Abstract
Background:
Abnormalities in hematologic, biochemical, and immunologic biomarkers have been shown to be associated with severity and mortality in Coronavirus Disease 2019 (COVID-19). Therefore, early evaluation and monitoring of both liver and kidney functions, as well as hematologic parameters, are pivotal to forecast the progression of COVID-19.
Objectives:
In this study, we performed a systematic review and meta-analysis to investigate the association between several complications, including acute kidney injury (AKI), acute liver injury (ALI), and coagulopathy, with poor outcomes in COVID-19.
Design:
Systematic review and meta-analysis
Setting:
Observational studies reporting AKI, ALI, and coagulopathy along with the outcomes of clinically validated death, severe COVID-19, or intensive care unit (ICU) care were included in this study. The exclusion criteria were abstract-only publications, review articles, commentaries, letters, case reports, non-English language articles, and studies that did not report key exposures or outcomes of interest.
Patients:
Adult patients diagnosed with COVID-19.
Measurements:
Data extracted included author, year, study design, age, sex, cardiovascular diseases, hypertension, diabetes mellitus, respiratory comorbidities, chronic kidney disease, mortality, severe COVID-19, and need for ICU care.
Methods:
We performed a systematic literature search from PubMed, SCOPUS, EuropePMC, and the Cochrane Central Database. AKI and ALI follow the definition of the included studies. Coagulopathy refers to the coagulopathy or disseminated intravascular coagulation defined in the included studies. The outcome of interest was a composite of mortality, need for ICU care, and severe COVID-19. We used random-effects models regardless of heterogeneity to calculate risk ratios (RRs) for dichotomous variables. Heterogeneity was assessed using I2. Random effects meta-regression was conducted for comorbidities and the analysis was performed for one covariate at a time.
Results:
There were 3615 patients from 15 studies. The mean Newcastle-Ottawa scale of the included studies was 7.3 ± 1.2. The AKI was associated with an increased the composite outcome (RR: 10.55 [7.68, 14.50], P < .001; I2: 0%). Subgroup analysis showed that AKI was associated with increased mortality (RR: 13.38 [8.15, 21.95], P < .001; I2: 24%), severe COVID-19 (RR: 8.12 [4.43, 14.86], P < .001; I2: 0%), and the need for ICU care (RR: 5.90 [1.32, 26.35], P = .02; I2: 0%). The ALI was associated with increased mortality (RR: 4.02 [1.51, 10.68], P = .005; I2: 88%) in COVID-19. Mortality was higher in COVID-19 with coagulopathy (RR: 7.55 [3.24, 17.59], P < .001; I2: 69%). The AKI was associated with the composite outcome and was not influenced by age (P = .182), sex (P = .104), hypertension (P = .788), cardiovascular diseases (P = .068), diabetes (P = .097), respiratory comorbidity (P = .762), and chronic kidney disease (P = .77).
Limitations:
There are several limitations of this study. Many of these studies did not define the extent of AKI (grade), which may affect the outcome. Acute liver injury and coagulopathy were not defined in most of the studies. The definition of severe COVID-19 differed across studies. Several articles included in the study were published at preprint servers and are not yet peer-reviewed. Most of the studies were from China; thus, some patients might overlap across the reports. Most of the included studies were retrospective in design.
Conclusions:
This meta-analysis showed that the presence of AKI, ALI, and coagulopathy was associated with poor outcomes in patients with COVID-19.
Keywords: acute kidney injury, acute liver injury, coagulopathy, COVID-19, multiorgan dysfunction
What was known before
Although most cases of Coronavirus Disease 2019 (COVID-19) are asymptomatic or relatively benign, several patients may be severely affected by the disease, leading to multiple organ failure. The susceptibility to clinical deterioration remains uncertain; however, the risk is higher in patients with specific comorbidities. The mechanism of COVID-19-induced multiorgan failure and the magnitude of the impact on prognosis remain uncertain.
What this adds
The presence of acute kidney injury, acute liver injury, or coagulopathy may be used as a marker for poor prognosis in COVID-19. Early identification and intervention are warranted, especially in the presence of acute kidney injury, which is associated with a 10-fold risk of poorer outcomes.
Introduction
The World Health Organization has declared Coronavirus Disease 2019 (COVID-19) as a global public health emergency and a worldwide pandemic. To date, more than 2 million cases and 140 000 deaths have been attributed to COVID-19.1 While most COVID-19 cases are asymptomatic or only display mild influenza-like symptoms, a significant number of patients may experience severe pneumonia, acute respiratory distress syndrome, multiple organ failure, and even death.2 Although it is known that patients with comorbidities are more susceptible to clinical deterioration, the underlying causes remain uncertain.3-6 Emerging evidence suggests that this virus may directly invade human extrapulmonary organs and tissues or induce hyperinflammation mediated by cytokine release, culminating in multiple organ dysfunction, including acute kidney injury (AKI), acute liver injury (ALI), or coagulopathy.7
Currently, there is no specific cure for COVID-19. Government officials around the globe highlighted the importance of old-style public health measures, such as isolation, quarantine, social distancing, and community containment.7 Meanwhile, abnormalities in hematologic, biochemical, and immunologic biomarkers are shown to be associated with severity and mortality in COVID-19.7 Therefore, early evaluation and monitoring of both liver and kidney functions, as well as hematologic parameters, are pivotal for predicting the progression of COVID-19. We, therefore, conducted a systematic review and meta-analysis to explore the association between several complications, including AKI, ALI, and coagulopathy, with poor outcomes in COVID-19. We hypothesized that AKI, ALI, and coagulopathy would be associated with mortality, the need for admission to intensive care, and severe COVID-19.
Methods
This systematic review and meta-analysis followed the Meta-analysis of Observational Studies in Epidemiology reporting guidelines.
Eligibility Criteria
The following types of articles were included: research articles in which samples were adult individuals with COVID-19 diagnosis who had information about AKI, ALI, or coagulopathy and the outcome of the clinically validated definition of death, severe COVID-19, or intensive care unit (ICU) care. Both published studies and preprints were included in this systematic review. We excluded abstract-only publications, review articles, commentaries, letters, case reports, and non-English language articles. Studies that did not report key exposures or outcomes of interest were excluded.
Search Strategy and Study Selection
A comprehensive systematic literature search was conducted using PubMed, SCOPUS, EuropePMC, and the Cochrane Central Database with keywords (1) “COVID-19” OR “SARS-CoV-2” AND “Characteristics,” (2) “COVID-19” OR “SARS-CoV-2” AND “Acute kidney injury,” (3) “COVID-19” OR “SARS-CoV-2” AND “liver injury,” (4) “COVID-19” OR “SARS-CoV-2” AND “coagulopathy” on April 11, 2020. The PubMed (MEDLINE) search strategy was (“COVID-19”[All Fields] OR “SARS-CoV-2”[All Fields] AND “Characteristics”[All Fields]) OR (“COVID-19”[All Fields] OR “SARS-CoV-2”[All Fields] AND (“Acute kidney injury”[All Fields] OR “Renal dysfunction”[All Fields]) OR (“COVID-19”[All Fields] OR “SARS-CoV-2”[All Fields] AND “liver injury”[All Fields]) OR (“COVID-19”[All Fields] OR “SARS-CoV-2”[All Fields] AND “coagulopathy”[All Fields]). The complete search strategy is shown in supplemental Table S1. Data searching from several preprint databases (medRvix, ResearchSquare, Preprints) and hand searching were also conducted. Duplicate articles were removed, and the titles and abstracts of the remaining articles were independently screened by 2 authors based on the inclusion and exclusion criteria. Both literature searchers are medical doctors who are experienced in performing systematic reviews and meta-analyses.
Data Extraction
Data extraction was conducted by 2 independent authors using standardized extraction forms that included author, year, study design, age, sex, cardiovascular diseases, diabetes mellitus, hypertension, respiratory comorbidities, chronic kidney disease (CKD), mortality, severe COVID-19, and the need for ICU care.
Both AKI and ALI follow the definition of the included studies. Coagulopathy refers to the included studies’ defined coagulopathy or disseminated intravascular coagulation.
The outcome of interest was a composite of mortality, ICU care, and severe COVID-19. The definition of acute respiratory distress syndrome was established based on the World Health Organization interim guidance of severe acute respiratory infection of COVID-19, which includes acute onset, chest imaging and origin of pulmonary infiltrates, and impairment of oxygenation.7 Severe COVID-19 was diagnosed if individuals had (1) respiratory distress (≥30 breaths per min), (2) resting oxygen saturation ≤93%, (3) ratio of the partial pressure of arterial oxygen (PaO2) to the fractional concentration of oxygen inspired air (fiO2) ≤300 mm Hg, or (4) critical complication (respiratory failure, septic shock, and multiple organ dysfunction/failure).9
The risk of bias of the included studies was assessed using the Newcastle-Ottawa Score by 2 independent authors and discrepancies were resolved via discussion.
Statistical Analysis
Review Manager 5.3 (Cochrane Collaboration) and Stata version 16 were used for statistical analysis. The Mantel-Haenszel formula with random-effects models regardless of heterogeneity was used for dichotomous variables to calculate risk ratios (RRs). All P values were 2-tailed, and statistical significance was set at ≤.05. Heterogeneity was assessed using I2, with a value of >50% or P < .10, indicating a statistically significant heterogeneity. Random effects meta-regression was conducted using a restricted-maximum likelihood for age, sex, cardiovascular disease, hypertension, diabetes mellitus, and respiratory comorbidities; the analysis was performed for one covariate at a time to avoid overfitting. In assessing the small study effect, a regression-based Harbord’s test for binary outcome was performed. An inverted funnel plot analysis was conducted to evaluate the risk of publication bias.
Results
Study Selection and Characteristics
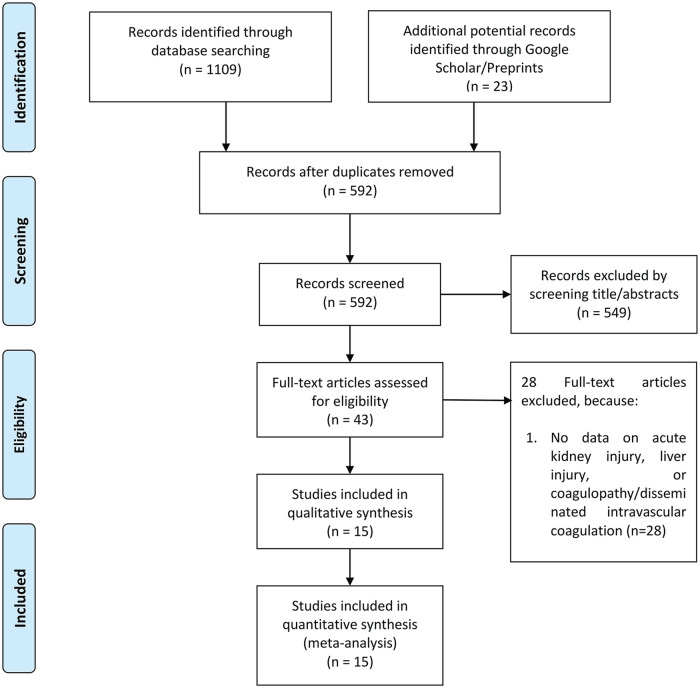
There were a total of 1132 records, and 592 remained after the removal of duplicates. A total of 549 records were excluded after screening the titles and abstracts because the records were (1) review articles, letters, and case reports; (2) non-English language articles; or (3) studies that did not report in terms of the outcome of interest. After assessing 43 full-text articles for eligibility, we excluded 28 full-text articles because there were no data on AKI, ALI, or coagulopathy/disseminated intravascular coagulation. We included 15 studies in the qualitative synthesis and meta-analysis (Figure 1). A total of 3615 patients from 15 studies were collected.10,11,12-24 The characteristics of the included studies are displayed in Table 1. A total of 7 of the 15 studies were preprints. The characteristics and definition of exposure and the outcome of interest are presented in Table 2. The mean Newcastle-Ottawa scale of the included studies was 7.3 ± 1.2 indicating a moderate risk of bias (Supplemental Table S2).
Figure 1.
PRISMA flow chart.
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of the Included Studies.
| Study | Study design | Preprint | Subjects | Male | Overall age (mean/median), y | Hypertension | Coronary artery disease/cardiovascular disease | Diabetes | Chronic kidney disease | Respiratory comorbidities | Baseline creatinine (mean/median), μmol/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bai et al.10 | Retrospective Cohort | Yes | 36 vs 91 | 28/36 vs 52/91 | 67 vs 50 | 15/36 vs 21/91 | 2/36 vs 1/91 | 5/36 vs 10/91 | N/A | N/A | N/A |
| Cao et al.11 | Retrospective Cohort | No | 17 vs 85 | 13/17 vs 40/85 | 72 vs 53 | 11/17 vs 17/85 | 3/17 vs 2/85 | 6/17 vs 5/85 | 3/17 vs 1/85 | 4/17 vs 6/85 | N/A |
| Chen et al.12 | Retrospective Cohort | Yes | 31 vs 92 | 22/31 vs 39/92 | 72 vs 53 | 15/31 vs 26/92 | 8/31 vs 7/92 | 6/31 vs 8/92 | 2/31 vs 5/92 | 3/31 vs 3/92 | 150 vs 67.8 |
| Chen et al.13 | Retrospective Cohort | No | 113 vs 161 | 83/113 vs 88/161 | 68.0 vs 51.0 | 54/113 vs 39/161 | 16/113 vs 7/161 | 24/113 vs 23/161 | 4/113 vs 1/161 | 11/113 vs 7/161 | 88 vs 66 |
| Luo et al.14 | Retrospective Cohort | Yes | 100 vs 303 | 57/100 vs 136/303 | 71 vs 49 | 60/100 vs 53/303 | 16/100 vs 20/303 | 25/100 vs 32/303 | 3/100 vs 4/303 | 17/100 vs 11/303 | 82 vs 68 |
| Yang et al.23 | Retrospective Cohort | No | 32 vs 20 | 21/32 vs 14/20 | 64.6 vs 51.9 | N/A | 3/32 vs 2/20 | 7/32 vs 2/20 | N/A | 2/32 vs 2/20 | 80.7 vs 76.3 |
| Zhou et al.15 | Retrospective Cohort | No | 54 vs 137 | 38/54 vs 81/137 | 69.0 vs 52.0 | 26/54 vs 32/137 | 13/54 vs 2/137 | 17/54 vs 19/137 | 2/54 vs 0/137 | 4/54 vs 2/137 | N/A |
| Guan16 | Retrospective Cohort | No | 173 vs 926 | 100/173 vs 537/926 | 52.0 vs 45.0 | 41/173 vs 124/926 | 10/173 vs 17/926 | 28/173 vs 53/926 | 3/173 vs 5/926 | 6/173 vs 6/926 | N/A |
| Hu et al.17 | Retrospective Cohort | Yes | 172 vs 151 | 91/172 vs 75/151 | 65 vs 56 | 66/172 vs 39/151 | 33/172 vs 8/151 | 33/172 vs 14/151 | 3/172 vs 4/151 | 6/172 vs 0/151 | N/A |
| Li et al.24 | Retrospective Cohort | Yes | 26 vs 299 | 20/26 vs 147/299 | 65 vs 49 | 12/26 vs 66/299 | 5/26 vs 13/299 | 5/26 vs 25/299 | 2/26 vs 2/299 | 2/26 vs 2/299 | 80 vs 62 |
| Liu et al.18 | Prospective Cohort | Yes | 17 vs 44 | 10/17 vs 21/44 | 56 vs 41 | 6/17 vs 6/44 | 1/17 vs 0/44 | 3/17 vs 2/44 | N/A | 3/17 vs 2/44 | 64 vs 56.5 |
| Wan et al.20 | Retrospective Cohort | No | 40 vs 95 | 21/40 vs 52/95 | 56 vs 44 | 4/40 vs 9/95 | 6/40 vs 1/95 | 9/40 vs 3/95 | N/A | 1/40 vs 0/95 | 63.5 vs 66 |
| Zhang et al.19 | Retrospective Cohort | Yes | 55 vs 166 | 35/55 vs 73/166 | 62 vs 51 | 26/55 vs 28/166 | 13/55 vs 9/166 | 7/55 vs 15/166 | 5/55 vs 1/166 | 4/55 vs 2/166 | 75 vs 67 |
| Huang, et al.22 | Retrospective Cohort | No | 13 vs 28 | 11/13 vs 19/28 | 49.0 vs 49.0 | 2/13 vs 4/28 | 3/13 vs 3/28 | 1/13 vs 7/28 | N/A | 1/13 vs 0/28 | 79 vs 73.3 |
| Wang et al.21 | Retrospective Cohort | 36 vs 102 | 36 vs 102 | 22/36 vs 53/102 | 66 vs 51 | 21/36 vs 22/102 | 9/36 vs 11/102 | 8/36 vs 6/102 | 2/36 vs 2/102 | 3/36 vs 1/102 | 80 vs 71 |
Note. Data are presented stratified by those with outcome of interest vs. those without outcome of interest. N/A = not available.
Table 2.
Characteristics of Exposures and Outcome of the Included Studies.
| Study | Defintion of confirmed COVID-19 | Outcome of interest | Definition of severe COVID-19 | Started in ICU, % | AKI | Definition of AKI | ALI | Definition of ALI | Coagulopathy | Definition of coagulopathy | Newcastle-Ottawa scale |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bai et al.10 | + RT-PCR SARS-CoV-2 | Mortality | N/A | N/A | 12/36 vs 0/91 | KDIGO | 9/36 vs 1/91 | N/A | 14/36 vs 0/91 | N/A | 9 |
| Cao et al.11 | + RT-PCR SARS-CoV-2 | Mortality | N/A | 17.6 | 15/17 vs 5/85 | N/A | 13/17 vs 21/85 | N/A | N/A | N/A | 7 |
| Chen et al.12 | + RT-PCR SARS-CoV-2 | Mortality | N/A | N/A | 15/31 vs 3/92 | KDIGO | 6/31 vs 13/92 | Increased ALT >2× UNL without preexisting chronic liver disease and Drug-induced liver injury | 24/31 vs 18/92 | Abnormal PT, APTT, D-dimer, and platelet, excluding anticoagulant effect | 9 |
| Chen et al.13 | + RT-PCR SARS-CoV-2 | Mortality | Severe: Chinese NHC (not defined) | N/A | 28/113 vs 1/161 | KDIGO | 10/113 vs 3/161 | Jaundice with a total bilirubin level of ≥3 mg/dL and increased ALT ≥5× UNL and/or increased ALP ≥2 UNL |
19/113 vs 2/161 | DIC | 7 |
| Luo et al.14 | + RT-PCR SARS-CoV-2 | Mortality | N/A | N/A | 43/100 vs 14/303 | N/A | 71/100 vs 16/303 | N/A | N/A | N/A | 7 |
| Yang et al.23 | + RT-PCR SARS-CoV-2 | Mortality | N/A | 100 | 12/32 vs 3/20 | KDIGO | 9/32 vs 6/20 | N/A | N/A | N/A | 7 |
| Zhou et al.15 | + RT-PCR SARS-CoV-2 | Mortality | Severe: Chinese NHC (not defined) | 26 | 27/54 vs 1/137 | KDIGO | N/A | N/A | 27/54 vs 10/137 | 3-second extension of PT or 5-second extension of APTT | 9 |
| Guan16 | + RT-PCR SARS-CoV-2 | Severe COVID-19 | Severe: American Thoracic Society (on admission) | 5 | 5/173 vs 1/926 | KDIGO | N/A | N/A | 5/173 vs 1/926 | DIC | 6 |
| Hu et al.17 | Clinical with Radiological and/or + RT-PCR SARS-CoV-2 | Severe COVID-19 | Severe: Chinese- World Health Organization Joint Mission (on admission) | N/A | 15/172 vs 2/151 | N/A | N/A | N/A | N/A | N/A | 8 |
| Li et al.24 | + RT-PCR SARS-CoV-2 | Severe COVID-19 | Severe: Chinese NHC (not defined) | N/A | 7/26 vs 12/299 | KDIGO | N/A | N/A | N/A | N/A | 6 |
| Liu et al.18 | Clinical with Laboratory Confirmation | Severe COVID-19 | Severe: Chinese NHC (at the end of study) | 13.1 | N/A | N/A | N/A | N/A | N/A | N/A | 9 |
| Wan et al.20 | + RT-PCR SARS-CoV-2 | Severe COVID-19 | Severe: Chinese NHC (not defined) | N/A | 1/40 vs 4/95 | N/A | N/A | N/A | N/A | N/A | 6 |
| Zhang et al.19 | + RT-PCR SARS-CoV-2 | Severe COVID-19 | Severe: Chinese NHC (on admission) | 31.9 | 8/55 vs 2/166 | KDIGO | N/A | N/A | N/A | N/A | 6 |
| Huang, 2020 | + RT-PCR SARS-CoV-2 | ICU Care | N/A | N/A | 3/13 vs 0/28 | KDIGO | N/A | N/A | N/A | N/A | 7 |
| Wang et al.21 | + RT-PCR SARS-CoV-2 | ICU Care | N/A | N/A | 3/36 vs 2/102 | KDIGO | N/A | N/A | N/A | N/A | 7 |
Note. Data are presented stratified by those with outcome of interest vs. those without outcome of interest. The mean Newcastle-Ottawa scale of the included studies was 7.3 ± 1.2 indicating a moderate risk of bias. COVID-19 = Coronavirus Disease 2019; ICU = intensive care unit; AKI = acute kidney injury; ALI = acute liver injury; RT-PCR = reverse transcriptase polymerase chain reaction; SARS-CoV-2 = Severe Acute Respiratory Syndrome Coronavirus-2; N/A = not available; KDIGO = Kidney Disease Improving Global Outcomes; ALT = alanine aminotransferase; UNL = upper normal limit; PT = prothrombin time; APTT = activated partial thromboplastin time; NHC = National Health Commission; DIC = disseminated intravascular coagulation.
AKI and Poor Outcome
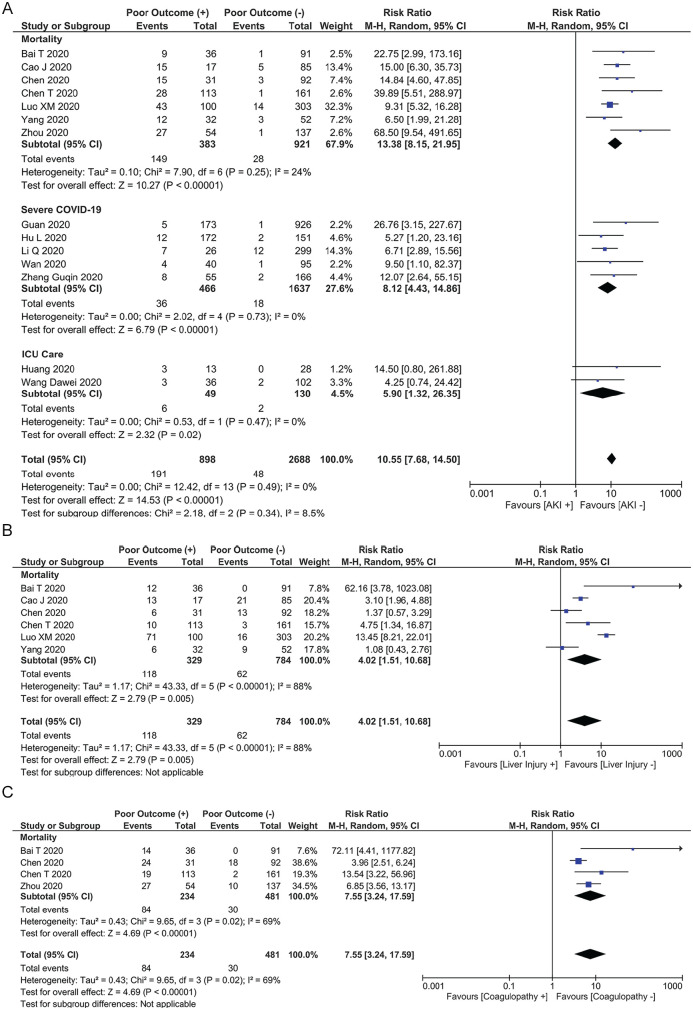
Acute kidney injury was associated with increased the composite outcome (RR: 10.55 [7.68, 14.50], P < .001; I2: 0%, P = .49) (Figure 2A). Subgroup analysis demonstrated that AKI was associated with increased mortality (RR: 13.38 [8.15, 21.95], P < .001; I2: 24%, P = .25), severe COVID-19 (RR: 8.12 [4.43, 14.86], P < .001; I2: 0%, P = .73), and the need for ICU care (RR: 5.90 [1.32, 26.35], P = .02; I2: 0%, P = .49). Acute kidney injury was still associated with the composite outcome after exclusion of studies that did not report baseline serum creatinine (RR: 9.19 [6.36, 13.27], P < .001; I2: 0%, P = .74) in patients with AKI.
Figure 2.
(A) Acute kidney injury was associated with increased the composite outcome, (B) acute liver injury was associated with increased mortality, and (C) mortality was higher in Coronavirus Disease 2019 patients with coagulopathy.
Note. CI = confidence interval.
ALI and Mortality
Acute liver injury was associated with increased mortality (RR: 4.02 [1.51, 10.68], P = .005; I2: 88%, P < .001) in COVID-19 patients (Figure 2B).
Coagulopathy
Mortality was higher in COVID-19 patients with coagulopathy (RR: 7.55 [3.24, 17.59], P < .001; I2: 69%, P = .02) (Figure 2C).
Meta-Regression
The association between AKI and increased the composite outcome was not influenced by age (P = .182) (Figure 3A), sex (P = .104), hypertension (P = .788), cardiovascular diseases (P = .068) (Figure 3B), diabetes (P = .097), respiratory comorbidity (P = .762), and CKD (P = .77).
Figure 3.
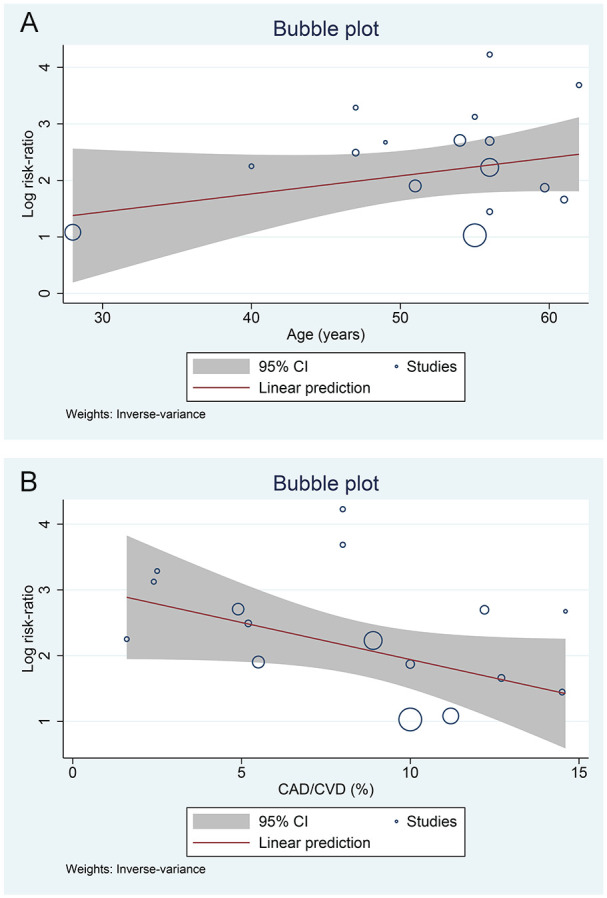
Meta-regression analysis. The association between acute liver injury and increased the composite outcome was not influenced by (A) age or (B) cardiovascular disease.
Note. CI = confidence interval; CAD = coronary artery disease; CVD = cardiovascular disease.
Publication Bias
The funnel plot analysis demonstrated an asymmetrical shape for AKI (Figure 4). There was no indication of small-study effects for the association between AKI and increased composite outcome (P = .117). There was no indication of small-study effects for the association of ALI (P = .122) and coagulopathy (P = .227) with mortality.
Figure 4.
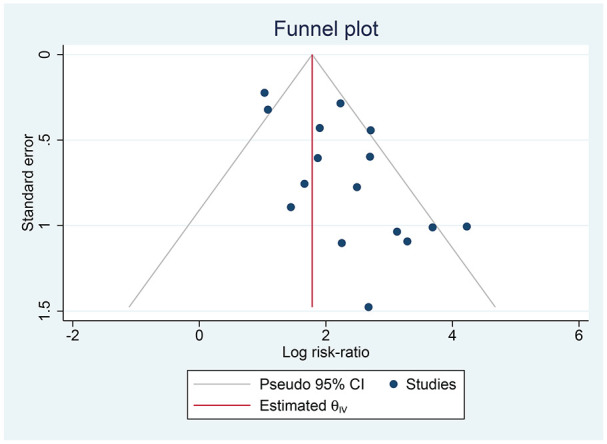
Publication bias. The funnel plot analysis showed an asymmetrical inverted funnel plot shape for acute kidney injury.
Note. CI = confidence interval.
Discussion
This meta-analysis showed that AKI was associated with poor composite outcomes, including mortality, severe COVID-19, and the need for ICU care in COVID-19 patients. This association was not significantly influenced by gender, age, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and CKD. Moreover, ALI and coagulopathy were also associated with increased mortality in patients with COVID-19. Five studies did not report baseline serum creatinine levels. Nevertheless, the exclusion of the aforementioned studies in the sensitivity analysis showed a consistent conclusion. The heterogeneity of ALI and coagulopathy was probably caused by the varying definitions used in the studies, which were not reported.
Qualitative risk of bias analysis indicated a moderate to high risk of bias in the included studies. These were mostly attributed to low comparability; the included studies were not specifically designed to evaluate AKI as a risk factor for mortality. Many of the studies only reported their findings in COVID-19 patients and were not geared toward determining whether the risk factors are independent of each other. Furthermore, we also recognized that the number of events/exposures in the studies was low; hence, the adjusted analysis might cause model overfitting. The quantitative risk of bias analysis showed the possibility of publication bias for the AKI outcome. There was no indication of small-study effects for any outcome.
Although SARS-CoV-2 primarily invades the human respiratory tract, there are indications of extrapulmonary organ involvement. Such manifestations may be directly caused by SARS-CoV-2 or due to medications used in the treatment of the infection. COVID-19 has 3-dimensional structure spike proteins, which are tightly bound to angiotensin-converting enzyme 2 (ACE2) following the activation of spike protein by transmembrane protease serine 2 (TMPRSS2).25,26 Hence, cells that express ACE2 become target cells that are prone to viral intrusion. Vulnerable cells include, but are not limited to, pulmonary alveolar cells type II, kidney tubular epithelial cells, liver endothelial cells, cardiac epithelial cells and artery smooth muscle cells, intestinal epithelial cells, and the gastrointestinal system. Therefore, it is plausible to assume multiorgan viral invasion in COVID-19, viremia may cause pneumonia, acute cardiac injury, diarrhea, ALI, and AKI.27-30 Furthermore, microvesicular steatosis and liver injury have been reported in the liver biopsy of some patients with COVID-19 pneumonia, suggesting possible damage from either viral invasion or drug-induced hepatic injury.31
Podocytes and proximal straight tubule cells are identified as kidney host cells for COVID-19 infection with the expression of ACE2 receptors, and TMPRSS2 genes were no less in kidney cells than in the lung, esophagus, small intestine, and colon.26,32,33 Playing a crucial role in urine filtration, reabsorption, and excretion, these cells are susceptible to both bacterial and viral damage, and podocyte injury swiftly causes heavy proteinuria.32-34 Viral-induced cytopathic effects may cause AKI in COVID-19, particularly in patients with evidence of viremia. This suggests the importance of early monitoring of kidney function and careful handling of laboratory specimens from COVID-19 patients with AKI to prevent accidental transmission.26
Cytokine release syndrome, rather than active viral replication in the kidney, is thought to be one of the pathomechanisms underlying AKI in COVID-19. Increased viral load in pneumocytes leads to remarkable immune responses, which generate a large number of cytokines leading to multiple organ dysfunction. It is also reasonable to assume that COVID-19 may infect the human kidney directly, causing AKI and massive viral invasion spreading in the body.35
Liver injury in COVID-19 might be due to direct viral invasion in liver cells, drug hepatotoxicity, or immune-mediated inflammation, including cytokine storm and hypoxia-related pneumonia.36,37 Both liver endothelial cells and bile duct epithelial cells express ACE2, with the latter expressing far higher levels of ACE2 than the former, but to a level comparable with that of type II alveolar cells in the lung.37 Bile duct epithelial cells play a significant role in immune response and liver regeneration, suggesting that the virus may affect bile duct cells rather than liver cells.38 Multiple studies have reported that patients with severe COVID-19 seem to have higher rates of liver dysfunction, as indicated by abnormal levels of alanine aminotransferase and aspartate aminotransferase accompanied by slightly elevated bilirubin and occasionally decreased albumin during disease progression.2,23,22,39-42 Although liver damage in mild COVID-19 cases is mostly transient and reversible, severe and critical cases tend to develop more advanced liver injury and even progress to liver failure. Such conditions require intervention to protect liver function and inhibit inflammatory responses.38,43
Severe COVID-19 pneumonia may coexist with coagulopathy, such as disseminated intravascular coagulation and venous thromboembolism.44 Endothelial cell dysfunction, together with the activation of platelets and leukocytes, leads to excessive thrombin production and fibrinolysis shutdown, which occurs both systemically and locally in the respiratory tract of patients with severe pneumonia, causing fibrin deposition along with subsequent tissue injury and microangiopathic pathology.45 Moreover, hypoxia occurring in severe pneumonia may promote thrombosis via increased blood viscosity and the hypoxia-induced transcription factor–dependent signaling pathway. These mechanisms might explain the hypercoagulable state observed in patients with severe COVID-19 pneumonia.44 Severe COVID-19 is also associated with increased platelet count, markedly elevated D-dimer values, longer prothrombin time, and lower fibrinogen and antithrombin activity.44-46 These findings suggest that abnormal coagulation parameters during the course of the disease are associated with poor prognosis.46
Formulating from the implications drawn from this systematic review and meta-analysis, we suggest several strategies for clinical practice; the presence of AKI, ALI, or coagulopathy may be used as markers for poor prognosis in COVID-19. Early identification and intervention are suggested, especially in the presence of AKI, where its presence is associated with a 10-fold risk of poor outcome. Regular monitoring for early identification and timely intervention of these complications is suggested, especially in treating patients with severe COVID-19. We suggest specific supportive measures in critically ill patients, including tight control of fluid balance and circulatory support, nutritional therapy, and deep vein thrombosis prophylaxis.47 Until solid evidence regarding specific drug therapy in COVID-19 emerge, we also recommend the use of fewer medications and withhold any drugs that could induce hepatic or kidney injury.
There are several limitations of this study. Many of these studies did not define the extent of AKI (grade), which may affect the outcome. Liver injury and coagulopathy were not defined in most of the studies. The definition of severe COVID-19 differs across studies. Several articles included in the study were published at preprint servers, which are not yet peer-reviewed. Most of the studies were from China; thus, some patients might overlap across the reports. Most of the included studies were retrospective in design.
Conclusions
This meta-analysis showed that the presence of AKI, ALI, and coagulopathy was associated with the composite outcome in patients with COVID-19. Due to the high risk of bias and poor comparability of the included studies, the effect estimate may not reflect the magnitude of the true effect. Other comorbidities or complications that may lead to poor outcomes, potentially confounding the effect estimate. Future research is encouraged to provide adjustment for their analysis and create a prediction model that includes AKI, ALI, and coagulopathy. We also encourage future research to investigate the risk factors associated with AKI, ALI, and coagulopathy in COVID-19 patients to aid drug considerations. Finally, we encourage studies to investigate the efficacy of additional measures for patients with multiorgan dysfunction, such as anticoagulants.
Supplemental Material
Supplemental material, Supplementary_Table for Multiorgan Failure With Emphasis on Acute Kidney Injury and Severity of COVID-19: Systematic Review and Meta-Analysis by Michael Anthonius Lim, Raymond Pranata, Ian Huang, Emir Yonas, Arto Yuwono Soeroto and Rudi Supriyadi in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: Not Applicable.
Consent for Publication: All authors consented to the publication of this manuscript.
Availability of Data and Materials: All data are available on reasonable request.
Author Contributions: I.H. and M.A.L. developed the concept and drafted the manuscript. I.H., M.A.L., and R.P. performed data acquisition and data analysis. E.Y., A.Y.S., and R.S. provided substantial analysis or interpretation of data for the article and critically revised the content to fit the reviewer’s comment. R.P. and I.H. performed the statistical analysis. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michael Anthonius Lim  https://orcid.org/0000-0001-7631-6835
https://orcid.org/0000-0001-7631-6835
Raymond Pranata  https://orcid.org/0000-0003-3998-6551
https://orcid.org/0000-0003-3998-6551
Ian Huang  https://orcid.org/0000-0003-1189-8453
https://orcid.org/0000-0003-1189-8453
Emir Yonas  https://orcid.org/0000-0002-8604-405X
https://orcid.org/0000-0002-8604-405X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. World Health Organization. Coronavirus Disease 2019 (COVID-19). Situation Report-95. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4. Accessed June 19, 2020.
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21(2):147032032092689. doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19—systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pranata R, Soeroto AY, Ian H, Lim MA, Santoso P, Permana H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis 2020. doi:10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 7. Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020;1-21. [Google Scholar]
- 9. World Health Organization. Report of the Who-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19). Accessed June 19, 2020.
- 10. Bai T, Tu S, Wei Y, et al. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: an analysis of 127 patients in Wuhan, China. https://poseidon01.ssrn.com/delivery.php?ID=882123066117004087071030000021013104049037064079033004076061037046052004057121010106035085055005018089009092043016071003091092061099113124125068024002102115106056044020005116124074125029114102086085015102115029088126098104096019103016104070100105&EXT=pdf. Accessed June 19, 2020.
- 11. Cao J, Tu W-J, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with Corona Virus Disease 2019 in Wuhan, China [published online ahead of print April 2, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen M, Fan Y, Wu X, et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-Coronavirus Infected Disease (COVID-19) in Wuhan, China. https://ssrn.com/abstract=3546069. Accessed June 19, 2020.
- 13. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with Coronavirus Disease 2019: retrospective study. BMJ. 2020;1091:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo X, Xia H, Yang W, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020. doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan W-J, Ni Z -Y, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382: 1-13. https://www.nejm.org/doi/full/10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu L, Ph D, Chen S, et al. Risk factors associated with clinical outcomes in 323 COVID-19 Patients in Wuhan, China. medRxiv. 2020. doi: 10.1101/2020.03.25.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020. 807. doi: 10.1101/2020.02.10.20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020. doi: 10.1101/2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-485. doi: 10.1016/S2213-2600(20)30079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Q, Ling Y, Zhang J, et al. Clinical characteristics of SARS-CoV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq. 2020;1-15. https://www.researchsquare.com/article/rs-18699/v1
- 25. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-280.e8. doi: 10.1016/J.CELL.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu D, Zhang H, Gong H, et al. Identification of a potential mechanism of acute kidney injury during the Covid-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou X, Chen K, Zou J, Han P, Haj J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. doi: 10.1007/s11684-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19—systematic review and meta-analysis. Postgr Med J. 2020:1-5. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santoso A, Pranata R, Wibowo A, Jibril Al-Farabi M, Huang I, Antariksa B. Cardiac Injury Is Associated With Mortality and Critically Ill Pneumonia in COVID-19: A Meta-Analysis. Am J Emerg Med. 2020. Epub ahead of print. doi: 10.1016/j.ajem.2020.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. doi: 10.1016/S2213-2600(20)30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jefferson JA, Nelson PJ, Najafian B, Shankland SJ. Podocyte disorders: core curriculum 2011. Am J Kidney Dis. 2011;58:666-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv. 2020. doi: 10.1101/2020.02.18.20023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perico L, Benigni A, Remuzzi G. Should COVID-19 concern nephrologists? why and to what extent? the emerging impasse of angiotensin blockade. Nephron. 2020;144:213-221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020. doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 38. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guan W, Ni Z-Y, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020. doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 40. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19 related liver damage. medRxiv. 2020. doi: 10.1101/2020.02.26.20026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. medRxiv. 2020. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 42. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Shi L, Wanga FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2 [published online ahead of print April 3, 2020]. J Thromb Thrombolysis 2020;50(1):72-81. doi: 10.1007/s11239-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li L, Li R, Wu Z, et al. Therapeutic strategies for critically ill patients with COVID-19. Ann Intensive Care. 2020;10:45. doi: 10.1186/s13613-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Table for Multiorgan Failure With Emphasis on Acute Kidney Injury and Severity of COVID-19: Systematic Review and Meta-Analysis by Michael Anthonius Lim, Raymond Pranata, Ian Huang, Emir Yonas, Arto Yuwono Soeroto and Rudi Supriyadi in Canadian Journal of Kidney Health and Disease