Abstract
Tinnitus is a widespread auditory disorder affecting approximately 10-15% of the population, often with debilitating consequences. Although tinnitus commonly begins with damage to the auditory system due to loud-noise exposure, aging, or other etiologies, the exact neurophysiological basis of chronic tinnitus remains unknown. Many researchers point to a central auditory origin of tinnitus; however, a growing body of evidence also implicates other brain regions, including the limbic system. Correspondingly, we and others have proposed models of tinnitus in which the limbic and auditory systems both play critical roles and interact with one another. Specifically, we argue that damage to the auditory system generates an initial tinnitus signal, consistent with previous research. In our model, this “transient” tinnitus is suppressed when a limbic frontostriatal network, comprised of ventromedial prefrontal cortex and ventral striatum, successfully modulates thalamocortical transmission in the auditory system. Thus, in chronic tinnitus, limbic-system damage and resulting inefficiency of auditory-limbic interactions prevents proper compensation of the tinnitus signal. Neuroimaging studies utilizing connectivity methods like resting-state fMRI and diffusion MRI continue to uncover tinnitus-related anomalies throughout auditory, limbic, and other brain systems. However, directly assessing interactions between these brain regions and networks has proved to be more challenging. Here, we review existing empirical support for models of tinnitus stressing a critical role for involvement of “non-auditory” structures in tinnitus pathophysiology, and discuss the possible impact of newly refined connectivity techniques from neuroimaging on tinnitus research.
Keywords: Tinnitus, auditory, limbic, frontostriatal, MRI, connectivity
1. Introduction
Chronic subjective tinnitus is a common auditory disorder in which patients experience ringing or buzzing “in the ear” in the absence of an external source of that perceived sound. There is a wealth of evidence linking tinnitus to dysfunction throughout the auditory system (Eggermont and Roberts, 2004; Roberts et al., 2010). However, an ever-growing number of studies, typically utilizing neuroimaging in humans, have identified tinnitus-related differences in function and anatomy outside central auditory pathways, particularly in structures considered to be part of the limbic system. Even if one were to assume that these limbic changes are the consequence (not the cause) of tinnitus, it seems that understanding central auditory dysfunction alone may not be sufficient to understand chronic tinnitus. We have previously proposed that chronic tinnitus is, in fact, caused by compromised limbic fronto-striato-thalamic circuits, which result in disordered evaluation of the tinnitus sensation’s perceptual relevance and, thus, disordered gain control of the tinnitus percept within thalamo-cortical auditory networks [Figure 1; (Leaver et al., 2011; Mühlau et al., 2006; Rauschecker et al., 2010)]. Although fronto-striatal circuits and other limbic structures may also regulate emotion and mood (Bar, 2009; Blood et al., 1999; Ressler and Mayberg, 2007), their involvement in tinnitus pathophysiology suggests they may be part of a more general “appraisal network,” determining which sensations are of value, and ultimately affecting how (or whether) those sensations are experienced (Breiter et al., 2001; Kable and Glimcher, 2009). Although details vary, several other prominent theories of tinnitus pathophysiology also propose network-level disturbances involving brain regions both within and outside of the central auditory system (De Ridder et al., 2011; Eggermont and Roberts, 2004; Jastreboff, 1990; Levine et al., 2003; Møller, 2003). Most of the underlying data, however, consist of (highly variable) localized activations, so, clearly, there is a need for research examining the potentially complex interactions between brain regions and networks.
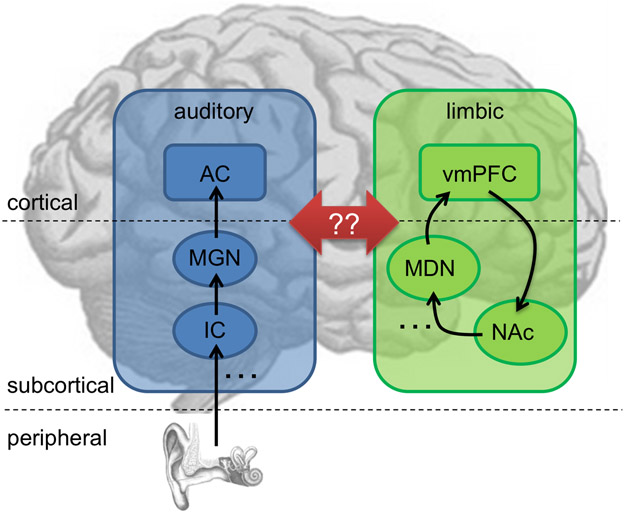
Figure 1. A schematic model of auditory-limbic interactions in tinnitus.
In our model of tinnitus, dysregulation of the auditory system by specific structures of the limbic system is what causes subjective tinnitus to become chronic (see Rauschecker et al., 2010; Leaver et al., 2011). Specifically, peripheral deafferentation of the central auditory pathway (shown in blue) causes increased activity leading to tinnitus via lesion-induced plasticity (Rauschecker, 1999). Typically, transient tinnitus can be assessed by limbic frontostriatal networks (green) as an unwanted and/or irrelevant stimulus (Leaver et al., 2011), and thus suppressed. In patients with chronic tinnitus, this regulatory mechanism does not function properly (Rauschecker et al., 2010): a volume loss is consistently found in the ventromedial prefrontal cortex (vmPFC; Mühlau et al., 2006; Leaver et al., 2011, 2012), and hyperactivity is found in the nucleus accumbens (NAc; Leaver et al., 2011). However, as indicated by the red arrows, exactly how and whether the auditory and limbic networks interact in the context of tinnitus remains to be determined. The initial tinnitus signal could enter limbic networks via projections from the auditory thalamus (MGN, medial geniculate nucleus) and/or auditory cortex (AC) to the amygdala and NAc, which is a part of the ventral striatum (LeDoux et al., 1991)], but may also enter through projections between AC and vmPFC (Romanski et al., 1999)]. Similarly, limbic structures could suppress auditory activity via projections between the vmPFC and MGN [via the thalamic reticular nucleus, (Zikopoulos and Barbas, 2006)]; however, suppression may also occur via the medial dorsal nucleus [MDN; (Pandya et al., 1994; Tanibuchi and Goldman-Rakic, 2003)]. Studies are sorely needed to test this and other models of tinnitus pathophysiology. Note that the placement of brain regions on this schematic is approximate and not intended to be anatomically accurate. Left hemisphere is shown; posterior is on the left; anterior on the right.
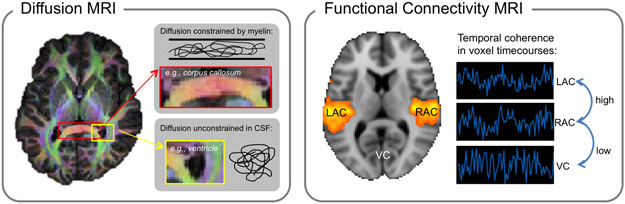
Connectivity analyses of human neuroimaging data will be critical for testing these current models of tinnitus, and for ultimately achieving a network-level understanding of tinnitus neuropathophysiology. Diffusion and functional resting-state connectivity magnetic resonance imaging (MRI) are relatively new techniques that allow inferences about anatomical (diffusion) and functional (resting-state) connections and relationships between brain structures (Figure 2). Diffusion MRI measures water diffusion to infer direction and density of white matter tracts in vivo (Le Bihan, 2003; Pierpaoli et al., 1996); functional connectivity MRI measures temporal coherence in brain activity to infer functional connections between brain areas (Fox and Raichle, 2007). Similar functional connectivity analyses are also applied to EEG and MEG data, in which relationships are measured between brain regions, albeit with coarser spatial resolution. There has been an explosion in the use of both of these techniques in tinnitus research in recent years (Boyen et al., 2014; Crippa et al., 2010; Husain and Schmidt, 2014; Mahoney et al., 2011; Maudoux et al., 2012a, 2012b; Seydell-Greenwald et al., 2014b). However, although connectivity studies support existing evidence of anatomical and functional anomalies in specific isolated regions, using these techniques to verify the complex network dysfunction between regions proposed by current tinnitus models continues to present significant challenges. Therefore, it remains unclear what influence, if any, tinnitus-related anomalies in limbic and other non-auditory brain structures have on auditory-system dysfunction in chronic tinnitus. In this review, we first outline current evidence from human neuroimaging supporting the involvement of auditory and non-auditory structures in tinnitus pathophysiology, with emphasis placed on our own contributions, as supported by the Tinnitus Research Consortium for this Special Issue of Hearing Research. Then, we discuss the extent to which this and other evidence supports the idea that tinnitus pathophysiology involves disordered connections between auditory, limbic, and other brain systems, including a final discussion of the impact of ever-evolving techniques for connectivity neuroimaging and analysis.
Figure 2. Methodological approaches to connectivity MRI.
Diffusion MRI (left) measures the strength and directionality of water diffusion. Color overlaid on the brain at left indicates the strongest direction of diffusion. For example, red marks strong diffusion in the left-right direction through major white matter tracts of the corpus callosum where axons are oriented in the same direction (top inset). Regions that do not have a color indicate instances of relatively unconstrained diffusion, for example through cerebrospinal fluid (CSF) in ventricles (bottom inset). Functional connectivity MRI (right) identifies regions with temporally coherent (i.e., correlated) fMRI activity. For example, activity in left and right auditory cortex (LAC and RAC, respectively) is typically highly coherent, as indicated by the orange color overlaid on the brain image. By contrast, fMRI activity in auditory cortex and visual cortex (VC) will have lower temporal coherence. To illustrate this relationship, example voxel time-courses are shown at right, where LAC and RAC time-courses are more correlated with each other than with the VC time-course.
2. Neuroimaging evidence for central auditory system dysfunction in tinnitus
The etiology of tinnitus often involves damage to auditory hair cells due to loud noise exposure, aging, drugs, and other factors. Correspondingly, a body of research is devoted to understanding peripheral mechanisms of tinnitus (Kaltenbach et al., 2002), as well as restoring peripheral function to treat tinnitus and hearing loss (Cox et al., 2014; Mizutari et al., 2013). However, as tinnitus may also originate at points further along the auditory pathways [e.g., from somatosensory interference at the brainstem; (Levine et al., 2003; Shore et al., 2007; Shore, 2011)], and because the mechanisms of tinnitus chronicity are thought to involve the central nervous system (Eggermont and Roberts, 2004; Jastreboff, 1990; Møller, 2003), there is much interest in understanding the series of events that generate and perpetuate the tinnitus signal within central auditory pathways.
Generally speaking, deafferentation caused by peripheral damage is thought to elicit increases in spontaneous and synchronous neuronal activity (Eggermont and Roberts, 2004; Llinás et al., 1999; Roberts et al., 2010) and/or reorganization of tonotopic maps (Eggermont and Komiya, 2000; Irvine et al., 2003; Langers et al., 2012; Mühlnickel et al., 1998; Rajan et al., 1993; Weisz et al., 2005; Wienbruch et al., 2006) in central auditory structures, which is perceived as tinnitus. In human neuroimaging research using blood-oxygenation-level-dependent (BOLD) fMRI, this central auditory dysfunction is typically measured using stimulus-evoked activity. Our own work has demonstrated increased stimulus-evoked BOLD responses in auditory cortex in two separate cohorts of tinnitus patients. Specifically, when presented with simple, narrow-band sounds matched to the center frequency of their tinnitus, patients exhibited moderately increased BOLD responses, or “hyperactivity,” in early auditory cortex on or near medial Heschl’s gyrus (i.e. primary-like or core areas of auditory cortex) compared to control volunteers without tinnitus and/or hearing loss (Leaver et al., 2011; Seydell-Greenwald et al., 2012). We have also reported complementary effects in the auditory system with diffusion MRI of white-matter microstructure, indicating increased white-matter integrity near medial Heschl’s gyrus and inferior colliculus in tinnitus patients (Seydell-Greenwald et al., 2014b). Our results are in line with MRI studies from other groups reporting similar effects in the central auditory system, including sound-evoked hyperactivity in auditory cortex (Gu et al., 2010) and inferior colliculus (Melcher et al., 2009), as well as reduced grey matter in auditory cortex [(Schneider et al., 2009), cf. (Boyen et al., 2012)] and inferior colliculus (Landgrebe et al., 2009). Taken together, these imaging results support several possible auditory-system contributions to tinnitus pathophysiology identified in previous studies. Hyperactivity and increased white-matter integrity in higher central auditory structures are consistent with increased input and/or spontaneous firing in neurons of these regions, perhaps caused by deafferentation of the cochlear nuclei. Decreased grey matter is also consistent with increased firing of neurons resulting in excitotoxicity and cell death.
The consensus across these studies points to tinnitus-related hyperactivity to sound in auditory cortex and inferior colliculus, though effects in the inferior colliculus might be better explained by hyperacusis (Gu et al., 2010), which has not always been controlled thoroughly (Landgrebe et al., 2009; Leaver et al., 2011; Melcher et al., 2009; Seydell-Greenwald et al., 2014b, 2012). However, it is notable that not all fMRI studies of tinnitus demonstrate sound-evoked hyperactivity in the auditory system (Boyen et al., 2014). Furthermore, it is difficult to determine whether hyperactivity on any level represents the actual tinnitus signal. For example, auditory cortex dysfunction could merely be a consequence of a tinnitus signal generated at lower levels of the auditory pathway (e.g., the cochlear nucleus) not captured by these neuroimaging studies. Conversely, hyperactivity in the inferior colliculus might be relayed back from auditory cortex. Thus, the location and nature of dysfunction that ultimately generates the chronic tinnitus percept may differ from the site and nature of initial damage, which itself may vary across patients (Henry et al, 2005). So, although converging evidence in both human and animal research points to increased spontaneous and sound-evoked activity in tinnitus throughout the auditory system, the precise series of events leading to tinnitus has yet to be elucidated. In the following sections, we discuss two major additional challenges facing fMRI tinnitus research.
2.1. Interpreting stimulus-evoked fMRI in tinnitus research
Studying stimulus-evoked neural activity using BOLD fMRI allows researchers to noninvasively examine the functional consequences of tinnitus in the brain, as the tinnitus percept itself is difficult to measure objectively. However, there are several factors to consider when interpreting the results of these studies. First, stimulus-evoked activity is unlikely to be equivalent to activity corresponding to the tinnitus itself. Unlike invasive electrophysiology or positron emission tomography (PET), which measure absolute levels of neural activity and/or metabolism directly, BOLD fMRI is restricted to making inferences regarding neural activity by comparing stimulus-evoked and baseline levels of signal (Logothetis, 2008). Thus, BOLD fMRI is unable to detect continuously elevated levels of baseline activity as one would predict in chronic tinnitus and as clearly demonstrated in animal research (Eggermont and Roberts, 2004; Eggermont, 2012; Roberts et al., 2010). In addition, stimulus-evoked BOLD activity may be differentially influenced by other factors in tinnitus patients and controls. Attentional resources may be allocated differently for tinnitus patients (Roberts et al., 2013), who must ignore their tinnitus in order to perform auditory tasks, even during simple “passive listening” tasks. MRI scanner noise, as well as the auditory stimuli used in tinnitus research, can both have variable effects on patients’ tinnitus sensations (Tyler et al., 2008), which potentially complicates the interpretation of stimulus-evoked fMRI even further.
These factors were demonstrated quite clearly in our most recent fMRI study conducted by Seydell-Greenwald et al. (2012). In this study, we replicated stimulus-evoked hyperactivity in auditory cortex of tinnitus patients (Gu et al., 2010; Leaver et al., 2011). However, in a simple yet important departure from previous tinnitus fMRI studies, we measured BOLD signal as it unfolded over several seconds in response to auditory stimuli. During each task trial, volunteers were either presented with a brief auditory stimulus or a stimulus-absent trial where no sound was presented. Unexpectedly, control participants exhibited significantly larger BOLD responses to stimulus-absent trials in auditory cortex than tinnitus patients, while the amplitude of BOLD responses during trials with auditory stimuli did not differ between groups. Thus, tinnitus-related “hyperactivity” in auditory cortex (i.e., a larger difference between the BOLD response in trials with and without auditory stimulation) was actually driven by decreased BOLD signal during silent, stimulus-absent trials in patients. BOLD fMRI responses in stimulus-absent trials have been elicited in previous studies in both the visual [e.g., (Kastner et al., 1999)] and auditory domains [e.g., (Seydell-Greenwald et al., 2014a)] and most likely reflect attentional modulation of neural activity in sensory cortices. Other studies previously reporting auditory-cortex hyperactivity (Gu et al., 2010; Leaver et al., 2011) might also be affected by this phenomenon.
This pattern of results could be explained in multiple ways, which are not mutually exclusive. BOLD responses to “missing” sound during stimulus-absent trials could be dampened by increases in ongoing spontaneous activity due to tinnitus sensations, which reduce the overall responsiveness of auditory cortex in tinnitus patients. This increased “baseline” spontaneous activity could similarly dampen BOLD responses to sound trials, thus also obscuring true hyper-responsiveness to sound in tinnitus patients. Attenuated BOLD responses to silent trials could also reflect taxed attentional resources in tinnitus patients; top-down modulation of auditory cortex activity corresponding to the anticipation of task trials could be greater in controls than in patients. In both cases, however, it is unclear why BOLD responses were not similarly reduced during trials in which sounds were presented in patients relative to controls. Future work studying individuals with intermittent tinnitus, or using imaging techniques that are able to measure neural and/or metabolic activity directly (e.g., PET or arterial-spin-labeled perfusion MRI), may be of use in this regard.
2.2. Addressing the effects of hearing loss and age
Accounting for the possible influences of hearing loss, age, and hyperacusis is critical to tinnitus research; the potential effects of hearing loss on tinnitus-related anomalies in the auditory system are particularly important to assess. Hearing loss can be interpreted as a correlate of peripheral or central auditory system damage and/or dysfunction, the latter of which is a critical component of all current theories of tinnitus pathophysiology. Therefore, we and others have found it critical to statistically control for levels of hearing impairment in both tinnitus patients and controls. However, standard audiometry of even an extended range of frequencies (i.e. >8 kHz) may not capture all types of hearing impairment [e.g., (Weisz et al., 2006)], and it is difficult to know what aggregate measure from pure-tone audiometry is most appropriate [e.g., average of entire spectrum or over certain frequency ranges, (Melcher et al., 2012)]. Also, the prevalence of tinnitus increases with age, possibly but not necessarily due to increased incidences of hearing loss (Heller 2003; Eggermont and Roberts 2004). This means that these factors are often intercorrelated in study participants and can therefore affect the validity of statistical models that include them both as covariates (Farrar and Glauber, 1967). Indeed, we have even identified reduced white-matter integrity in early auditory cortex near medial Heschl’s gyrus specific to mean hearing loss when controlling for tinnitus status (Seydell-Greenwald et al., 2014b). Melcher and colleagues (2012) have further demonstrated that hearing loss can affect neuroimaging results that could otherwise be attributed to tinnitus. By separating the mean hearing loss measure into different frequency bins (i.e., <2, 2-8, >8 kHz), this study suggested that vmPFC grey-matter reductions in tinnitus patients, which had also been observed in other studies (Boyen et al., 2012; Leaver et al., 2012, 2011; Mühlau et al., 2006), may not be wholly attributable to tinnitus but also to negative correlations with hearing thresholds >8 kHz [perhaps particularly so in tinnitus patients; (Melcher et al., 2012)]. Clearly, controlling for heterogeneity in hearing impairment is critical in tinnitus research. This may only be possible in multi-site studies where sample sizes are large enough to accommodate statistical models with multiple nuisance covariates regarding hearing loss, age, hyperacusis, and other measures.
3. Neuroimaging evidence of limbic anomalies in tinnitus
From among the first neuroimaging studies in this field, researchers have identified tinnitus-related anomalies outside the auditory system (Lockwood et al., 1998; Mirz et al., 2000; Shulman et al., 1995), often in structures connected with the “limbic system” (see definition below). In our hands, extra-auditory tinnitus effects most consistently fall within ventromedial prefrontal cortex (Leaver et al., 2012, 2011; Mühlau et al., 2006; Seydell-Greenwald et al., 2014b, 2012), but also ventral striatum (Leaver et al., 2011), posterior thalamus (Mühlau et al., 2006), and dorsal prefrontal regions (Leaver et al., 2012; Seydell-Greenwald et al., 2012). Other groups have reported complementary tinnitus-related effects in these same regions [e.g., ventromedial prefrontal cortex (Boyen et al., 2012; Schlee et al., 2009); striatum (Cheung and Larson, 2010; Maudoux et al., 2012b; Reyes et al., 2002)]. However, several other brain regions have been implicated in tinnitus as well, including parahippocampal regions (Landgrebe et al., 2009; Maudoux et al., 2012b; Mirz et al., 2000; Ueyama et al., 2013) and posterior cingulate cortex (Maudoux et al., 2012a; Ueyama et al., 2013). The challenge in interpreting these extra-auditory tinnitus effects lies in determining whether they are associated with the generation and chronification of the tinnitus signal itself, or whether they are associated with tinnitus-related reactions and/or compensations on the part of patients (or other factors often concomitant with tinnitus, like hearing loss and hyperacusis, as discussed above). In particular, given that a large percentage of these extra-auditory effects fall within limbic structures, careful assessment of tinnitus-related distress and symptoms of depression and anxiety is an important consideration in human tinnitus research as well.
3.1. Examining the relationship between distress and tinnitus in neuroimaging research
The term “limbic system” was originally defined as a circuit supporting emotion, connecting the hippocampal formation, hypothalamus, cingulate and adjacent prefrontal cortex (MacLean, 1949, 1952). It is now also used to refer to other regions implicated in emotion processing like the amygdala and ventral striatum [e.g. LeDoux et al., 1991 (Catani et al., 2013)]. Many of the same limbic regions implicated in tinnitus research listed above have also been implicated in the experience and regulation of affect, emotion, and mood (de Gelder et al., 2011; Drevets et al., 1997; Mayberg, 1997). Perhaps not surprisingly then, many models of tinnitus pathophysiology propose that negative or emotional reactions to tinnitus may be involved in causing the disorder to become chronic (De Ridder et al., 2011; Jastreboff, 1990). Undoubtedly, tinnitus can be associated with stress and negative mood (De Ridder et al., 2011; Dobie, 2003; Jastreboff, 1990; Schecklmann et al., 2011; Sullivan et al., 1988) and increased incidence of depression or dysphoria (Folmer et al., 1999). However, there is substantial variability in the extent to which tinnitus patients experience stress and/or mood disturbances (Eggermont and Roberts, 2004; Heller, 2003), and levels of distress do not successfully predict the sensory severity of the tinnitus itself [e.g., loudness or intermittent presence; (Leaver et al., 2012; Meyer et al., 2014)]. Furthermore, given that few neuroimaging studies of tinnitus have adequately assessed emotional symptoms and/or recruited study samples with sufficiently broad levels of distress, the role of the limbic system in tinnitus perception vs. tinnitus distress is far from clear.
In our research, we have attempted to address this issue by distinguishing the extent to which tinnitus-related differences in limbic anatomy, function, and connectivity are correlated with suffering and depression concomitant with tinnitus, from their correlation with perceptual characteristics of the tinnitus itself independent of tinnitus distress. This is of particular importance to the tinnitus-related differences we have identified in ventromedial prefrontal cortex and ventral striatum (Leaver et al., 2012, 2011; Mühlau et al., 2006; Seydell-Greenwald et al., 2014b, 2012). Both these structures have been targets of deep brain stimulation for major depression (Lozano et al., 2008; Schlaepfer et al., 2008), and both are thought to play major roles in the neural basis of mood disorders along with other regions and networks (Bar, 2009; Gotlib and Joormann, 2010; Price and Drevets, 2012; Ressler and Mayberg, 2007). With regard to their role in tinnitus, we have demonstrated reduced grey matter in the ventromedial prefrontal cortex in three separate cohorts of tinnitus patients (Leaver et al., 2012, 2011; Mühlau et al., 2006). In Mühlau et al. (2006) and Leaver et al. (2011), measures of tinnitus-related distress or anxiety/depression scores were not available; therefore, it was impossible to determine from those studies whether anatomical differences were due to chronic tinnitus, or to increased levels of stress, anxiety, and/or depression in tinnitus patients (though these two cohorts were matched in other domains like age, sex, and mean hearing loss). Therefore, in our more recent cohort, we measured levels of tinnitus distress [Tinnitus Handicap Inventory, (Newman et al., 1996)], anxiety [Generalized Anxiety Disorder scale, GAD-7 (Spitzer et al., 2006)], and depression [Patient Health Questionnaire 9, PHQ-9 (Spitzer et al., 1999)], in order to examine the extent to which tinnitus-related neuroimaging findings could be explained by these factors.
In Leaver et al. (2012), decreased grey matter in ventromedial prefrontal cortex of tinnitus patients could not be explained by ongoing tinnitus distress, depression, or anxiety scores. In this same cohort, we also demonstrated differences in stimulus-evoked activity and white-matter microstructure in the same region of ventromedial prefrontal cortex (Seydell-Greenwald et al., 2014b, 2012). In all three studies, the effects in ventromedial prefrontal cortex were correlated with patients’ ratings of tinnitus loudness, and not with measures of tinnitus distress, depression, or anxiety. The latter scores were, however, correlated with brain markers in other regions. Most notably, we identified a negative correlation between depression and anxiety scores and cortical thickness in a separate region near subgenual anterior cingulate cortex (Leaver et al., 2012): regardless of tinnitus status, participants with higher depression/anxiety scores had thinner cortex among both tinnitus patients and controls, compatible with studies reporting similar decreases in patients with major depressive disorder (Drevets et al., 1997; Mayberg, 1997). Other parts of the brain are similarly related to measures of tinnitus-related distress and do not exhibit large differences between tinnitus patients and controls, like the anterior insula (Leaver et al., 2012; Vanneste et al., 2010) and anterior and posterior cingulate cortex (Joos et al., 2012; Maudoux et al., 2012a; Ueyama et al., 2013; Vanneste et al., 2010).
Preliminarily, our results suggest that the neural basis of tinnitus-related suffering seems to be separate from the neural basis of the tinnitus signal itself. If this is correct, the acoustic-perceptual characteristics of tinnitus and tinnitus-related suffering should be targeted separately during treatment. However, future research specifically targeting cohorts with concomitant tinnitus and high levels of tinnitus-related distress and/or mood disorder are needed to properly address this hypothesis.
3.2. Cognitive and attentional compensation in tinnitus neuroimaging
Other cognitive and attentional processes beyond stress and affect are clearly relevant to tinnitus. As mentioned in Section 2.1, patients must deal with the tinnitus percept as it interferes with incoming auditory stimuli in their daily lives. Indeed, some have argued that the tinnitus signal is under attentional control (Roberts et al., 2013), and some therapies teach patients to use attentional and cognitive strategies to lessen the impact of tinnitus (Jastreboff, 2007). Without a doubt, neuroimaging findings in regions outside the ascending auditory pathway may support these processes. For example, we have reported correlations between patient-reported tinnitus loudness and increases in stimulus-evoked fMRI signal in lateral prefrontal cortex (Seydell-Greenwald et al., 2012). Given the role the lateral prefrontal cortex plays in conscious and effortful cognitive control (Miller and Cohen, 2001; Miyake et al., 2000), this effect most likely represents the increased effort needed to ignore a loud tinnitus signal during our auditory task. As another example, our group and others have noted a relationship between tinnitus and increased activity in posterior auditory cortex (Giraud et al., 1999; Leaver et al., 2011; Lockwood et al., 2001; Reyes et al., 2002), which has been implicated in separating multiple auditory signals [e.g., listening to a single voice at a cocktail party; (Alain et al., 2005; Wilson et al., 2007)]. In these examples, different cognitive processes (and associated neural substrates) direct attention away from the tinnitus signal to other sensory events and may temporarily attenuate the tinnitus percept. However, these mechanisms are likely different from those governing the modulation of the tinnitus signal in cases of increased stress or relaxation. We would further argue that such attentional shifts are fundamentally different from modulation of the gain of sensory activity responsible for perpetuating chronic subjective tinnitus as well, like those posed by our tinnitus model (Rauschecker et al., 2010). Clearly, there are several layers of cognitive control to consider in the context of tinnitus research, but a more thorough discussion of models of attention and cognitive control as they relate to tinnitus are outside the scope of the current review. Future studies that carefully control the contribution of these factors will be needed to test these models and hypotheses.
4. Neuroimaging auditory-limbic interactions in tinnitus
We have outlined evidence from our group and from others clearly demonstrating differences between tinnitus patients and controls in both the auditory system and in extra-auditory, mostly limbic-related brain systems. Some of this evidence is taken from studies using connectivity neuroimaging techniques, including diffusion MRI and resting-state fMRI. However, even studies using these connectivity techniques can be limited in their ability to directly measure the complex interactions purported to underlie tinnitus pathophysiology in current models of tinnitus. In this section, we describe current knowledge regarding tinnitus-related anomalies using connectivity MRI, with emphasis on our own work, and take a critical look at the extent to which current findings and techniques can speak to the role of auditory-limbic interactions in chronic tinnitus.
4.1. Contributions of diffusion MRI
Two general approaches have been taken when using diffusion MRI to analyze white matter. First, the strength and directionality of water diffusion can be used to infer white-matter integrity, either analyzed voxelwise or in predefined regions of interest (e.g., inferior colliculus) overlapping white matter. Second, the strength and directionality of diffusion can be used to reconstruct white-matter tracts, and inferences can be made from the reconstruction success regarding how well two regions are connected. With standard diffusion MRI, this latter approach can be challenging. Often, if fiber-tracking algorithms are not constrained anatomically, white-matter tracts can be “successfully” tracked into neurobiologically implausible paths (e.g., through ventricles or grey matter). Thus, fiber tracking using standard diffusion MRI may be best served when restricted to well-characterized white-matter tracts [e.g., (Crippa et al., 2010)]. However, even when making quantitative inferences using reconstructed tracts, or voxelwise in regions of interest, multiple interpretations of diffusion differences must be considered.
In our own diffusion MRI data (Seydell-Greenwald et al., 2014b), we reported tinnitus-related effects in both auditory and limbic regions; however, these results may not be directly indicative of altered auditory-limbic interactions in tinnitus. For example, we demonstrated a negative correlation between markers of white-matter integrity in ventromedial prefrontal cortex and patients’ ratings of tinnitus loudness. This regional effect is compatible with reduced output from ventromedial prefrontal cortex to the striatum and thalamus, and ultimately to the auditory system, as we have proposed previously; however it is impossible to discern the directionality or specificity of the connections underlying this effect. On the other hand, tinnitus-related effects we identified within major white-matter tracts may be more apt to speak to long-range connectivity. For example, negative correlations between tinnitus loudness and white-matter integrity (i.e., fractional anisotropy) in the anterior thalamic radiation (Seydell-Greenwald et al., 2014b) may reflect white-matter insufficiencies in patients with loud tinnitus (though no group difference was present) in tracts connecting anterior or medial thalamic nuclei and ventromedial prefrontal cortex. We identified this same relationship in the anterior/superior corona radiata, which could reflect a continuation of the effect in the anterior thalamic radiation, perhaps extending even further to the local effect within the ventromedial prefrontal cortex region of interest. Taken together, these data could indicate differences in thalamo-frontal connectivity related to tinnitus loudness, consistent with our model. Again, however, this effect cannot be tied to specific thalamic nuclei or prefrontal regions without more refined techniques.
4.2. Contributions of resting-state fMRI
Analyzing intrinsic activity during the “resting state” is a common way to assess functional brain networks in various disorders (Damoiseaux et al., 2012; Greicius et al., 2007; Raichle et al., 2001). In these studies, brain activity is measured while volunteers rest and do not perform an experimental task, and temporal coherence in the functional activity of brain regions is measured (Power et al., 2010; Smith et al., 2009). In the context of tinnitus research, resting-state fMRI could potentially be used to capture brain activity related to the tinnitus percept itself, because patients’ sensory-perceptual experience “at rest” is quite different from that of control volunteers who do not experience tinnitus. In this way, resting-state fMRI could theoretically identify the functional networks underlying the experience of chronic tinnitus, insofar as intrinsic activity within these networks was temporally coherent/correlated. Furthermore, because this approach compares patterns of relative coherent brain activity (as opposed to averaged activity) between tinnitus patients and controls, resting-state fMRI is not limited by the fact that BOLD fMRI must be used as a relative, and not absolute, measure of neural activity, as described above in Section 2.1 (Logothetis, 2008). Resting-state fMRI research could thus offer complementary information to previous fMRI studies of tinnitus, which rely on stimulus-evoked activity to make inferences regarding tinnitus-related activity (Gu et al., 2010; Leaver et al., 2011; Melcher et al., 2009, 2000; Seydell-Greenwald et al., 2012), and to EEG/MEG studies, which can also identify patterns of synchronous brain activity at rest albeit with coarser spatial resolution.
Despite the obvious importance of functional connectivity research to tinnitus, no clear consensus has yet emerged. Resting-state fMRI, EEG, and MEG studies have identified altered functional connectivity in several parts of the brain, including in auditory cortex (Burton et al., 2012; Kim et al., 2012; Maudoux et al., 2012a, 2012b; Vanneste et al., 2011a, 2011b), basal ganglia (Maudoux et al., 2012b), prefrontal cortex (Kim et al., 2012; Maudoux et al., 2012b; Schlee et al., 2009), parahippocampal regions (Maudoux et al., 2012a, 2012b; Schmidt et al., 2013; Vanneste et al., 2011a), and insula (Burton et al., 2012; Vanneste et al., 2011b). However, these results are not consistent across studies [e.g., see (Husain and Schmidt, 2014) for a recent review], and still others have found no differences in functional connectivity between tinnitus patients and controls (Davies et al., 2014; Wineland et al., 2012).
Divergent methodological approaches taken across studies might in part explain these inconsistencies. In resting-state studies, patterns of functional connectivity are compared between groups in networks of interest, defined either using a “seed” region [e.g., auditory cortex, (Burton et al., 2012; Schmidt et al., 2013; Wineland et al., 2012)] or using blind source-separation techniques like independent component analysis [e.g., auditory network, (Davies et al., 2014; Maudoux et al., 2012a, 2012b)]. In both cases, a given network of interest represents a group of brain regions with temporally coherent/correlated brain activity, and group differences are identified in brain regions that are more or less functionally connected to the network of interest. In this way, studies can differ both in their approach to defining networks of interest (seed region vs. independent component analysis) and in the assumptions made a priori regarding which brain regions and networks are most relevant to tinnitus. Approaches to preprocessing and noise reduction also vary, e.g., considering the effects of subject motion, vascular, respiratory, and other artifacts are of critical relevance, especially for seed-based analyses (Cole et al., 2010; Murphy et al., 2013). Furthermore, the resting-state networks studied may not necessarily reflect the precise networks hypothesized to be affected in tinnitus, but rather large-scale standardized networks like the entire auditory system or the “default-mode network,” which overlaps regions implicated in tinnitus like medial prefrontal and hippocampal regions (Raichle et al., 2001). EEG and MEG studies are more likely to use blind source-separation techniques to identify unique networks relevant for tinnitus [e.g., (Vanneste et al., 2014)]; however, many of these studies still rely on the same prior assumptions regarding regions of interest, and coarse spatial resolution is a concern. It also remains to be determined what relationship, if any, resting-state fMRI has with functional phenomena identified using other approaches (e.g., increased spontaneous firing of neurons, tonotopic map reorganization, or synchronous activity at finer timescales measured with EEG/MEG). Though clearly promising, findings from resting-state fMRI and other “functional connectivity” studies will be most informative when contextualized with respect to complementary findings in other modalities and model systems.
5. Conclusions and future directions
Although we have couched this review in terms of our own work and tinnitus model for this Special Issue of Hearing Research for the Tinnitus Research Consortium, the studies and issues discussed also have direct bearing on other models of tinnitus that involve the limbic system and extra-auditory regions (De Ridder et al., 2011; Dobie, 2003; Jastreboff, 1990; Landgrebe et al., 2009; Lockwood et al., 1998; Møller, 2003; Simpson and Davies, 2000). Despite the many challenges facing the pursuit of a network-level understanding of disorders like tinnitus, the field will continue to progress as tinnitus-related effects continue to be identified in the same brain regions and networks across methodologies, cohorts, and study sites. At the same time, methods for analyzing neuronal connectivity are improving rapidly and will spur future progress in tinnitus research, including cluster analysis (Pestilli et al., 2014; Wig et al., 2014) and graph theory (Maudoux et al., 2012a), which assess more complex metrics of network connectivity beyond relative temporal coherence. Multi-echo MRI sequences allow sampling at ultra-high resolution within shorter scan times than standard diffusion sequences [i.e., increased diffusion directions and spatial/temporal resolution (Uğurbil et al., 2013)], thus improving on characterization of white-matter connections in vivo and of effective directional functional relationships amongst network regions. New histological techniques like CLARITY allow visualization of neuronal connections in post-mortem tissue that is made transparent using acrylamide-based hydrogels (Chung et al., 2013). Combined with imaging it can render brain connectivity with even greater fidelity than studies using conventional neuroanatomical tracing.
Finally, it should be noted that, given variability in MRI scanners, sequence parameters, statistical approaches, and other methodological considerations across studies, some inconsistency in the brain regions linked to tinnitus by the neuroimaging literature is perhaps to be expected [e.g., (Adjamian et al., 2014)]. This only underscores the importance of emphasizing those brain regions exhibiting tinnitus-related effects across multiple imaging modalities, patient cohorts, study sites, and model systems (i.e., human vs. other animals). By continuing to identify commonalities across studies, by engaging in multi-site collaborative research, and by incorporating newly developing techniques, we will continue to refine our understanding of tinnitus pathophysiology in order to advance its treatment and, with hope, a cure.
Highlights.
We review evidence supporting auditory and limbic contributions to tinnitus pathophysiology
Assessing auditory-limbic interactions with connectivity neuroimaging is a challenge
Recent findings and new connectivity methods will impact models of chronic tinnitus
Acknowledgements:
This work was funded by the Tinnitus Research Consortium, National Institutes of Health (RC1-DC010720), American Tinnitus Association, and Tinnitus Research Initiative.
Abbreviations
- AC
auditory cortex
- BOLD
blood oxygenation level dependent
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- fMRI
functional magnetic resonance imaging
- GAD-7
generalized anxiety disorder questionnaire 7
- IC
inferior colliculus
- LAC
left auditory cortex
- MDN
mediodorsal nucleus
- MEG
magnetoencephalography
- MGN
medial geniculate nucleus
- MRI
magnetic resonance imaging
- NAc
nucleus accumbens
- PET
positron emission tomography
- PHQ-9
patient health questionnaire 9
- RAC
right auditory cortex
- VC
visual cortex
- vmPFC
ventromedial prefrontal cortex
References
- Adjamian P, Hall DA, Palmer AR, Allan TW, Langers DRM, 2014. Neuroanatomical abnormalities in chronic tinnitus in the human brain. Neurosci. Biobehav. Rev 45, 119–33. doi: 10.1016/j.neubiorev.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Reinke K, McDonald KL, Chau W, Tam F, Pacurar A, Graham S, 2005. Left thalamo-cortical network implicated in successful speech separation and identification. Neuroimage 26, 592–599. [DOI] [PubMed] [Google Scholar]
- Bar M, 2009. A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci 13, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC, 1999. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci 2, 382–387. [DOI] [PubMed] [Google Scholar]
- Boyen K, de Kleine E, van Dijk P, Langers DRM, 2014. Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear. Res 312, 48–59. doi: 10.1016/j.heares.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Boyen K, Langers DRM, de Kleine E, van Dijk P, 2012. Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hear. Res doi: 10.1016/j.heares.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P, 2001. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30, 619–639. [DOI] [PubMed] [Google Scholar]
- Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF, 2012. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. 13, 3. doi: 10.1186/1471-2202-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Dell’acqua F, Thiebaut de Schotten M, 2013. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev 37, 1724–37. doi: 10.1016/j.neubiorev.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Cheung SW, Larson PS, 2010. Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169, 1768–1778. [DOI] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim S-Y, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K, 2013. Structural and molecular interrogation of intact biological systems. Nature 497, 332–7. doi: 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF, 2010. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci 4, 8. doi: 10.3389/fnsys.2010.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen D-H, Chalasani K, Steigelman KA, Fang J, Rubel EW, Cheng AG, Zuo J, 2014. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141, 816–29. doi: 10.1242/dev.103036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa A, Lanting CP, van Dijk P, Roerdink JB, 2010. A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag J 4, 16–25. doi: 10.2174/1874440001004010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD, 2012. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol. Aging 33, 828.e19–30. doi: 10.1016/j.neurobiolaging.2011.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Gander PE, Andrews M, Hall DA, 2014. Auditory network connectivity in tinnitus patients: a resting-state fMRI study. Int. J. Audiol 53, 192–8. doi: 10.3109/14992027.2013.846482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder B, van Honk J, Tamietto M, 2011. Emotion in the brain: of low roads, high roads and roads less travelled. Nat. Rev. Neurosci 12, 1–2. doi: 10.1038/nrn2920-c1 [DOI] [PubMed] [Google Scholar]
- De Ridder D, Elgoyhen AB, Romo R, Langguth B, 2011. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. U. S. A 108, 8075–80. doi: 10.1073/pnas.1018466108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie RA, 2003. Depression and tinnitus. Otolaryngol Clin North Am 36, 383–388. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr., Todd RD, Reich T, Vannier M, Raichle ME, 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, 2012. Hearing loss, hyperacusis, or tinnitus: What is modeled in animal research? Hear. Res doi: 10.1016/j.heares.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H, 2000. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res 142, 89–101. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE, 2004. The neuroscience of tinnitus. Trends Neurosci 27, 676–682. [DOI] [PubMed] [Google Scholar]
- Farrar DE, Glauber RR, 1967. Multicollinearity in Regression Analysis: The Problem Revisited. Rev. Econ. Stat 49, 92–107. [Google Scholar]
- Folmer RL, Griest SE, Meikle MB, Martin WH, 1999. Tinnitus severity, loudness, and depression. Otolaryngol Head Neck Surg 121, 48–51. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–11. doi: 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Giraud AL, Chery-Croze S, Fischer G, Fischer C, Vighetto A, Gregoire MC, Lavenne F, Collet L, 1999. A selective imaging of tinnitus. Neuroreport 10, 1–5. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, 2010. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol 6, 285–312. doi: 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF, 2007. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–37. doi: 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR, 2010. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol 104, 3361–3370. doi:jn.00226.2010 [pii] 10.1152/jn.00226.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AJ, 2003. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am 36, 239–248. [DOI] [PubMed] [Google Scholar]
- Husain FT, Schmidt SA, 2014. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res 307, 153–62. doi: 10.1016/j.heares.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, Smith S, 2003. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol 467, 354–374. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, 2007. Tinnitus retraining therapy. Prog Brain Res 166, 415–423. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, 1990. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8, 221–254. [DOI] [PubMed] [Google Scholar]
- Joos K, Vanneste S, De Ridder D, 2012. Disentangling depression and distress networks in the tinnitus brain. PLoS One 7, e40544. doi: 10.1371/journal.pone.0040544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW, 2009. The neurobiology of decision: consensus and controversy. Neuron 63, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M, 2002. Cisplatin-Induced Hyperactivity in the Dorsal Cochlear Nucleus and Its Relation to Outer Hair Cell Loss: Relevance to Tinnitus. J Neurophysiol 88, 699–714. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG, 1999. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22, 751–61. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim Y, Lee S, Seo J-H, Song H-J, Cho JH, Chang Y, 2012. Alteration of functional connectivity in tinnitus brain revealed by resting-state fMRI?: A pilot study. Int. J. Audiol 51, 413–417. doi: 10.3109/14992027.2011.652677 [DOI] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G, 2009. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46, 213–218. [DOI] [PubMed] [Google Scholar]
- Langers DRM, de Kleine E, van Dijk P, 2012. Tinnitus does not require macroscopic tonotopic map reorganization. Front. Syst. Neurosci 6, 2. doi: 10.3389/fnsys.2012.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, 2003. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci 4, 469–80. doi: 10.1038/nrn1119 [DOI] [PubMed] [Google Scholar]
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP, 2011. Dysregulation of limbic and auditory networks in tinnitus. Neuron 69, 33–43. doi:S0896–6273(10)00987–6 [pii] 10.1016/j.neuron.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Seydell-Greenwald A, Turesky TK, Morgan S, Kim HJ, Rauschecker JP, 2012. Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci 6, 21. doi: 10.3389/fnsys.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM, 1991. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci. Lett 134, 139–144. doi: 10.1016/0304-3940(91)90526-Y [DOI] [PubMed] [Google Scholar]
- Levine RA, Abel M, Cheng H, 2003. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res 153, 643–648. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP, 1999. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A 96, 15222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW, 1998. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50, 114–120. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Wack DS, Burkard RF, Coad ML, Reyes SA, Arnold SA, Salvi RJ, 2001. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology 56, 472–480. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, 2008. What we can do and what we cannot do with fMRI. Nature 453, 869–78. doi: 10.1038/nature06976 [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH, 2008. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64, 461–7. doi: 10.1016/j.biopsych.2008.05.034 [DOI] [PubMed] [Google Scholar]
- MacLean PD, 1952. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (Visceral brain). Electroencephalogr. Clin. Neurophysiol 4, 407–418. doi: 10.1016/0013-4694(52)90073-4 [DOI] [PubMed] [Google Scholar]
- MacLean PD, 1949. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom. Med 11, 338–53. [DOI] [PubMed] [Google Scholar]
- Mahoney CJ, Rohrer JD, Goll JC, Fox NC, Rossor MN, Warren JD, 2011. Structural neuroanatomy of tinnitus and hyperacusis in semantic dementia. J. Neurol. Neurosurg. Psychiatry 82, 1274–8. doi: 10.1136/jnnp.2010.235473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudoux A, Lefebvre P, Cabay J-E, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A, 2012a. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 1485, 10–21. doi: 10.1016/j.brainres.2012.05.006 [DOI] [PubMed] [Google Scholar]
- Maudoux A, Lefebvre P, Cabay J-E, Demertzi A, Vanhaudenhuyse A, Laureys S, Soddu A, 2012b. Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS One 7, e36222. doi: 10.1371/journal.pone.0036222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, 1997. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9, 471–481. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Knudson IM, Levine RA, 2012. Subcallosal brain structure: Correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear. Res 295, 1–8. doi: 10.1016/j.heares.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B, 2009. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257, 63–74. doi:S0378–5955(09)00196–8 [pii] 10.1016/j.heares.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ Jr., Levine RA, 2000. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83, 1058–1072. [DOI] [PubMed] [Google Scholar]
- Meyer M, Luethi MS, Neff P, Langer N, Büchi S, 2014. Disentangling tinnitus distress and tinnitus presence by means of EEG power analysis. Neural Plast. 2014, 468546. doi: 10.1155/2014/468546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Ishizu K, Pedersen CB, 2000. Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol 543, 241–243. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, 2000. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn. Psychol 41, 49–100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge ASB, 2013. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58–69. doi: 10.1016/j.neuron.2012.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AR, 2003. Pathophysiology of tinnitus. Otolaryngol Clin North Am 36, 249–66, v–vi. [DOI] [PubMed] [Google Scholar]
- Mühlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschläger AM, Simon F, Etgen T, Conrad B, Sander D, 2006. Structural brain changes in tinnitus. Cereb Cortex 16, 1283–1288. [DOI] [PubMed] [Google Scholar]
- Mühlnickel W, Elbert T, Taub E, Flor H, 1998. Reorganization of auditory cortex in tinnitus. Proc. Natl. Acad. Sci. U. S. A 95, 10340–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA, 2013. Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–59. doi: 10.1016/j.neuroimage.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CW, Jacobson GP, Spitzer JB, 1996. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 122, 143–148. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Rosene DL, Doolittle AM, 1994. Corticothalamic connections of auditory-related areas of the temporal lobe in the rhesus monkey. J. Comp. Neurol 345, 447–71. doi: 10.1002/cne.903450311 [DOI] [PubMed] [Google Scholar]
- Pestilli F, Yeatman JD, Rokem A, Kay KN, Wandell BA, 2014. Evaluation and statistical inference for human connectomes. Nat. Methods 11, 1058–1063. doi: 10.1038/nmeth.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G, 1996. Diffusion tensor MR imaging of the human brain. Radiology 201, 637–48. doi: 10.1148/radiology.201.3.8939209 [DOI] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE, 2010. The development of human functional brain networks. Neuron 67, 735–748. doi:S0896–6273(10)00627–6 [pii] 10.1016/j.neuron.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC, 2012. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci 16, 61–71. doi: 10.1016/j.tics.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A 98, 676–82. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF, Wise LZ, Heil P, 1993. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol 338, 17–49. doi: 10.1002/cne.903380104 [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Mühlau M, 2010. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 66, 819–826. doi:S0896–6273(10)00325–9 [pii] 10.1016/j.neuron.2010.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS, 2007. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10, 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH, 2002. Brain imaging of the effects of lidocaine on tinnitus. Hear Res 171, 43–50. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA, 2010. Ringing ears: the neuroscience of tinnitus. J. Neurosci 30, 14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Husain FT, Eggermont JJ, 2013. Role of attention in the generation and modulation of tinnitus. Neurosci. Biobehav. Rev 37, 1754–73. doi: 10.1016/j.neubiorev.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP, 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci 2, 1131–6. doi: 10.1038/16056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Landgrebe M, Poeppl TB, Kreuzer P, Männer P, Marienhagen J, Wack DS, Kleinjung T, Hajak G, Langguth B, 2011. Neural correlates of tinnitus duration and Distress: A positron emission tomography study. Hum Brain Mapp. doi: 10.1002/hbm.21426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V, 2008. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368–77. doi: 10.1038/sj.npp.1301408 [DOI] [PubMed] [Google Scholar]
- Schlee W, Mueller N, Hartmann T, Keil J, Lorenz I, Weisz N, 2009. Mapping cortical hubs in tinnitus. BMC Biol 7, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SA, Akrofi K, Carpenter-Thompson JR, Husain FT, 2013. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS One 8, e76488. doi: 10.1371/journal.pone.0076488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Andermann M, Wengenroth M, Goebel R, Flor H, Rupp A, Diesch E, 2009. Reduced volume of Heschl’s gyrus in tinnitus. Neuroimage 45, 927–939. doi:S1053–8119(08)01304–9 [pii] 10.1016/j.neuroimage.2008.12.045 [DOI] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Greenberg AS, Rauschecker JP, 2014a. Are you listening? Brain activation associated with sustained nonspatial auditory attention in the presence and absence of stimulation. Hum. Brain Mapp 35, 2233–52. doi: 10.1002/hbm.22323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Leaver AM, Turesky TK, Morgan S, Kim HJ, Rauschecker JP, 2012. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res. 1485, 22–39. doi: 10.1016/j.brainres.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Raven EP, Leaver AM, Turesky TK, Rauschecker JP, 2014b. Diffusion imaging of auditory and auditory-limbic connectivity in tinnitus: preliminary evidence and methodological challenges. Neural Plast. 2014, 145943. doi: 10.1155/2014/145943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S, 2007. Neural mechanisms underlying somatic tinnitus. Prog. Brain Res 166, 107–23. doi: 10.1016/S0079-6123(07)66010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, 2011. Plasticity of somatosensory inputs to the cochlear nucleus--implications for tinnitus. Hear. Res 281, 38–46. doi: 10.1016/j.heares.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman A, Strashun AM, Afriyie M, Aronson F, Abel W, Goldstein B, 1995. SPECT Imaging of Brain and Tinnitus-Neurotologic/Neurologic Implications. Int Tinnitus J 1, 13–29. [PubMed] [Google Scholar]
- Simpson JJ, Davies WE, 2000. A review of evidence in support of a role for 5-HT in the perception of tinnitus. Hear Res 145, 1–7. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF, 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A 106, 13040–5. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, 1999. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 282, 1737–1744. doi:joc90770 [pii] [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B, 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166, 1092–1097. doi:166/10/1092 [pii] 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Katon W, Dobie R, Sakai C, Russo J, Harrop-Griffiths J, 1988. Disabling tinnitus. Association with affective disorder. Gen Hosp Psychiatry 10, 285–291. [DOI] [PubMed] [Google Scholar]
- Tanibuchi I, Goldman-Rakic PS, 2003. Dissociation of spatial-, object-, and sound-coding neurons in the mediodorsal nucleus of the primate thalamus. J. Neurophysiol 89, 1067–77. doi: 10.1152/jn.00207.2002 [DOI] [PubMed] [Google Scholar]
- Ueyama T, Donishi T, Ukai S, Ikeda Y, Hotomi M, Yamanaka N, Shinosaki K, Terada M, Kaneoke Y, 2013. Brain regions responsible for tinnitus distress and loudness: a resting-state FMRI study. PLoS One 8, e67778. doi: 10.1371/journal.pone.0067778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, Strupp J, Sapiro G, De Martino F, Wang D, Harel N, Garwood M, Chen L, Feinberg DA, Smith SM, Miller KL, Sotiropoulos SN, Jbabdi S, Andersson JLRR, Behrens TEJJ, Glasser MF, Van Essen DC, Yacoub E, 2013. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage 80, 80–104. doi: 10.1016/j.neuroimage.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Congedo M, De Ridder D, 2014. Pinpointing a highly specific pathological functional connection that turns phantom sound into distress. Cereb. Cortex 24, 2268–82. doi: 10.1093/cercor/bht068 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Van de Heyning P, De Ridder D, 2011a. Contralateral parahippocampal gamma-band activity determines noise-like tinnitus laterality: a region of interest analysis. Neuroscience 199, 481–90. doi: 10.1016/j.neuroscience.2011.07.067 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Plazier M, van der Loo E, Van de Heyning P, Congedo M, De Ridder D, 2010. The neural correlates of tinnitus-related distress. Neuroimage 52, 470–80. doi: 10.1016/j.neuroimage.2010.04.029 [DOI] [PubMed] [Google Scholar]
- Vanneste S, van de Heyning P, De Ridder D, 2011b. The neural network of phantom sound changes over time: a comparison between recent-onset and chronic tinnitus patients. Eur. J. Neurosci 34, 718–31. doi: 10.1111/j.1460-9568.2011.07793.x [DOI] [PubMed] [Google Scholar]
- Weisz N, Wienbruch C, Dohrmann K, Elbert T, 2005. Neuromagnetic indicators of auditory cortical reorganization of tinnitus. Brain 128, 2722–2731. [DOI] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE, 2006. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. Neuroimage 33, 180–194. [DOI] [PubMed] [Google Scholar]
- Wig GS, Laumann TO, Petersen SE, 2014. An approach for parcellating human cortical areas using resting-state correlations. Neuroimage 93 Pt 2, 276–91. doi: 10.1016/j.neuroimage.2013.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EC, Melcher JR, Micheyl C, Gutschalk A, Oxenham AJ, 2007. Cortical FMRI activation to sequences of tones alternating in frequency: relationship to perceived rate and streaming. J Neurophysiol 97, 2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wineland AM, Burton H, Piccirillo J, 2012. Functional connectivity networks in nonbothersome tinnitus. Otolaryngol. Head. Neck Surg 147, 900–6. doi: 10.1177/0194599812451414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H, 2006. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 26, 7348–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]