Abstract
In prior research, intrathecal tigecycline was successfully used to treat central nervous system infection by extensively drug-resistant Acinetobacter baumannii. However, little is known about its safe dose and adverse reactions. This study reports the case of a 28-year-old male patient who was diagnosed with central nervous system infection by extensively drug-resistant A. baumannii after the removal of a ventriculoperitoneal shunt. Intravenous and intrathecal tigecycline were administrated simultaneously. Spinal arachnoiditis was discovered after nine doses of intrathecal tigecycline. Spinal arachnoiditis was resolved after discontinuation of the antibiotic. This is the first report of an adverse reaction to intrathecal tigecycline. The case was complicated by spinal arachnoiditis, which obstructed the assessment of cerebrospinal fluid. The appropriate dose and administration schedule of intrathecal tigecycline remain to be determined.
Keywords: Tigecycline, adverse reaction, intrathecal therapy, Acinetobacter baumannii, extensively drug-resistant, central nervous system, infection
Introduction
Central nervous system (CNS) infection by extensively drug-resistant Acinetobacter baumannii (XDRAB) is associated with high mortality. The current treatment of choice is colistin.1 However, it was reported that 21.7% of patients experience neurotoxicity as an adverse reaction to this therapy.2 Tigecycline has proven activity against XDRAB. However, because of its poor blood–brain barrier permeability, intravenous (IV) tigecycline is not recommended for treating CNS infection by XDRAB.3 The intrathecal (IT) route is not routinely used to administer tigecycline. The first case report of IT tigecycline was presented in 2019.4 This represents the second case report of IT tigecycline for the treatment of CNS infection.
Case report
A 28-year-old male patient presented with a high fever and rigidity in his extremities. He was discharged 1 month prior because of CNS infection. The patient was diagnosed with grade I astrocytoma in the third ventricle 2 years previously when he underwent ventriculoperitoneal shunting (VPS) to treat acute hydrocephalus. He did not recover from the coma (Glasgow coma score, E4V2M3) after the surgery.
Relapsed CNS infection was suspected. Empirical IV linezolid (Zyvox, 600 mg q12h, Pfizer, New York, NY, USA) and meropenem (Merrem, 2000 mg q8h, Dainippon Sumitomo Pharma Co., Ltd, Osaka, Japan) were used according to the previous admission information. The symptoms subsided on the seventh day. To prevent recurrent infection, the shunt was removed on the ninth day. The patient developed a high fever and seizure after the surgery. Lumbar puncture (LP) was performed, and an examination of cerebrospinal fluid (CSF) revealed leukocytosis (22,592 × 106/L) accompanied by hypoglycorrhachia (0.11 mmol/L). CSF culture implied the presence of XDRAB that was only sensitive to colistin (minimum inhibitory concentration < 0.5 µg/mL) and tigecycline (minimum inhibitory concentration = 2 µg/mL). Therefore, IV tigecycline (Tygacil, 100 mg q12h, Pfizer) and cefotaxime/sulbactam (3000 mg twice daily) were applied.
IT tigecycline was administered simultaneously with IV tigecycline. LP was performed once daily. The L3/4 and L4/5 intervertebral spaces were chosen as the insertion sites alternatively. Five milligrams of tigecycline were diluted in saline to a total volume of 5 mL. After releasing 30 to 50 mL of CSF, the antibiotics were slowly injected using the withdraw/inject technique to achieve an even distribution. The patient was kept in the horizontal position for 4 to 6 hours.
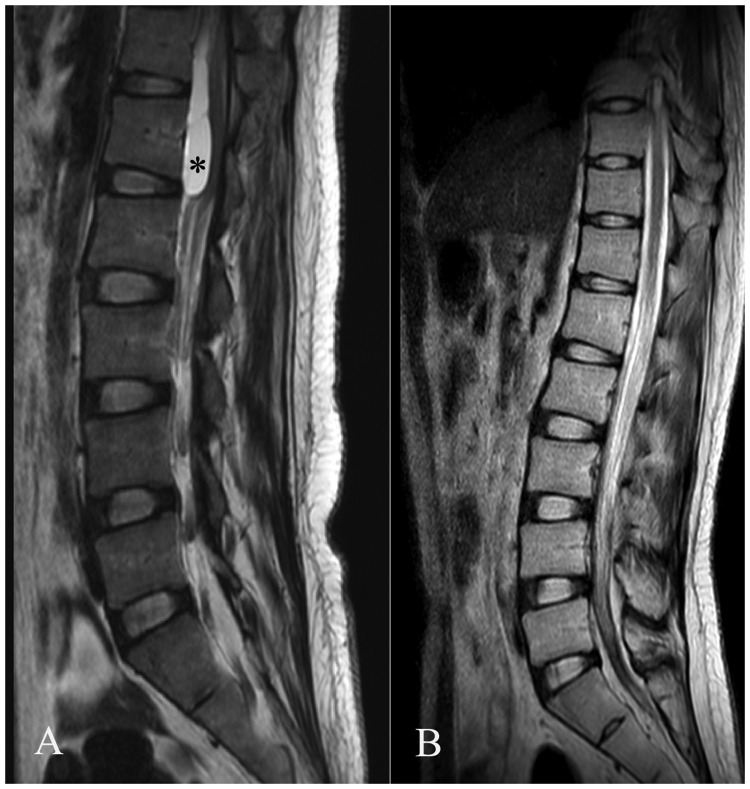
IT tigecycline was discontinued after 7 days of persistently negative CSF cultures. The results of CSF analysis were improved (neutrophil cell count = 1246 × 106/L, CSF glucose level = 2.88 mmol/L). In total, IV tigecycline was administered for 53 days, and nine doses of IT tigecycline were delivered. Dramatically, the patient’s fever relapsed 10 days after the withdrawal of antibiotics. LP was performed, but no CSF was obtained. Non-enhanced lumbar magnetic resonance imaging (MRI) revealed a subdural T2-WI hyperintense sac located above the L1 level (Figure 1a). Spinal arachnoiditis (SA) was suspected, but the patients’ family refused to allow the patient to undergo thoracic MRI. Thus, the extent of the lesion and its transverse section image were not accessible.
Figure 1.
(a) Lumbar magnetic resonance imaging revealed a subdural sac (asterisk) compressing the conus medullaris and causing obstructive hydrocephalus. (b) The sac had disappeared after 12 months of follow-up.
The patient’s fever was ameliorated by 18 additional days of IV tigecycline administration. LP was not reattempted. CSF was sampled transcutaneously through the bulging right frontal surgical incision. The CSF test suggested that the CNS infection was resolved. Enhanced thoracic and lumbar MRI indicated that SA was resolved after 12 months of follow-up (Figure 1b).
All procedures performed in article were in accordance with the ethical standards of the local review board.
Discussion
IT administration is a sophisticated technique for antibiotics with reduced blood-brain-barrier permeability. Deng et al.4 successfully treated an XDRAB CNS infection by switching intraventricular tigecycline (4 mg twice daily) to IT tigecycline (4 mg once daily). However, little is known about the safe dose and treatment schedule of IT tigecycline.
SA usually presents with radiculopathy. In this case, because the patient was comatose, no obvious symptom or sign had been observed. The abnormality was identified only after the failure of LP. Although no special management was required, this obstructed the CSF examination, making it difficult to evaluate the stage of infection.
SA denotes inflammation of the pia-arachnoid and encapsulation of the nerve roots.5 In this case, three pathophysiologic mechanisms should be considered, namely an adverse reaction to IT antibiotics, the complication of CNS infection, and injury caused by LP.
The safety of IT administration for various antibiotics such as vancomycin and amikacin has been established.6–8 The most common complications of IT antibiotics are ventriculitis and seizure.9 The adverse effects of IV tigecycline commonly involve the gastrointestinal tract, whereas those of IT tigecycline are unknown.10 In this case, SA occurred rapidly after prolonged IT tigecycline administration, and this complication disappeared after treatment was halted. There was a possibility that the SA was the result of its toxicity and the subsequent reactive inflammation.
Mycotic11 and tuberculous12 CNS infection can have spinal involvement. The condition is mostly observed in immunocompromised patients with post-infectious inflammatory response syndrome in response to prolonged infection and the exudate.13 However, most lesions present as extradural or leptomeningeal granuloma. Further, SA related to A. baumannii has not been described in the literature.
Repeated LP may cause chronic inflammation of the dura and arachnoid mater. Rarely, it can lead to intrathecal hematoma that presents as SA.14,15 However, SA will develop at the puncture site in such cases, and it should present near the corresponding spinal level (L3–L5). In our case, the subdural sac was much higher than the site of puncture. In addition, SA displayed no relationship with the entrance pathway. It was extremely unlikely that SA resulted from direct injury caused by LP in this case.
To our knowledge, this is the first report of an adverse reaction to IT tigecycline. This case was complicated by SA that obstructed CSF assessment. The appropriate dose and treatment schedule of IT tigecycline remain to be determined.
Abbreviations
CNS = Central nervous system
CSF = Cerebrospinal fluid
IT = Intrathecal
IV = Intravenous
LP = Lumbar puncture
VPS = Ventriculoperitoneal shunting
XDRAB = Extensively drug-resistant Acinetobacter baumannii.
Authors’ contributions
Zheng Wen-Jian interpreted the clinical findings. Shi Shang-Wen was a major contributor to writing the manuscript. Li Liang-Ming was the chief physician primarily managing the patient. All authors read and approved the final manuscript.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Consent was not required as there was no patient’s private information in the paper.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Wen-Jian Zheng https://orcid.org/0000-0001-5490-3852
References
- 1.Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis 2009; 9: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karaiskos I, Galani L, Baziaka F, et al. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents 2013; 41: 499–508. [DOI] [PubMed] [Google Scholar]
- 3.Ray L, Levasseur K, Nicolau DP, et al. Cerebral spinal fluid penetration of tigecycline in a patient with Acinetobacter baumannii cerebritis. Ann Pharmacother 2010; 44: 582–586. [DOI] [PubMed] [Google Scholar]
- 4.Deng ZW, Wang J, Qiu CF, et al. A case report of intraventricular and intrathecal tigecycline infusions for an extensively drug-resistant intracranial Acinetobacter baumannii infection. Medicine (Baltimore) 2019; 98: e15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooij JJ. Spinal arachnoiditis: disease or coincidence? Acta Neurochir (Wien) 1980; 53: 151–160. [DOI] [PubMed] [Google Scholar]
- 6.Remes F, Tomas R, Jindrak V, et al. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg 2013; 119: 1596–1602. [DOI] [PubMed] [Google Scholar]
- 7.Luer MS, Hatton J. Vancomycin administration into the cerebrospinal fluid: a review. Ann Pharmacother 1993; 27: 912–921. [DOI] [PubMed] [Google Scholar]
- 8.Tsimogianni A, Alexandropoulos P, Chantziara V, et al. Intrathecal or intraventricular administration of colistin, vancomycin and amikacin for central nervous system infections in neurosurgical patients in an intensive care unit. Int J Antimicrob Agents 2017; 49: 389–390. [DOI] [PubMed] [Google Scholar]
- 9.Khan SA, Waqas M, Siddiqui UT, et al. Intrathecal and intraventricular antibiotics for postoperative Gram-negative meningitis and ventriculitis. Surg Neurol Int 2017; 8: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton JE, Curran MP. Tigecycline. Drugs 2005; 65: 2623–2635; discussion 36-7. [DOI] [PubMed] [Google Scholar]
- 11.Stein SC, Corrado ML, Friedlander M, et al. Chronic mycotic meningitis with spinal involvement (arachnoiditis): a report of five cases. Ann Neurol 1982; 11: 519–524. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava T, Kochar DK. Asymptomatic spinal arachnoiditis in patients with tuberculous meningitis. Neuroradiology 2003; 45: 727–729. [DOI] [PubMed] [Google Scholar]
- 13.Panackal AA, Komori M, Kosa P, et al. Spinal arachnoiditis as a complication of Cryptococcal meningoencephalitis in non-HIV previously healthy adults. Clin Infect Dis 2017; 64: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gürbüz MS, Erdoğan B, Yüksel MO, et al. Postlumbar puncture arachnoiditis mimicking epidural abscess. BMJ Case Rep 2013; 2013: bcr2013200169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy-Gash F, Engrand N, Lecarpentier E, et al. Intrathecal hematoma and arachnoiditis mimicking bacterial meningitis after an epidural blood patch. Int J Obstet Anesth 2017; 32: 77–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.