Abstract
Intravenous drug use has increased substantially over the past decade, with heroin abuse more than doubling. Injection drug use-related infective endocarditis hospitalizations have similarly increased over the same period. Right-sided infective endocarditis is strongly associated with intravenous drug use, and 90% of right-sided endocarditis involves the tricuspid valve. During the period of the opioid epidemic, tricuspid-related endocarditis rates have increased, while the incidence of surgery for tricuspid endocarditis has increased as much as five-fold. Within this context, optimizing surgical technique for valve repair is increasingly important. In this report, we examine the indications for tricuspid valve surgery for endocarditis, describe specific techniques for tricuspid valve leaflet repair and augmentation, and assess postoperative care and surgical outcomes after both tricuspid valve repair and replacement for infective endocarditis.
Keywords: Tricuspid valve, Endocarditis, Valve repair
Introduction
Intravenous drug use (IVDU) has increased substantially over the past decade, with heroin abuse more than doubling between 2006 and 20131 and a corresponding increase in injection drug use-related infective endocarditis (IE) hospitalizations during the same period.2–5 Right-sided IE is strongly associated with IVDU, and 90% of right-sided IE involves the tricuspid valve, predominantly through Staphylococcus aureus infections.4,6–10 In addition to IVDU, tricuspid IE may result in patients with pacemaker or defibrillator leads, congenital heart disease, and vascular access for dialysis.6,10–14 Multiple analyses have reported an increase in tricuspid-related IE corresponding to the increase in IVDU during the same period.4,12,15,16
While the incidence of all types of tricuspid valve surgery has more than doubled,17 some institutional surgical volumes for right-sided IE have increased as much as five-fold during the opioid crisis.18 Accordingly, optimizing surgical technique for valve repair in these challenging patients is increasingly important, as the opioid crisis and incidence of right-sided IE show no signs of slowing.
Surgical candidates should meet the appropriate Duke criteria for diagnosis of IE19 prior to consideration for surgical intervention. Whereas consensus indications for surgery are clear for left-sided IE, intervention for right-sided IE is not as well defined.7,20–22 According to European guidelines7 (all class IIa recommendations), indications for surgery in the setting of right-sided IE include any of the following: (1) right heart failure secondary to severe tricuspid regurgitation with poor response to diuretic therapy; (2) tricuspid valve vegetations greater than 20 mm that persist after recurrent pulmonary emboli with or without concomitant right heart failure; and (3) IE caused by organisms that are difficult to eradicate (eg, persistent fungi) or bacteremia for at least 7 days despite adequate antimicrobial therapy. The most recent American Heart Association/American College of Cardiology guidelines are similar, but issue a class IIb indication for early surgery in patients with native valve endocarditis and mobile vegetations greater than 10 mm in length, rather than 20 mm, and notably do not stratify recommendations for left- and right-sided IE.20,21 Within the North American surgical community, the American Association for Thoracic Surgery consensus indications for surgery addressing right-sided IE are failure to control the infection, prevention of further septic pulmonary embolism, and, less often, severe tricuspid valve regurgitation.22
Special considerations for managing tricuspid IE in active IVDUs are required, since these patients often have more advanced disease due to delayed presentation, multi-valve involvement, polymicrobial infection involving resistant organisms, and poor compliance.12 Although the overall rate of recurrent IE is low, durability of repair in the IVDU population carries increased importance, since these patients are at risk of relapse into IVDU, recurrent IE, and subsequent requirement for reoperation.12,14 Multiple repair techniques have been described in the literature, including ring and suture annuloplasty,12,14,23,24 the Kay bicuspidalization,24–27 and valvectomy without replacement.26,28,29
Although some have suggested an advantage to avoiding prosthetic materials such as an annuloplasty ring in the setting of infective endocarditis,26 there has been no evidence to show lower infection rates, whereas several studies have shown ring annuloplasty to be superior to DeVega suture annuloplasty in avoiding recurrent tricuspid regurgitation (TR) or reoperation.30–34 In our experience, ring annuloplasty in conjunction with leaflet repair confers the best overall valve repair, with no increase in infection rate and superior freedom from residual TR. Leaflet repair can effectively be achieved with or without a repair patch, depending on the extent of leaflet destruction. When feasible, vegetations can either be debrided or resected (depending on level of leaflet involvement) followed by bicuspidization by reapproximating the anterior and posterior leaflets with 4–0 Ethibond sutures (Video). In this scenario, a ring annuloplasty is still performed, while a repair patch is not necessary.
Preoperative management of tricuspid endocarditis centers on diagnosis, antibiotic treatment, and diuresis. While not necessary to diagnose tricuspid endocarditis, we recommend a transesophageal echocardiogram to fully evaluate involvement of other valves. Second, we recommend microorganism identification and antibiotic treatment for at least 48 hours preoperatively. Finally, as with any patient with severe TR, we support aggressive diuresis prior to operating. While timing of surgery will vary by individual, the ability to repair the valve is important in the context of severe TR. Therefore, every effort should be made to safely repair the valve before valve destruction has progressed to the extent of precluding repair. This report details each step of complex tricuspid valve leaflet repair for tricuspid valve endocarditis, as typically performed at our institution (Figures 1–9).
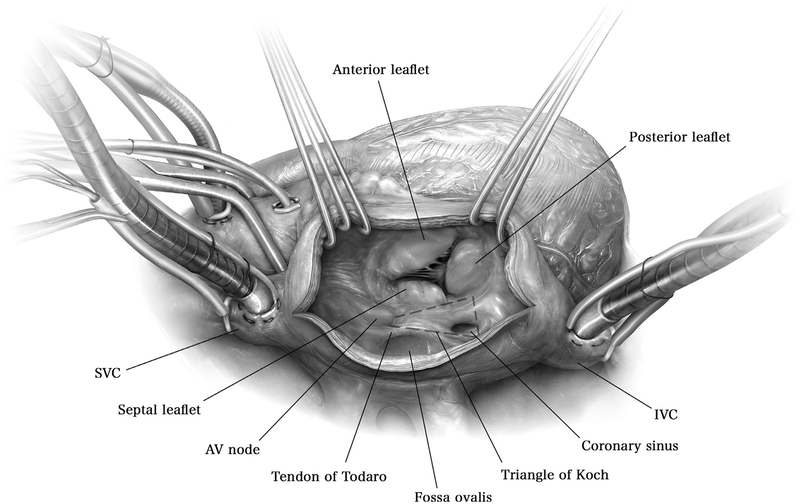
Figure 1.
A median sternotomy is made, the pericardium is incised vertically, and standard ascending aortic and bicaval venous cannulation is performed with cannulae in the superior and inferior vena cavae. Cardiopulmonary bypass is initiated and the perfusate is cooled to achieve a nasopharyngeal temperature of 34°C. From the surgeon’s view, a transverse incision in the right atrium is performed and retractors are placed by the assistant to expose the tricuspid valve. The anterior, posterior, and septal leaflets of the valve are identified, as well as the adjacent coronary sinus.
AV = atrioventricular; IVC = inferior vena cava; SVC = superior vena cava.
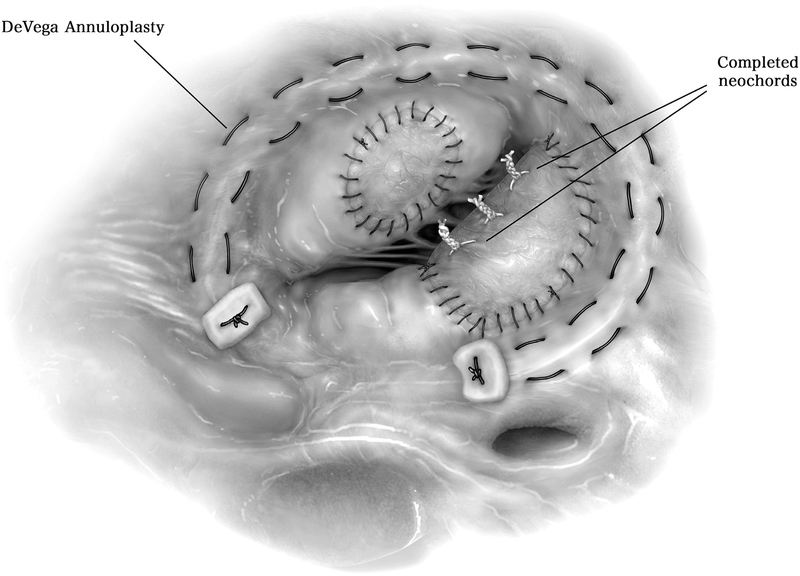
Figure 9.
Another alternative to prosthetic ring annuloplasty is the DeVega suture annuloplasty, in which a 3–0 prolene is used to suture 2 rows in the tricuspid annulus, around the perimeter of the tricuspid valve along the same distribution as a partial annuloplasty ring (10 o’clock to 6 o’clock). A pledget is utilized on either end of the rows of suture and the ends tied down and cut.
Outcomes
An analysis of over 900 North American patients undergoing tricuspid surgery for IE between 2002 and 2009 found no difference between perioperative mortality between repair and replacement (7.6% vs 6.3%, P = 0.34), while patients with treated rather than active IE demonstrated lower complication rates, length of stay, and a trend toward decreased mortality.35 However, a more recent meta-analysis of 12 retrospective observational studies through 2016 found a 59% median repair proportion among studies and reported associations between valve repair and lower recurrent IE, lower reoperation, lower need for permanent pacemaker, with no difference in long-term survival after repair or replacement.23
While tricuspid replacement was historically performed more than repair,36 more contemporary series have shown that valve repair is preferred over replacement,14,37 especially in IVDUs who are predominantly young, noncompliant, and carry a higher risk of recurrent infection, thromboembolic and bleeding complications, and reoperation with valve replacement.6,14,37 The Society of Thoracic Surgeons Clinical Practice Guideline for surgical management of IE includes a class I recommendation for tricuspid valve repair for native tricuspid IE, when surgery is indicated.38 Similar to recently published series, we support making every effort to avoid replacing the tricuspid valve, with replacement performed only if repair is impossible.14,24,37,39–41
Postoperative Management
All endocarditis consultations at our institution are seen in conjunction with other members of the multidisciplinary endocarditis team. Among those who undergo cardiac surgery, postoperative considerations depend on the underlying cause of endocarditis. For IVDU patients, we require each patient to sign a contract prior to surgery, committing to abstinence from continued drug use and attending drug rehabilitation after recovery from surgery. Patients remain in the hospital until recovery after surgery and completion of IV antibiotic therapy as decided by the multidisciplinary endocarditis team.
Conclusions
In conclusion, tricuspid valve leaflet repair is an important operative technique for every practicing cardiac surgeon as the incidence of right-sided and resulting tricuspid valve endocarditis continues to increase during the current opioid epidemic. When possible, tricuspid repair should be performed over replacement, and medical, surgical, and social factors must be considered for this challenging population.
Supplementary Material
Figure 2.
A large vegetation is shown on the posterior leaflet of the TV. Vegetations are often companied by “mirror” vegetations on the corresponding leaflet that is contacted with each cardiac cycle. A smaller mirror vegetation is appreciated on the TV anterior leaflet.
TV = tricuspid valve.
Figure 3.
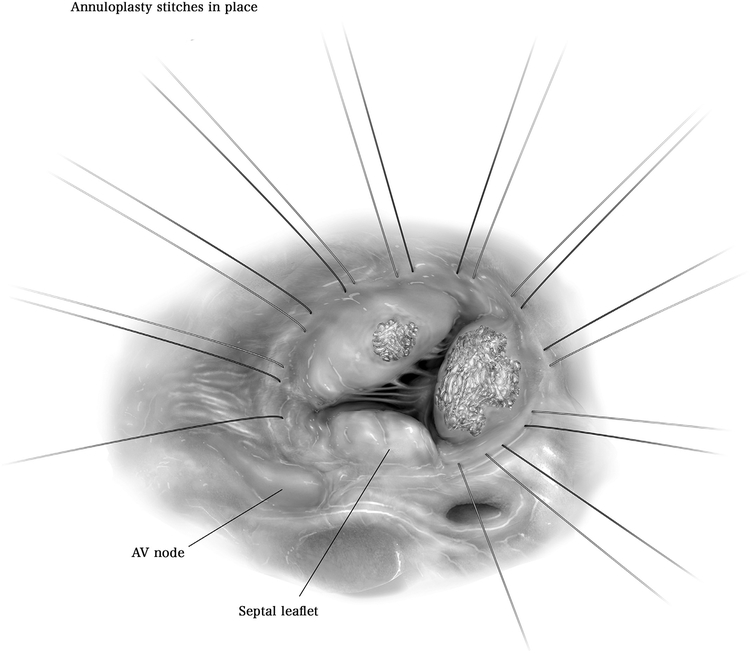
Before any leaflet intervention, annuloplasty sutures are placed. Approximately 10 interrupted 2–0 Ethibond sutures are placed from the 10 o’clock position to the 6 o’clock position from the surgeon’s view within the tricuspid valve annulus, taking care to avoid the atrioventricular node. These 10 interrupted sutures are left untied as attention is then turned to the leaflet pathology.
AV = atrioventricular
Figure 4.
In addressing the large vegetation shown, the majority of the posterior leaflet is excised, while the mirror vegetation on the anterior leaflet is also excised circumferentially, with consideration given to maintaining leaflet margins adequate to sew a repair patch.
Figure 5.
Bovine pericardium is utilized to create 2 oversized patches. The larger patch is sewn to the tricuspid annulus adjacent to the remainder of the posterior leaflet with 4–0 prolene suture and joined with the remaining septal leaflet to functionally “bicuspidalize” the tricuspid valve. The smaller patch is then sewn directly onto the anterior leaflet, with suture placement substantially far away from the resection hole. Most importantly, the patches should be sufficiently oversized to be sewing into non-compromised tissue.
Figure 6.
To further restore function to the now bicuspidalized tricuspid valve posteroseptal leaflet, e-PTFE neochords are added. We create freehand loops with the e-PTFE suture, anchored in each papillary muscle. These neochords are left untied at this stage, but are eventually tied according to the height of the annulus.
e-PTFE = expanded polytetrafluoroethylene
Figure 7.
The untied annuloplasty sutures are passed through a semi-rigid partial annuloplasty ring, tied into place, and cut. Importantly, this step occurs before tying down the e-PTFE neochords, since the resultant geometry of the tricuspid annulus changes after securing the partial annuloplasty ring. Subsequently, the neochords are tied into place at the height of the annulus. Neochord height should approximate the height of nearby chords. Importantly, neochords being slightly too long rather than too short is much better for adequate coaptation.
AV = atrioventricular; e-PTFE = expanded polytetrafluoroethylene
Figure 8.
An alternative to prosthetic ring annuloplasty is the Kay bicuspidization technique. Through this technique, the posterior leaflet is eliminated and the valve contains only an anterior and septal leaflet. The suture used to reshape the annulus is brought through a pledget on both sides and tied down, as shown.
Conflict of interest statement and sources of funding:
A.A.B. is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123).
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.optechstcvs.2019.09.002.
References
- 1.National Survey on Drug Use and Health: Summary of National Findings n.d. 2013. Results from the.
- 2.Wurcel AG, Anderson JE, Chui KKH, et al. : Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 3:157, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronan MV, Herzig SJ: Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff 35:832–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman L, Barnes E, Bachmann L, et al. : Opiate injection-associated infective endocarditis in the Southeastern United States. Am J Med Sci 352:603–608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischauer AT, Ruhl L, Rhea S, et al. : Hospitalizations for endocarditis and associated health care costs among persons with diagnosed drug dependence - North Carolina, 2010–2015. MMWR Morb Mortal Wkly Rep 66:569–573, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain ST, Witten J, Shrestha NK, et al. : Tricuspid valve endocarditis. Ann Cardiothorac Surg 6:255–261, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habib G, Lancellotti P, Antunes MJ, et al. : Document Reviewers. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36:3075–3128, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Murdoch DR, Corey GR, Hoen B, et al. : Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miró JM, del Río A, Mestres CA: Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin 21:167–184, 2003. 10.1016/s0733-8651(03)00025-0 [DOI] [PubMed] [Google Scholar]
- 10.Baraki H, Saito S, Al Ahmad A, et al. Surgical treatment for isolated tricuspid valve endocarditisn.d doi: 10.1253/circj.CJ-12-1364. [DOI] [PubMed] [Google Scholar]
- 11.Ostovar R, Schroeter F, Kuehnel R-U, et al. : Endocarditis: an ever increasing problem in cardiac surgery. Thorac Cardiovasc Surg 2019. 10.1055/s-0039-1688475 [DOI] [PubMed] [Google Scholar]
- 12.Yong MS, Coffey S, Prendergast BD, et al. : Surgical management of tricuspid valve endocarditis in the current era: A review. Int J Cardiol 202:44–48, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Sridhar ARM, Lavu M, Yarlagadda V, et al. : Cardiac implantable electronic device-related infection and extraction trends in the U.S. Pac Clin Electro-physiol 40:286–293, 2017. 10.1111/pace.13009 [DOI] [PubMed] [Google Scholar]
- 14.Witten JC, Hussain ST, Shrestha NK, et al. : Surgical treatment of right-sided infective endocarditis. J Thorac Cardiovasc Surg 157:1418–1427, 2019. e14 [DOI] [PubMed] [Google Scholar]
- 15.Seratnahaei A, Leung SW, Charnigo RJ, et al. : The changing “face” of endocarditis in Kentucky: An increase in tricuspid cases. Am J Med 127:786.e1–786.e6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferraris VA, Sekela ME: Missing the forest for the trees: The world around us and surgical treatment of endocarditis. J Thorac Cardiovasc Surg 152:677–680, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Vassileva CM, Shabosky J, Boley T, et al. : Tricuspid valve surgery: The past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg 143:1043–1049, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Wallen TJ, Szeto W, Williams M, et al. : Tricuspid valve endocarditis in the era of the opioid epidemic. J Card Surg 33:260–264, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. : Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, et al. : 2014. AHA/ACC guideline for the management of patients with valvular heart disease. Circulation 129, 2014 10.1161/cir.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 21.Nishimura RA, Otto CM, Bonow RO, et al. : 2017 AHA/ACC Focused Update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017. 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 22.AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS, et al. : 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 153:1241–1258, 2017 e29 [DOI] [PubMed] [Google Scholar]
- 23.Yanagawa B, Elbatarny M, Verma S, et al. : Surgical management of tricuspid valve infective endocarditis: A systematic review and meta-analysis. Ann Thorac Surg 106:708–714, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Gottardi R, Bialy J, Devyatko E, et al. : Midterm follow-up of tricuspid valve reconstruction due to active infective endocarditis. Ann Thorac Surg 84:1943–1948, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kay JH, Maselli-Campagna G, Tsuji KK: Surgical treatment of tricuspid insufficiency. Ann Surg 162:53–58, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akinosoglou K, Apostolakis E, Koutsogiannis N, et al. : Right-sided infective endocarditis: Surgical management. Eur J Cardiothorac Surg 42:470–479, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Filsoufi F, Salzberg SP, Abascal V, et al. : Surgical management of functional tricuspid regurgitation with a new remodeling annuloplasty ring. Mt Sinai J Med 73:874–879, 2006 [PubMed] [Google Scholar]
- 28.Protos AN, Trivedi JR, Whited WM, et al. : Valvectomy versus replacement for the surgical treatment of tricuspid endocarditis. Ann Thorac Surg 106:664–669, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Arbulu A, Holmes RJ, Asfaw I: Surgical treatment of intractable right-sided infective endocarditis in drug addicts: 25 years experience. J Heart Valve Dis 2:129–137, 1993. discussion 138–9 [PubMed] [Google Scholar]
- 30.McCarthy PM, Bhudia SK, Rajeswaran J, et al. : Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 127:674–685, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hata H, Fujita T, Miura S, et al. : Long-term outcomes of suture vs. ring tricuspid annuloplasty for functional tricuspid regurgitation. Circ J 81:1432–1438, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Murashita T, Okada Y, Kanemitsu H, et al. : Long-term outcomes of tricuspid annuloplasty for functional tricuspid regurgitation associated with degenerative mitral regurgitation: Suture annuloplasty versus ring annuloplasty using a flexible band. Ann Thorac Cardiovasc Surg 20:1026–1033, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama K, Matsumoto M, Sugita T, et al. : De Vega annuloplasty and Carpentier-Edwards ring annuloplasty for secondary tricuspid regurgitation. J Heart Valve Dis 10:520–524, 2001 [PubMed] [Google Scholar]
- 34.Tang GHL, David TE, Singh SK, et al. : Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation 114:I577–I581, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Gaca JG, Sheng S, Daneshmand M, et al. : Current outcomes for tricuspid valve infective endocarditis surgery in North America. Ann Thorac Surg 96:1374–1381, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Gaca JG, Sheng S, Daneshmand MA, et al. : Outcomes for endocarditis surgery in North America: A simplified risk scoring system. J Thorac Cardiovasc Surg 141:98–106, 2011 e1–2 [DOI] [PubMed] [Google Scholar]
- 37.Dawood MY, Cheema FH, Ghoreishi M, et al. : Contemporary outcomes of operations for tricuspid valve infective endocarditis. Ann Thorac Surg 99:539–546, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Byrne JG, Rezai K, Sanchez JA, et al. : Surgical management of endocarditis: The society of thoracic surgeons clinical practice guideline. Ann Thorac Surg 91:2012–2019, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Musci M, Siniawski H, Pasic M, et al. : Surgical treatment of right-sided active infective endocarditis with or without involvement of the left heart: 20-year single center experience☆. Eur J Cardio-Thorac Surg 32:118–125, 2007. 10.1016/j.ejcts.2007.02.034 [DOI] [PubMed] [Google Scholar]
- 40.Jiang S-L, Li B-J, Zhang T, et al. : Surgical treatment of isolated right-sided infective endocarditis. Tex Heart Inst J 38:639–642, 2011 [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss AJ, Anyanwu AC: Commentary: Goals of surgical therapy in tricuspid valve endocarditis. J Thorac Cardiovasc Surg 157:1430–1431, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.