Abstract
Purpose:
The optimal management of CNS relapse of rhabdomyosarcoma is unclear. We examined diagnosis, management, and outcomes of rhabdomyosarcoma patients developing CNS relapse.
Methods:
Records of 23 patients diagnosed with CNS relapse between 1999–2016 were reviewed. Median age at presentation of CNS relapse was 15 years (range, 1–34 years). High-risk features at initial presentation were as follows: 16 alveolar patients, 13 Stage IV, and 13 with primary tumor in parameningeal locations.
Results:
CNS relapse occurred at a median 12 months (range, 1–23 months) from diagnosis and most common presenting symptoms were headache (n=9), nausea/vomiting (n=8), visual difficulty (n=5), and none (n=5). Leptomeningeal metastases were detected in 21 patients while only 2 developed parenchymal metastases without leptomeningeal involvement. Fifteen patients received CNS-directed RT, including craniospinal irradiation to a median 36 Gy (range, 18–36 Gy) and/or whole brain radiotherapy to a median 30 Gy (range, 6–41.4 Gy). Three patients received concurrent chemotherapy. Follow-up MRI was conducted in 13 patients after RT initiation with 8 demonstrating improvement, 2 with stable disease, and 3 with progression. Twelve patients were tested for reactivity to I-131-labeled monoclonal antibody 8H9, and three tested positive and received at least 1 intra-Ommaya dose; all three lived >12 months post-CNS relapse. Twenty-one patients died of CNS disease and two of metastatic disease at other sites. Median survival post-CNS relapse was 5 months (range, 0.1–49 months).
Conclusions:
The prognosis for rhabdomyosarcoma patients with CNS relapse remains poor. Treatment including CNS-directed RT should be considered and investigation into preventative therapies is warranted.
Keywords: rhabdomyosarcoma, radiation therapy, CNS relapse, immunotherapy
Introduction
Rhabdomyosarcoma (RMS) is a malignant tumor arising from undifferentiated skeletal muscle cells and most often occurs in the head and neck, genitourinary system, and extremities. CNS relapse is a particular concern for RMS originating at parameningeal or paraspinal sites, given ease of access by direct extension. [1–4] However, CNS relapse has also been noted for RMS originating at sites outside of the head and neck, suggesting hematogenous spread. [5–7] While RMS commonly presents with metastases to the lungs, pleura, bones, and marrow, metastasis to the CNS at diagnosis is virtually unheard of. [7–10]
Even with treatment, patients with CNS relapse are unlikely to survive >10 weeks from diagnosis. [4] Optimal management of CNS relapse is difficult, but a multimodal approach using a combination of surgery, stereotactic radiosurgery (SRS), radiotherapy, or systemic or intrathecal chemotherapy is generally offered. [11,12] Radiation treatment may involve whole brain RT (WBRT) and/or craniospinal irradiation (CSI). Though studies from large cooperative groups have characterized the development of CNS relapse in RMS, little information is available on outcomes after treatment. We therefore report our institutional experience on the diagnosis, management, and outcomes of RMS patients developing CNS relapse.
Methods
Patient population
This is a single-institution cohort of RMS patients diagnosed with CNS relapse between 1999 and 2016 after initial presentation without CNS involvement. Only patients with alveolar or embryonal histology were included. After approval by our Institutional Review Board, 23 patients were identified.
The basic characteristics of the 23 patients are shown in Table 1. On initial presentation, the median age was 14 years (range, 1–33 years) and 8 patients were ≥21 years of age. Twelve patients were male and eleven were female. Histology was alveolar in 16 patients and embryonal in 7 patients. Staging was performed using the Intergroup Rhabdomyosarcoma Study Group (IRSG) pretreatment clinical staging system; ten patients were Stage III and thirteen were Stage IV, none with CNS metastasis at diagnosis. Thirteen patients had parameningeal primaries, all with negative cytology on lumbar puncture (LP) at presentation; of these, twelve had intracranial extension and ten had ≥1 cranial neuropathies. One patient presented with an intradural extramedullary embryonal tumor in the spinal canal below the spinal cord. The remaining patients had alveolar tumors: 6 extremity, 1 paraspinal, 1 upper abdominal wall, and 1 with unknown primary location. All patients with parameningeal, spinal canal, and paraspinal primary tumors had negative MRI brain ± spine and CSF cytology at diagnosis. Patients with tumors at other primary sites did not routinely receive MRI brain/spine or CSF cytology but had complete staging with CT chest/abdomen/pelvis and whole body PET/CT.
Table 1:
Patient population characteristics
| Characteristuc | n (%) |
|---|---|
| Total patients | 23 |
| Age at CNS relapse diagnosis (y) | |
| Median (range) | 15 (1–34) |
| Sex | |
| Female | 11 (46%) |
| Histology | |
| Alveolar | 16 (70%) |
| Embryonal | 7 (30%) |
| Primary site | |
| Parameningeal | 13 (57%) |
| Intracranial extension* | 12 (92%) |
| Cranial neuropathy* | 10 (77%) |
| Extremity | 6 (26%) |
| Spinal/paraspinal | 2 (9%) |
| Abdominal wall | 1 (4%) |
| Unknown | 1 (4%) |
| Stage* | |
| III | 10 (43%) |
| IV | 13 (57%) |
| Clinical group* | |
| 3 | 10 (42%) |
| 4 | 13 (57%) |
on initial presentation
Management of primary disease
Of the 23 patients, 4 received upfront subtotal or total resection of primary tumor. All 23 patients received multi-agent chemotherapy and 22 were treated on or as per institutional or Children’s Oncology Group (COG) intermediate- and high-risk protocols, which featured more aggressive modifications to the VAC (vincristine, dactinomycin, and cyclophosphamide) backbone conventionally used in RMS treatment. [13–16] One patient was treated with 3 cycles of ifosfamide/doxorubicin before presenting to our institution and receiving a revised pathologic diagnosis of RMS. Fourteen patients had completed all cycles of initial treatment with chemotherapy prior to CNS relapse while nine patients relapsed before completion. Due to toxicity, 11 patients experienced chemotherapy delays of >1 week and 19 patients required at least 1 chemotherapy dose reduction.
Twenty patients received RT for their primary tumor to a median 50.4 Gy (range, 36–50.4 Gy). Nineteen patients were able to receive the full prescribed RT dose and one patient had transportation difficulties and received hypofractionated RT to a dose of 47.5 Gy in lieu of 50.4 Gy. In 2 parameningeal patients who had achieved a complete response to initial cycles of chemotherapy, reduced doses of 36 and 45 Gy were used. The median duration between starting chemotherapy and radiotherapy was 15 weeks (range, 2–22 weeks; Table 2) for all patients and 14 weeks (range, 2–22 weeks) for parameningeal RMS patients. Eighteen patients completed RT without interruptions while two patients required treatment breaks (2 and 5 days) for chemotherapy-related infection. Of the 3 patients not receiving RT as part of upfront therapy for primary tumor, 1 had diffuse metastases on initial presentation with unclear primary tumor site, 1 relapsed in the CNS upon presentation to our institution and had not yet received definitive RT, and 1 declined RT.
Table 2:
Characteristics of RMS patients developing CNS metastases
| Age*/Sex | Primary site | Stage / Clinical group* | Histology* | Intra-cranial extension* | Cranial neuro-pathy* | Upfront RT-chemo start interval (wk) | Site(s) of CNS relapse | Time to CNS relapse from dx (mo) | Method of CNS relapse detection** | CNS-directed treatment | Follow-up MRI response after RT initiated | Time to death from CNS relapse (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameningeal primary | ||||||||||||
| 2/F | Petrous bone | III/3 | Embryonal | Yes | Yes | 2 | L | 9 | MRI | None | 0.3 | |
| 3/M | Temporal bone | IV/4 | Embryonal | Yes | Yes | 14 | L | 16 | MRI, LP | CSI | Worsened | 3 |
| 2/F | Infratemporal fossa | IV/4 | Embryonal | Yes | No | 22 | L | 9 | MRI | Chemo | 2 | |
| 4/F | Infratemporal fossa | IV/4 | Embryonal | Yes | No | 14 | L+P | 6 | MRI, Bx | None | 0.3 | |
| 7/F | Infratemporal fossa | III/3 | Embryonal | Yes | Yes | 15 | L | 13 | MRI | None | 0.2 | |
| 6/M | Ethmoid sinus | III/3 | Embryonal | Yes | Yes | 3 | L | 10 | MRI, LP | None | 3 | |
| 31/M | Ethmoid sinus | III/3 | Alveolar | No | No | 10 | L+P | 23 | MRI, LP | WBRT | Improved | 5 |
| 14/F | Maxillary sinus | III/3 | Alveolar | Yes | Yes | 17 | L | 7 | MRI, LP | Intrathecal chemo | 0.5 | |
| 24/F | Maxillary sinus | III/3 | Alveolar | Yes | Yes | 17 | L | 16 | MRI | Chemo, CSI | Improved | 11 |
| 33/M | Frontal sinus | III/3 | Alveolar | Yes | No | 15 | L+P | 13 | MRI, Bx | Surgery, CSI, SRS, 8H9 | Improved | 13 |
| 19/M | Paranasal sinus | III/3 | Alveolar | Yes | Yes | 12 | L,P† (6 mo) | 18 | MRI | CSI | Improved | 8 |
| 24/M | Paranasal sinus + orbit | IV/4 | Alveolar | Yes | Yes | 11 | L+P | 10 | MRI | WBRT (partial course) | 0.2 | |
| 29/F | Paranasal sinus | IV/4 | Alveolar | Yes | Yes | 14 | L | 12 | MRI | Chemo, CSI | Worsened | 5 |
| Extremity primary | ||||||||||||
| 1/M | Buttock | III/3 | Alveolar | 16 | P | 12 | MRI | WBRT | Improved | 11 | ||
| 1/F | Anterior thigh | III/3 | Alveolar | 21 | P,L† (6 mo) | 12 | CT, MRI, Bx | Surgery, CSI, WBRT | Stable | 5 | ||
| 20/M | Hand | IV/4 | Alveolar | 15 | L | 11 | MRI | WBRT | 2 | |||
| 17/M | Forearm | IV/4 | Alveolar | 17 | L+P | 22 | MRI, Bx | Surgery, CSI, WBRT, 8H9 | Improved | 18 | ||
| 27/M | Forearm | IV/4 | Alveolar | 14 | L | 10 | MRI, LP | CSI | Improved | 11 | ||
| 22/M | Forearm | IV/4 | Alveolar | 29 | L | 10 | MRI, LP | CSI | Worsened | 5 | ||
| Other primary | ||||||||||||
| 1/F | Spinal canal | IV/4 | Embryonal | (No RT) | L | 1 | MRI, LP | CSI | Stable | 7 | ||
| 4/F | Paraspinal | IV/4 | Alveolar | 17 | P | 21 | MRI, Bx | Surgery, WBRT, chemo, 8H9 (1 dose) | Improved | 50 | ||
| 13/F | Unknown | IV/4 | Alveolar | (No RT) | L | 17 | Clinical | None | 0.1 | |||
| 26/M | Abdominal wall | IV/4 | Alveolar | (No RT) | L | 11 | MRI | None | 0.1 | |||
On initial presentation.
Only methods for which positive findings were noted are listed.
Developed sequential relapse, displayed in order of presentation with duration in between relapses denoted in parenthesis.
L = leptomeningeal relapse, P = parenchymal relapse, L+P = combined relapse at presentation. Bx = brain biopsy, MRI = magnetic resonance imaging, LP = lumbar puncture, CSI = craniospinal irradiation, WBRT = whole brain radiotherapy, SRS = stereotactic radiosurgery, 8H9 = Intra-Ommaya 131I-8H9, wk = weeks, mo = months.
Diagnosis of CNS relapse
Work-up for patients suspected of having CNS relapse consisted of MRI of the brain and/or spine, which was performed in all but 2 patients: 1 presented with hemorrhagic parenchymal lesions on non-contrast head CT and 1 could not be scanned due to prohibitive anxiety and died shortly after clinical diagnosis. Confirmatory pathology by CSF cytology and/or brain biopsy was performed in 16 patients.
Statistical analysis
Statistical analysis was performed with Stata Version 13.0 (StataCorp, College Station, TX, USA). Survival post-CNS relapse was calculated from the time of diagnosis of CNS relapse to death of any cause. The Kaplan-Meier method was used to assess survival post-CNS relapse. Survival curves among different patient subgroups (age<10 vs. ≥ 10 years, histology, use of CNS-directed RT, use of radioimmunotherapy) were compared with the log-rank test. A p-value of ≤0.05 was used to determine statistical significance for all comparisons.
Results
Development of CNS relapse
Patient-level details regarding CNS relapse are shown in Table 2. CNS relapse first occurred at a median 12 months (range, 1–23 months) after initial diagnosis. Time to CNS relapse was not significantly different between parameningeal and non-parameningeal patients.
The most common presenting symptoms were headache (n=9), nausea/vomiting (n=8), visual difficulties (n=5), speech difficulty (n=3), pain (n=3), seizures (n=3), and weakness (n=3). Five patients had no symptoms and were diagnosed after routine MRI. Twenty-one patients developed leptomeningeal relapse, of which seven also developed parenchymal relapse. Two patients developed parenchymal relapse only. CSF cytology was analyzed for 14 patients with 7 testing positive. Brain biopsy was positive in all 5 patients for whom it was performed.
In 5 patients, CNS relapse occurred at the same time as or shortly after relapse at another site, suggestive of active systemic disease: 1 at the primary site (infratemporal fossa), 1 at distant nodes, 1 at osseous sites, 1 at bilateral breasts, and 1 at multiple osseous, pulmonary, and nodal sites. For the remaining 18 patients who presented without evidence of systemic disease, 9 had not yet completed all planned cycles of chemotherapy as part of definitive management at the time of CNS relapse while 9 patients had completed definitive treatment.
At our institution, the incidences of CNS relapse for parameningeal and extremity RMS patients from 1999–2016 were 18% and 13%, respectively. Two patients with spinal canal and paraspinal region primary sites presented to our institution after CNS relapse.
Management of CNS relapse
An overview of patient-level management is displayed in Table 2. Of the 15 patients who presented with leptomeningeal disease without parenchymal involvement at the time of first CNS relapse, 4 received CSI, 1 received CSI + parietal lobe boost, 2 received CSI + concurrent chemotherapy, 1 received WBRT, 1 received intrathecal etoposide but expired 1 week after starting treatment, 1 received a 5-day course of systemic cyclophosphamide and etoposide and expired 3 weeks after completing treatment, and 5 expired before any CNS-directed therapy. Of the 3 patients who presented with parenchymal disease without leptomeningeal involvement at the time of first CNS relapse, 1 received parenchymal surgical excision + CSI + posterior fossa boost, 1 received parenchymal surgical excision + WBRT + concurrent chemotherapy + 1 dose of 131I-8H9, and 1 received WBRT. Of the 5 patients who presented with combined leptomeningeal and parenchymal disease, 1 received parenchymal surgical excision + CSI + SRS + 2 doses of 131I-8H9, 1 received parenchymal surgical excision + CSI + frontal lobe boost + 2 doses of 131I-8H9, 1 received WBRT + temporal lobe boost, 1 received a partial WBRT course, and 1 expired before any CNS-directed therapy.
Median doses for craniospinal RT, WBRT, and RT boost were 36 Gy (range, 18–36 Gy), 30 Gy (range, 6–41.4 Gy), and 48.6 Gy (range, 36–50.4 Gy). Agents used for concurrent chemotherapy are as follows: 1 patient received oral etoposide, 1 received cyclophosphamide, vinorelbine, and temsirolimus, and 1 received irinotecan and temozolomide. Among 15 total patients receiving CNS-directed RT, follow-up MRI was conducted in 13 patients. The median duration to follow-up MRI was 8 weeks (range, 1–15 weeks) after RT initiation. Eight patients demonstrated radiographic improvement, 2 had stable disease, and 3 had continued progression. B7-H3 expression was tested for using direct tissue sampling from parenchymal biopsy in 5 patients and using CSF sampling in all 7 patients with positive cytology. Of these, 3 (60%) tested positive on parenchymal biopsy and 0 tested positive on CSF sampling. Patients testing positive received intra-Ommaya monoclonal antibody 131I-8H9; of these, 2 received the complete course of 2 doses and 1 patient received 1 dose due to persistent thrombocytopenia. These patients received 131I-8H9 at 11, 19, and 20 months after completion of upfront RT and 1.6, 1.1, and 2.4 months after completion of CNS-directed RT.
Clinical outcomes
Twenty-one patients expired due to CNS metastatic disease. Median survival post-CNS relapse was 5 months (range, 0.1–49 months; Figure 1). Survival post-CNS relapse at 12 months was 13% (n=3); two patients experienced combined leptomeningeal and parenchymal relapse, and one experienced parenchymal relapse only. All 3 had surgical resection of parenchymal lesions, CNS-directed RT, and at least 1 dose of intra-Ommaya 131I-8H9.
Figure 1:
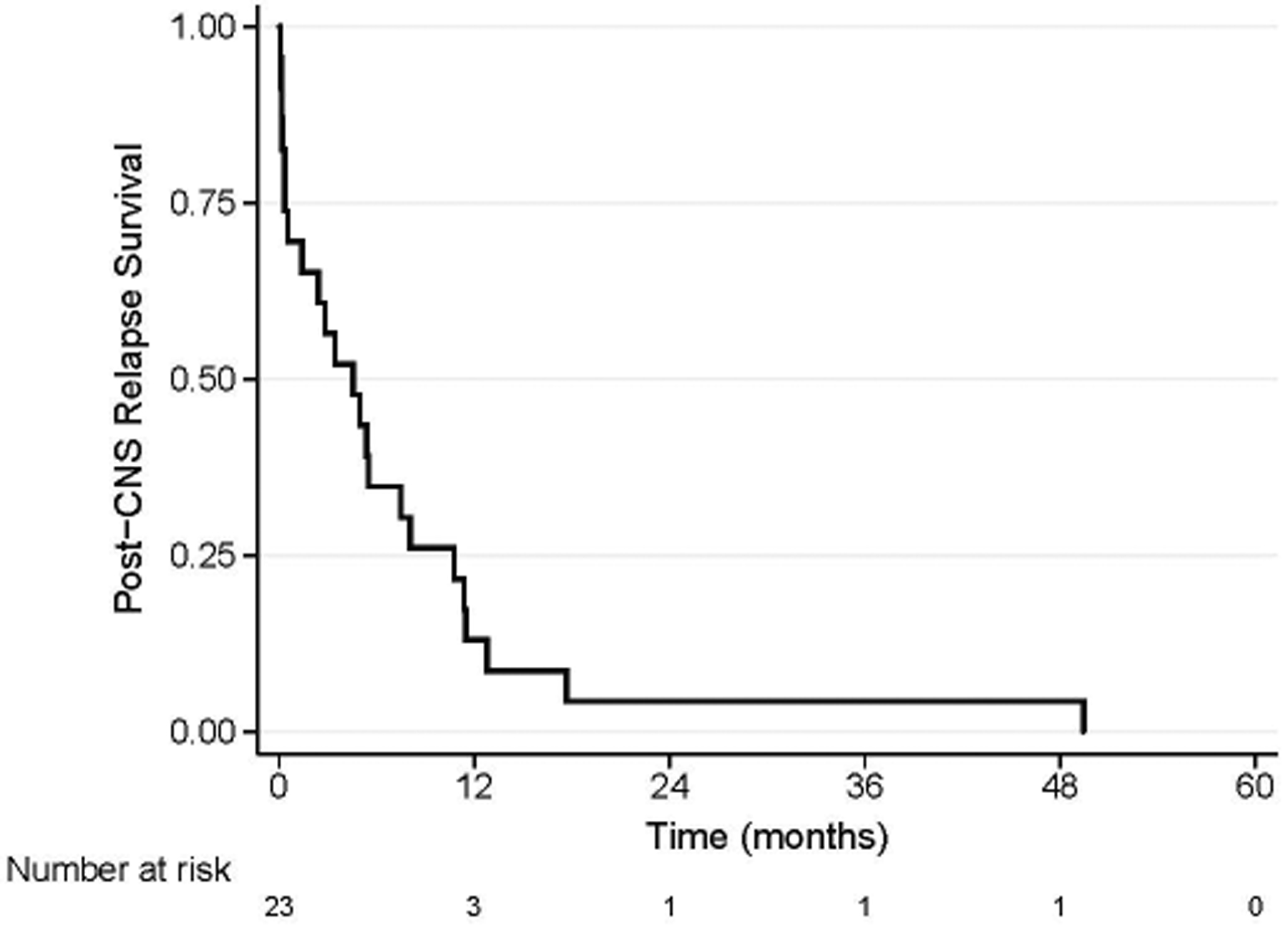
Kaplan-Meier curves for all patients included in study. Median survival post-CNS relapse was 5 months (range, 0.1–49 months).
Two patients with extremity primaries died after widespread systemic recurrence with apparently controlled CNS disease; one patient who received intra-Ommaya 131I-8H9 developed widespread recurrence after leptomeningeal metastasis but did not demonstrate CNS disease on post-mortem analysis and the other patient expired 18 months after CNS relapse due to septic shock following bone marrow transplant for relapsed treatment-associated acute myelogenous leukemia (t-AML).
On log-rank analysis, superior survival was noted for patients receiving intra-Ommaya monoclonal antibody 131I-8H9 (p=0.003) and CNS-directed RT (p=0.00004) (Figure 2). Differences in survival post-CNS relapse across age, stage, histology, and primary location were not significant. Patients with parameningeal primary tumors were re-treated to a median maximal cumulative brain dose of 81 Gy (range, 56.4–86.4 Gy); no short-term complications of RT were noted.
Figure 2:
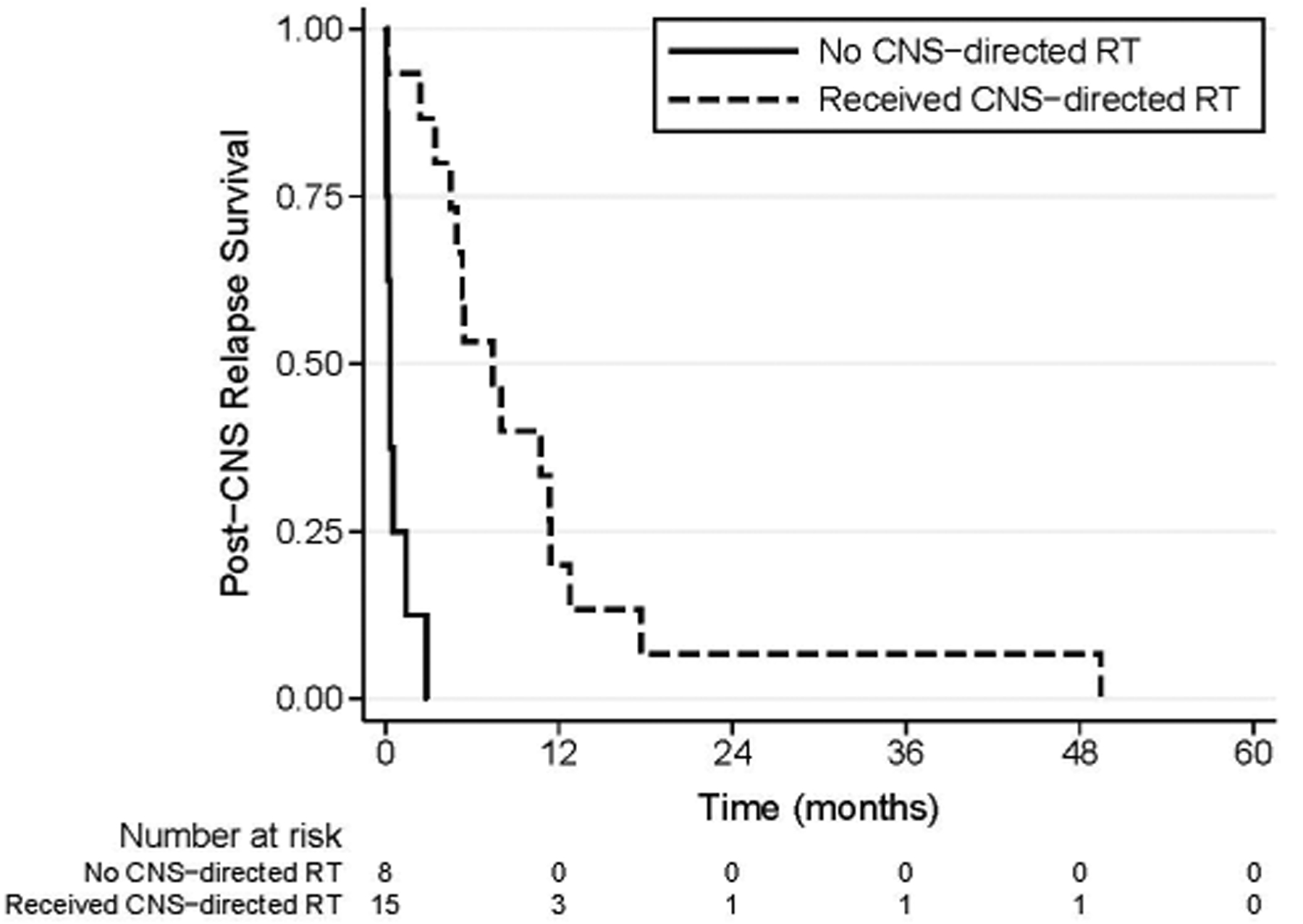
Survival post-CNS relapse in patients receiving CNS-directed RT versus those not receiving CNS-directed RT.
Discussion
Parameningeal and extremity locations are considered unfavorable primary sites for RMS. The Intergroup Rhabdomyosarcoma Study Group reports that <7% of localized parameningeal RMS patients develop CNS relapse, usually in the setting of initial high risk features of intracranial extension and cranial nerve palsy. [4] In our sample, most patients with parameningeal primaries initially presented with intracranial extension (92%) and cranial nerve palsies (77%), reinforcing the prognostic importance of these attributes. We report incidences of CNS relapse for parameningeal and extremity RMS of 18% and 13%, respectively, concordant with previous reports from our institution. [3,17] Several factors may explain a high rate of CNS relapse of RMS at our institution as compared to published results from IRS II-IV protocols [4], such as inclusion of patients with Stage IV/Group 4 disease in our analysis, higher risk patients (e.g. greater proportion of parameningeal patients with intracranial extension and/or cranial neuropathies, outside referrals with suboptimal RMS management based on an incorrect diagnosis, etc.), and inclusion of adults. For RMS, adults are known to have a consistently worse prognosis than children. Potential reasons for a high rate of CNS relapse in adults include more frequent tumor location at an unfavorable site, lower tolerance to intensive treatment, and more pronounced expression of multidrug-resistance proteins, which may undermine the efficacy of chemotherapy. [18,19]
While chemotherapy interruptions and dose reductions were common in the current study, few patients receiving RT for primary disease required breaks or dose reduction, suggesting that inadequate primary tumor treatment did not contribute significantly to CNS relapse. For parameningeal patients, the recommended timing for RT varies according to clinical protocols and continues to be studied in ongoing cooperative group trials. [11,20–22] Despite the presence of high-risk features, including intracranial extension, parameningeal patients in the current study started RT at a median 14 weeks after starting chemotherapy; it is unclear how this contributed to CNS relapse. Further data from ongoing protocols are needed to clarify the impact of delaying RT on CNS relapse.
Our results illustrate varied presentation of CNS relapse of RMS. While 18 patients (78%) presented with symptoms, many of these were non-specific, including headache, nausea/vomiting, and pain. More specific neurologic signs such as new-onset cranial nerve palsies, seizures, and weakness were seen in a minority of patients. Furthermore, 5 patients in our sample (22%) were asymptomatic and found to have CNS metastases on routine MRI. Given that many cases present without characteristic neurologic findings, a high index of suspicion may be needed to make a prompt diagnosis of CNS relapse.
Definitive diagnosis of CNS relapse can be challenging given that some patients experience particularly rapid decline; 7 of the 23 patients in our sample expired within 1 month of CNS relapse. The majority of diagnoses were made on MRI of the brain and/or spine but diagnosis was made clinically in 1 emergent case and on the basis of hemorrhagic lesions seen on non-contrast CT in another. Neurosurgical biopsy was positive in all 5 cases in which it was performed, but CSF cytology was positive in only 7 of the 14 patients who received LP. This suggests that LP may be poorly sensitive for diagnosis of leptomeningeal disease, and that clinical and MRI findings should still be considered in the absence of positive cytology.
In this cohort, treatment of parenchymal brain metastasis generally consisted of surgical resection, SRS, WBRT, and/or CSI for patients with coexisting leptomeningeal disease. Treatment of leptomeningeal disease generally consisted of CSI and/or systemic or intrathecal chemotherapy. Patients showed favorable response to both modalities of CNS-directed RT as evidenced by follow-up MRI. Patients who received CNS-directed RT did significantly better than those who did not (Figure 2), though patients not receiving RT may have been selected against due to rapidly declining medical conditions and demise before planned therapeutic intervention. However, these findings taken together in the context of lack of sequelae and significant experience from other institutions [12] support the use of CNS-directed RT in a timely manner when possible, with the caveat that no treatment, including CNS-directed RT, was associated with an increased chance of cure.
Choice of RT dose and field at CNS relapse can be challenging, particularly for patients who have already received RT to a parameningeal site. In our cohort, patients with diffuse leptomeningeal metastases ± parenchymal metastases were generally treated with CSI to 36 Gy. Patients with diffuse parenchymal metastases were offered WBRT to 30–36 Gy. Areas of gross parenchymal disease were boosted with an additional 14–20 Gy. In addition to CSI, one patient with discrete, unresectable parenchymal metastases received SRS to 18 Gy, an approach that has shown promise in oligometastatic sarcomatous CNS disease. [23] Use of radioimmunotherapy did not influence RT dose or field, and no RT-related complications were noted in patients who received both treatments. RT fields at relapse were chosen to minimize overlap with previous fields, particularly in the brainstem. However, given the poor prognosis and often emergent condition of these patients, greater consideration was given to palliation of symptoms than to potential long-term complications. While no serious acute complications related to brain RT were identified after cumulative doses up to 86.4 Gy, the majority of patients (8/13) survived less than 3 months, thus making it impossible to evaluate the longer-term effects of such high doses. As improved treatments enable longer-term survival, further investigation will be needed to determine safe dose thresholds to avoid serious complications of RT such as myelitis and necrosis. Conformal RT techniques, such as proton CSI, may help limit overlap and reduce toxicities; their use warrants consideration in these patients. [24,25]
For patients testing positive for B7-H3 expression, a surface immunomodulatory glycoprotein preferentially expressed on some solid tumors, intra-Ommaya monoclonal antibody 131I-8H9 has been proposed as a potentially effective therapy and a Phase I trial examining its use in CNS/leptomeningeal neoplasms is underway (NCT00089245). [26] Several studies have examined rates of B7-H3 expression on primary tumor tissue samples of RMS; one reported that 67% (6/9) expression and another study from our institution reported 97% (28/29) expression. [27,28] The proportion of patients in our study who tested positive for B7-H3 expression was 60% (3/5) on parenchymal biopsy and 0% (0/7) on CSF sampling, for a combined rate of 25% (3/12) -- considerably lower than historical controls. One explanation for the observed discrepancy is that LP was used to assess for antibody reactivity in the majority of patients in our study. This technique may have inadequately sampled cells, resulting in a poorly sensitive assay which did not yield any positive results. Conversely, a greater proportion of patients receiving direct tissue sampling via parenchymal biopsy tested positive. While studies have shown high expression of B7-H3 on primary RMS, it remains unclear whether B7-H3 expression is conserved for metastatic RMS; reduced expression on metastatic RMS would potentially limit the applicability of 131I-8H9 for RMS patients with CNS relapse. Future protocols may benefit from direct tumor sampling at the time of CNS relapse or from refinement of the assay to permit studying paraffin embedded tumor samples rather than requiring fresh tissue. In addition, the role of B7-H3 testing at the time of initial diagnosis and the conservation of its expression at relapse also needs further investigation.
The 3 patients who received at least one dose of intra-Ommaya monoclonal antibody 131I-8H9 lived longer than those who did not, with all surviving >1 year after CNS relapse. However, these patients may have been selected for based on favorable response to RT, surgically amenable disease, and less aggressive disease course, enabling survival long enough to be receive additional treatment. All 3 patients received surgical resection of well-demarcated parenchymal metastases as well as CNS-directed RT, and showed favorable response on MRI. Nonetheless, continued investigation into the efficacy and safety of intra-Ommaya monoclonal antibody 131I-8H9 is needed to further characterize its benefit to RMS patients with CNS relapse.
Previously, WBRT and/or high-dose intrathecal chemotherapy were used upfront to prevent CNS relapse in patients presenting with parameningeal RMS with high-risk features. [8] However, data showing treatment-related toxicities in patients receiving intrathecal chemotherapy and unclear benefit of whole brain RT led to discontinuation of CNS-directed prophylaxis. [29,30] In the context of the high fatality of CNS relapse in RMS patients, we encourage continued investigation into novel CNS-directed therapies for high-risk patients.
While aggressive treatment may be appropriate for some patients, palliative care is important for all patients with CNS relapse of RMS. Studies examining end-of-life care in pediatric CNS malignancies highlight the impact of debilitating symptoms including drowsiness, loss of consciousness, poor communication, focal neurologic deficits, seizures, dysphagia, and headaches. Potential interventions include steroids, anti-epileptic drugs, analgesics, hydration, anti-emetics, urinary catheterization, CSF diversion, and palliative sedation. [31,32] Palliation of symptoms with chemotherapy and/or RT may be considered for pulmonary metastases and RT may be considered in cases of cord compression or bony metastases. [33,34] Treating clinicians need to delicately discuss symptoms and potential treatments to inform goals of care discussions. Multidisciplinary care involving palliative medicine physicians, nurses, pain specialists, and therapists may help to support the physical, emotional, social, and spiritual needs of patients and their families. [35]
Limitations of our study include the small sample size, retrospective design, heterogeneous patient population and management, selection bias, and probable confounders. The true clinical impact of CNS-directed RT and intra-Ommaya 131I-8H9 is difficult to assess through this retrospective analysis. However, the data presented raise interest for future investigation via a prospective study and may guide clinicians seeking to discuss treatment options with patients and their families. Treatment including CNS-directed RT should be considered in RMS patients demonstrating CNS relapse and further investigation into preventative therapies is warranted.
Acknowledgements
Funding was provided by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748). This work is also supported by a gift from Jack and Susan Rudin. This study was presented at the 99th Annual Meeting of the American Radium Society (ARS), Colorado Springs, CO on May 6-9, 2017.
Abbreviations used:
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CSI
Craniospinal irradiation
- LP
Lumbar puncture
- MRI
Magnetic resonance imaging
- RMS
Rhabdomyosarcoma
- RT
Radiation therapy
- SRS
Sterotactic radiosurgery
- WBRT
Whole brain radiotherapy
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- [1].Gerson JM, Jaffe N, Donaldson MH Tefft M. Meningeal seeding from rhabdomyosarcoma of the head and neck with base of the skull invasion: recognition of the clinical evolution and suggestions for management. Med Pediatr Oncol 1978;5:137–144. [DOI] [PubMed] [Google Scholar]
- [2].Salunke P, Sura S, Gupta K, Tripathi M Aggarwal A. Middle ear rhabdomyosarcoma infiltrating the petrous with diffuse leptomeningeal spread in a child. J Pediatr Neurosci 2012;7:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang JC, Wexler LH, Meyers PA Wolden SL. Parameningeal rhabdomyosarcoma: outcomes and opportunities. Int J Radiat Oncol Biol Phys 2013;85:e61–66. [DOI] [PubMed] [Google Scholar]
- [4].Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978–1997. Med Pediatr Oncol 2002;38:22–32. [DOI] [PubMed] [Google Scholar]
- [5].Kline RM, Oseas RS, Jolley SG, et al. Leptomeningeal metastasis from a paraspinal rhabdomyosarcoma with a der(13)t(1;13)(q23;q32) in a 14-month-old boy. Cancer Genet Cytogenet 1997;98:97–101. [DOI] [PubMed] [Google Scholar]
- [6].Arush MW, Kollender Y, Issakov J, et al. Unusual leptomeningeal dissemination in a child with extracranial metastatic alveolar rhabdomyosarcoma. Pediatr Hematol Oncol 2009;26:473–478. [DOI] [PubMed] [Google Scholar]
- [7].Shweikeh F, Bukavina L, Saeed K, et al. Brain metastasis in bone and soft tissue cancers: a review of incidence, interventions, and outcomes. Sarcoma 2014;2014:475175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raney RB Jr., Tefft M, Newton WA, et al. Improved prognosis with intensive treatment of children with cranial soft tissue sarcomas arising in nonorbital parameningeal sites. A report from the Intergroup Rhabdomyosarcoma Study. Cancer 1987;59:147–155. [DOI] [PubMed] [Google Scholar]
- [9].Kebudi R, Ayan I, Gorgun O, et al. Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol 2005;71:43–48. [DOI] [PubMed] [Google Scholar]
- [10].Raney RB Jr., Tefft M, Maurer HM, et al. Disease patterns and survival rate in children with metastatic soft-tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer 1988;62:1257–1266. [DOI] [PubMed] [Google Scholar]
- [11].Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys 2004;59:1027–1038. [DOI] [PubMed] [Google Scholar]
- [12].Parasuraman S, Langston J, Rao BN, et al. Brain metastases in pediatric Ewing sarcoma and rhabdomyosarcoma: the St. Jude Children’s Research Hospital experience. J Pediatr Hematol Oncol 1999;21:370–377. [DOI] [PubMed] [Google Scholar]
- [13].Dharmarajan KV, Wexler LH Wolden SL. Concurrent radiation with irinotecan and carboplatin in intermediate- and high-risk rhabdomyosarcoma: a report on toxicity and efficacy from a prospective pilot phase II study. Pediatr Blood Cancer 2013;60:242–247. [DOI] [PubMed] [Google Scholar]
- [14].Irinotecan and Carboplatin as Upfront Window Therapy in Treating Patients With Newly Diagnosed Intermediate-Risk or High-Risk Rhabdomyosarcoma: https://ClinicalTrials.gov/show/NCT00077285.
- [15].Group CsO Institute NC. High-Dose Combination Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Metastatic Rhabdomyosarcoma or Ectomesenchymoma: https://ClinicalTrials.gov/show/NCT00354744, 2006.
- [16].Group CsO Institute NC. Combination Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Rhabdomyosarcoma: https://ClinicalTrials.gov/show/NCT00354835, 2006.
- [17].Gerber NK, Wexler LH, Singer S, et al. Adult rhabdomyosarcoma survival improved with treatment on multimodality protocols. Int J Radiat Oncol Biol Phys 2013;86:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol 2009;27:3391–3397. [DOI] [PubMed] [Google Scholar]
- [19].Komdeur R, Klunder J, van der Graaf WT, et al. Multidrug resistance proteins in rhabdomyosarcomas: comparison between children and adults. Cancer 2003;97:1999–2005. [DOI] [PubMed] [Google Scholar]
- [20].Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys 2013;87:512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Douglas JG, Arndt CA Hawkins DS. Delayed radiotherapy following dose intensive chemotherapy for parameningeal rhabdomyosarcoma (PM-RMS) of childhood. Eur J Cancer 2007;43:1045–1050. [DOI] [PubMed] [Google Scholar]
- [22].Puri DR, Wexler LH, Meyers PA, et al. The challenging role of radiation therapy for very young children with rhabdomyosarcoma. Int J Radiat Oncol Biol Phys 2006;65:1177–1184. [DOI] [PubMed] [Google Scholar]
- [23].Flannery T, Kano H, Niranjan A, et al. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys 2010;76:513–519. [DOI] [PubMed] [Google Scholar]
- [24].Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016;17:287–298. [DOI] [PubMed] [Google Scholar]
- [25].Song S, Park HJ, Yoon JH, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol 2014;53:1158–1164. [DOI] [PubMed] [Google Scholar]
- [26].Orentas RJ, Lee DW Mackall C. Immunotherapy Targets in Pediatric Cancer. Front Oncol 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gregorio A, Corrias MV, Castriconi R, et al. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008;53:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Modak S, Kramer K, Gultekin SH, Guo HF Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001;61:4048–4054. [PubMed] [Google Scholar]
- [29].Gasparini M, Lombardi F, Gianni MC, et al. Questionable role of CNS radioprophylaxis in the therapeutic management of childhood rhabdomyosarcoma with meningeal extension. J Clin Oncol 1990;8:1854–1857. [DOI] [PubMed] [Google Scholar]
- [30].Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major Lessons From the IRS-I Through IRS-IV Studies as Background for the Current IRS-V Treatment Protocols. Sarcoma 2001;5:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Walbert T Khan M End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. J Neurooncol 2014;117:217–224. [DOI] [PubMed] [Google Scholar]
- [32].Kuhlen M, Hoell J, Balzer S, Borkhardt A Janssen G. Symptoms and management of pediatric patients with incurable brain tumors in palliative home care. Eur J Paediatr Neurol 2016;20:261–269. [DOI] [PubMed] [Google Scholar]
- [33].Casey DL, Wexler LH, Meyers PA, et al. Radiation for bone metastases in Ewing sarcoma and rhabdomyosarcoma. Pediatr Blood Cancer 2015;62:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grimer R, Judson I, Peake D Seddon B. Guidelines for the Management of Soft Tissue Sarcomas. Sarcoma 2010;2010:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol;8:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]


