Abstract
Electric light has enabled humans to conquer the night, but light exposure at night can disrupt the circadian timing system and is associated with a diverse range of health disorders. To provide adequate lighting for visual tasks without disrupting the human circadian timing system, a precise definition of circadian spectral sensitivity is required. Prior attempts to define the circadian spectral sensitivity curve have used short (≤90-min) monochromatic light exposures in dark-adapted human subjects or in vitro dark-adapted isolated retina or melanopsin. Several lines of evidence suggest that these dark-adapted circadian spectral sensitivity curves, in addition to 430- to 499-nm (blue) wavelength sensitivity, may include transient 400- to 429-nm (violet) and 500- to 560-nm (green) components mediated by cone- and rod-originated extrinsic inputs to intrinsically photosensitive retinal ganglion cells (ipRGCs), which decay over the first 2 h of extended light exposure. To test the hypothesis that the human circadian spectral sensitivity in light-adapted conditions may have a narrower, predominantly blue, sensitivity, we used 12-h continuous exposures of light-adapted healthy human subjects to 6 polychromatic white light-emitting diode (LED) light sources with diverse spectral power distributions at recommended workplace levels of illumination (540 lux) to determine their effect on the area under curve of the overnight (2000–0800 h) salivary melatonin. We derived a narrow steady-state human Circadian Potency spectral sensitivity curve with a peak at 477 nm and a full-width half-maximum of 438 to 493 nm. This light-adapted Circadian Potency spectral sensitivity permits the development of spectrally engineered LED light sources to minimize circadian disruption and address the health risks of light exposure at night in our 24/7 society, by alternating between daytime circadian stimulatory white light spectra and nocturnal circadian protective white light spectra.
Keywords: circadian, light spectrum, melatonin, human, spectral sensitivity
The human circadian timing system evolved while our ancestors spent most of their days outside in bright daylight and their nights in the dark from sunset to sunrise (Moore-Ede et al., 1982). Today, in industrialized societies, less than 8% of time is spent outdoors and more than 87% indoors (Klepeis et al., 2001), where humans are exposed to thousand-fold dimmed light during the day (e.g., 100 lux indoors versus up to 100,000 lux outdoors) and thousand-fold brightening during the night (e.g., 100 lux or more indoors versus <0.1 lux outside in the pre-electric world).
Insufficient light during the day and excessive light exposure during evening and night hours disrupts the human circadian timing system and may cause impaired sleep, immunity, and mood, and it is associated with increased risks of obesity and diabetes as well as breast, prostate, and other neuroendocrine-sensitive cancers (Bass and Lazar, 2016; Panda, 2016; Stevens et al., 2014). In 2010, the World Health Organization International Agency for Research on Cancer classified nightshift work with circadian disruption as a probable (group 2A) human carcinogen (International Agency for Research on Cancer, 2010), and in 2018, the US National Toxicology Program expert panel concluded that circadian disruption caused by excessive light at night and insufficient light during the day is reasonably anticipated to be a human carcinogen (U.S. National Toxicology Program, 2018). Yet, our modern 24/7 society depends on the use of electric lighting at night and lowered indoor illumination levels during the day.
A potential spectral engineering solution to this problem was raised by the discovery that the circadian timing system primarily responds to short-wavelength visible light (Thapan et al., 2001; Brainard et al., 2001), mediated by several subtypes of intrinsically photosensitive retinal ganglion cells (ipRGCs) containing the photopigment melanopsin (Opn4; Bailes and Lucas, 2013), some of which also receive rod- and cone-initiated photoresponses (Mure et al., 2019). The ipRGCs and ON bipolar cells presynaptic to the ipRGCs can remain continuously active when exposed to blue light (Wong, 2012). The M1 and M2 subtypes of ipRGCs transmit the day-night pattern of retinal illuminance via a retinohypothamic tract directly to neurons in the circadian pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus (Fernandez et al., 2016). In turn, the SCN communicates circadian temporal information to ensure internal synchronization of the multioscillator circadian timing system via neuroendocrine pathways including the timing of melatonin release from the pineal gland, which, when measured as saliva or plasma melatonin, serves as a useful indicator of the timing of the circadian system (Pandi-Perumal et al., 2007).
The spectral sensitivity of the circadian timing system across the visible light spectral range (380-780 nm) has previously been assessed using short monochromatic light exposures in dark-adapted human subjects with pharmacologically dilated pupils. Thapan et al. (2001) blindfolded subjects for 0.5 to 3.5 h and Brainard et al. (2001, 2008) blindfolded them for 2 h prior to exposure to monochromatic lights of different visible wavelengths for 30 min and 90 min, respectively, during late evening hours. In vitro studies using short monochromatic light exposures of small pieces of human retinae (Mure et al., 2019) and of human melanopsin expressed in human embryonic kidney cells (HEK-293; Bailes and Lucas, 2013) have also been used to define circadian spectral sensitivity. The circadian spectral sensitivity curves derived from these in vivo and in vitro studies span a broad band of 400 to 429 nm (hereinafter referred to as violet), 430 to 499 nm (hereinafter blue), and 500 to 560 nm (hereinafter green) wavelengths, but their reported peak sensitivities (λmax 457-479 nm) are distinct from the spectral sensitivity curves of rod (λmax 498 nm) and S-cone (λmax 420 nm), M-cone (λmax 534 nm) and L-cone (λmax 564 nm) pigments (Brainard et al., 2001; Mure et al., 2019).
However, there are important differences between the short ≤90-min exposures of dark-adapted retinae to monochromatic lights previously used to derive the circadian spectral sensitivity curve and the typical light-adapted human exposure to natural and/or electric polychromatic white light for up to ~16 continuous hours.
Several lines of evidence suggest that the circadian spectral sensitivity under normal light-adapted working conditions may have a narrower wavelength distribution than that defined by the previous studies. When human subjects are illuminated by polychromatic white light for 12 h at night at workplace levels of light intensity (600-1000 lux table top), Rahman et al. (2011) and Gil-Lozano et al. (2016) have reported near complete restoration of melatonin levels when <500-nm dichroic cutoff filters are used to eliminate violet and blue, but not green, wavelengths. Similarly, when Gooley et al. (2010) exposed previously dark-adapted human subjects to extended 6-h exposures of either monochromatic green (555 nm) or blue (460 nm) lights, both green and blue light had similar suppressive effects on melatonin over the first 30 min, but green light–induced melatonin suppression rapidly decayed over the next 2 h, whereas blue light maintained a sustained suppression of melatonin throughout the 6-h exposure. Gooley et al. (2010) suggested that transitory extrinsic input to ipRGCs from M-cones may account for the sensitivity to green wavelengths for the first 1 to 2 h after dark adaptation, but under normal extended polychromatic light exposures, these green wavelengths (500-575 nm) do not contribute significantly to the light-adapted circadian spectral sensitivity curve.
A similar phenomenon may occur from transitory extrinsic input to ipRGCs from S-cones (λmax 420 nm), with violet light exposure. A comparison of melatonin suppression with different durations of monochromatic violet (420-424 nm) light exposure in subjects who have been dark adapted by wearing blindfolds in a dark room revealed that 30 min of violet light exposure produced 95% melatonin suppression (Thapan et al., 2001) whereas 90 min of violet light exposure produced only 27% of maximum melatonin suppression (Brainard et al., 2008). Furthermore, Souman et al. (2018) found that a 3-h light exposure with peak emission at 420 nm caused minimal melatonin suppression.
The reduced circadian sensitivity to violet and green wavelengths with extended light exposures suggests that the circadian spectral sensitivity curve under a normal duration (8-16 h) polychromatic white light exposure in light-adapted subjects may have a narrower spectral range than previously reported.
To test this hypothesis, we measured the circadian spectral sensitivity using (1) a light exposure time of 12 h instead of brief ≤90-min light exposures; (2) a comparison of 6 different polychromatic overhead ceiling white light-emitting diode (LED) light sources with diverse spectral power distributions to probe circadian spectral sensitivity, instead of the monochromatic light stimuli previously used; (3) exposures to illumination levels recommended by the IES Lighting Handbook for health care and manufacturing work surfaces (540 lux = 50 foot candles, horizontally at table top; DiLaura et al., 2011), providing 254 to 323 lux vertical illuminance at the cornea of the eye; and (4) measurements of the total 12-h nocturnal (2000-0800 h) suppression of melatonin (area under the curve) as a circadian disruption biomarker (Mirick and Davis, 2008) that has direct relevance to health outcomes (Hill et al., 2015).
Materials and Methods
Human Subject Selection
Adult human subjects were recruited through local advertisements and screened to ensure they had regular sleep patterns with nocturnal sleep from 2300 h to 0700 h (±1 h), were nonsmokers, and were medication free. Enrollment exclusion criteria included recent history of shift work, recent travel history across time zones, history of sleep disorders, color blindness (as determined by the Ishihara Test for Color Blindness; Nakajima et al., 1960), history of ocular/vision disorders, depression as measured on the Center for Epidemiological Studies (Radloff, 1977) and other self-reported medical or psychiatric illnesses as screened by a general health questionnaire.
Thirty-four subjects (26 men, 8 women) aged 18 to 41 years (mean age 28 years) were enrolled in the study. Each participant gave written informed consent after receiving a full explanation of the study purpose, design, and procedures. The study protocol and consent forms were reviewed and approved by the Circadian Institutional Review Board (IRB 00001522) registered for federal wide assurance with the Office for Human Research Protections of the Department of Health and Human Services.
Research Protocol and Data Collection
For 7 days prior to each overnight study, all participants were instructed to maintain a preset sleep schedule (2300 h to 0700 h ± 30 min) and to maintain sleep diaries, which were confirmed with actigraphy. On the day of the study, the individual subjects awoke at their habitual time at home, pursued their normal daytime activities, and arrived at the Circadian Light Research Center at 1800 h for the overnight study session. All simulated night shifts were conducted with the subjects seated around a conference room table in a light-controlled workplace simulation area of the research center, illuminated by overhead lighting at a ceiling height of 8 feet.
During each simulated night shift condition (2000 h to 0800 h), groups of 8 to 13 subjects were continuously illuminated by 1 of the 6 polychromatic white LED light sources with the spectral power distributions labelled A-F in Figure 1 (top), and reported in 1-nm increments between 360 nm and 760 nm in Supplementary Table S1, each adjusted to provide 50-foot candles (540 lux) of horizontal table-top polychromatic white illuminance at the level of the conference room table around which the subjects were seated. Table-top and corneal eye-level spectral irradiance (spectrophotometer at a seated eye level directed toward the wall, 17 inches above and parallel to the table) was measured, and the total and photopigment-weighted (α-opic) irradiance values for each light source as well as total photon irradiance and photopic lux were calculated with the CIE S026:2018 Toolbox (March 2019 S026 testing version issued to tutorial participants; CIE, 2018). Table 1 shows the total visible (380-760 nm) irradiance, illuminance, log photon irradiance, and photopigment-weighted illuminance values for each LED light source (A-F) using the eye-level vertical irradiance measurements. In addition, we exposed subjects to 1 fluorescent light source, with the spectral power distribution shown in Figure 1 and illuminance and irradiance values shown in Table 1, under the same experimental protocol and conditions.
Figure 1.
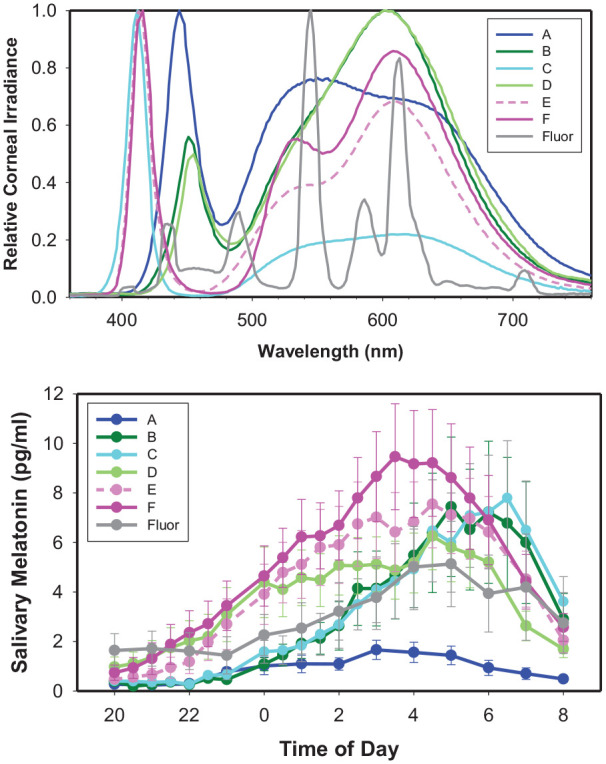
Spectral power distributions of polychromatic white light sources and resulting curves of overnight salivary melatonin levels. (Top) Normalized spectral power distributions of 6 polychromatic white light-emitting diode light sources (A-F) and 1 fluorescent light source (Fluor) measured at the cornea (eye level) of human subjects seated around a conference table illuminated at 540 lux table top. (Bottom) Salivary melatonin levels in pg/mL (mean ± SEM) measured overnight (2000 h to 0800 h) from subjects under each light source (A-F and Fluor).
Table 1.
Characteristics of the eye-level (corneal) illumination delivered by the 6 light-emitting diode light sources (A, B, C, D, E, F) and the fluorescent light all adjusted to provide 540 lux (50-foot candles) of table-top horizontal illumination.
| Light Source | Light Characteristics (Unweighted) |
Photopigment-Weighted (α-Opic) Irradiances (µW/cm2) |
||||||
|---|---|---|---|---|---|---|---|---|
| Irradiance (µW/cm2) | Illuminance (lux) | Log Photon Irradiance (log/cm2/s) | S-Cone-Opic | M-Cone-Opic | L-Cone-Opic | Rhodopic | Melanopic | |
| LED A | 105.01 | 300.69 | 14.477 | 17.47 | 39.85 | 48.48 | 32.74 | 27.19 |
| LED B | 78.27 | 254.25 | 14.360 | 7.77 | 29.90 | 41.74 | 19.70 | 15.09 |
| LED C | 114.96 | 253.82 | 14.492 | 10.77 | 30.25 | 41.32 | 19.36 | 14.04 |
| LED D | 83.81 | 269.34 | 14.394 | 7.19 | 31.37 | 44.29 | 20.25 | 15.43 |
| LED E | 113.09 | 320.67 | 14.507 | 8.77 | 36.81 | 52.86 | 22.91 | 16.55 |
| LED F | 108.41 | 322.60 | 14.495 | 7.25 | 36.44 | 52.82 | 21.03 | 14.08 |
| Fluor | 85.06 | 304.77 | 14.380 | 10.64 | 38.23 | 49.26 | 27.65 | 21.64 |
The overnight shift work simulation, when the subjects were not being tested, consisted of playing board games, with one of the research investigators in the room at all times making sure that all subjects kept continuously awake with their eyes open. Subjects were instructed to remain seated (with eyes about 17 inches above the table), to look straight ahead with the direction of gaze parallel to the table surface as much as possible during their activities, and to avoid looking directly into the ceiling lights at all times while in the simulation test room. They wore <500-nm cutoff dichroic blue-filtering glasses (Rahman et al., 2011) during brief bathroom breaks, which were not allowed during the 20 min prior to each testing time (lighting in hallway and bathroom was dimmed). From arrival at the facility to entering the night-shift simulation area at 2000 h, subjects were seated under standard fluorescent office light at approximately 540 lux table top. They were provided dinner at 1900 h and then 3 isocaloric meals every 4 h (after saliva sampling at 2300 h, 0300 h, and 0700 h). Water was the only beverage allowed during the simulation nights and available ad libitum, except for the 20 min prior to each saliva sampling time. Subjects were instructed to refrain from caffeinated beverages after breakfast and to not consume alcohol on the day prior to each simulated night shift.
Salivary melatonin samples were collected using the Salivette method (Sarstedt AG & Co, Nürmbrecht Germany), at set intervals of 30 or 60 min during each simulated night shift between 2000 h and 0800 h and immediately frozen. The samples were assayed for melatonin by SolidPhase Inc (Portland, ME) using the Bühlmann Direct Saliva Melatonin Radioimmunoassay (Bühlmann Diagnostics, Amherst, NH), which has a functional sensitivity of 0.9 pg/mL and an analytical sensitivity of 0.2 pg/mL, an intra-assay precision of 2.6% to 20.1% coefficient of variation (CV), and an interassay precision of 6.6% to 16.7% CV. The mean and standard error of the mean salivary melatonin results in pg/mL collected under each light source are shown in Supplemental Table S2.
Data Analysis
The area under the curve (AUC) of melatonin levels during the nocturnal rise and fall of melatonin concentration between 2000 h and 0800 h was calculated for each individual subject night, using the trapezoid method (Notari, 1980). The trapezoid AUC method used the normalized units h∙pg/mL, taking into account the exact time interval between 2 consecutive data points (i.e., 30 min or 60 min) and allowing comparability of the data regardless of sampling interval. The mean ± SEM melatonin AUC was determined for each lighting condition, as shown in Supplemental Table S2.
A data analyst (R.G.) who was unacquainted with, and blind to, previously published circadian spectral sensitivity curves was asked to independently derive the best curve fit between the melatonin AUC data and the relative spectral power distribution of each of the 6 LED light sources. Because our goal was to maintain the photopic illuminance levels required for adequate visual acuity while minimizing circadian disruption, we used as the optimization parameter the ratio between the total relative Circadian Potency spectral sensitivity based on total melatonin AUC suppression and the total relative photopic power380-760 (circadian potency/phototopic ratio [CPPR]). The photopic function with a peak at 555 nm and a full width at half maximum (FWHM) of 100 nm between 510 and 610 nm describes the brightness of light perceived by the human eye based on the properties of the visual cones in the retina (CIE, 2018). For each of the 6 LED light sources, the normalized spectral power distribution between 380 and 760 nm was multiplied by the photopic power at each wavelength to calculate the total relative photopic power between 380 and 760 nm (total relative photopic power380-760).
Rather than using a simple Gaussian curve fit, we allowed for negatively and positively skewed solutions by independently optimizing 3 parameters: (1) peak Circadian Potency spectral sensitivity wavelength (µ) and 2 separate Gaussian functions ( , where P = relative circadian potency, λ = wavelength, µ = peak Circadian Potency wavelength, σ = standard deviation, σ2 = variance) for (2) wavelengths less than µ, and (3) wavelengths greater than µ. The final asymmetric curve was assembled using the values from the short-wavelength Gaussian curve for λ ≤ µ and using the values from the long-wavelength Gaussian curve for λ > µ. Before assembling, the optimized the best-fit Circadian Potency curves for both sides of the peak Circadian Potency wavelength value were scaled so that the maximum relative Circadian Potency µ was equal to 1.0.
Results
Figure 1 (bottom) shows the mean and SEM melatonin curves measured overnight from 2000 h to 0800 h under the 6 LED light sources (A-F) and 1 fluorescent light source (Fluor) with the spectral power distributions shown in Figure 1 (top). We found a greater than 5-fold variation in the mean nocturnal melatonin AUC between the different LED light sources at the same light intensity. The melatonin AUC ranged between 11.2 and 64.3 h∙pg/mL depending on the spectral power distribution of the white light source, even though each light source was maintained at the same 540 lux table-top light intensity continuously during the 12-h test session.
The CPPR ranged from 0.11 to 0.33 for the 6 tested LED light sources. Analysis of variance calculations indicated a statistical difference in the AUC values among the LED light conditions (Kruskal-Wallis one-way analysis of variance on ranks: P = 0.02). Figure 2 (top) shows the optimized best-fit regression function between CPPR and melatonin AUC, which had an R2 coefficient of 0.991. Figure 2 (bottom) displays the optimized Circadian Potency spectral sensitivity curve (function, which provided the best-fit melatonin AUC versus CPPR linear regression line shown in Figure 2 (top). For wavelengths shorter than the 477-nm peak Circadian Potency spectral sensitivity wavelength, the optimized curve fit was , and for wavelengths longer than the 477-nm peak, it was .
Figure 2.
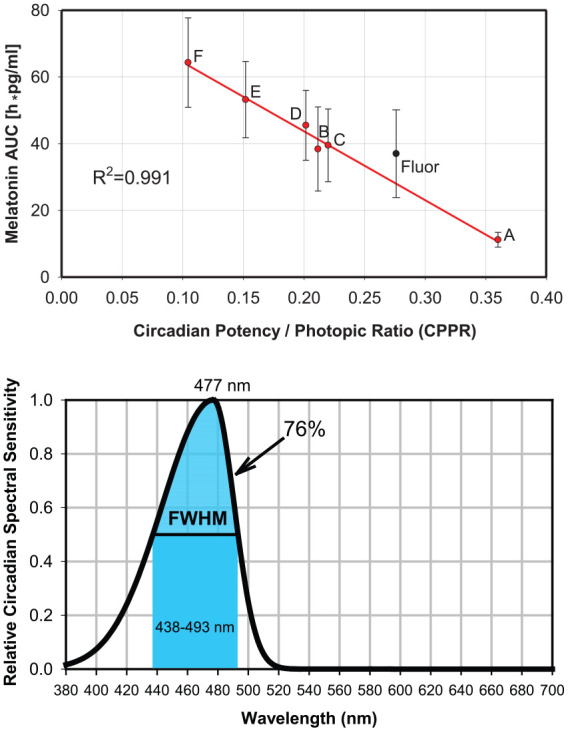
Derivation of optimized spectral sensitivity of normalized Circadian Potency for 12-h exposure to polychromatic white light-emitting diode (LED) light. (Top) Optimized linear regression fit between the circadian potency/photopic ratio (CPPR) of each LED light source and mean ± SEM melatonin total area under the curve. The result from the fluorescent light source (Fluor) is also shown. (Bottom) Optimized Circadian Potency spectral sensitivity curve that provided the best linear regression fit in Figure 2 (top), with 76% of spectral sensitivity falling in the full-width half maximum 438- to 493-nm blue band depicted with the color of 477 nm blue.
The Circadian Potency spectral sensitivity curve had a right width at half maximum of 16 nm and a left width at half maximum of 39 nm. Thus, the Circadian Potency curve had an FWHM of 55 nm from 438 nm to 493 nm that was negatively skewed. Seventy-six percent of the total Circadian Potency spectrally sensitive irradiant power fell within the 438- to 493-nm FWHM band.
To characterize the transient behavior of circadian spectral sensitivity, we compared the steady-state Circadian Potency spectral sensitivity curve derived from this study (with 12-h light exposures) with the best-fit curves for the published experimentally derived data for 30-min light exposures (Thapan et al., 2001) and for 90-min light exposures (Brainard et al., 2001; Brainard et al., 2008) obtained using the same 3-parameter optimized curve-fitting methodology (Fig. 3). As light exposure is extended, there is a progressive transition of the circadian spectral sensitivity curve. The decay of the S-cone violet and M-cone green transient spectral responses to light appears to be associated with a progressive right shift of the maximum circadian sensitivity from 430 nm (30-min exposure) to 440 nm (90-min exposure) to 477 nm (720-min exposure) and a progressive narrowing of the circadian sensitivity curve from an FWHM of more than 100 nm (30-min exposure) to 85 nm (90-min exposure) to 55 nm (720-min exposure).
Figure 3.
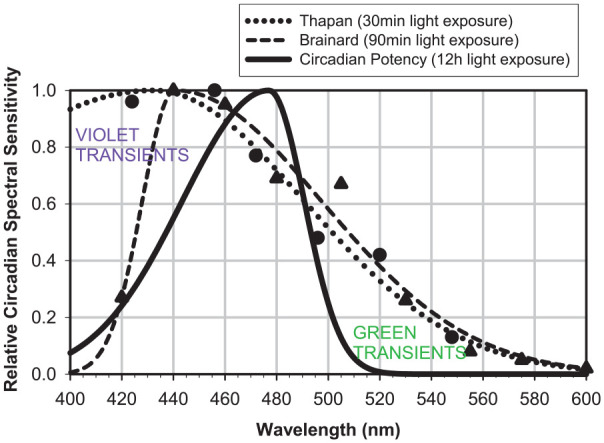
Comparison of normalized circadian spectral sensitivity curves of 30-min, 90-min, and 12-h light exposures. Raw data from Thapan et al. (2001) for 30-min (•) and Brainard et al. (2001, 2008) for 90-min (▲) light exposures plus their best-fit asymmetric Gaussian functions. After violet and green transient decay, the residual steady-state Circadian Potency spectral sensitivity curve is narrower, right shifted, and negatively skewed.
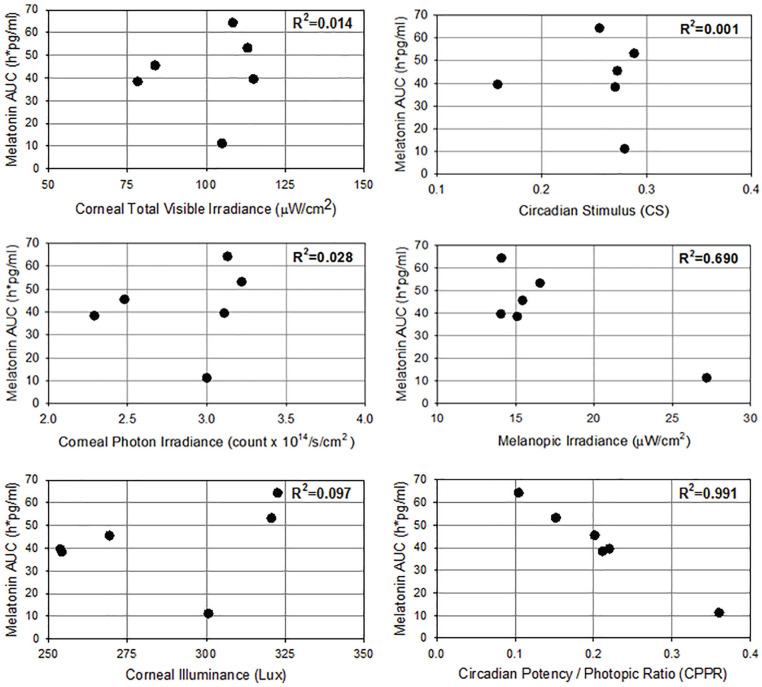
Because all light sources were adjusted to provide the same horizontal table-top level of illumination (the standard measure that lighting designers use to illuminate indoor spaces), and because the spectral power distributions of the chosen LED light sources were significantly diverse, the intensity of total visible light entering the eye (measured vertically at eye level with the subjects seated at the conference table) varied between the light sources. This provided the opportunity to determine if the melatonin AUC was influenced by corneal light metrics (irradiance in µW/cm2, photon irradiance in photons/cm2/s, photopic illuminance in lux). Individual pupil sizes and macular pigment optical density were not measured and were not taken into account. As Figure 4 (left) shows, there was no significant correlation between light intensity by any of these metrics and the melatonin AUC results.
Figure 4.
Relationships between melatonin area under the curve and corneal total visible irradiance (top left), corneal photon irradiance (middle left), corneal illuminance in lux (bottom left), circadian stimulus (CS; top right), CIE melanopic irradiance (middle left), and circadian potency/photopic ratio (bottom left).
We next examined how well the most commonly used circadian lighting calculators, the circadian stimulus (CS; Rea and Figueiro, 2018), CIE melanopic irradiance (CIE, 2018), and the closely related equivalent melanopic lux (EML) calculator (Lucas et al., 2014), correlated with the 12-h overnight melatonin AUC data measured in light-adapted human subjects under the 6 polychromatic white LED light sources. Figure 4 (right) compares the R2 coefficient of determination for melatonin AUC for (top) CS (R2 = 0.001), (middle) melanopic irradiance (R2 = 0.690), and (bottom) CPPR (R2 = 0.991). The CS, CIE, and also EML calculators derived primarily by using short monochromatic light exposures of dark-adapted retinae did not predict the 12-h overnight melatonin AUC results as accurately as the Circadian Potency spectral sensitivity curve reported here.
The one fluorescent light source we tested under this experimental protocol with the spectral power distribution shown in Supplementary Table S1 was calculated to have a CPPR value of 0.276 and a projected melatonin AUC of 28 h∙pg/mL using our LED light–derived Circadian Potency spectral sensitivity curve. The 12-h exposure to the fluorescent light at 540 lux resulted in a melatonin AUC of 37.0 ± 13.4 h∙pg/mL (Fig. 2A), and thus, the CPPR-predicted melatonin AUC of 28 h∙pg/mL fell within the measured SEM.
Discussion
In contrast to the previously reported circadian spectral sensitivity curves derived using short exposures of dark-adapted retinae to monochromatic lights, the Circadian Potency spectral sensitivity curve derived in this study with extended 12-h exposures to polychromatic white LED lights in light-adapted subjects had a peak at 477 nm and a narrower spectral range of circadian active predominantly blue wavelengths (FWHM 438-493 nm) with substantially less contributions from violet (400-429 nm) and green (500-560 nm) wavelengths. This finding is consistent with the hypothesis of Gooley et al. (2010) that the initial circadian system sensitivity to green wavelengths in dark-adapted subjects is most likely the consequence of transitory extrinsic input to ipRGCs from M-cones (λmax = 534 nm and potentially rods with λmax = 498 nm). Similarly, it would appear that the initial sensitivity to violet wavelengths in dark-adapted subjects may be a result of transitory extrinsic input to ipRGCs from S-cones (λmax = 420nm), which dissipates with extended violet (400-429 nm) light exposure.
The Circadian Potency spectral sensitivity for extended polychromatic light exposures that is largely confined to blue wavelengths explains the near complete restoration of melatonin levels in humans (Rahman et al., 2011; Gil-Lozano et al., 2016) and attenuation of circadian phase resetting in rats (Gladanac et al., 2019) when <500-nm dichroic cutoff filters are used to eliminate violet and blue, but not green, wavelengths in extended 12-h exposures to polychromatic white light. Similarly, the narrow Circadian Potency spectral sensitivity explains the findings of Souman et al. (2018) of melatonin restoration in violet-enriched blue-depleted light. Furthermore, it explains how a 12-µW/cm2 monochromatic 480-nm blue light source near the peak of the Circadian Potency spectral sensitivity curve with pharmacologically dilated eyes could produce a comparable-amplitude circadian phase-response curve as a 3000-µW/cm2 polychromatic white light source (Rüger et al., 2013).
The peak Circadian Potency wavelength (λmax = 477 nm) found in this study of light-adapted human subjects under extended 12-h exposures to polychromatic light is close to the peak sensitivity (λmax ~ 479 nm) of human melanopsin in vitro (Bailes and Lucas, 2013), supporting an intrinsic ipRGC melanopsin-mediated sustained circadian response. However, the spectral sensitivity curve determined by exposing human melanopsin expressed in human embryonic kidney cells (HEK-293) to monochromatic light flashes and measuring the dynamics of the first minute of intracellular calcium mobilization (Bailes and Lucas, 2013) showed a broader sensitivity to violet and green wavelengths than the Circadian Potency spectral sensitivity. Melanopsin, however, is tristable (Hughes et al., 2016), with each state having a different spectral sensitivity curve (Emmanuel and Do, 2015), so this may account in part for the sustained narrow circadian spectral sensitivity we observed in light-adapted subjects.
There are differences in the method we used to determine circadian spectral sensitivity under light-adapted conditions as compared with the classic photobiology methods used in the dark-adapted state, where the dose-response curves have been used to derive circadian spectral sensitivities. Rather than fixing the response using the half-maximal effective dose (EC50), we fixed the input using approximately photon-matched lights. Most photobiological modeling of cones, rods, and ipRGCs is based on the dark-adapted retina and may not be directly applicable, so we have taken an empirical systems approach to derive the overall circadian sensitivity of the human circadian timing system in the light-adapted retina using total overnight melatonin as an output function, which is a well-validated circadian disruption and health risk biomarker.
With mounting evidence that artificial light sources can disrupt circadian rhythms and lead to myriad negative health outcomes, there is a dire need for biologically healthy light sources (Zielinska-Dabkowska, 2018). There are several problems with using the previously reported circadian spectral sensitivity curve from short exposures of dark-adapted subjects to monochromatic light to spectrally engineer healthy white light sources for day and night applications. First, the previously reported circadian spectral sensitivity from dark-adapted subjects spans a broad band of violet, blue, and green wavelengths (400-560 nm), and when all of these wavelengths are removed from the polychromatic 380- to 780-nm white light spectrum, the result is an unattractive yellow-orange light not suitable for most human uses. Second, the experimentally determined spectral sensitivity curves, and the circadian calculators derived from them, CS (Rea and Figueiro, 2018), melanopic irradiance (CIE, 2018) and EML (Lucas et al., 2014), are significantly inconsistent, leading to confusion in the lighting engineering and design communities (Clark and Lesniak, 2017). Third, the sizeable recent gains in energy efficiency of lighting (from 5-10 lumens/watt of incandescents to the 100-200 lumens/watt efficacy of blue pump GaN-based LED dies) are currently not feasible to attain when this broad band of violet, blue, and green spectral wavelengths is removed to spectrally engineer nocturnal lighting, because the most energy-efficient dies are violet or blue. Fourth, there has previously been a lack of empirical circadian spectral sensitivity data derived from humans living and working in electrically illuminated workplace environments under normal intensities of polychromatic white light.
The steady-state Circadian Potency spectral sensitivity curve reported here should provide better clarity for defining the optimal design specifications required by circadian lighting. We recognize the precise shape of the Circadian Potency spectral sensitivity curve may be modified by interindividual differences such as ethnicity, age, and sex. A wide range of polynomial and other functions could potentially be used to define the steady-state Circadian Potency curve. However, the R2 coefficient of determination of 0.991 derived using the optimized Gaussian functions for short and long wavelengths and the peak sensitivity wavelength provides a sufficiently precise curve fit to describe the optimum Circadian Potency spectral sensitivity curve.
In comparison, the overnight 12-h melatonin AUC values under the 6 LED light sources are not explained by corneal light intensity metrics including total visible irradiance (R2 = 0.014), total photon irradiance (R2 = 0.028), or photopic lux (R2 = 0.097). We recognize that the range of irradiance, photon irradiance, and photopic illuminance (lux) values that could be examined with these data was limited, but the variation represents typical photometric ranges of lighting in the workplace.
Our findings from the overnight melatonin AUC suppression data under the 6 LED light sources (A-F) could not be adequately explained by the previously proposed circadian melanopic calculators, melanopic irradiance (CIE, 2018); EML (Lucas et al., 2014), which had an R2 = 0.690; and CS (Rea and Figueiro, 2018), which had a R2 = 0.001, which were primarily derived using data from short (≤90 min) monochromatic light exposures. This conclusion is supported by recent studies showing that the CS calculator accurately predicts the response to the first hour of light after a dark-adapted state but is less effective at predicting the responses to longer light exposures of 2 to 3 h (Nagare et al., 2019).
Normally the eyes of humans are fully dark adapted during the nocturnal hours of sleep in a dark room, and the first light exposure occurs with dawn or when the bedroom electric lights are switched on in the morning. Since the M-cone mediated extrinsic green and the S-cone mediated extrinsic violet, and inputs to the ipRGCs would be largely dissipated by the time an individual reaches their workplace (>2 h after first morning exposure to light), we propose that our steady-state predominantly blue Circadian Potency spectral sensitivity be used for designing workplace LED lighting, whereas the initial circadian spectral sensitivity to light described by the CS, CIE, and EML calculators may be more applicable to the design of morning lighting in the home environment.
The definition of the steady-state Circadian Potency spectral sensitivity curve makes it feasible to design and build blue-rich white LED chips to optimize Circadian Potency during daytime hours and blue-depleted white LED chips to minimize Circadian Potency from sunset to sunrise. Because the steady-state Circadian Potency spectral sensitivity is narrower than the previously reported circadian spectral sensitivity curves for dark-adapted conditions, it permits the use of dies and phosphors that emit violet and green (plus yellow and red) wavelengths at night to produce attractive and energy-efficient white electric light, which minimizes circadian disruption.
When we examined the response to a fluorescent light source under the same experimental protocol, the melatonin AUC was suppressed to 37 ± 13.4 h∙pg/mL, which fell approximately midway in the 11.2- and 64.3-h∙pg/mL range of responses to the 6 LED light sources (Fig. 1B). However, because the fluorescent light source was calculated to have a CPPR value of 0.276 using our LED-derived Circadian Potency function and an expected melatonin AUC of 28 h∙pg/mL, the Circadian Potency curve somewhat overestimated the melatonin-suppressive effects of this fluorescent light source, although it fell within 1 SEM of the measured value. It would require testing multiple fluorescent lights with different spectral power distributions to determine whether this is a general effect of fluorescent lights, but based on this result, we conclude that until such testing is done, the Circadian Potency function should be applied only to LED lights.
One particularly interesting feature of the Circadian Potency spectral sensitivity curve is the relative insensitivity to spectral wavelengths less than 420 nm. This permits the incorporation of 2 important features into nocturnal circadian lighting. First, violet LED dies with peak wavelengths of 410 to 420 nm can be used to replace the typical ~450-nm blue peak emissions of conventional LEDs to spectrally engineer colorimetrically balanced attractive white-colored light. Second, short-wavelength light is known to have alerting-, performance-, and mood-enhancing properties (Rahman et al., 2014; Viola et al., 2008), leading to the proposal that blue-rich light should be used at night to reduce human error, but this has the unfortunate consequence that it could significantly increase the risk of circadian disruption and health disorders. However, the alerting effect of short-wavelength light can be retained in lights spectrally engineered to remove circadian-disruptive blue wavelengths by replacing them with ~420-nm violet wavelengths, especially since there is evidence that the alerting effects of 420-nm violet light are even greater than 440-nm or 470-nm blue light (Revell et al., 2006).
Another application of the Circadian Potency spectral sensitivity curve is to phase shift the human circadian system to treat circadian sleep disorders or aid in the adjustment to work schedules or travel across time zones. Phase-response curves describe how circadian entrainment is achieved through the Circadian Potency wavelengths, alternatively phase advancing or phase delaying the circadian system depending on the circadian phase when high CPPR light is detected by the ipRGCs. The Rüger et al. (2013) type 1 phase-response curve for monochromatic blue 480-nm light serves as the closest available approximation to the likely Circadian Potency phase-response curve. Using the Circadian Potency function makes it possible to spectrally engineer efficient white lights suitable for normal visual acuity while achieving the phase-resetting goal.
Why might evolutionary selection result in such a narrow band of blue wavelengths defined by the Circadian Potency spectral sensitivity curve being used to communicate the environmental day-night signal? We suggest it may be because life forms have been exposed to steady-state blue wavelengths during the day ever since the origin of life deep in the primitive oceans. Below a depth of 200 m, all other visible spectral wavelengths except for ~475-nm light are absorbed by seawater (Denton, 1990). From single-cell marine organisms such as Gonyaulax (Hastings, 2001) to humans, the fundamental apparatus consisting of (1) blue photoreceptors maximally sensitivity at ~475 nm, (2) circadian clocks, and (3) melatonin release as the internal temporal signal has been conserved for billions of years to equip life for successful predictive adaptation to a rotating planet (Moore-Ede, 1986).
Supplemental Material
Supplemental material, Circadan_Potency_Supplementary_Material_March_11_2020 for Circadian Potency Spectrum with Extended Exposure to Polychromatic White LED Light under Workplace Conditions by Martin Moore-Ede, Anneke Heitmann and Rainer Guttkuhn in Journal of Biological Rhythms
Acknowledgments
We thank M. Smith, U. Trutschel, D. Platika, A. Aguirre, H. Rao, K. Appleman, B Chacko, P. Lever, A. Moore-Ede, and J. Luciani for helpful discussions; I. Sauer for data collection support; SolidPhase Inc. for performing melatonin radioimmunoassays; R. Casper and F. Turek for prepublication review and helpful comments on the article; and L.J.M. Schlangen and L. Price for access to the beta version of the CIE S 026 Toolbox. Studies in the Circadian Light Research Center were supported by in part by National Institutes of Health SBIR grants R43HL110769 and R44HL110769.
Supplementary material is available for this article online.
Footnotes
Author Contributions: M.M.-E. and A.H. conceived the idea and designed the studies, A.H. was the principal investigator on the SBIR grants and conducted the human subject studies, R.G. undertook the CPPR optimization analysis, and M.M.-E. and A.H. wrote and edited the manuscript.
Conflict of Interest Statement: M.M.-E., A.H., and R.G. are inventors on patent application PCT/US2019/041728 assigned to Circadian ZircLight Inc., which discloses circadian optimized spectrally engineered light sources including day and night LEDs using the Circadian Potency function described in this article, and issued patents US9827440, CN105265025, EP2982224, JP6391669, CA2908659, KR101986700, AU2018241062. M.M.E. is the chairman and CEO and holds equity and receives compensation from Circadian ZircLight, Inc. and Circadian Technologies Inc. R.G. and A.H. are employees of Circadian Technologies, Inc.
ORCID iDs: Martin Moore-Ede  https://orcid.org/0000-0002-9557-0609
https://orcid.org/0000-0002-9557-0609
Anneke Heitmann  https://orcid.org/0000-0002-9560-2906
https://orcid.org/0000-0002-9560-2906
References
- Bailes HJ, Lucas RJ. (2013) Human melanopsin forms a pigment maximally sensitive to blue light (λmax ~479 nm) supporting activation of Gq/11 and Gi/o signalling cascades. Proc R Soc B 280:20122987. 10.1098/rspb.2012.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Lazar MA. (2016) Circadian time signatures of fitness and disease. Science 354:994-998. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21:6405-6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Jasser S, Gerner E, Rollag MD. (2008) Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms 23:379-386. [DOI] [PubMed] [Google Scholar]
- Commission Internationale de L’Eclairage (2018) CIE International Standard (CIE S 026/E:2018) system for metrology of optical radiation for ipRGC-influenced responses to light. Vienna (Austria): Commission Internationale de L’Eclairage, Central Bureau; http://www.cie.co.at/publications/international-standards [Google Scholar]
- Clark E, Lesniak N. (2017, December 20) Circadian lighting solutions are real and important—why aren’t they being used? Metropolis Magazine; https://www.metropolismag.com/design/circadian-lighting-survey/ [Google Scholar]
- Denton EJ. (1990) Light and vision at depths greater than 200 meters. In: Herring PJ, Campbell AK, Whitfield M, Maddock L, editors. Light and life in the sea. Cambridge (UK): Cambridge University Press. [Google Scholar]
- DiLaura DL, Houser W, Mistrick RG, Steffy GR. (2011) The lighting handbook. 10th ed. Illuminating Engineering Society. [Google Scholar]
- Emmanuel AJ, Do MTH. (2015) Melanopsin tristability for sustained and broadband phototransduction. Neuron 85:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Chang YT, Hattar S, Chen SK. (2016) Architecture of retinal projections to the central circadian circadian pacemaker. Proc Natl Acad Sci USA 113:6047-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Lozano M, Hunter PM, Behan L-A, Gladanac B, Casper RF, Brubaker PL. (2016) Short-term sleep deprivation with nocturnal light exposure alters time dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab 310:E41-E50. [DOI] [PubMed] [Google Scholar]
- Gladanac B, Jonkman J, Shapiro CM, Brown TJ, Ralph MR, Casper RF, Rahman SA. (2019) Removing short wavelengths from polychromatic white light attenuates circadian phase resetting in rats. Front Neurosci 13:954-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. (2010) Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2:31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW. (2001) Cellular and molecular mechanisms of circadian regulation in the unicellular dinoflagellate gonyaulax polyhedra. In: Takahashi JS, Turek FW, Moore RY. editors. Circadian clocks. Vol 12, Handbook of behavioral neurobiology. New York: Plenum; p. 321-331. [Google Scholar]
- Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al. (2015) Melatonin: an inhibitor of breast cancer. Endocrine Relat Cancer 22:R183-R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Jagannath A, Rodgers J, Hankins MW, Pierson SN, Foster RG. (2016) Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye 30:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2010) Working Group on the Evaluation of Carcinogenic Risks to Humans: shift work. Painting, Firefighting, and Shiftwork 98:563-764. [PMC free article] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. (2001) The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol 11:231-252. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. (2014) Measuring and using light in the melanopsin age. Trends Neurosci 37:1-9. https://www.cell.com/cms/10.1016/j.tins.2013.10.004/attachment/baab3719-3769-4c31-886b-4fe56da7c66d/mmc1.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirick DK, Davis S. (2008) Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Biomarkers Prev 17:3306-3313. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC. (1986) Physiology of the circadian timing system: predictive versus reactive homeostasis. Thirtieth Annual Bowditch Lecture. Am J Physiol 19:R735-R752. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. (1982) The clocks that time us: physiology of the circadian timing system. Cambridge (MA): Harvard University Press. [Google Scholar]
- Mure LS, Vinberg F, Hanneken A, Panda S. (2019) Functional diversity of human intrinsically photosensitive retinal ganglion cells. Science 366:1251-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagare R, Rea MS, Plitnick B, Figueiro MG. (2019) Nocturnal melatonin suppression by adolescents and adults for different levels, spectra, and durations of light exposure. J Biol Rhythms 34:178-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Ichikawa H, Nakagawa O, Majima A, Watanabe M. (1960) Ishihara test in color-vision defects: studies on a statistical method for evaluation of the screening efficiency of several plates. Am J Opthamol 49:921-929. [PubMed] [Google Scholar]
- Notari RE. (1980) Biopharmaceutics and clinical pharmacokinetics: an introduction. New York: M Dekker. [Google Scholar]
- Panda S. (2016) Circadian physiology of metabolism. Science 354:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, Kayumov L. (2007) Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Progr Neuro Psychopharm Biol Psych 31:1-11. [DOI] [PubMed] [Google Scholar]
- Radloff LS. (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement 1:385-401. [Google Scholar]
- Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. (2014) Diurnal spectral sensitivity of the acute alerting effects of light. Sleep 37(2):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SA, Marcu S, Shapiro CM, Brown TJ, Casper RF. (2011) Spectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposure. Am J Physiol Endocrinol Metab 300:E518-E527. [DOI] [PubMed] [Google Scholar]
- Rea MS, Figueiro MG. (2018) Light as a circadian stimulus for architectural lighting. Light Res Technol 50:497-510. [Google Scholar]
- Revell VL, Arendt J, Fogg LF, Skene DJ. (2006) Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett 399:96-100. [DOI] [PubMed] [Google Scholar]
- Rüger M, St Hilaire MA, Brainard GC, Khalsa SBS, Kronauaer RE, Cziesler CA, Lockley SW. (2013) Human phase response curve to a single 6.5 hour pulse of short wavelength light. J Physiol 591:353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souman JL, Borra T, de Goijer I, Schlangen JLM, Vlaskamp BNS, Lucassen MP. (2018) Spectral tuning of white light allows for strong reduction in melatonin suppression without changing illumination level or color temperature. J Biol Rhythms 33:420-431. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. (2014) Breast cancer and circadian disruption from electric lighting in the modern world. Ca Cancer J Clin 64:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 15:261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US National Toxicology Program (2018) Draft report on carcinogens monograph on night shift work and light at night. US National Institute for Environmental Health Sciences. https://ntp.niehs.nih.gov/ntp/about_ntp/monopeerrvw/2018/october/landraftmonograph20180824.pdf
- Viola AU, James LM, Schlangen LJM, Dijk D-J. (2008) Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health 34(4):297-306. [DOI] [PubMed] [Google Scholar]
- Wong Y. (2012) A retinal ganglion cell that can signal irradiance continuously for 10 hours. J Neurosci 32:11478-11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska-Dabkowska KM. (2018) Make lighting healthier. Nature 553:274-276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Circadan_Potency_Supplementary_Material_March_11_2020 for Circadian Potency Spectrum with Extended Exposure to Polychromatic White LED Light under Workplace Conditions by Martin Moore-Ede, Anneke Heitmann and Rainer Guttkuhn in Journal of Biological Rhythms