvant applications, for example, to improve performance of mucosal animal vaccines (Gerdts et al., 2006).
Thermostable flagellin may also have application for nonspecific protection against various threats. Flagellin treatment is not associated with severe inflammatory pathology yet induces strong innate responses in epithelial cells that provide non-specific protection against a variety of challenges including bacterial, chemical, viral, and radiation treatments in mice (Vijay-Kumar et al., 2008). A single injection of an engineered Salmonella flagellin deletion (CBLB502, N- and C-terminal domains separated by flexible linker) improved survival of mice and rhesus monkeys against total body irradiation (Burdelya et al., 2008).
As well, flagellin fusion vaccines (Huleatt et al., 2007) have been developed for a variety of specific antigens, including plague (flagellin-F1/V; Mizel et al., 2009) and Influenza (flagellin-HA1 head domain; Song et al., 2008). These flagellin fusion proteins retained TLR5 activation and may additionally target the fused protein to TLR5 expressing cells to improve immune responses. However, they do not retain flagellin solubility and require solubilization from inclusion bodies (Mizel et al., 2009; Song et al., 2008). The pfMBP carrier may have application to improve solubility, thermostability and production of these product-specific flagellin fusion proteins, as was obtained with the high yield production of soluble thermostable HN-MBP-flagellin fusion protein.
5. Conclusions
In summary, we report the development and application of pVEX expression vectors for high yield fermentation and efficient downstream purification of thermostable vaccine antigens (HN-pfMBP fusions) and adjuvant (HN-flagellin). The pfMBP-based TST platform has utility to engineer proteins with improved thermostability. pfMBP may be applied to stabilize vaccine antigens, or candidate innate immune agonist adjuvant proteins, such as the TLR2 activating Neisseria meningitidis PorB (Singleton et al., 2005) or TLR4 agonist type 1 fimbria fimH adhesion protein (Mossman et al., 2008).
Acknowledgments
We thank Sheryl Anderson and Angela Schukar for cleaning, batching and operating fermentors and Kim Hanson for purifying the plasmid DNA used in this study.
Abbreviations
- A450
absorbance at 450 nm
- A600
absorbance at 600 nm
- EU
endotoxin units
- HA
hemagglutinin
- IMAC
immobilized metal affinity chromatography
- IN
intranasal
- MBP
maltose or maltodextrin-binding protein
- pfMBP
Pyrococcus furiosus maltodextrin-binding protein
- TLR
toll-like receptor
- TST
thermostable tag
Footnotes
Conflict of interest statement
JML, AEC, CPH and JAW have an equity interest in Nature Technology Corporation.
References
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV,Fort FL,Osterman AL,Didonato JA,Feinstein E,Gudkov AV, 2008. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Kristensen D, 2009. Opportunities and challenges of developing thermostable vaccines. Expert Rev. Vaccines 8, 547–557. [DOI] [PubMed] [Google Scholar]
- Chiu FF, Venkatesan N, Wu CR, Chou AH, Chen HW, Lian SP, Liu SJ, Huang CC, Lian WC, Chong P, Leng CH, 2009. Immunological study of HA1 domain of hemagglutinin of influenza H5N1 virus. Biochem. Biophys. Res. Commun. 383, 27–31. [DOI] [PubMed] [Google Scholar]
- de Marco A, Casatta E, Savaresi S, Geerlof A, 2004. Recombinant proteins fused to thermostable partners can be purified by heat incubation. J. Biotechnol. 107, 125–133. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Palmer DR, Simmons M, Sun P, Bisbing J, McClain S, Mani S, Burgess T, Gunther V, Sun W, 2007. Potential role for Toll-like receptor 4 in mediating Escherichia coli maltose-binding protein activation of dendritic cells. Infect. Immun. 75, 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JD, Routzahn KM, Bucher MH, Waugh DS, 2003. Maltodextrin-binding proteins from diverse bacteria and archaea are potent solubility enhancers. FEBS Lett. 537, 53–57. [DOI] [PubMed] [Google Scholar]
- Fox JD, Waugh DS, 2003. Maltose-binding protein as a solubility enhancer. Methods Mol. Biol. 205, 99–117. [DOI] [PubMed] [Google Scholar]
- Gerdts V, Mutwiri GK, Tikoo SK, Babiuk LA, 2006. Mucosal delivery of vaccines in domestic animals. Vet. Res. 37, 487–510. [DOI] [PubMed] [Google Scholar]
- Honko AN, Mizel SB, 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 72, 6676–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honko AN, Sriranganathan N, Lees CJ, Mizel SB, 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74, 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Liu J, de Marco A, 2006. Induced fit of passenger proteins fused to Archaea maltose binding proteins. Biochem. Biophys. Res. Commun. 344, 25–29. [DOI] [PubMed] [Google Scholar]
- Huleatt JW, Jacobs AR, Tang J, Desai P, Kipp EB, Huang Y, Song L, Nakaar V, Powell TJ, 2007. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25, 763–775. [DOI] [PubMed] [Google Scholar]
- Kink JA, Williams JA, 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect. Immun. 66, 2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahira A, Das P, Chakravortty D, 2008. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine 26, 6777–6783. [DOI] [PubMed] [Google Scholar]
- Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE, 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel SB, Graff AH, Siranganathan N, Ervin S, Lees CJ, Lively MO, Hantgan RR, Thomas MJ, Wood J, Bell B, 2009. A fusion protein, flagellin/F1/V, is an effective plague vaccine in mice and two species of nonhuman primates. Clin. Vaccine Immunol. 16, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KL, Mian MF, Lauzon NM, Gyles CL, Lichty B, Mackenzie R, Gill N, Ashkar AA, 2008. Cutting edge: FimH adhesion of type 1 fimbriae is a novel TLR4 ligand. J. Immunol. 181, 6702–6706. [DOI] [PubMed] [Google Scholar]
- Murthy KGK, Deb A, Goonesekera S, Szabo C, Salzman AL, 2004. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J. Biol. Chem. 279, 5667–5675. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Silber KR, Sauer RT, 1990. Carboxy-terminal determinants of intracellular protein degradation. Genes Dev. 4, 277–286. [DOI] [PubMed] [Google Scholar]
- Ramos HC, Rumbo M, Sirard JC, 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12, 509–517. [DOI] [PubMed] [Google Scholar]
- Rebl A, Goldammer T, Seyfert HM, 2010. Toll-like receptor signaling in bony fish. Vet. Immunol. Immunopathol. 134, 139–150. [DOI] [PubMed] [Google Scholar]
- Ren X, Yu D, Yu L, Gao G, Han S, Feng Y, 2007. A new study of cell disruption to release recombinant thermostable enzyme from Escherichia coli by thermolysis. J. Biotechnol. 129, 668–673. [DOI] [PubMed] [Google Scholar]
- Singleton TE, Massari P, Wetzler LM, 2005. Neisserial porin-induced dendritic cell activation is myd88 and TLR2 dependent. J. Immunol. 174, 3545–3550. [DOI] [PubMed] [Google Scholar]
- Skountzou I, Martin MD, Wang B, Ye L, Koutsonanos D, Weldon W, Jacob J, Compans RW, 2010. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine 28, 4103–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, Jacobs A, Liu G, Huang Y, Desai P, Maksymiuk G, Takahashi V, Umlauf S, Reiserova L, Bell R, Li H, Zhang Y, McDonald WF, Powell TJ, Tussey L, 2008. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS ONE 3, e2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen HP, Mortensen KK, 2005. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 115, 113–128. [DOI] [PubMed] [Google Scholar]
- Trepod CM, Mott JE, 2000. Modification of the carboxy-terminal amino acid sequence alters the Escherichia coli expression of a gene encoding multiple repeats of a bovine growth hormone releasing factor analog. J. Biotechnol. 84, 273–284. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT, 2008. Flagellin treatment protects against chemicals, bacteria, viruses and radiation. J. Immunol. 180, 8280–8285. [DOI] [PubMed] [Google Scholar]
- Williams JA, Langeland JA, Thalley B, Skeath JB, Carroll SB, 1994. Production and purification of polyclonal antibodies against proteins expressed in E. coli In: DNA Cloning: Expression Systems. IRL Press. [Google Scholar]
- Xu Y, Foong FC, 2008. Characterization of a cellulose binding domain from Clostridium cellulovorans endoglucanase-zylanase D and its use as a fusion partner for soluble protein expression in Escherichia coli. J. Biotechnol. 135, 319–325. [DOI] [PubMed] [Google Scholar]
- Zimmerman T, Frère CP, Satzger M, Raba M, Weisbach M, Döhn K, Popp A, Donzeau M, 2006. Simultaneous metal chelate affinity purification and endotoxin clearance of recombinant antibody fragments. J. Immunol. Methods 314, 67–73. [DOI] [PubMed] [Google Scholar]
- Zou Z, Cao L, Zhou P, Su Y, Sun Y, Li W, 2008. Hyper-acidic protein fusion partners improve solubility and assist correct folding of recombinant proteins expressed in Escherichia coli. J. Biotechnol. 135, 333–339. [DOI] [PubMed] [Google Scholar]