Abstract
Previous research has found lower serum levels of dehydroepiandrosterone (DHEA) or its sulfated form, DHEA-S, in women diagnosed with Hypoactive Sexual Desire Disorder (HSDD). Given that DHEA and DHEA-S have multiple direct actions on the brain as well as anti-glucocorticoid properties, it is possible that lower levels of DHEA directly impact women’s sexual functioning. To date, the significance of the lower DHEA levels remains unclear. To our knowledge, there has been no empirical study of stress hormones as markers of HPA dysregulation in women with HSDD. To attend to this gap, the present study utilized several measures of HPA axis function – morning and evening cortisol and DHEA, the cortisol awakening response (CAR), diurnal cortisol slope, and cortisol:DHEA ratio – and examined their relationship with sexual functioning in N=275 women with (n=137) and without (n=138) HSDD. Results demonstrated multiple hormonal markers of HPA dysregulation in women diagnosed with HSDD compared to control participants, specifically, lower AM cortisol and AM DHEA levels, a flatter diurnal cortisol slope, and a lower CAR. Overall, results of the present study indicate that persistently low sexual desire in women is associated with HPA axis dysregulation, with both cortisol and DHEA alterations potentially detrimental to sexual desire.
Keywords: Dehydroepiandrosterone, Cortisol, Sexual dysfunction, Sexual desire, Hypoactive sexual desire disorder
1. Introduction
The experience of distressing low sexual desire (termed Hypoactive Sexual Desire Disorder [HSDD] in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders; DSM-IV-TR; American Psychiatric Association, 2000) occurs in approximately 10%–14% of women (West et al., 2008; Lutfey et al., 2009; Mitchell et al., 2009). Studies have explored possible roles for testosterone and dihydrotestosterone in promoting or mediating sexual desire (see Korkidakis and Reid, 2017 for a review), however, no significant association between these androgenic hormones and sexual desire has typically been observed (Davis et al., 2005; Santoro et al., 2005; Cappelletti and Wallen, 2016; Jones et al., 2018). This lack of association has been supported in recent studies utilizing newer more sensitive and accurate methods, including mass spectrometry (Owen et al., 2016) to assay testosterone (Basson, 2010; Wåhlin-Jacobsen et al., 2015, 2017) and to measure androgen metabolites (Labrie et al., 2017), such as androstene glucuronide (ADT-G) (Adler 2008; Basson et al., 2010; Wåhlin-Jacobsen et al., 2015, 2017). There is variable support for estrogen levels influencing desire in premenopausal women (Roney and Simmons, 2013; Grebe et al., 2016; Jones et al., 2018). Despite the marked reduction of estrogen post menopause, the prevalence of disorders of low sexual desire does not increase (West et al., 2008). Data from a large study on sexual ideation, sexual function, and sexual problems showed no difference between women with and without past bilateral oophorectomy (Erekson et al., 2012). Ideation was chosen, as having thoughts about sex is less likely to be affected by contextual details, such as the sexual relationship, than is sexual function or motivation for partnered sex. In addition, research has generally shown negative effects of progesterone on desire (Jones et al., 2018).
In contrast to the findings on testosterone and estrogen, many studies have found lower serum levels of dehydroepiandrosterone (DHEA) in women reporting low or absent sexual desire (e.g., Davis et al., 2005; Basson, 2010; Randolph et al., 2015; Wåhlin-Jacobsen et al., 2015). DHEA and its sulfate ester (DHEA-S) are the most abundant steroid hormones in women (and in men), with adult levels declining progressively by some two-thirds between the ages of 30 and 70 (Kiechl et al., 2000). Adrenal glands produce DHEA along with cortisol in response to stress. Long-term stress, especially in childhood, can lead to hypothalamic-pituitary-adrenal (HPA) dysregulation in adult life (Kuras et al., 2017), with abnormal basal and stress levels of the HPA hormones, including cortisol and DHEA. Specifically, dysregulation in cortisol activity has been observed in individuals reporting a history of childhood sexual abuse (CSA; e.g., Trickett et al., 2010). Research has shown that the degree of cortisol decrease in response to sexual stimuli is associated with sexual desire (Hamilton et al., 2008; van Anders, 2012) and that CSA survivors typically demonstrate a smaller decrease in cortisol during sexual arousal than the non-sexually abused (Rellini et al., 2009). In contrast, the significance of low DHEA in women with low desire is unclear but an etiological role is scientifically plausible. In addition to being a prohormone for the intracellular production of testosterone and estrogen, DHEA is known to have multiple direct actions, including the modulation of various receptors and synaptic transmission in the brain where its concentration can be six times higher than in serum (Dong and Zheng, 2012; Pluchino et al., 2015). Previous findings that distressing low sexual desire (i.e., HSDD) is associated with lower serum DHEA levels, but similar serum levels of estrogen, testosterone and androgen metabolites compared to controls, suggests that any etiological role of low DHEA levels in HSSD is likely through non-androgenic mechanisms, possibly via DHEA’s known but minimally researched actions on the brain. Moreover, low DHEA may simply or additionally be a marker of past chronic stress or adversity in youth and the past adversity then plays a major role in adult sexual dysfunction. Further, it is possible that low DHEA is a marker of HPA dysregulation. Importantly, DHEA has anti-glucocorticoid properties and thus may counteract detrimental effects of stress (Kaminska et al., 2000). Indeed, as cortisol has many neurotoxic, and DHEA many neuroprotective, actions, it has been suggested that any disruption of the dynamic balance of the two may increase risk to mental and physical health (Kamin and Kertes, 2017).
The HPA hormones have a diurnal pattern of secretion such that cortisol levels are high at awakening, reach a peak 30 min after awakening and then decrease throughout the day to their lowest levels at approximately midnight (Edwards et al., 2001). Currently the most common measures of this diurnal rhythm are the cortisol awakening response (CAR), which reflects the rise in cortisol during the first 30 min after awakening (Clow et al., 2004), and the diurnal cortisol slope, which is the difference in cortisol levels from morning to evening (Adam and Kumari, 2009). A flattened CAR and/or a flattened diurnal cortisol slope are the most consistent markers of HPA axis dysfunction. For example, adults who experienced childhood adversity have lower morning cortisol (Kuras et al., 2017), a blunted cortisol diurnal slope (van der Vegt et al., 2009; Kuras et al., 2017), and lower cortisol levels throughout the day (van der Vegt et al., 2009). Research into a possible etiological role of cortisol in women with low desire is limited. Within-subject changes in desire have not been found to correlate with cortisol in non-clinical samples of women (Grebe et al., 2016; Jones et al., 2018). It is possible that lower cortisol levels might well simply reflect past chronic stress and adversity that in turn may have limited healthy development of sexual desire.
While the adverse effects of chronic or repeated elevations in cortisol are well documented, fewer studies have investigated the relationship between prolonged psychological stress and DHEA-S or DHEA levels (Yehuda et al., 2006). One relatively large study of patients with clinical ‘burnout’ who met the criteria for “stress-related exhaustion” (Jeckel et al., 2009) found DHEA-S levels to be some 25% lower than in controls (Lennartsson et al., 2015). However, evidence that DHEA may oppose or offset the actions of cortisol has stimulated interest in examining both DHEA levels and the cortisol:DHEA ratio as an alternative index of adrenocortical function and the net effects of cortisol (Kamin and Kertes, 2017). Investigation of the cortisol:DHEA ratio demonstrated that higher cortisol:DHEA ratios may be seen in non-medicated depressed patients compared to controls (Young et al., 2002), and that higher morning cortisol:DHEA ratios predict shorter time to recurrence of major depressive disorder (Mocking et al., 2015). In addition, the cortisol:DHEA ratio was found to increase in association with work days compared to non-work days (Kim et al., 2010), numbers of stressful life events in the previous year (Heaney et al., 2014), and living with HIV (Qiao et al., 2017).
To our knowledge, there has been no empirical study of stress hormones as markers of HPA dysregulation in women diagnosed with HSDD. The present study was designed to fill this gap. Building on our previous research that found lower morning DHEA in non-depressed women diagnosed with HSDD (Basson et al., 2010), the present study extended our measures of HPA axis function – morning and evening cortisol and DHEA, the CAR, diurnal cortisol slope, and cortisol:DHEA ratio. We examined the relationship between sexual functioning, measured both categorically and continuously (i.e. on a continuum/scale), and HPA function/regulation. We hypothesized that the HSDD group would demonstrate more evidence of HPA axis dysregulation compared to the control group. Additionally, given evidence of a relationship between sexual assault history and cortisol dysregulation (e.g., Rellini, 2008), we expected that sexual assault history would impact CAR in both control and HSDD subjects.
2. Methods
2.1. Participants & procedure
Eligible participants were required to be between the ages of 19 and 65, have no major medical illnesses known to impact sexual functioning, be non-smokers/non-drug users, not have a current diagnosis of depression, not be using medications with known side-effects on sexual functioning (e.g., antidepressants), topical or oral DHEA products, or hormonal contraception or menopausal hormone therapy. Additionally, participants were excluded if they reported a body mass index (BMI) of less than 18.5 or higher than 29.9 or current pregnancy. We also excluded women for whom their low desire symptoms were entirely attributed to daily stress or to severe relationship discord. Finally, women were excluded if they reported pain during penetrative sex that was not relieved by an external lubricant or reported that low desire had been present for less than one year.
Participants were recruited via posting advertisements online (i.e., Craigslist, university paid studies list, hospital research institute list-serves), in local newspapers, and on flyers throughout the community. Primary care providers accepting patients with sexual health concerns were also made aware of the study and encouraged to post an ad in their clinics. Advertisements initially targeted women with and without sexual concerns. At a later date, advertisements were posted to target women with sexual desire concerns specifically asking: “Are you a woman between the ages of 19–65 who experiences low or absent sexual desire?”
Women who responded to advertisements were scheduled to speak over the phone to a trained research assistant. During their initial phone screen interview, prospective participants were assessed for operational HSDD criteria and asked a series of questions assessing inclusion and exclusion criteria. In addition to meeting the single criterion for HSDD according to the DSM-IV-TR (American Psychiatric Association, 2000) which was “lack of interest in sexual activity and reduced or absent sexual thoughts/fantasies” prospective low sexual desire participants were also required to endorse that these symptoms were causing significant personal or interpersonal distress.
Contact information was collected from participants who met inclusion criteria and they were emailed and asked to review the consent form for the full study. If, after reviewing the consent form, the respondent still wished to participate in the study, an in-person meeting with a research assistant was scheduled to review the study procedures in more detail. During the meeting, participants were provided with a saliva sampling kit, a saliva collection log form, a checklist, detailed instructions for saliva collection procedures, and a link to an instructional video.
Participants were instructed to collect saliva samples at home through the passive drool method. Briefly, participants were instructed to allow saliva to pool in the mouth and deposit it (guided through a straw) into 1.6 ml vials at four time points in the diurnal cycle: at awakening, 30 min and 60 min after waking, and immediately before bedtime, on three separate, typical weekdays (note: collection days were not required to be consecutive). To prevent sample contamination, participants were asked to avoid consuming foods (on sample collection days) that may impact hormone levels of interest to this study, such as chocolate, alcohol, and caffeine, and to avoid eating, brushing teeth, flossing teeth, consuming beverages (other than water), using mouthwash, chewing gum, or eating a large meal within an hour of collecting a saliva sample. Participants were also asked to perform a cold-water rinse prior to all saliva sample collections except upon waking. Samples were briefly stored in the participant’s freezer (at approximately −15 °C) until sample collection was completed and then were transported to the Weinberg laboratory for analysis.
Following her in-person meeting, each participant was emailed a link to an on-line battery of questionnaires which she was asked to complete at a time of her choosing. After completing her on-line questionnaire and saliva collection, each participant was invited back to the lab to meet with a research assistant trained in diagnostic interviewing for completion of the Decreased Sexual Desire Screener (DSDS; Clayton et al., 2009). During this in-person meeting, the portion of the Structured Clinical Interview for DSM-IV Axis 1 disorders (nonpatient version; (SCID-I/NP) that assesses major depressive disorder was also administered to ensure participants were not experiencing depression (despite not having a formal diagnosis).
A total of 856 women expressed interest in the study. Following the initial screening stage, 485 women were either found to be ineligible, or did not follow through with subsequent procedures. The most common reason for ineligibility was presence of pain during intercourse that was not relieved by an external lubricant. Of the 371 women who met inclusion criteria and agreed to participate, 96 withdrew from participation prior to completing all components of the study. The most common reason for participant withdrawal was increased life demands. Participants who withdrew from the study prior to submitting their saliva samples were not included in analyses, leaving a total N = 275 for the present analyses.
The sample was comprised of a control group (n = 138), which consisted of healthy women with no indications of sexual dysfunction, and an experimental group (n = 137), which consisted of women who met diagnostic criteria for HSDD. Groups were compared on several demographic variables including: age, ethnicity, highest education level, sexual orientation, relationship status, and relationship duration. No significant group differences on any of these demographic variables was observed (all p’s > .05; Table 1).
Table 1.
Participant Demographic Characteristics for women with Hypoactive Sexual Desire Disorder (HSDD) and Healthy Controls.
| HSDD (n = 137) | Control (n = 138) | ||||
|---|---|---|---|---|---|
| Variable | M | SD | M | SD | |
| Age | 33.01 | 11.68 | 31.81 | 12.05 | |
| RelationshipDuration (months) | 68.55 | 97.73 | 50.90 | 88.48 | |
| n | % | n | % | ||
| Race/Ethnicity | |||||
| Euro-Caucasian | 83 | 60.6 | 81 | 58.7 | |
| East Asian | 25 | 18.2 | 35 | 25.4 | |
| South Asian | 9 | 6.6 | 9 | 6.5 | |
| First Nations | 2 | 1.5 | 1 | 0.7 | |
| Middle Eastern | 5 | 3.6 | 3 | 2.1 | |
| African-Canadian | 2 | 1.5 | 1 | 0.7 | |
| Other | 11 | 8.0 | 8 | 5.8 | |
| Education | |||||
| Highschool | 13 | 9.5 | 25 | 18.1 | |
| College/Technical/Trade School | 24 | 17.5 | 19 | 13.8 | |
| Undergraduate Degree | 55 | 40.1 | 55 | 39.9 | |
| Masters Degree | 29 | 21.2 | 23 | 16.7 | |
| Doctoral Degree | 6 | 4.4 | 9 | 6.5 | |
| Other | 10 | 7.3 | 7 | 5.1 | |
| Employment Status | |||||
| Full-Time | 54 | 39.4 | 50 | 36.2 | |
| Part-Time | 22 | 16.1 | 21 | 15.2 | |
| Self-Employed | 6 | 4.4 | 8 | 5.8 | |
| Unemployed | 2 | 1.5 | 1 | .7 | |
| Retired | 5 | 3.6 | 2 | 1.4 | |
| Student | 39 | 28.5 | 52 | 37.7 | |
| Homemaker | 3 | 2.2 | 1 | 0.7 | |
| Other | 6 | 4.4 | 3 | 2.2 | |
| Sexual Orientation | |||||
| Heterosexual | 115 | 83.9 | 114 | 82.6 | |
| Lesbian | 4 | 2.9 | 4 | 2.9 | |
| Bisexual | 12 | 8.8 | 15 | 10.9 | |
| Other | 6 | 4.4 | 5 | 3.6 | |
| Relationship Status | |||||
| Partnered | 105 | 76.6 | 94 | 68.12 | |
| Not Partnered | 32 | 23.4 | 44 | 31.89 | |
Note: Demographic variables did not differ significantly by group.
2.2. Measures
2.2.1. Demographics
In addition to collecting the demographic information listed above, participants were asked background questions including: household income, employment status, number of children, number of pregnancies, number of deliveries, height/weight, menopausal status, medical and medication history, and sexual history (treatment history, age of first intercourse, experiences of non-consensual sexual contact, and frequency of sexual activity in past month).
2.2.2. Diagnostic interview of HSDD
For the purposes of group placement (i.e., HSDD vs. control), the Decreased Sexual Desire Screener (DSDS) was administered to each participant, in person. The DSDS is 5-item clinician administered diagnostic brief screener for generalized acquired HSDD in women (Clayton et al., 2009). Participants were presented with a set of four yes/no questions related to sexual desire (e.g., level of, satisfaction with). When all four items were endorsed, a fifth question was asked for the purpose of ruling out potentially confounding causes for decreased desire (i.e., medical illness, relationship factors, medications, obstetric or gynecological factors, or stress and/or fatigue). When respondents answered “yes” to questions 1–4 and “no” to all factors in question five, she received a diagnosis of HSDD. Respondents who answered “yes” to items 1–4 and “yes” to factors in item five also received a diagnosis of HSDD if a further assessment showed those factors did not point to another primary diagnosis. The DSDS shows 85.2% diagnostic accuracy and high sensitivity and specificity, with point estimates of .84 and .88, respectively (Clayton et al., 2009).
2.2.3. Continuous measure of sexual functioning
To obtain a continuous measure of sexual functioning, two items from the Sexual Interest and Desire Inventory-Female (SIDI-F; Clayton et al., 2006) were summed. Two items—(1) Desire (Over the past month, how frequently and how strongly have you wanted to engage in some kind of sexual activity, either with or without a partner? (one score) and (2) Distress (Over the past month, when you thought about sex or were approached for sex, how distressed (worried, concerned, guilty) were you about your level of desire?)—were chosen on the basis that they map directly onto the HSDD diagnostic criteria. The possible total range was from 0 to 9, with lower scores indicating a lower level of sexual functioning. Cronbach’s alpha for the two items was 0.77 in the present sample.
2.3. Salivary cortisol and DHEA
Measures of HPA axis regulation included morning (AM) and evening (PM) cortisol levels, diurnal cortisol slope, AM and PM DHEA levels, the cortisol:DHEA ratio, and the cortisol awakening response (CAR). As cortisol levels at 60 min post awakening are considered the true morning levels (Clow et al., 2004), salivary cortisol concentrations at 60 min post-waking, averaged across the three days, were used in the analyses to reflect AM cortisol levels. PM cortisol concentrations, averaged across three days, were used in the analyses for evening cortisol levels. Cortisol slope was calculated by subtracting mean PM cortisol levels from mean AM (60-minute post-waking measure) cortisol levels. The CAR was assessed by measuring cortisol levels and pattern of secretion at awakening and at 30 min and 60 min post-awakening and by calculating area under the curve.
To assay salivary cortisol concentrations, samples were vortexed and centrifuged at 1400 g for 10 min at 18 °C. Salivary cortisol was measured using the commercially available High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics Assays, 1–3002, State College, PA) according to the standard protocol. The minimum amount of saliva required by this assay is 25 μl, and intra- and inter-assay coefficients of variation were 4.6% and 6.0%, respectively.
To measure salivary DHEA concentrations, aliquots from each of the morning saliva samples (awakening, 30 min and 60 min post-awakening) were pooled to provide an average AM DHEA level on each day [as there is no DHEA awakening response comparable to the cortisol awakening response (Clow et al., 2010)], and values were then averaged across the three days. AM and PM saliva samples were vortexed and centrifuged at 1400 g for 10 min at 18 °C. Salivary DHEA was measured using the commercially available Salivary DHEA Enzyme Immunoassay Kit (Salimetrics Assays, 1–1202; State College, PA) according to the standard protocol. The minimum amount of saliva required by this assay is 50 μl. Intra- and inter-assay coefficients of variation were 5.6% and 8.2%, respectively. For both the cortisol and DHEA assays, plates were read at 450 nm (secondary filter correction at 490 nm) using an ELX808 ultra microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
2.4. Data analytic plan
Analyses were designed a priori and intended to examine the relationship between sexual functioning in women (measured categorically and continuously) and HPA axis regulation. To compare levels of HPA regulation between groups (i.e., HSDD vs. control), Independent Samples t tests were carried out on each hormone measure. In cases wherein HPA axis regulation showed unequal variance (AM DHEA and PM Cortisol), Welch’s t-test was conducted. We also examined sexual functioning measured continuously and its relationship to HPA axis regulation using simple linear regressions. To examine the relationship between CAR and sexual functioning, we carried out a Between-Within Repeated Measures Analysis of Variance (ANOVA) with group as the between-subjects’ factor and time as the within-subjects’ factor. Due to the presence of mild heteroskedasticity, a linear mixed effects model was carried out for comparison. Area under the curve, using the trapezoid rule (GraphPad Prism 7, La Jolla CA) was also computed.
3. Results
3.1. Salivary cortisol levels
When sexual functioning was measured categorically, a significant relationship between morning cortisol and sexual functioning was observed, with HSDD participants (M =8.20 nmol/L, SD=4.25) showing lower, on average, AM cortisol levels compared to control participants (M =9.36 nmol/L, SD=4.06), t(273)=2.31, p = .02 (Fig. 1). When sexual function was measured continuously, no significant relationship with AM cortisol was observed (see Table 2). AM cortisol levels explained only 1% of the proportion of variance in the continuous measure of sexual functioning.
Fig. 1.
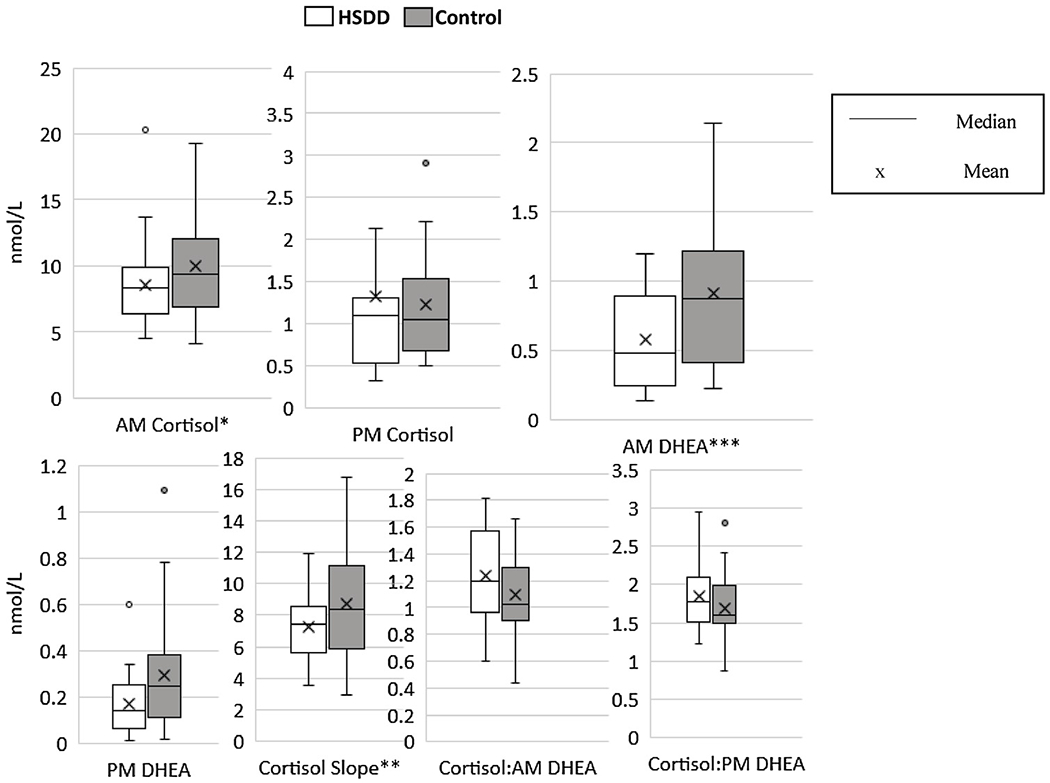
Measures of HPA axis regulation compared by group.
* p < .05; ** p < .01; *** p = .001.
Table 2.
Summary of Continuous Measure of Sexual Functioning Regressed onto Measures of HPA Axis Functioning.
| Variable | B | SE B | t | P | R2 |
|---|---|---|---|---|---|
| AM Cortisol | .06 | .03 | 1.87 | .06 | .01 |
| PM Cortisol | −.08 | .07 | −1.18 | .24 | .01 |
| Cortisol Slope* | .08 | .03 | 2.48 | .01 | .02 |
| AM DHEA* | .59 | .24 | 2.47 | .01 | .02 |
| PM DHEA | .42 | .51 | .82 | .42 | .00 |
| Cortisol:AM DHEA | −.17 | .40 | −.42 | .67 | .00 |
| Cortisol:PM DHEA | .23 | .34 | .63 | .53 | .00 |
p ≤ .01.
By contrast, the relationship between PM cortisol and sexual function did not reach statistical significance, irrespective of whether sexual functioning was measured categorically, t(273) = −1.00, p = .32 (Welch’s test; Fig. 1) or continuously (see Table 2).
3.2. Diurnal cortisol slope
There was a significant relationship between cortisol slope and sexual functioning, irrespective of whether sexual functioning was measured categorically or continuously. An Independent Samples t test revealed a significantly steeper cortisol slope in control participants (M =7.87 nmol/L, SD = 3.92) compared to HSDD participants (M = 6.44 nmol/L, SD = 4.41), t(273) = 2.85, p = .005 (Fig. 2). A simple linear regression revealed that cortisol slope significantly predicted scores on the continuous measure of sexual functioning; however the coefficient of determination indicated that cortisol slope explained less than 2% of the variance in this measure (see Table 2).
Fig. 2.
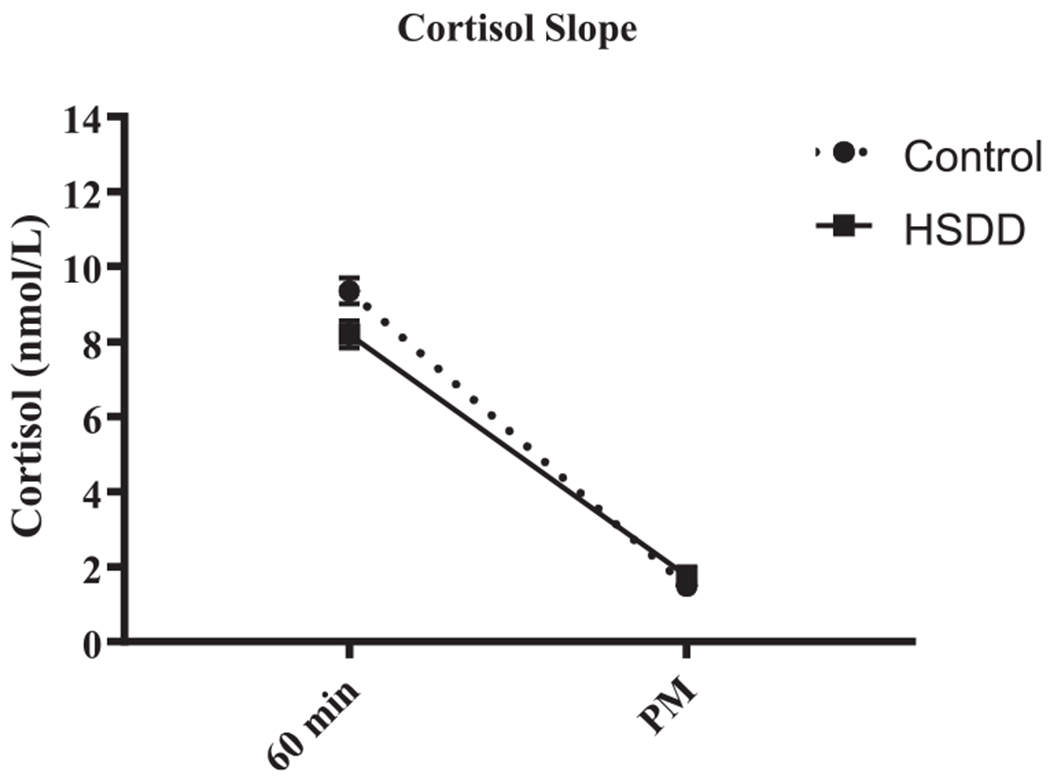
Salivary cortisol levels at 60 min after waking and in the evening (PM) are shown (mean ± SEM). Overall, the diurnal cortisol slope is flatter in HSDD compared to control participants (p < .005).
3.3. Cortisol awakening response (CAR)
To explore the relationship between sexual functioning and the CAR, a repeated measures ANOVA was carried out. Due to the presence of mild heteroskedasticity, a linear mixed effects model was run for comparison. Results obtained by fitting a linear mixed effects model to the data were essentially identical with the results obtained from the repeated measures ANOVA and thus, only the results of the linear mixed effects model are reported here.
Although the group x time interaction for the CAR was not significant, F(2, 544) = .87, p = .42, we found significant main effects of group, F(1, 272) = 5.35, p = .02, and time, F(2, 546) = 54.41, p < .001 (Fig. 3). Pairwise comparisons revealed that overall, mean cortisol levels at 30 min post-waking were higher by 2.51 nmol/L (SE = 0.26, p < 0.001) than levels upon waking, while mean cortisol levels at 60 min post-waking were not significantly different from waking levels (higher by 0.27 nmol/L; SE = 0.26, p = 0.3). Moreover, the average CAR for the HSDD group was smaller by 0.99 nmol/L (SD =0.43, p = .02) than that of the control group, with significant differences between groups at both 30 min (p = .04) and 60 min (p = .02), but not at awakening (p = .17; Fig. 3). The estimates of random effects were approximately normal with range (−6.81, 10.97), and an estimated standard deviation of the random effects across the respondents of 3.04. In support of the significant ANOVA findings, analysis of area under the curve for the CAR indicated that HSDD participants had a lower overall AUC compared to control participants, t(272) = 2.30, p = .02; Fig. 3.
Fig. 3.
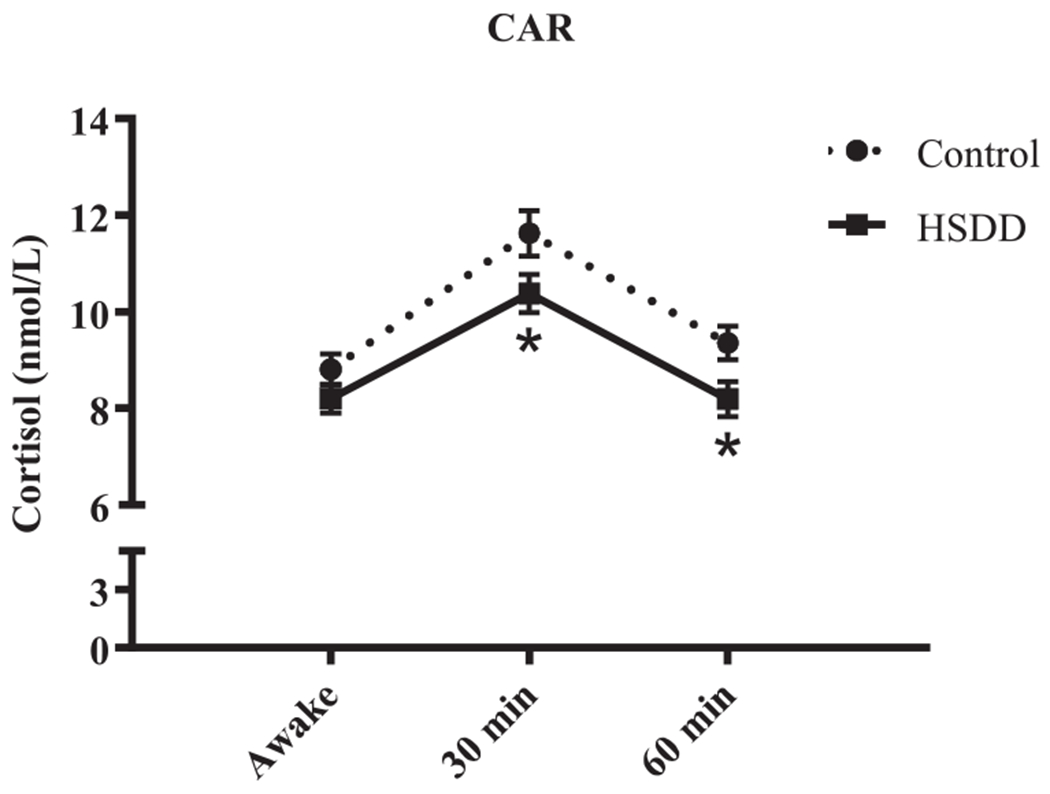
Cortisol awakening response (CAR). Salivary cortisol levels at awakening (awake), and 30 min and 60 min after waking are shown (mean ± SEM). The “*” indicates a significant post-hoc comparison to the control group at the indicated time point (p < .05). In addition, both the average CAR and area under the curve (AUC) are lower in HSDD compared to control participants (p < .05).
We next used an adjusted model, including a dichotomous variable of a yes/no indication of sexual assault as a covariate, to explore the association between self-reported history of sexual abuse and CAR. Analysis of the adjusted and unadjusted models yielded the same results; overall, the data indicate that women with a history of sexual assault showed a lower CAR (by .89 nmol/L, p = .05) than those with no history of sexual assault, independent of sexual functioning.
3.4. Salivary DHEA levels
Next, we explored the relationship between sexual functioning and DHEA levels. For AM DHEA, there was a significant relationship with sexual functioning measured categorically, with the HSDD group (M = .86 nmol/L, SD = .52) showing lower, on average, morning DHEA levels, compared to the control group (M =1.11 nmol/L, SD = .69; t (273) = 3.38, p = .001 (Welch’s test; Fig. 1). Similarly, when sexual functioning was measured continuously, lower levels of morning DHEA significantly predicted lower levels of sexual function; however, the coefficient of determination indicated that less than 2% of the proportion of variance in the continuous measure of sexual functioning was explained by morning DHEA levels (see Table 2).
When sexual function was measured categorically, the relationship with PM DHEA levels failed to reach statistical significance, t (273) = 1.50, p = .14 (Fig. 1). Likewise, PM DHEA levels were not significantly predictive of a continuous measure of sexual function (see Table 2).
Given the known impacts of age (e.g., Kiechl et al., 2000) and BMI (e.g., Mazza et al., 1999) on DHEA levels, all analyses were re-run to control for age and BMI. The results adjusted for age and BMI did not differ from the unadjusted results.
3.5. Cortisol:DHEA ratio
To examine the relationship between sexual functioning and the cortisol:DHEA ratio, we calculated values to represent both the AM cortisol:DHEA and the PM cortisol:DHEA ratios as per Young et al., 2002. The value for the AM cortisol:DHEA ratio was calculated by dividing the mean (across three days) AM (60-min post-waking) cortisol levels by the mean AM DHEA (averaged across awakening, 30 min post- and 60 min post-awakening time points per day, and this measure then averaged across the three days), levels. Similarly, for the PM cortisol:DHEA ratio, we divided the mean (across 3 days) PM cortisol levels by the mean (across three days) PM DHEA levels. Due to significant skewness in both the AM cortisol:DHEA (15.84, SE = .15) and PM cortisol:DHEA (8.10, SE = .15) ratios, log base 10 transformations were conducted to normalize the distributions.
An Independent Samples t test was conducted to explore the relationship between sexual functioning, measured categorically, and the AM cortisol:DHEA ratio. No significant difference between the HSDD (M =1.00nmol/L, SD = .34) and control (M =.99nmol/L, SD = .38) participants, t(273) = −.40, p = .69 was indicated; Fig. 1. Similarly, no significant relationship between sexual functioning measured continuously and AM cortisol:DHEA was observed (see Table 2). Consistent with these findings, there were no significant group differences in the PM cortisol:DHEA ratio between the HSDD and control participants, regardless of whether sexual functioning was measured categorically [HSDD (M =1.45 nmol/L, SD = .43), control (M =1.47nmol/L, SD = .41; t(273) = .29, p = .77); Fig. 1] or continuously (see Table 2).
4. Discussion
In this study we have identified, for the first time, multiple hormonal markers of HPA dysregulation in women diagnosed with low sexual desire (i.e., HSDD). We found that compared to control participants, women with HSDD had lower AM cortisol and AM DHEA levels, a flatter diurnal cortisol slope and a lower CAR. This study also found that women with a history of sexual assault had a lower CAR regardless of their current level of sexual desire. Overall, the present study demonstrates that in women, persistently low sexual desire causing distress is associated with HPA axis dysregulation.
4.1. Low cortisol and HSDD
The finding that cortisol levels were lower in women with HSDD than in controls is not unexpected. Cortisol secretion is increased by acute stress, and in the short term, high levels of cortisol enable the individual to respond to and cope with the stressor. However, HPA function can become dysregulated under conditions of chronic or prolonged stress (Egeland et al., 2015). For example, low basal cortisol levels have been reported in children following early life stress/trauma including sexual abuse (King et al., 2001), and maltreatment or bullying (Ouellet-Morin et al., 2011). A recent meta-analysis found an association between early-life adversity and blunted cortisol responses to social stress, with the greatest effects in adulthood (Bunea et al., 2017). Blunting of the cortisol response with prolonged stress is thought to be beneficial to the individual from a physiological perspective, as ongoing high cortisol levels could have serious adverse effects on multiple aspects of physiology, including immune and metabolic function (Fries et al., 2005). However, low basal levels and a blunted HPA response may predispose one to ‘stress-associated disorders’ such as post-traumatic stress disorder, atypical depression, fibromyalgia, chronic fatigue, and chronic pain syndromes (Heim et al., 2000; Roberts, 2004). This can be understood in the context of the “match-mismatch” model (Gluckman et al., 2007), which suggests that altered HPA regulation in early life might result in inappropriate responses to stressors encountered later in life. Thus, HPA alterations resulting in blunted HPA activity may be adaptive during exposure to ongoing early life adversity, but may became maladaptive in later life. For example, in a positive or nurturing environment, robust responses to stressors are needed to maintain physiological homeostasis or balance, and low cortisol levels or blunted HPA activity could result in increased vulnerability to diseases or disorders, including mental health problems (Daskalakis et al., 2013) and potentially including sexual disorders. Thus, while low cortisol in women with HSDD may simply reflect a past stressful life—the latter itself predisposing to low sexual desire—the resulting hormonal dysregulation may be an independent risk factor for HSDD.
4.2. Low DHEA and HSDD
Like cortisol, DHEA levels were also lower in women with HSDD than in controls. Studies have shown that DHEA and DHEA-S have neuroprotective, antioxidative, anti-inflammatory, and anti-glucocorticoid effects (Maninger et al., 2009), and thus DHEA may protect against the adverse effects of high cortisol levels/prolonged stress (Kamin and Kertes, 2017). Consistent with this, a high cortisol:DHEA ratio has been associated with chronic stress (Jeckel et al., 2009), depression (Young et al., 2002), and cognitive disorders (Ferrari et al., 2001). We did not replicate the abnormal cortisol:DHEA ratio previously reported in depression and other disorders, suggesting that, at least in relation to endocrine function/dysregulation, HSDD may be distinct from these disorders.
DHEA concentrations are known to be particularly high in the brain, and to be synthesized de novo in brain glial cells (Friess et al., 2000). Thus, in addition to providing one index of HPA axis dysregulation, low serum DHEA may reflect a similar cerebral DHEA deficit. Given that DHEA is known to have multiple direct actions, including the modulation of various receptors and synaptic transmissions in the brain (Pluchino et al., 2015), both the hormonal deficit and the past stress that it reflects may contribute to HSDD. Its action on sigmoid receptors to modulate mood is of particular interest in the context of low sexual desire. Even when clinical depression is excluded, negative mood may impair sexual function (West et al., 2008).
4.3. Diurnal cortisol slope and altered CAR in HSDD
Two additional indices of HPA dysregulation, a flattened diurnal cortisol slope reflecting a blunted circadian rhythm, and a lower CAR, were observed in women with HSDD compared to control participants. Because cortisol exerts widespread and potent influences throughout the brain and body, including metabolism, immune function, vascular reactivity, skeletal muscle function, secretion of other hormones, cognition/attention/alertness (Chrousos and Gold, 1992), and mood and sexual behavior (Sapolsky et al., 2000), a flatter slope could have broad implications for physical and mental health and psychological wellbeing. Indeed, a recent review and meta-analysis provides evidence that reduced diurnal variation in cortisol is related to a wide range of negative health outcomes (Adam et al., 2017), including worse overall health and worse outcome for immune dysregulation, fatigue, cancer, obesity/BMI/adiposity, externalizing and internalizing symptoms, and depressive symptoms, plus increased mortality. These findings have important implications for understanding possible links between HPA dysregulation, psychosocial stress, and mental and/or physical health outcomes in women with HSDD.
While the specific functional implications of the CAR are still not fully understood, it has been proposed that the CAR may have an important role in a number of critical awakening-induced processes (e.g., consciousness, alertness, and cognitive function), as well as changes in other hormones and in immune system balance, mobilization of motor function (Clow et al., 2010), and an individual’s orientation about self in time and space as well as anticipation of the demands of the day (Fries et al., 2009; Wilhelm et al., 2007). Moreover, depending on those anticipated demands, the magnitude of the CAR may vary (Clow et al., 2010; Fries et al., 2009). Blunting of the CAR, as observed in the present study, would thus be detrimental to physical, physiological, and behavioral functioning.
Of note, all aspects of the HPA axis are dynamic and may vary with the timing and methodology of measurement. Recently identified are different patterns of abnormality of cortisol secretion depending on whether short or long-term measures are examined in depression (Herane-Vives et al., 2018). This is of relevance to our findings here and suggests that a definitive characterization of the endocrine aspects of HSDD requires further study.
4.4. Cortisol awakening response and self-reported history of sexual assault
Our prediction that, irrespective of current sexual functioning, a history of sexual assault would be related to the CAR was supported. Women with a self-reported history of sexual assault had a lower CAR overall than women with no self-reported history of sexual assault. This finding is consistent with meta-analyses showing that individuals with a history of PTSD and sexual abuse have a lower CAR (Chida and Steptoe, 2009). Of note, a blunted CAR has been observed in individuals with cardiovascular, autoimmune, atopic, allergic, and psychiatric disorders, particularly depression, compared to healthy controls (Stetler and Miller, 2005). However, an increased CAR has been observed in association with low socioeconomic status and in individuals reporting chronic stress, work overload, and social stress, although data have not been entirely consistent (reviewed in Fries et al., 2009). Further research is needed to understand more fully the relationship between the CAR and life stressors/childhood adversity.
Of note, morning cortisol was related to sexual desire when women were classified as HSDD or control, but not when sexual desire was measured continuously as it was with AM DHEA. Given the possibility that there is a ‘normal’ distribution of levels of desire, with a somewhat arbitrary cut off, this difference is interesting. This finding suggests that further research should examine women’s low sexual desire both categorically and continuously in order to obtain a more complete picture of the issue.
4.5. Clinical implications of findings
These findings support the need for inquiry into past non-sexual as well as sexual experiences when assessing sexual desire. Learned adaptive strategies that formerly helped in dealing with a stressful life situation may become maladaptive for adult (sexual) life. Cognitive therapies are the mainstay of treatment for low desire, both traditional cognitive behavioral therapy (CBT) (Günzler and Berner, 2012) and more recently mindfulness-based cognitive therapy (MBCT) (Brotto and Basson, 2014; Paterson et al., 2017). Given the potential direct contribution of HPA dysregulation to HSDD etiology, research into therapies additional to behavioral therapy in women with sexual desire disorder is needed. In terms of hormonal therapy, traditional menopausal hormone therapy focuses on estrogen (and progesterone). A recent review of studies over the past four decades concludes that estrogen therapy to peri-ovulatory levels can increase desire (Cappelletti and Wallen, 2016). However current updated guidelines from the International Society for Sexual Medicine state that data do not support the use of systemic estrogen for any postmenopausal sexual dysfunction adding that vaginal estrogen’s benefit to dryness and dyspareunia may increase sexual motivation (Santoro et al., 2016). A later study suggests that transdermal, rather than oral route, may benefit low sexual desire (Taylor et al., 2017), but only on a temporary basis.
Although testosterone deficiency has not been demonstrated in women diagnosed with sexual dysfunction, randomized trials of testosterone therapy have been conducted, mostly in postmenopausal women. The eligibility criteria for these trials did not meet either former (HSDD) or current (Sexual Interest/Arousal Disorder -SIAD) diagnostic criteria for a sexual disorder and showed conflicting results. Testosterone was given in the form of a transdermal patch in one series of studies and a gel in a later series. While delivery of 300 but not 150 or 450 μg/day via patch showed modest improvement— ‘satisfactory sexual encounters’ increased from some 2–3 per month to 5 per month in women on active drug and to 4 per month in women receiving placebo (Braunstein et al., 2005)—when 300 μg/day was delivered by gel it was without benefit (Snabes et al., 2012). Recent review concludes that only supraphysiological levels of testosterone affect desire (Cappelletti and Wallen, 2016). However, given DHEA’s multiple non-androgenic actions, it is possible that DHEA supplementation may be of some benefit. While reviews of DHEA supplementation have not supported its use to increase sexual desire, recruitment has not focused on women diagnosed with sexual disorder with or without relatively low serum DHEA (Panjari and Davis, 2007; Genazzani et al., 2011). Recent research revealed benefits to genital sexual sensitivity reduced by oral contraceptives from 50 mg DHEA daily (van Lunsen et al., 2018). Clinical trials of recently FDA-approved vaginal DHEA allowing local conversion to testosterone and estrogen without their systemic increase, and only very small increases in serum DHEA, showed not only benefit to the targeted vaginal lubrication and coital comfort, but easier and more intense orgasms, possibly from restored genital sexual sensitivity, as well as increased desire (Labrie et al., 2016). Of note, the diagnostic criteria for Sexual Interest/Arousal Disorder (SIAD) which, in 2013 replaced HSDD in the DSM-5 (American Psychiatric Association, 2013), include reduced genital sexual sensitivity. SIAD also focuses on reduced pleasure and subjective sexual arousal/excitement both strongly linked to mood, supporting the need for further systemic DHEA trials in mid-life and older women with SIAD.
4.6. Strengths
This study is highly novel, being the first to identify multiple hormonal markers of HPA regulation/dysregulation in women diagnosed with HSDD. We were able to meet our target sample size despite the challenge of depression or antidepressant use as exclusion criteria. Diagnoses were made using validated questionnaires and both in-person and telephone structured interviews. Salivary samples were taken over 3 days to increase accuracy and stability of both cortisol and DHEA measures, and multiple measures of HPA axis activity and regulation provided a more comprehensive view of the involvement of HPA function in HSDD.
4.7. Limitations
This study was initiated when HSDD was still the accepted diagnosis of low desire according to the DSM-IV-TR; however, midway through the study, the DSM-5 was released and proposed a new definition of low desire defined by SIAD. Given that the criteria for diagnosing SIAD are broader than for HSDD (O’Loughlin et al., 2017), some of the current HSDD participants may not have been experiencing difficulties with sexual pleasure, subjective arousal and genital sexual sensitivity, symptoms included in SIAD. How the stress hormonal profiles of women with SIAD differ from women with HSDD and from sexually healthy women should be a focus of future research. Secondly, because we excluded women with a current diagnosis of depression and current use of antidepressants, our cohort of women may not have been fully representative of the majority of women with low desire. Thirdly, our continuous measure of sexual functioning was based on only two items from the SIDI given that so many of the women were not sexually active, thus precluding us from using the SIDI total score. Additionally, some of our HPA measures were not entirely independent of each other. For example, the diurnal slope, the CAR, and the cortisol:DHEA ratio are all derived from the same set of measures of cortisol and DHEA. On the positive side, the HPA parameters reported in this manuscript generally capture different aspects of HPA function and capture multiple aspects of physiological regulation/ dysregulation. Moreover, due to overlap in the cortisol and DHEA pathways, these hormones are expected to be correlated to some extent. While there is no clear strategy for dealing with the overlap in measures from a statistical perspective, as different types of analyses were utilized for each of the measures, nevertheless the overlap in measures should be acknowledged. Lastly, as participants were not asked to report menstrual cycle stage at the time of saliva collection, we cannot assess stage of menstrual cycle for our participants. However, since the saliva collection spanned multiple days, salivary hormone levels are unlikely to be representative of a very narrow window within the menstrual cycle. Moreover, as noted above, estrogen, and therefore stage of menstrual cycle, likely play a minor, if any, role in sexual desire (West et al., 2008; Erekson et al., 2012).
One final limitation concerns the fact that the effects of chronic stress are complex, and that numerous factors may influence whether exposure to stressors at different times in the life course results in increased or blunted HPA responsiveness. The cortisol concentrations observed in women with HSDD in the present study were within the normal range of cortisol values generally reported. Importantly, however, our suggestion that blunted HPA activity, including lower AM cortisol and DHEA levels, a flatter diurnal cortisol slope, and a lower CAR, suggests HPA dysregulation in these women is supported by numerous studies in the literature. For example, an extensive meta-analysis of the effects of chronic stress in adulthood (Miller et al., 2007) reported that exposure to stressors including combat/war experience, abuse/assault, death or loss of a major relationship, caregiving experiences, natural disasters, and job loss and/or unemployment was associated with significantly lower concentrations of morning cortisol, more pronounced suppression of cortisol following dexamethasone challenge, greater concentrations of afternoon/evening cortisol, a flatter diurnal rhythm, and a higher daily volume of cortisol, collectively indicating a dysregulated pattern of hormone secretion. However, some studies did report increased HPA activity following chronic stress. Miller and colleagues (2007) suggest that whether chronic stress results in increased or decreased HPA activity may depend on factors such as the time elapsed since stressor onset, the nature of the threat posed, the core emotions likely to be elicited by the stressor, the controllability of the stressor, and the psychiatric characteristics of the person. Overall, effects of early life adversity are complex, and, as noted above, long-term effects may depend on multiple factors, which, in the case of early life stressors, may include current psychiatric status, current life stress or adversity, age, and genetic and epigenetic factors (Tarullo and Gunnar, 2006). Nevertheless, our finding of blunted HPA activity in women with HSDD is consistent with a large body of literature indicating that HPA dysregulation may be associated with a decreased or blunted HPA response, and may be manifest in several different measures of HPA function.
5. Conclusion
Utilizing multiple measures of HPA function, we examined HPA activity and regulation in women with HSDD compared to sexually healthy control partcipants. We found strong evidence of HPA axis dysregulation in women with HSDD. Women with HSDD had lower morning cortisol and DHEA levels, a flatter diurnal cortisol slope and a blunted CAR. Both past stress as reflected in the hormonal changes and the hormonal alterations per se may contribute to low sexual desire in adult life. Research is needed on DHEA supplementation in women with both low sexual desire and low serum DHEA.
Acknowledgements
We wish to thank Yvonne Erskine for assistance with project administration, Melissa Moses and Chanel Wood for assistance in recruitment and data collection, Kelly Smith, Miriam Driscoll and Erin Breckon for assistance with clinical/diagnostic interviews, Wayne Yu for running hormone assays, and John Petkau, Biljana Stojkova and Elena Shchurenkova for assistance with statistical analysis.
Funding
This work was supported by the Canadian Institutes for Health Research [grant number F11-01015]. The research in Dr. Weinberg’s laboratory was supported by NIH/National Institute on Alcohol Abuse and Alcoholism grants R37 AA007789 and R01 AA022460
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- Adam EK, Kumari M., 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE, 2017. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorder, 4th ed. text rev Washington, DC: Author. [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorder, 5th ed. Arlington, VA: Author. [Google Scholar]
- Basson R, 2010. Review: testosterone therapy for reduced libido in women. Ther. Adv. Endocrinol. Metab 1, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson R, Brotto LA, Petkau AJ, Labrie F, 2010. Role of androgens in women’s sexual dysfunction. Menopause. 17, 962–971. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, 2005. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch. Intern. Med 165, 1582–1589. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Basson R, 2014. Group mindfulness-based therapy significantly improves sexual desire in women. Behav. Res. Ther 57, 43–54. [DOI] [PubMed] [Google Scholar]
- Bunea IM, Szentágotai-Tătar A, Miu AC, 2017. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl. Psychiatry 7, 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Wallen K, 2016. Increasing women’s sexual desire: the comparative effectiveness of estrogens and androgens. Horm. Behav 78, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2009. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol 80, 265–278. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW, 1992. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. J. Am. Med. Assoc 267, 1244–1252. [PubMed] [Google Scholar]
- Clayton AH, Segraves RT, Leiblum S, Basson R, Pyke R, Cotton D, Lewis-D’Agostino D, Evans KR, Sills TL, Wunderlich GR, 2006. Reliability and validity of the Sexual Interest and Desire Inventory-Female (SIDI-F), a scale designed to measure severity of female hypoactive sexual desire disorder. J. Sex Marital Ther 32, 115–135. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Goldfischer ER, Goldstein I, Derogatis L, Lewis-d’Agostino DJ, Pyke R, 2009. Validation of the decreased sexual desire screener (DSDS): a brief diagnostic instrument for generalized acquired female hypoactive sexual desire disorder (HSDD). J. Sex. Med 6, 730–738. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F, 2004. The awakening cortisol response: methodological issues and significance. Stress 7, 29–37. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Thorn L, 2010. The cortisol awakening response in context. Int. Rev. Neurobiol 93, 153–175. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER, 2013. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38, 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Davison SL, Donath S, Bell RJ, 2005. Circulating androgen levels and self-reported sexual function in women. J. Am. Med. Assoc 294, 91–96. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zheng P, 2012. Dehydroepiandrosterone sulphate: action and mechanism in the brain. J. Neuroendocrinol 24, 215–224. [DOI] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F, 2001. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 68, 2093–2103. [DOI] [PubMed] [Google Scholar]
- Egeland M, Zunszain PA, Pariante CM, 2015. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci 16, 189–200. [DOI] [PubMed] [Google Scholar]
- Erekson EA, Martin DK, Zhu K, Ciarleglio MM, Patel DA, Guess MK, Ratner ES, 2012. Sexual function in older women after oophorectomy. Obstet. Gynecol 120, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E, Cravello I, Muzzoni B, Casarotti D, Paltro M, Solerte SB, Fioravanti M, Cuzzoni G, Pontiggia B, Magri F, 2001. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur. J. Endocrinol 144, 319–329. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C, 2009. The cortisol awakening response (CAR): facts and future directions. Int. J. Psychophysiol 72, 67–73. [DOI] [PubMed] [Google Scholar]
- Friess E,, Schiffelholz T, Steckler T, Steiger A, 2000. Dehydroepiandrosterone–a neurosteroid. Eur. J. Clin. Invest 30, 46–50. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Stomati M, Valentino V, Pluchino N, Potì E, Casarosa E, Merlini S, Giannini A, Luisi M, 2011. Effect of 1-year, low-dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric. 14, 661–668. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS, 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol 19, 1–19. [DOI] [PubMed] [Google Scholar]
- Grebe NM, Thompson ME, Gangestad SW, 2016. Hormonal predictors of women’s extra-pair vs. In-pair sexual attraction in natural cycles: implications for extended sexuality. Horm. Behav 78, 11–219. [DOI] [PubMed] [Google Scholar]
- Günzler C, Berner M, 2012. Efficacy of psychosocial interventions in men and women with sexual dysfunctions - a systematic review of controlled clinical trials. J. Sex. Med 9, 3108–3125. [DOI] [PubMed] [Google Scholar]
- Hamilton LD, Rellini AH, Meston CM, 2008. Cortisol, sexual arousal, and affect in response to sexual stimuli. J. Sex. Med 5, 2111–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney JLJ, Carroll D, Phillips AC, 2014. Physical activity, life events stress, cortisol, and DHEA: preliminary findings that physical activity may buffer against the negative effects of stress. J. Aging Phys. Act 22, 465–473. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH, 2000. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25, 1–35. [DOI] [PubMed] [Google Scholar]
- Herane-Vives A, Fischer S, de Angel V, Wise T, Cheung E, Chua KC, Arnone D, Young AH, Cleare AJ, 2018. Elevated fingernail cortisol levels in major depressive episodes. Psychoneuroendocrinology 88, 17–23. [DOI] [PubMed] [Google Scholar]
- Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon IIDL, Stein LM, Bauer ME, 2009. Neuroendocrine and immunological correlates of chronic stress in “strictly healthy” populations. Neuroimmunomodulation. 17, 9–18. [DOI] [PubMed] [Google Scholar]
- Jones BC, Hahn AC, Fisher CI, Wang H, Kandrik M, DeBruine LM, 2018. General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women’s hormonal status. Psychoneuroendocrinology 88, 153–157. [DOI] [PubMed] [Google Scholar]
- Kamin HS, Kertes DA, 2017. Cortisol and DHEA in development and psychopathology. Horm. Behav 89, 69–85. [DOI] [PubMed] [Google Scholar]
- Kaminska M, Harris J, Gijsbers K, Dubrovsky B, 2000. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP: implications for depression. Brain Res. Bull 52, 229–234. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Bonora E, Schwarz S, Xu Q, 2000. No association between dehydroepiandrosterone sulfate and development of atherosclerosis in a prospective population study (Bruneck Study). Arterioscler. Thromb. Vase. Biol 20, 1094–1100. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee YJ, Ahn RS, 2010. Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Med. J 51, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Mandansky D, King S, Fletcher KE, Brewer J, 2001. Early sexual abuse and low cortisol. Psychiatry Clin. Neurosci 55, 71–74. [DOI] [PubMed] [Google Scholar]
- Korkidakis KA, Reid RL, 2017. Testosterone in women: measurement and therapeutic use. J. Obstet. Gynaecol. Can 39, 124–130. [DOI] [PubMed] [Google Scholar]
- Kuras YI, Mclnnis CM, Thoma MV, Chen X, Hanlin L, Gianferante D, Rohleder N, 2017. Increased alpha-amylase response to an acute psychosocial stress challenge in healthy adults with childhood adversity. Dev. Psychobiol 59, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Cote I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur E, 2016. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 23, 243–256. [DOI] [PubMed] [Google Scholar]
- Labrie F, Martel C, Belanger A, Pelletier G, 2017. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J. Steroid Biochem. Mol. Biol 168, 9–18. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Sjörs A, Währborg P, Ljung T, Jonsdottir IH, 2015. Burnout and hypocortisolism - a matter of severity? A study on acth and cortisol responses to acute psychosocial stress. Front. Psychiatry 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfey KE, Link CL, Rosen RC, Wiegel M, McKinlay JB, 2009. Prevalence and correlates of sexual activity and function in women: results from the Boston area community health (BACH) survey. Arch. Sex. Behav 38, 514–527. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol 30, 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza E, Maccario M, Ramunni J, Gauna C, Bertagna A, Barberis AM, Patroncini S, Messina M, Ghigo E, 1999. Dehydroepiandrosterone sulfate levels in women. Relationships with age, body mass index and insulin levels. J. Endocrinol. Invest 22, 681–687. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psycho Bull. 133, 25–45. [DOI] [PubMed] [Google Scholar]
- Mitchell KR, Mercer CH, Wellings K, Johnson AM, 2009. Prevalence of low sexual desire among women in Britain: associated factors. J. Sex. Med 6, 2434–2444. [DOI] [PubMed] [Google Scholar]
- Mocking RJT, Pellikaan CM, Lok A, Assies J, Ruhe HG, Koeter MW, Visser I, Bockting CL, Olff M, Schene AH, 2015. DHEAS and cortisol/DHEAS-ratio in recurrent depression: state, or trait predicting 10-year recurrence? Psychoneuroendocrinology. 59, 91–101. [DOI] [PubMed] [Google Scholar]
- O’Loughlin JI, Basson R, Brotto LA, 2017. Women with hypoactive sexual desire disorder versus sexual interest/arousal disorder: an empirical test of raising the bar. J. Sex Res 55, 734–746. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, Arseneault L, 2011. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol. Psychiatry 70, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen LJ, Wu FC, Biittler RM, Keevil BG, 2016. A direct assay for the routine measurement of testosterone, androstenedione, dihydrotestosterone and dehydroepiandrosterone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem 53, 580–587. [DOI] [PubMed] [Google Scholar]
- Panjari M, Davis SR, 2007. DHEA therapy for women: effect on sexual function and wellbeing. Hum. Reprod. Update 13, 239–248. [DOI] [PubMed] [Google Scholar]
- Paterson LQP, Handy AB, Brotto LA, 2017. A pilot study of eight-session mindfulness-based cognitive therapy adapted for women’s sexual interest/arousal disorder. J. Sex Res 54, 850–861. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR, 2015. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol 145, 273–280. [DOI] [PubMed] [Google Scholar]
- Qiao S, Li X, Zilioli S, Chen Z, Deng H, Pan J, Guo W, 2017. Hair measurements of cortisol, DHEA, and DHEA to cortisol ratio as biomarkers of chronic stress among people living with HIV in China: known-group validation. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph JF, Zheng H, Avis NE, Greendale GA, Harlow SD, 2015. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J. Clin. Endocrinol. Metab 100, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellini A, 2008. Review of the empirical evidence for a theoretical model to understand the sexual problems of women with a history of CSA. J. Sex. Med 5, 31–46. [DOI] [PubMed] [Google Scholar]
- Rellini AH, Hamilton LD, Delville Y, Meston CM, 2009. The cortisol response during physiological sexual arousal in adult women with a history of childhood sexual abuse. J. Trauma. Stress 22, 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ADL, 2004. Salivary cortisol response to awakening in chronic fatigue syndrome. Br. J. Psychiatry 184, 136–141. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL, 2013. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm. Behav 63, 636–645. [DOI] [PubMed] [Google Scholar]
- Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G, 2005. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J. Clin. Endocrinol. Metab 90, 4836–4845. [DOI] [PubMed] [Google Scholar]
- Santoro N, Worsley R, Miller KK, Parish SJ, Davis SR, 2016. Role of estrogens and estrogen-like compounds in female sexual function and dysfunction. J. Sex. Med 13, 305–316. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Snabes MC, Zborowski J, Simes S, 2012. Libigel® (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire disorder in postmenopausal women. J. Sex. Med 9, 171. [Google Scholar]
- Stetler C, Miller GE, 2005. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J. Abnorm. Psychol 114, 697–705. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR, 2006. Child maltreatment and the developing HPA axis. Horm. Behav 50, 632–639. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Tal A, Pal L, Li F, Black DM, Brinton EA, Budoff MJ, Cedars MI, Du W, Hodis HN, Lobo RA, 2017. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern. Med 177, 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW, 2010. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psyychopathol 22, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, 2012. Testosterone and sexual desire in healthy women and men. Arch. Sex. Behav 41, 1471–1484. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJM, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H, 2009. Early neglect and abuse predict diurnal cortisol patterns in adults. A study of international adoptees. Psychoneuroendocrinology. 34, 660–669. [DOI] [PubMed] [Google Scholar]
- van Lunsen RHW, Zimmerman Y, Coelingh Bennink HJT, Termeer HMM, Appels N, Fauser BCJM, Laan E, 2018. Maintaining physiologic testosterone levels during combined oral contraceptives by adding dehydroepiandrosterone: II. Effects on sexual function. A phase II randomized, double-blind, placebo-controlled study. Contraception. 98, 56–62. [DOI] [PubMed] [Google Scholar]
- Wåhlin-Jacobsen S, Pedersen AT, Kristensen E, Laesspe NC, Lundqvist M, Cohen AS, Hougaard DM, Giraldi A, 2015. Is there a correlation between androgens and sexual desire in women? J. Sex. Med 12, 358–373. [DOI] [PubMed] [Google Scholar]
- Wåhlin-Jacobsen S, Kristensen E, Pedersen AT, Laessoe NC, Cohen AS, Hougaard DM, Lundqvist M, Giraldi A, 2017. Androgens and psychosocial factors related to sexual dysfunctions in premenopausal women. J. Sex. Med 14, 366–379. [DOI] [PubMed] [Google Scholar]
- West SL, D’Aloisio AA, Agans RP, Kalsbeek WD, Borisov NN, Thorp JM, 2008. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch. Intern. Med 168, 1441–1449. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S, 2007. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 32, 358–366. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, Yang RK, 2006. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr. Scand 114, 187–193. [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Porter RJ, 2002. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am. J. Psychiatry 159, 1237–1239. [DOI] [PubMed] [Google Scholar]


