Abstract
Background:
Regorafenib (REG) is an oral multikinase inhibitor used in colorectal cancer, gastrointestinal stromal tumour and hepatocellular carcinoma. Several adverse events (AEs) are commonly reported during REG administration, and strategies for managing AEs in everyday clinical practice include supportive care, dose modifications and, when necessary, treatment withdrawal. We performed a systematic review and meta-analysis to assess the schedule treatment modifications of REG associated with AEs across randomized controlled clinical trials (RCTs).
Methods:
Eligible studies included RCTs assessing standard dose REG versus placebo. Outcomes of interest included: AE-related permanent discontinuation, dose interruptions and dose reductions.
Results:
We retrieved all the relevant RCTs through PubMed/Med, Cochrane library and EMBASE: 7 eligible studies involving a total of 2099 patients (Regorafenib: 1362; placebo: 737) were included in our analysis. The use of REG was associated with higher incidence and risk of all outcomes of interest when compared with placebo. The incidences of permanent discontinuation, dose interruptions and dose reductions in patients receiving REG were 9.7%, 57.2% and 47%, respectively, versus 3.3%, 16.7% and 7.7% of placebo group; compared with placebo, the summary relative risks (RRs) of permanent discontinuation, dose interruptions and dose reductions in REG arm were 2.80 (95% CI 1.85–4.22), 3.21 (95% CI 2.59–3.99) and 6.02 (95% CI 3.28–11.03), respectively.
Conclusions:
Treatment with REG at the standard dose of 160 mg is associated with a significant increase in AE-related permanent discontinuation, dose interruptions and dose reductions. Prompt identification and management of AEs seem mandatory to obtain maximal benefit from REG treatment. In the current landscape, dose personalization of REG may have the potential to improve quality of life, minimize treatment discontinuation and maximize patient outcomes.
Keywords: colorectal cancer, gastrointestinal stromal tumours, GIST, meta-analysis, regorafenib
Introduction
Regorafenib (REG) is an orally bio-available multikinase inhibitor (MKI) that blocks the activity of several protein kinases, including those associated with proliferation (KIT, BRAF, RAF-1 and RET), tumour angiogenesis (VEGFR-1, VEGFR-2, VEGFR-3 and TIE2) and tumour microenvironment signalling (PDGFR and FGFR).1,2 Clinical benefits from the administration of REG were initially observed in metastatic colorectal cancer (CRC) and gastrointestinal stromal tumour (GIST), as stated by the three randomized controlled clinical trials (RCTs) CORRECT, GRID and CONCUR.3–5 More recently, REG gained approval in hepatocellular carcinoma (HCC), on the basis of data from the RESORCE trial,6 where REG showed a survival benefit in patients affected by advanced or metastatic HCC who progressed on sorafenib treatment. Currently, REG is approved as a single agent for the treatment of CRC, GIST and HCC at a dose of 160 mg orally once daily on days 1–21 of each 28 days cycle.7 There are currently ongoing trials aimed to evaluate the efficacy of REG as monotherapy or in combination with other anticancer agents and the number of indications and patients receiving REG is supposed to further increase in the near future.8,9 REG has undoubtedly modified clinical practice for patients affected by several malignancies, however various drug-related adverse events (AEs), such as fatigue, hypertension, hand–foot skin reaction (HFSR), rash and diarrhoea have been reported.10–12 Moreover, REG-related AEs frequently require treatment modifications and may also result in early discontinuation of treatment.13,14 As consequence, this management may interfere with the efficacy and long-term outcomes of patients. Prompt identification of AEs is fundamental to guarantee that patients can be treated as safely as possible and, in order to ensure that anticancer treatment is effective, maintaining the optimal dose levels represents a major issue.15,16
In the current study, we performed a systematic review and meta-analysis to examine AE-related permanent discontinuation of treatment, AE-related dose interruptions and AE-related dose reductions in patients receiving REG, across the seven available RCTs.
Materials and methods
Search strategies
All phase II and III clinical trials published from 15 June 2008 to 29 January 2020 regarding the clinical role of REG therapy in advanced malignancies were retrieved. Keywords used for searching on PubMed/ Medline, Cochrane Library and EMBASE were: “Regorafenib” OR “Stivarga” OR “BAY 73-4506” AND “phase 2 trial” OR “phase 3 trial”; only articles published in peer-reviewed journals and written in English language were considered. Furthermore, proceedings of the main international oncological meetings (American Society of Clinical Oncology, European Society of Medical Oncology, European Council of Clinical Oncology and American Association for Cancer Research) were also searched from 2005 onward for relevant abstracts.
The search and review of the articles were evaluated by two authors (AR and MN) independently.
Aims of the systematic review and meta-analysis
The aims of the systematic review and meta-analysis were:
to evaluate incidence and risk of AE-related permanent discontinuation in RCTs comparing REG treatment versus placebo;
to evaluate incidence and risk of AE-related dose interruptions in RCTs comparing REG treatment versus placebo;
to evaluate incidence and risk of AE-related dose reductions in RCTs comparing REG treatment versus placebo.
Selection criteria
Studies selected from first analysis were then restricted to: (a) prospective phase II or III RCTs in advanced malignancies; (b) participants enrolled in REG treatment or placebo; (c) studies with available data about AE-related dose reductions and/or AE-related dose interruptions and/or AE-related permanent discontinuation of treatment.
Data extraction and quality assessment
The following data were extracted for each publication: (a) study general information (author, year, phase, carry out country, inclusion criteria); (b) primary site; (c) interventions; (d) formulation of REG therapy; (e) number of patients; (f) available outcomes in terms of AE-related dose reductions, dose interruptions and/or permanent discontinuation. Two separate authors (AR and MN) conducted the search and identification independently.
We assessed the methodological quality of the included trials using Cochrane Collaboration tool. Studies examined were graded as having a ‘low risk’, ‘high risk’, or ‘unclear risk’ of bias across the seven specified domains. This meta-analysis was conducted according to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.17
Assessment of risk of bias
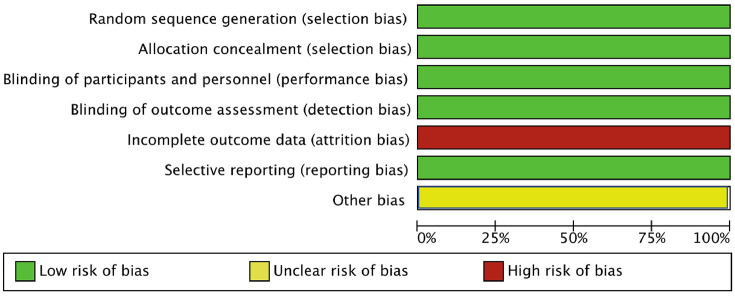
Risk of bias in the seven selected studies was assessed independently by two authors (AR and MN) using the tool of The Cochrane Collaboration for assessing risk of bias and therefore including selection, performance, detection, attrition and reporting bias.18 The lists of outcomes reported in the published paper were compared with those from study protocols or trials registries. The results were summarized in a risk of bias graph ( Figure 1 ).
Figure 1.
Risk of bias graph: authors’ judgements on each risk of bias item presented as percentages across all included studies.
Types of outcome measures
We examined three outcomes: AE-related dose reductions, AE-related dose interruptions and AE-related permanent discontinuation. Dose interruptions were defined as AE-related transitory discontinuation of treatment. All data were obtained from full text or supplementary material of each study.
Statistical design
All statistical analyses were performed using R studio. For the calculation of incidence rate (IR), the number of patients with AE-related dose reductions, AE-related dose interruptions and AE-related permanent discontinuation and the total number of patients being treated with REG and placebo were determined from each trial. The proportions of patients and 95% CIs were derived.
Relative risks (RRs) were used to analyse dichotomous variables, including AE-related dose reductions, dose interruptions and permanent discontinuation: RRs were combined with the Mantel–Haenszel method. Statistical heterogeneity between studies was examined using the chi-squared test and the I2 statistic: substantial heterogeneity was considered to exist when the I2 value was greater than 50% or there was a low p value (<0.10) in the chi-squared test.
Results
Studies selected
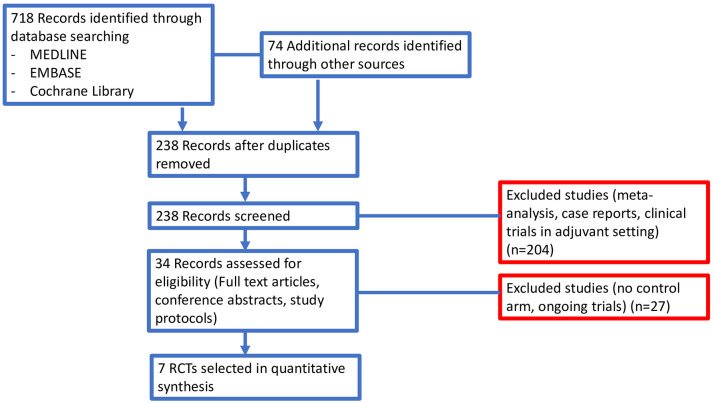
In our search, we identified 792 potentially relevant reports, which were subsequently restricted to seven.19–25 We excluded 785 records as non-pertinent reports (meta-analysis and systematic reviews, review articles, editorials, case reports, pre-clinical studies, retrospective studies, non-randomized studies, no placebo-controlled arm trials, ongoing trials/trials in progress), as shown in Figure 2 . All studies included in our analysis were published in full manuscript.
Figure 2.
Study flow diagram.
Of the seven eligible studies, two trials compared REG treatment versus placebo in pretreated patients affected by advanced or metastatic CRC.19–21 The same comparison was made in the other five trials, comparing REG versus placebo in advanced or metastatic GIST,20 gastric cancer/gastroesophageal junction (GC/GEJ),22 HCC,24 osteosarcoma25 and soft tissue sarcoma (STS).23 Four trials were phase III studies,19–21,24 whereas three studies were phase II trials.22,23,25
The seven studies shared several characteristics: they were all randomized, double-blind, multicentre, placebo-controlled trials including patients whose disease had progressed after treatment with one to two previous lines of chemotherapy for advanced or metastatic disease. In all these RCTs only patients with an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1 were enrolled.19–25
A total of 2099 patients were available for the meta-analysis (REG: 1362; placebo: 737); REG dosage and schedule was as follows: 160 mg orally on days 1 to 21 of each 28-day cycle. A summary of the included RCTs is presented in the Table 1 .
Table 1.
Summary of the included studies.
| Author/ yearreference | Phase of CT | Primary site | Carry out country | Number in intervention/control group | ECOG-PS | Type of treatment | ADE-related outcomes included in analysis |
|---|---|---|---|---|---|---|---|
| Grothey / 2013 19 | III | CRC | 16 countries in North America, Europe, Asia, and Australia | 500/253 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle + BSC versus placebo + BSC | Dose reductions Dose interruptions Permanent discontinuation |
| Demetri / 2013 20 | III | GIST | 17 countries in North America, Europe, and Asia | 132/66 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle + BSC versus placebo + BSC | Dose reductions Permanent discontinuation |
| Li / 2015 21 | III | CRC | China, Hong Kong, South Korea, Taiwan, and Vietnam | 136/68 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle + BSC versus placebo + BSC | Dose reductions Dose interruptions Permanent discontinuation |
| Pavlakis / 2016 22 | II | GC / GEJ | Australia, New Zealand, Canada, Korea | 56/29 | 0,1 | Oral regorafenib 160 mg daily on days 1–21 each 28-day cycle + BSC versus oral placebo + BSC | Permanent discontinuation |
| Mir / 2016 23 | II | STS | France, Austria | 26/12 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle versus placebo | Dose reductions Dose interruptions Permanent discontinuation |
| Bruix / 2017 24 | III | HCC | 21 countries in North America, South America, Europe, Asia, and Australia | 379/194 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle + BSC versus placebo + BSC | Dose reductions Permanent discontinuation |
| Duffaud / 2019 25 | II | Osteosarcoma | France | 89/92 | 0,1 | Oral regorafenib 160 mg once daily on days 1–21 each 28-day cycle + BSC versus placebo + BSC | Dose reductions Dose interruptions Permanent discontinuation |
ADE, adverse drug event; BSC, best supportive care; CRC, colorectal cancer; CT, clinical trial; ECOG-PS, Eastern Cooperative Oncology Group performance status; GC, gastric cancer; GEJ, gastroesophageal junction; GIST, gastrointestinal stromal tumour; HCC, hepatocellular carcinoma; STS, soft tissue sarcoma.
All seven RCTs reported data on AE-related permanent discontinuation;19–25 four trials reported data on AE-related dose interruption19,21,23,25 whereas six studies reported data on AE-related dose reductions during treatment.19–21,23–25
Incidence and RR of AE-related permanent discontinuation
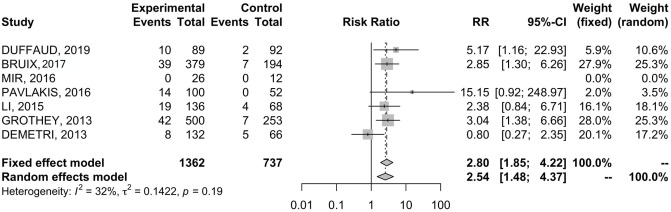
Table 2 shows the pooled IRs of all outcomes included in our analysis. In order to evaluate AE-related permanent discontinuation, data were extracted from all seven RCTs comprising 2099 patients.19–25 The incidence of AE-related permanent discontinuation during REG treatment and placebo were 9.7% and 3.3%, respectively. Patients treated with REG showed higher risk of permanent discontinuation (RR = 2.80; 95% CI 1.85–4.22) ( Figure 3 ); results were associated with low heterogeneity (I2 = 32%), therefore a fixed effects model was used.
Table 2.
Incidence rate of drug-related AE outcomes resulting from regorafenib treatment and placebo.
| Drug-related AE outcomes | Number of events/sample size |
Incidence rate % (95% CI) |
||
|---|---|---|---|---|
| Regorafenib | Placebo | Regorafenib | Placebo | |
| Permanent discontinuation | 132/1362 | 25/737 | 9.7% (8.1–11.2) | 3.3% (2.1–4.6) |
| Transient interruptions | 430/751 | 71/425 | 57.2% (53.7–60.8) | 16.7% (13.1-20.2) |
| Dose reductions | 593/1262 | 53/685 | 47% (44.2–49.7) | 7.7% (5.7–9.7) |
AE, adverse event.
Figure 3.
Forest plot of comparison between regorafenib treatment and placebo: the outcome was risk ratio of permanent discontinuation.
CI, confidence interval.
Incidence and RR of AE-related dose interruption
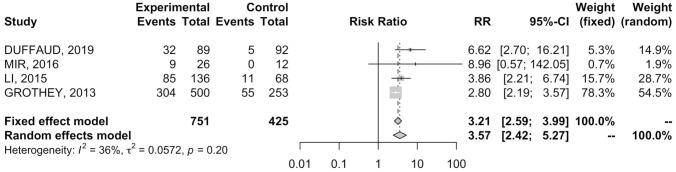
Four RCTs19,21,23,25 provided data on AE-related dose interruption in 501 patients. REG treatment was associated with a pooled IR of dose interruption of 57.2% versus 16.7% in the placebo group ( Table 2 ). Patients receiving REG showed an increased RR of AE-related dose interruption (RR = 3.21; 95% CI 2.59–3.99) ( Figure 4 ); results were associated to low heterogeneity (I2 = 36%), therefore a fixed effects model was used.
Figure 4.
Forest plot of comparison between regorafenib treatment and placebo: the outcome was risk ratio of dose interruption.
CI, confidence interval.
Incidence and RR of AE-related dose reduction
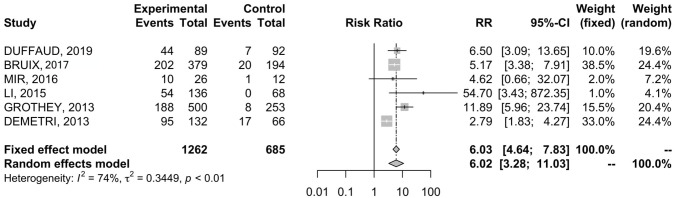
Data were available in six RCTs19–21,23–25 comprising 646 patients. The incidence of AE-related dose reduction in REG arm and placebo arm was 47% and 7.7%, respectively ( Table 2 ). REG was associated with higher RR of dose reduction when compared with placebo (RR = 6.02; 95% CI 3.28–11.03) ( Figure 5 ). The analysis was associated with significant heterogeneity between trials (I2 = 74%), so a random-effects model was used for the OS analysis.
Figure 5.
Forest plot of comparison between regorafenib treatment and placebo; the outcome was risk ratio of dose reduction.
CI, confidence interval.
Discussion
In recent years, the use of MKIs as a standard of care in several solid tumours has notably changed the landscape of cancer management.26,27 All MKIs, including REG at the recommended standard dose of 160 mg, are associated with various drug-related AEs that mainly occur within the first cycles of therapy and which can result in a relevant impact on the quality of life of patients and frequently lead to treatment dose reduction and drug discontinuation.28 A plethora of recent meta-analyses evidenced that standard-dose REG leads to a significant risk of developing several all-grade and grade 3–4 AEs in patients with advanced cancer.29–31
To the best of the authors’ knowledge, this is the first meta-analysis to investigate the incidence and risk of AE-related permanent discontinuation of treatment, dose interruptions and dose reductions associated with standard-dose REG in cancer patients. The meta-analysis was based on seven RCTs19–25 with a total of 2099 patients, 1362 of which received standard-dose REG. We noted that AE-related dose reductions, dose interruptions and permanent treatment interruptions were, as expected, more common in the REG group than in the placebo group (reductions: 47% versus 7.7%; dose interruptions: 57.2% versus 16.7%; permanent discontinuation: 9.7% versus 3.3%, respectively).
As stated previously, in placebo-controlled RCTs regarding REG, a large proportion of patients experienced either an AE-related dose reduction or interruption when receiving the standard schedule; moreover, a small but non-negligible proportion of participants (9.7%) had to permanently discontinue the treatment and withdrew from the trial as AE-related result. This data has even more relevance considering that all patients enrolled into RCTs must have good performance status (ECOG = 0, 1) and no significant comorbidities.32 To address this relevant source of bias, the European REBECCA trial recently evaluated the role of REG in metastatic CRC with a focus on efficacy and optimal safety in a real-world setting and revealing that progression-free survival, overall survival and safety profile were similar to those of CORRECT and CONCUR trial.33
Despite a steadily increased use of REG in advanced malignancies over the last years, real-world data on the role of non-standard schedules are scarce, often resulting from small study populations with short follow-up.34 In this scenario, balancing between treatment efficacy, tolerability and quality of life is of major interest. A German study by Mross et al., revealed that REG presented similar pharmacokinetic and pharmacodynamic effects for dose levels from 120 to 220 mg; moreover, no significant pharmacologic activity was shown at doses of less than 120 mg.35 As regards current clinical practice, the use of REG seems heterogeneous, with several authors which recommend REG at the starting dose of 80 or 120 mg and subsequent dose-escalation, and others which prefer shorter cycles at standard doses of 160 mg.36,37 In our recent retrospective Italian multicentre experience, we identified approximately 20 different strategies of treatment personalization in patients affected by advanced or metastatic GIST, including dose reduction from 160 to 120 mg, 120 mg as starting dose, the initial dose of 80 mg and several others.38 Of note, whatever was the strategy adopted, REG treatment personalization has led not only to a clinical benefit defined as complete or partial resolution of side effects in almost all patients, but especially positively affected the duration of REG treatment. In particular, a median duration of 9.9 months was observed, significantly greater than the 22.9 weeks of the GRID trial, with 23% of patients who exceeded 20 months and a mean duration of 32.14 months (range 20.50–53.67 months).
In a recent retrospective large study on 2376 Japanese patients affected by metastatic CRC, the initial dose of 80 mg or 120 mg REG had lower grade 3–4 AEs but similar efficacy when compared with recommended standard dose of 160 mg.39 Moreover, the results of the phase II REDOS RCT comparing a dose-escalation approach with the standard REG dosing strategy of 160 mg daily in 123 patients affected by metastatic CRC, are currently available.40 The study reported that the dose-escalation strategy starting from 80 to 160 mg daily via 40 mg increases over 3 weeks represents a safe and effective alternative to the standard dose, confirming the results from previous field-practice studies. There are currently ongoing trials, including the phase II trial REGOCC21, aimed to compare different schedules of REG treatment and which finals results will help to shed light on different dose-escalation approaches.41
In patients receiving 160 mg, an important number of AEs occur within the first cycles of therapy, suggesting that treatment adjustment during REG is not only a common event but also an early one.42 In REBECCA study, for example, the median time between the start of 160 mg treatment and first dose adjustment was 0.7 months;33 thus, early monitoring and effective management of AEs seems mandatory to reduce treatment discontinuation and to optimize clinical benefit. In addition, the risks associated with REG therapy may be increased by other underlying factors, including prior oncologic and non-oncologic therapies, concomitant medications and other comorbidities.43–46
From our point of view, modifications of REG dose and schedule should be more valorized and spread into everyday clinical practice, because it may positively affect patient’s compliance and enhance adherence to treatment. This inevitably leads to an undoubtful optimization of treatment duration, that clearly affects disease long-term outcome. Since the chronic and continuative MKI intake is crucial according to their mechanism of action, therapy optimization takes part for the treatment.
Our meta-analysis holds its own strengths and limitations. The strengths of our work regard the inclusions of only placebo-controlled RCTs, the total number of patients and the high quality of statistical analysis. However, the results of this meta-analysis should be interpreted with caution for some limitations. First, despite random-effects modelling was used in order to address heterogeneity, AE-related dose reduction analysis was burdened by substantial heterogeneity (I2 = 74%). Second, data were extracted from published clinical trial results and were not gathered from individual patient data; moreover, the seven RCTs included were industry funded. Third, no subgroup analysis has been performed. The selection of patients represents another key element. In fact, all these studies compared REG versus placebo in participants with ECOG-PS grade 0 or 1 and which can only partly be representative of all patients receiving REG for advanced malignancies in everyday clinical practice. Patients with comorbidities, including uncontrolled hypertension, impaired hepatic or renal function and/or history of venous thromboembolic events, were excluded from the RCTs as well as subjects with ECOG-PS ⩾2. Finally, geographical elements [single country (n = 1 RCT), two European countries (n = 1), multinational (n = 4), single continent (n = 1)], the impact of previous treatments and primary cancer site [GIST (n = 1), CRC (n = 2), GC/GEJ (n = 1), STS (n = 1), HCC (n = 1) and osteosarcoma (n = 1)] may represent other possible sources of heterogeneity.
Conclusion
To the best of the authors’ knowledge, this is the first meta-analysis addressing this issue, providing evidence that REG 160 mg is associated with a significant increased incidence and risk of AE-related permanent discontinuation, dose interruptions and dose reductions. Therefore, despite the several limitations owing to the substantial heterogeneity, our findings may assist and help clinicians to optimize REG treatment, since early recognition and management of AEs are mandatory in clinical practice. Ongoing trials on REG are focused on the identification of effective and personalized strategies helping clinicians to maximize treatment benefits and to minimize the need for therapy discontinuation in the fragile population of pretreated patients affected by advanced cancer.
Supplemental Material
Supplemental material, Rizzo_et_al_Revised_Supplementary_material for Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis by Alessandro Rizzo, Margherita Nannini, Marco Novelli, Angela Dalia Ricci, Valerio Di Scioscio and Maria Abbondanza Pantaleo in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Study concept and design: Alessandro Rizzo, Margherita Nannini and Maria Abbondanza Pantaleo; acquisition of data: Angela Dalia Ricci; statistical analysis: Alessandro Rizzo and Marco Novelli; analysis and interpretation of data: Alessandro Rizzo and Marco Novelli; drafting of the manuscript: Alessandro Rizzo, Angela Dalia Ricci and Valerio Di Scioscio; critical revision of the manuscript for important intellectual content: Margherita Nannini and Maria Abbondanza Pantaleo.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Department of Experimental, Diagnostic and Specialty Medicine, DIMES, Sant’Orsola-Malpighi University Hospital, Bologna, Italy.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Alessandro Rizzo  https://orcid.org/0000-0002-5257-8678
https://orcid.org/0000-0002-5257-8678
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alessandro Rizzo, Department of Specialized, Experimental and Diagnostic Medicine, Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
Margherita Nannini, Medical Oncology Unit, Sant’Orsola-Malpighi University Hospital, via Massarenti 9, Bologna, 40138, Italy.
Marco Novelli, Department of Statistical Sciences, University of Bologna, Bologna, Italy.
Angela Dalia Ricci, Department of Specialized, Experimental and Diagnostic Medicine, Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
Valerio Di Scioscio, Department of Statistical Sciences, University of Bologna, Bologna, Italy.
Maria Abbondanza Pantaleo, Department of Specialized, Experimental and Diagnostic Medicine, Sant’Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy.
References
- 1. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011; 129: 245–255. [DOI] [PubMed] [Google Scholar]
- 2. Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 2013; 12: 1322–1331. [DOI] [PubMed] [Google Scholar]
- 3. André T, Dumont SN, et al. Regorafenib approved in metastatic colorectal cancer. Bull Cancer 2013; 100: 1027–1029. [DOI] [PubMed] [Google Scholar]
- 4. de la Fouchardière C, et al. Regorafenib in the treatment of metastatic colorectal cancer. Future Oncol 2018; 14: 2239–2246. [DOI] [PubMed] [Google Scholar]
- 5. Mazzocca A, Napolitano A, Silletta M, et al. New frontiers in the medical management of gastrointestinal stromal tumors. Ther Adv Med Oncol 2019; 11: 175883591984194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forner A, Reig M, Bruix J, et al. Hepatocellular carcinoma. Lancet 2018; 391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 7. Rimassa L, Pressiani T, Personeni N, et al. Regorafenib for the treatment of unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther 2017; 17: 567–576. [DOI] [PubMed] [Google Scholar]
- 8. Cerrito L, Ponziani FR, Garcovich M, et al. Regorafenib: a promising treatment for hepatocellular carcinoma. Expert Opin Pharmacother 2018; 19: 1941–1948. [DOI] [PubMed] [Google Scholar]
- 9. Liu Z, Lin Y, Zhang J, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res 2019; 38: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Personeni N, Pressiani T, Bozzarelli S, et al. Targeted agents for second-line treatment of advanced hepatocellular carcinoma. World J Gastrointest Oncol 2019; 11: 788–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karthaus S, Falcone A, Van Cutsem, et al. Regorafenib dose modifications in patients with meta- static colorectal cancer in the phase III CORRECT study. Oncol Res Treat 2014; 37: 39. [Google Scholar]
- 12. Facciorusso A, Abd El, Aziz MA, Sacco R, et al. Efficacy of regorafenib in hepatocellular carcinoma patients: a systematic review and meta-analysis. Cancers (Basel) 2019; 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawakami K, Wakatsuki T, Soejima A, et al. Factors associated with regorafenib adherence with metastatic colorectal cancer. Patient Prefer Adherence 2019; 13:1745–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goel G, et al. Evolution of regorafenib from bench to bedside in colorectal cancer: is it an attractive option or merely a “me too” drug? Cancer Manag Res 2018; 10: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazzocca A, Napolitano A, Silletta M, et al. New frontiers in the medical management of gastrointestinal stromal tumours. Ther Adv Med Oncol 2019; 11: 1758835919841946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loree JM, Kopetz S, et al. Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol 2017; 9: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins J, Green S, et al. Cochrane Handbook for systematic reviews of interventions, version 5.1.0. Cochrane Collab, 2011. [Google Scholar]
- 19. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 20. Demetri GD, Reichardt P, Kang Y-K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with pre- viously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015; 16: 619–629. [DOI] [PubMed] [Google Scholar]
- 22. Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II Trial. J Clin Oncol 2016; 34: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mir O, Brodowicz T, Italiano A, et al. Safety of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 24. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 25. Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2019; 20: 120–133. [DOI] [PubMed] [Google Scholar]
- 26. Rimassa L, Pressiani T, Merle P, et al. systemic treatment options in hepatocellular carcinoma. Liver Cancer 2019; 8: 427–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhillon S, et al. Regorafenib: a review in metastatic colorectal cancer. Drugs 2018; 78: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 28. Verbrugghe M, Duprez V, Beeckman D, et al. Factors influencing adherence in cancer patients taking oral tyrosine kinase inhibitors: a qualitative study. Cancer Nurs 2016; 39: 153–162. [DOI] [PubMed] [Google Scholar]
- 29. Abdel-Rahman O, Fouad M, et al. Risk of cardiovascular toxicities in patients with solid tumors treated with sunitinib, axitinib, cediranib or regorafenib: an updated systematic review and comparative meta-analysis. Crit Rev Oncol Hematol 2014; 92: 194–207. [DOI] [PubMed] [Google Scholar]
- 30. Xie G, Gong Y, Wu S, et al. meta-analysis of regorafenib-associated adverse events and their management in colorectal and gastrointestinal stromal cancers. Adv Ther 2019; 36: 1986–1998. [DOI] [PubMed] [Google Scholar]
- 31. Wang Z, Xu J, Nie W, et al. Risk of hypertension with regorafenib in cancer patients: a systematic review and meta-analysis. Eur J Clin Pharmacol 2014; 70: 225–231. [DOI] [PubMed] [Google Scholar]
- 32. Kotecki N, Penel N, et al. Inappropriate dose of multitargeted tyrosine kinase inhibitors: the original sin. Curr Opin Oncol 2016; 28: 437–440. [DOI] [PubMed] [Google Scholar]
- 33. Adenis A, de La Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016; 16: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Del Prete S, Cennamo G, Leo L, et al. Adherence and safety of regorafenib for patients with metastatic colorectal cancer: observational real-life study. Future Oncol 2017; 13: 415–423. [DOI] [PubMed] [Google Scholar]
- 35. Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012; 18: 2658–2667. [DOI] [PubMed] [Google Scholar]
- 36. Tabchi S, Ghosn M. et al. Regorafenib: start low and go slow. Target Oncol 2015; 10: 445–447. [DOI] [PubMed] [Google Scholar]
- 37. Grothey A, George S, van Cutsem E, et al. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist 2014; 19: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nannini M, Nigro MC, Vincenzi B, et al. Personalization of regorafenib treatment in metastatic gastrointestinal stromal tumours in real-life clinical practice. Ther Adv Med Oncol 2017; 9: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakashima M, Ide K, Kawakami K. et al. comparison of standard initial dose and reduced initial dose regorafenib for colorectal cancer patients: a retrospective cohort study. Target Oncol 2019; 14: 295–306. [DOI] [PubMed] [Google Scholar]
- 40. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol 2019; 20: 1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kudo T, Kato T, Kagawa Y, et al. Phase II dose titration study of regorafenib for patients with unresectable metastatic colorectal cancer that progressed after standard chemotherapy. J Clin Oncol 2018; 36(Suppl. 4): 821. [Google Scholar]
- 42. Grothey A, Sobrero AF, Siena, et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol 2013; 31(Suppl. 15): 3637. [Google Scholar]
- 43. Van Cutsem E, Martinelli E, Cascinu S, et al. regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN Study. Oncologist 2019; 24: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Røed Skårderud M, Polk A, Kjeldgaard Vistisen K, et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2018; 62: 61–73. [DOI] [PubMed] [Google Scholar]
- 45. Jariwala PB, Pellock SJ, Goldfarb D, et al. Discovering the microbial enzymes driving drug toxicity with activity-based protein profiling. ACS Chem Biol 2020; 15: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ervin SM, Hanley RP, Lim L, et al. Targeting regorafenib-induced toxicity through inhibition of gut microbial β-glucuronidases. ACS Chem Biol 2019; 14: 2737–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Rizzo_et_al_Revised_Supplementary_material for Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis by Alessandro Rizzo, Margherita Nannini, Marco Novelli, Angela Dalia Ricci, Valerio Di Scioscio and Maria Abbondanza Pantaleo in Therapeutic Advances in Medical Oncology