Abstract
In this study, we aimed to investigate the antidiabetic effects of Euphorbia nivulia (En), native to Cholistan Desert area of Bahawalpur, Pakistan. First, we performed high-performance liquid chromatography analysis and found that this plant contains ferulic acid, gallic acid, quercetin, benzoic acid, polyphenols, and flavonoids. Then, we performed in vitro and in vivo studies to assess its effects on diabetic Wistar rat model. The experiments were performed and compared with control drug glibenclamide. The 70% hydroalcoholic extract of En exhibited 97.8% in vitro α-glucosidase inhibitory effect at a dose of 1.0 mg/mL. We orally administered the extract of En and control drug to the streptozotocin (STZ)-induced diabetic rats and analyzed its antidiabetic effects. We found that the extract of En with a dose of 500 mg/kg/body weight exhibited significant effect to reduce blood glucose in STZ-induced rats as compared with the control group (P < .001). Our histological data also showed that the extract significantly improved the histopathology of pancreas. Collectively, both in vitro and in vivo studies revealed that En possesses α-glucosidase inhibitory, antioxidant, and anti-hyperglycemic effect in STZ-induced diabetic rats.
Keywords: Euphorbia nivulia, antioxidants, flavonoids, medicinal plants, antidiabetic
Introduction
Diabetes mellitus (DM) is a metabolic disorder in which fasting blood glucose level increases up to 120 mg/dL, after 2 hours of any type of meal taken by an individual. Diabetes is also associated with other complications such as cardiac diseases, blindness, and renal failure.1,2 Diabetes is characterized by high blood glucose level which leads to the generation of reactive oxygen species which causes oxidative damage in the β-cells of pancreas, kidney, and liver.3 Diabetes is classified into 2 main types, type 1 and type 2 diabetes. In type 1 or insulin-dependent diabetes mellitus (IDDM), the β-cells of pancreas fail to produce insulin due to autoimmune response or genetic factors, while in type 2 diabetes or non-insulin-dependent diabetes mellitus (NIDDM), the pancreas produces insulin but its receptors are blocked or unable to perform its actions due to sedentary life style or other adaptive causes. Insulin-dependent diabetes mellitus establishes about 5% to 10% of total diabetic cases due to deficiency of insulin secretion,4 while NIDDM accounts for 90% to 95% of the cases due to the deficiency in its actions. In this type of disease, the insulin production by pancreas is sufficient, but there is no response by muscular tissue for insulin, this occurs mainly due to pancreatic disease, genetic defects in insulin action, surgery, infections, drugs, or chemicals and environmental factors.4 World Health Organization (WHO) has declared that diabetes will affect 439 million in 2030 worldwide.5
The previous report showed that the inhibitory enzyme such as α-glucosidase enzyme reduces the rate of carbohydrate digestion, and as a result, the hyperglycemia level decreases.6 The synthetic drugs have limited efficacy, severe adverse effects, and costly, and resistance is developing against them. According to the WHO, more than 150 herbs are being used for the management of DM7,8 and that also recommended the development of phytomedicine for the treatment of the disease.9 Globally, herbal drugs are popular these days and getting more attention in alternative medicine.5,10-17
There are number of medicinal plants/plant-derived medicines which are traditionally used for the treatment of diabetes.11 A few examples are Acacia arabica bark,18 Allium sativum bulb,3 Aloe barbadensis fresh leaf gel,19 Curcuma longa,20 and Nigella sativa seeds.21 Increased formation of free radicals and decreased antioxidant potential may be a reason in both IDDM and NIDDM.22 The antioxidant principles may be useful as organ-protective and antidiabetic agents. Some of the members of Euphorbiaceae family, such as E prostrata and E hirta (plants of the same genus) have been reported to have a hypoglycemic effect.23 These medicinal plants contain flavonoids, phenolic compounds, terpenoids, carotenoids, alkaloids, glycosides, coumarins, and other active constituents which have antidiabetic properties.24-26
One of the members of the family Euphorbiaceae, Euphorbia nivulia (En) Buch.–Ham gains the attention of the researchers due to its biological activities.27 This plant is reported to be involved in the treatment of skin disorders, ear infections, worm infection, and retention of urine.28-30 We presumed that this plant might have antidiabetic potential as it belongs to the same family that are known to have strong antidiabetic potential. We aimed to examine its hypoglycemic effect as this plant is available in Northern, central India as well as in Pakistan. Chemically, it contains tetracyclic triterpenes and 3 diterpenes.31 The latex also contains phenolic compounds, alkaloids, cyanogenic glycosides, terpenes, and tannins.28
The present study was aimed to analyze the phytoconstituents of En and to evaluate its antioxidant potential and α-glucosidase inhibitory and anti-hyperglycemic effects in STZ-induced diabetic rats. We used hydroalcoholic (70%) crude extract of En to investigate its hypoglycemic/antidiabetic potentials. We found that the administration of this plant extract to the rats results in the reduction of blood glucose. Our in vitro also shows its antioxidant activity. Thus, our in vitro study revealed that this plant has strong antioxidant potential that may be a reason for its in vivo antidiabetic effect.
Materials and Methods
Plant Material and Extract Preparation
Aerial parts (leaves, branches, stem, and flowers) of fresh, well-grown En plant (110 kg) were collected during the months of March and April 2015 from Hasilpur Road and adjoining areas of Bahawalpur region, Pakistan, and authenticated by taxonomist Ghulam Sarwar, Department of Botany, The Islamia University of Bahawalpur. Voucher specimen (No. EN-AP-05-12-041) of the plant was deposited in the herbarium of Pharmacology Research Lab, Department of Pharmacy, The Islamia University of Bahawalpur, Pakistan. The collected plant parts were chopped into pieces and spread on filter paper in shade for drying at room temperature for the period of about 40 days; these were powdered by an electric grinder and sieved through mesh no.60. Ten kilograms of dried powdered plant material was macerated in 70% aqueous ethanol at room temperature for 15 days with occasional stirring. Filtration was done by Whatman Grade-1 filter paper followed by 3 times soaking. The filtrate was then evaporated under reduced pressure (−760 mm Hg) and controlled temperature on the rotary evaporator (Heidholph Laborota 4000-efficient Germany and Buchi Rotavapor R-20). A thick and semisolid, dark brown gummy mass was obtained, which was then placed in oven (Memmert Beschichung Loading Model 100-800). The dried material was weighed, labeled, and then stored at 4 °C in refrigerator in airtight container. The percentage yield was calculated and the condensed extract was used for further experimentation.
Equipment and Chemicals
Spectrophotometer (DAD 8453 Agilent), laboratory centrifuge model (YJ03-043-4000), Solid 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma), ammonium molybdate, sodium phosphate, phosphate, sulfuric acid, potassium ferricyanide K3[Fe (CN)6], trichloroacetic acid, ferric chloride (FeCl3), methanol, ascorbic acid, quercetin, gallic acid, pyrogallol, brilliant green, FeSO4, H2O2, Na2CO3, microplate reader, dimethyl sulfoxide (DMSO), potassium phosphate, and Na2CO3. Analytical grade solvents and chemicals including DPPH used were purchased from Merck, Sigma-Aldrich, and B.D.H. Glibenclamide was purchased from local market (Euglucon, Martin Dow Ltd; Batch No. P05094) and streptozotocin (Lot no. 5K40517 from Bioshop Canada Inc).
Experimental Animals
Wistar rats (180-200 g) were used for experimentation. Guidelines of National Institute of Health, Islamabad, were strictly followed to conduct the experiment. The designed protocol was ratified (Bch#0265) by the ethical committee of Quaid-i-Azam University, Islamabad, Pakistan. Animals were kept at room temperature with a dark/light 12-hour cycle in aluminum cages and provided with water and standard chow/pellet diet supply ad libitum. The animals were acclimatized for 1 week under laboratory conditions.
Phytochemical Screening
Preliminary qualitative phytochemical screening of the crude extract to identify different phytoconstituents such as alkaloids, glycosides, flavonoids, tannins, saponins, phenols, and so on was carried out by using standard conventional procedures.32
Quantification of Total Phenolic Content
Total phenolic content (TPC) of En crude extract was determined using Folin–Ciocalteu method. Briefly, colorimetric method was used with some modifications. An aliquot of 0.3-mL (various concentrations) extract was mixed with 2.25 mL Folin–Ciocalteu phenol reagent. After 5 minutes, 20 to 25 mL of Na2CO3 (6%) was added. The solution was allowed to stand for 90 minutes. Afterward, absorbance was measured at 725 nm. Total phenolic content was calculated using the standard calibration curve (ranging from 0 to 200 µg/mL) and data were expressed as milligram of gallic acid equivalent per gram of dry extract (mg of GAE/g of DE). Assays were carried out in triplicates.33
Quantification of Total Flavonoid Content
Total flavonoid content (TFC) in En crude extract was measured by modified colorimetric method. A calibration curve was established using quercetin as standard, 1 mg/mL in methanol, ranging from 0 to 100 µL (0-100 µg). All solutions were made in methanol. A 100 µL of sample solution was mixed with 25 µL of 1% sodium nitrite solution and allowed to stand for 5 minutes, followed by the addition of 10 µL of 10% aluminum chloride solution and again allowed to react for 5 minutes. Finally, 35 µL of 4% sodium hydroxide solution was added and mixture was diluted with 30 µL of methanol. Absorbance was measured at 510 nm. Total flavonoid content was calculated using the calibration curve equation and expressed as milligram of quercetin equivalent per gram of dry extract (mg of QE/g of DE).34
High-Performance Liquid Chromatography Analysis of Phenolic Compounds
Hydrolysis of En crude extract was performed in Central Hi-Tech Laboratory, University of Agriculture, Faisalabad, Pakistan, as described previously by Pak-Dek et al.35 A 50 mg of each extract was dissolved in 24-mL methanol and was homogenized. Distilled water of 16 mL was added, followed by 10 mL of 6 M HCl. Mixture was then thermostated for 2 hours at 95 °C. The final solution was filtered using 0.45-µm nylon membrane filter (Biotech) prior to high-performance liquid chromatography (HPLC) analysis. Separation of plant sample on gradient HPLC (LC-10A, SHIMADZU) was performed using shim-pack CLC-ODS (C118), 25 cm × 4.6 mm, 5-µm column. Chromatographic separation was carried out using a mobile phase gradient: A (H2O: acetic acid-94:6, pH = 2.27), B (acetonitrile 100%). The gradient used was 15% solvent B (0-15 minutes), 45% solvent B (15-30 minutes), and 100% solvent B (35-45 minutes) with 1 mL/min flow rate. Ultraviolet (UV)–Visible detector (λ max 280 nm) was used for separation of phenolic compounds. Identification of phenolic compounds was established by comparing the retention time and UV-Visible spectra of the peaks with those previously obtained by injection of standards. Quantification was performed by external calibration.
In Vitro Antioxidant Activity
2,2-diphenyl-1-picrylhydrazyl antioxidant assay
The antioxidant activity of En crude extract was measured by DPPH reagent method. A mixture of 90 µL of 0.3 mM DPPH solution and 10 µL of sample solution dissolved in methanol (5 mg/mL) was mixed and incubated for 30 minutes at room temperature in dark in 96-well plate. Absorbance was measured at 517 nm by using LT-4500 96-well microplate reader, LabTech. Absorbance of blank and standard were also measured. Both positive and negative controls were included in the triplicate assays. The percentage of total inhibition of DPPH radicals was measured by using the following equation:
Antioxidant activity of active samples after suitable dilutions were determined and IC50 values were calculated using EZ-Fit Enzyme Kinetics Software (Perrella Scientific Inc).36
Assay of total antioxidant capacity/reducing power assay
Total antioxidant capacity of En crude extract was measured by ferric reducing antioxidant power (FRAP) method. This method is based on the ability of tissue in reducing Fe3+ to Fe2+ in the presence of TPTZ (2,4,6-tris(2-pyridyl)-s-triazine). The reaction between Fe2+ and TPTZ gives a blue complex with the maximum absorbance at 593 nm.37 Fe-reducing power of plant extract was determined by the method of Nile and Park (2015), with a slight modification. The reaction flask consists of 100 mL extract, 0.5% vol/vol DMSO, and 5 mL of potassium ferric cyanide (1 mM) solution which was incubated for 30 minutes in water bath. Finally, the reaction was terminated using 3 mL of trichloro acetic acid (10%) solution. The upper portion of the reaction mixture (5 mL) was mixed with 5-mL distilled water and 1-mL FeCl3 solution (0.01%). The absorbance of the sample was measured at 593 nm spectrophotometrically after cooling for 10 minutes at room temperature, using an appropriate blank solution. The calibration curve was constructed using Trolox (100-2000 lM), and the results were expressed in l mol Trolox/g extract. All values were taken in triplicates, and mean ± SD values were calculated.
α-Glucosidase Inhibition Assay
α-Glucosidase inhibition was carried out with slight changes as described by Pierre protocol.38 A 100 µL of the reaction mixture, consisting of 70-µL (50 mM) phosphate-buffered saline with pH 6.8, and 10-µL (0.5 mM) test compound was prepared. A 10-µL (0.057 units) of enzyme was added to it. All these contents were mixed, preincubated for 10 minutes at 37 °C and pre-read at 400 nm. Reaction started by the addition of 10-µL (0.5 mM) substrate (p-nitrophenyl glucopyranoside). Acarbose was used as positive control. After 30 minutes of incubation at 37 °C, absorbance was measured at 400 nm using Synergy HT (BioTek) 96-well microplate reader. Yellow color absorbance was produced due to the formation of p-nitrophenol. Percentage inhibition of the enzyme was calculated using the following equation:
EZ-Fit Enzyme Kinetics Software (Perrella Scientific Inc.) was used for the calculation of IC50 values. IC50 value is the concentration at which 50% enzyme activity is inhibited. The sample was dissolved in methanol and experiments were performed in triplicate (mean ±SEM, n = 3).
Induction of Diabetes by Streptozotocin
Streptozotocin single injection (55 mg/kg body weight (BW) dissolved in citrate buffer) was injected intraperitoneally to induce diabetes in overnight-fasted rats. Animals were monitored 12 to 24 hours and serum glucose level was measured by using glucometer (range of 400-500 mg/dL was considered as diabetic).
Anti-Hyperglycemic Effect in Rats
The experiment was performed in Wistar rats using 3 different doses of the extract (125, 250, and 500 mg/kg) during 28-day study. Rats were divided into 6 groups (n = 5).
Group I: normal control, received distilled water.
Group II: positive control, received glibenclamide 10 mg/kg BW followed by STZ 55 mg/kg BW.
Group III: diabetic control group STZ 55 mg/kg BW.
Group IV: 125 mg/kg BW of En extract followed by STZ 55 mg/kg BW.
Group V: 250 mg/kg BW of En extract followed by STZ 55 mg/kg BW.
Group VI: 500 mg/kg BW of En extract followed by STZ 55 mg/kg BW.
The experiment was performed on fasting rats, starved for 12 hours. The prepared solutions and dilutions of different extracts were administered to their respective groups. Blood samples were collected during 28 days dosing interval, and blood glucose was measured by using glucometer.39
Analysis of Lipid Profile
Animals were slaughtered at the end of treatment. Blood samples were collected for the analysis of different physiological parameters such as high-density lipoproteins (HDL), triglycerides (TG), and total cholesterol (TC).
Histopathology
A 10% formalin was used for storage of harvested tissues. Histopathology standard procedures were utilized for handling the tissues. The pancreas of the treated rats was collected and subjected to hematoxylin and eosin staining as per the manufacturer’s protocol.
Results
Preliminary Phytochemical Screening
The crude extract of En was subjected for phytochemical analysis. We found that the extract contains various phytochemicals such as alkaloids, glycosides, flavonoids, phenols, saponins, tannins, and carbohydrates are shown in Table 1.
Table 1.
Phytochemical Evaluation (Crude Euphorbia nivulia Extract).
| Test | Observation | Inference |
|---|---|---|
| Alkaloids | ||
| Hager test | Yellow ppt | Alkaloids present |
| Mayer Test | Creamy ppt | Alkaloids present |
| Wagner test | Reddish-brown ppt | Alkaloids present |
| Glycosides | ||
| Keller-Killani test | Brown ring (at junction) | Glycosides present |
| Tannins | ||
| Fe Cl3 test | Blackish color | Tannins present |
| Flavonoids | ||
| Test with alkali solution | Dark yellow color | Flavonoids present |
| Saponins | ||
| Froth test | Froth formation | Saponins present |
| Phenolic contents | ||
| Fe Cl3 test | Blackish color | Phenolic present |
| Carbohydrates | ||
| Fehling reagent | Brick-red color | Carbohydrates present |
Total Phenolic Content and Total Flavonoid Content
Then, we measured the TPC and TFC of En extract. Total phenolic content and TFC were quantified using gallic acid and quercetin as standard. We found that the crude extract possesses promising phenolic and flavonoid content as depicted in Tables 2 and 3. It yielded 125.6 ± 1.32 mg/g GA TPC and 69.8 ± 1.21 mg/g GQ TFC, respectively.
Table 2.
Total Phenolic Content of Euphorbia nivulia Crude (Encr) Extract and Fractions.
| Sr.#. | Sample | Total phenolics (mg GA equivalent/g of sample) |
|---|---|---|
| 1 | Euphorbia nivulia crude extract | 125.6 ± 1.32 |
Table 3.
Total Flavonoid Content (TFC) of Euphorbia nivulia (En) Crude Extract and Fraction.
| Sr.#. | Sample | TFC (equivalent to mg quercetin/g of sample) |
|---|---|---|
| 1 | En crude extract | 69.8 ± 1.21 |
HPLC Analysis of Phenolic Compounds
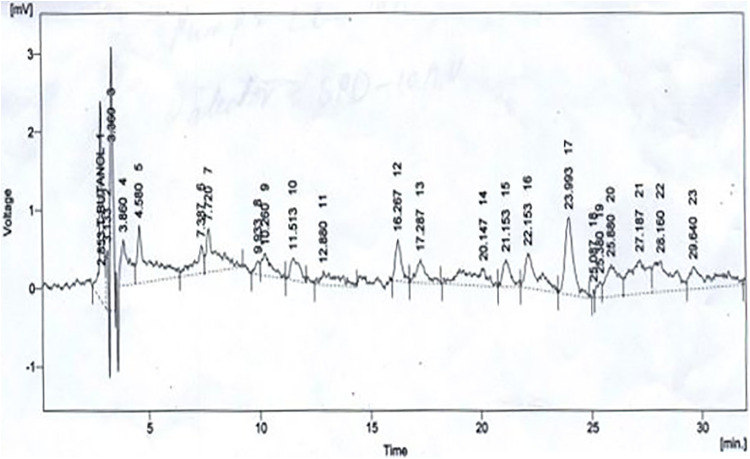
Next, we performed HPLC to analyze phenolic contents in the extract; HPLC confirmed the presence of 8 polyphenols: quercetin, gallic acid, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, and ferulic acid in the extract of En. Figure 1 shows the representative HPLC chromatograms generated for the detection of the polyphenols that were present in the extract of En. The Central Hi-Tech Laboratory, University of Agriculture, Faisalabad, used the library of external standards of polyphenols for calculation of quantities of polyphenols in the sample (extract).
Figure 1.
HPLC chromatogram of Euphorbia nivulia crude extract, standard gallic acid solution, and quercetin measured at 298 nm and mobile phase water:ethyl acetic acid (94:6).
In Vitro Antioxidant Activities
2,2-diphenyl-1-picrylhydrazyl scavenging assay and FRAP assay
The antioxidant potential of En extract was quantified (in vitro) by 2 ways, that is, by free radical scavenging activity (DPPH assay) and by FRAP assay. The results revealed that En extract showed excellent antioxidant potential at a concentration of 1.0 mg/mL in both experimental assays as shown in Table 4. The extract demonstrated DPPH radical scavenging activity of 91% ± 0.13% inhibition with IC50 0.14 ± 0.83 μg/mL. Whereas FRAP value of En crude extract was 708.32 μM.
Table 4.
Antioxidant Activity of Euphorbia nivulia (En) Crude Extract (Expressed as IC50).
| Sr.# | Sample | DPPH (% inhibition) | DPPH (IC50 μg/mL) | FRAP value (μM) |
|---|---|---|---|---|
| 1 | En crude extract | 91 | 0.14 | 708.32 |
| 2 | Rutin (std.) | 75.2 | 0.014 | – |
Abbreviation: FRAP, ferric reducing antioxidant power.
α-Glucosidase Inhibition Assay
En extract showed very promising α-glucosidase inhibitory potential as depicted in Table 5 at the concentration of 1 mg/mL. Results revealed that it exhibited 97.81% ± 1.87% inhibition of the enzyme with IC50 of 22.83 ± 1.53 μg/mL. These values were very close to the standard used.
Table 5.
Anti-α-Glucosidase Activity of Euphorbia nivulia (En) Crude Extract Expressed as IC50.
| Code | Inhibition (%) at 0.5 mg/mL | IC50 (μg/mL) |
|---|---|---|
| En Cr | 97.81 ± 1.87 | 22.83 ± 1.53 |
| Acarbose (std.) | 92.68 ± 0.19 | 37.49 ± 0.17 |
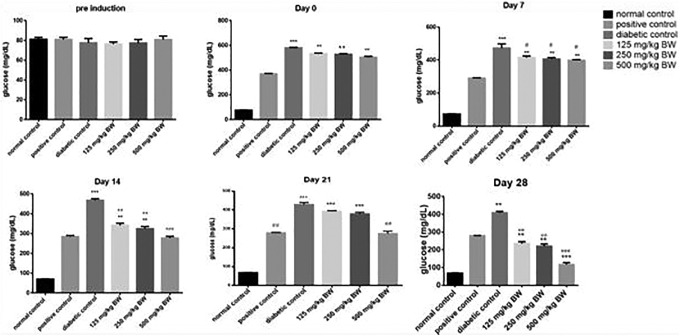
Antihyperglycemic Effect in Rats
After inducing diabetes with STZ, antihyperglycemic effect was measured in positive control, diabetic control and En extract treated (125, 250, and 500) mg/kg BW rats at days 0, 7, 14, 21, and 28. Orally administered 500 mg/kg BW of the extract showed a significant hypoglycemic effect in comparison to a positive control (P < .001) at day 28. Antihyperglycemic effect of 125 and 250 mg/kg BW of En extract was also significant (P < .01) in comparison to the positive control group. Results are presented in Figure 2. A dose-dependent decrease in glucose levels was observed, and this effect was significant at day 28.
Figure 2.
Effect of positive control (glibenclamide), diabetic control, and hydro-ethanolic Euphorbia nivulia extract at (125, 250, and 500 mg/kg) concentrations in diabetic rats (n = 5). Each value is expressed by taking mean ± SD and analyzed by 1-way analysis of variance. ***Indicates P < .001; **indicates P < .01; and *indicates P < .05 compared to positive control and ### indicates P < .001; ## indicates P < .01; and # indicates P < .05 compared to diabetic control.
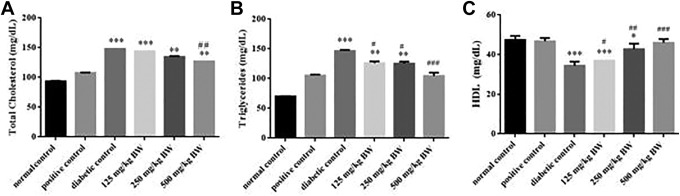
Analysis of Lipid Profile
Lipid profile was analyzed by comparing the positive control group (glibenclamide) with a diabetic control group and plant extract–treated groups at the concentration of 125, 250, and 500 mg/kg BW. The extract (500 mg/kg BW) significantly improved the TC, TG, and HDL levels at 500 mg/kg BW. Results are compared with both positive and diabetic control groups. Significant hypolipidemia was observed when treatments are compared with the diabetic control group. Results are depicted in Figure 3.
Figure 3.
Effect of positive control (glibenclamide), diabetic control, and hydro-ethanolic Euphorbia nivulia extract on (A) total cholesterol, (B) triglycerides, and (C) high density lipoproteins (HDL) at (125, 250, and 500 mg/kg) concentrations in diabetic rats (n = 5). Each value is expressed by taking mean ± SD and analyzed by 1-way analysis of variance. *** indicates P < .001; ** indicates P < .01; and * indicates P < .05 compared to positive control and ### indicates P < .001; ## indicates P < .01; # indicates P < .05 compared to diabetic control.
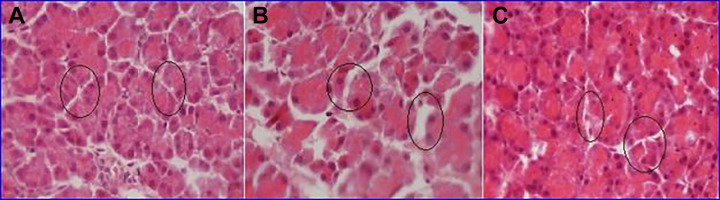
Histopathology
Histological analysis of pancreases revealed that STZ induces necrosis in the pancreas of the diabetic rats, while the plant extracts of En have significantly reduced the necrotic areas. Results are presented in Figure 4. Section from diabetes-induced group exhibits that most of the acinar cells were undergoing degenerative changes including coagulative necrosis (B) was improved markedly on treatment with En 500 mg/kg extract (C) when compared with control group (A).
Figure 4.
Photomicrograph of pancreas section of Wistar rats (H & E stain). A, Section from control group exhibiting no pathological changes. B, Section from diabetes-induced group exhibiting acinar cells were undergoing degenerative changes. C, Section from Euphorbia nivulia (500 mg/kg)–treated group showing normal acinar cells exhibiting improvement in pancreas. H&E indicates hematoxylin and eosin.
Discussion
Traditional medicinal practices formed the basis of most of the early medicines followed by subsequent clinical, pharmacological, and chemical studies.11-17,40-46 Plants have been well-documented for their medicinal uses for thousands of years.47 They have evolved and adapted over millions of years to withstand bacteria, insects, fungi, and weather to produce unique, structurally diverse secondary metabolites. Their ethno-pharmacological properties have been used as a primary source of medicines for early drug discovery.48,49 The current study was conducted to evaluate the antioxidant and antidiabetic potential of En (70% hydroalcoholic) extract against STZ-induced DM in Wistar rats. The plant contains phenolic compounds, such as alkaloids, flavonoids, terpenes, and tannins.28 The current investigation proved the presence of flavonoids and TPC in this plant. Prominent antioxidant capacity and profound hypoglycemic/antidiabetic ability of polyphenolic compounds such as gallic acid,50 chlorogenic acid,51 pyrocatechol,52 ferulic acid, and coumarin53-55 has been well documented. Quantitative determination of poly phenolics and flavonoids in the extract adds up to the previously reported claims regarding protective effects of these secondary metabolites against oxidative stress.11,40 Antioxidant potential of the extract was evaluated by using in vitro antioxidant DPPH scavenging assay. Well-known DPPH assay is widely practiced to evaluate the scavenging ability of any antioxidant moiety because DPPH is a stable and cell permeable free radical, which appears deep purple. Mechanism of the assay is based on hydrogen (H+) accepting ability of DPPH, wherein the proton is donated by scavenger antioxidant molecule, ultimately reducing DPPH to DPPH2. Color of reagent changes from purple to yellow at 515 nm.56 Maximum DPPH scavenging activity exhibited by En extract was 52.63% ± 1.13% inhibition at 1.0 mg/mL (IC50 482.74 ± 0.83 μg/mL). Euphorbia nivulia extract exhibited antioxidant potential that may be attributed to phenolics as well as flavonoids content. It was proposed that formation of free radicals has been associated with the induction of insulin and non-insulin-dependent diabetes.22 Euphorbia nivulia extract scavenges free radicals posing antioxidant effect which is also a proposed mechanism for the significant hypoglycemic activity.57 Nontoxic behavior of the extract was confirmed by acute toxicity investigation of 2 weeks, where no death was observed during this period. This investigation endorsed suitability and safety of the plant for in vivo studies. Thus, profound antioxidant potential and nontoxic nature of the extract was a sufficient reason to further evaluate antidiabetic potential against STZ-induced diabetes model.
The study revealed a potent inhibition of α-glucosidase by 97.81% ± 1.87%. The inhibitory concentration IC50 was found to be 22.83 ± 1.53 µg/mL. Polyphenols have the ability to increase the secretion of insulin along with decreased glucose output. They also increase the disposing of adipocyte glucose in type 2 DM. The present study explains an inhibition of α-glucosidase possibly because of the presence of polyphenols. It is suggested that (70% hydroalcoholic) extract of En inhibits α-glucosidase possibly by decreasing glucose transport through the intestine epithelial surface.58 α-glucosidase enzyme causes the degradation of oligosaccharides (branched chain) and categorized as a major source of hyperglycemia by releasing glucose.59 Inhibition of the enzyme can be a potential approach in maintaining the glucose level within the optimized normal range. Literature studies reveal that many medicinal plants have an inhibitory property for α-glucosidase enzyme and are categorized as potential antidiabetic agent.60 It is also reported in many studies that plants show α-glucosidase inhibition due to presence of the flavonoids as a chemical entity.61 Thus hypoglycemic potential of the extract may be due to high contents of flavonoids.
Sections from diabetes-induced group exhibiting cytoplasmic degenerations, in islets, were improved markedly on treatment with En 500 mg/kg. The extract possesses the potential to ameliorate the histopathological injuries provoked by STZ and has the ability for the restoration of biochemical and hematological parameters, revitalization of endogenous antioxidants, and suppression of inflammatory markers. All this justifies its protective and shielding capacity to abrogate the pancreatic damage due to oxidative stress.11,46 The defensive properties of En might probably be due to its phytochemical profile and antioxidant potential. Histology of STZ-intoxicated rats portrayed signs of severe toxicity due to necrosis, a high degree of damage in pancreatic cells by inducing inflammation, fibrosis, fatty degeneration, vacuolization, distortion of veins, cellular hypertrophy, hemorrhage, and steatosis. Administration of low and high dose of En ameliorated the toxic effects of STZ and decreased the damages induced with STZ intoxication. Protective effects displayed by the En might be attributed to the phytoconstituents such as flavonoids, terpenoids, sterols, tannins, and polyphenols.62 Findings of this study suggest that En is a strong protective agent with free radical scavenging potential in pancreas toxicity; pharmacological evaluation of the En extract for in vitro antioxidant and pancreas-protective potentials validate remarkable in vitro antioxidant profile of the plant.
A 70% hydroalcoholic En extract exhibits significant anti-hyperglycemic effect in comparison with positive control group. The results are attributed to high phenolic and flavonoid contents. High-performance liquid chromatography analysis exhibits the presence of quercetin, gallic acid, caffeic acid, syringic acid, coumaric acid, ferulic acid, and cinnamic acid which might result in lowering of blood glucose. The study is in comparison with previous findings where the plants of family Euphorbiaceae has shown hypoglycemic potential.30
Conclusion
Our study showed that En (70% hydroalcoholic) extract possesses the potential to ameliorate the histopathological injuries provoked by STZ and has the ability for the restoration of biochemical and hematological parameters, revitalization of endogenous antioxidants, and suppression of inflammatory markers. All this justifies its protective and shielding capacity to abrogate the pancreatic damage due to oxidative stress. The defensive properties of En might probably be due to its phytochemical profile and antioxidant potential. The antidiabetic role of En extract was found to be comparable with glibenclamide which might be due to the presence of polyphenols and/or flavonoids. We propose that this plant extract may further be subjected to bioassay-guided isolation to identify the active component(s). The extract may be a good candidate as antioxidant and/or antidiabetic agent for developing new formulations.
Acknowledgment
The authors are thankful to the chairman of the Department of Pharmacognosy, Faculty of Pharmacy & Pharmaceutical Sciences, University of Karachi, Karachi for financial and moral support. The authors are also thankful to the Department of Pharmacy, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad for providing necessary facilities to conduct experimentation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The chairman of the Department of Pharmacognosy, Faculty of Pharmacy & Pharmaceutical Sciences, University of Karachi, Karachi, financially supported this study.
ORCID iD: Muhammad Younus  https://orcid.org/0000-0003-4930-6687
https://orcid.org/0000-0003-4930-6687
Muhammad Rahil Aslam  https://orcid.org/0000-0003-0684-9147
https://orcid.org/0000-0003-0684-9147
References
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035–2038. [DOI] [PubMed] [Google Scholar]
- 2. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. [DOI] [PubMed] [Google Scholar]
- 3. Kazi S. Use of traditional plants in diabetes mellitus. Int J Pharm. 2014;4(4):283–289. [Google Scholar]
- 4. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American college of sports medicine and the American diabetes association: joint position statement. Diabetes care. 2010;33(12):e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dechandt CRP, Siqueira JT, de Souza DLP, et al. Combretum lanceolatum flowers extract shows antidiabetic activity through activation of AMPK by quercetin. Revista Brasileira de Farmacognosia. 2013;23(2):291–300. [Google Scholar]
- 6. Kim GN, Shin JG, Jang HD. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009;117(1):35–41. [Google Scholar]
- 7. Shelbaya S, Amer H, Seddik S, et al. Study of the role of interleukin-6 and highly sensitive C-reactive protein in diabetic nephropathy in type 1 diabetic patients. Eur Rev Med Pharmacol Sci. 2012;16(2):176–182. [PubMed] [Google Scholar]
- 8. Conget I. Diagnóstico, clasificación y patogenia de la diabetes mellitus. Revista Española de Cardiología. 2002;55(5):528–535. [DOI] [PubMed] [Google Scholar]
- 9. Bodeker G, Bhat K, Burley J, Vantomme P. Medicinal Plants for Forest Conservation and Health Care. FAO; 1997. [Google Scholar]
- 10. Mondal P, Bhuyan N, Das S, Kumar M, Borah S, Mahato K. Herbal medicines useful for the treatment of diabetes in north-east India: a review. Int J Pharmacy Biol Sci. 2013;3(1):575–589. [Google Scholar]
- 11. Nawaz A, Mehmood A, Kanatani Y, et al. Sirt1 activator induces proangiogenic genes in preadipocytes to rescue insulin resistance in diet-induced obese mice. Sci Rep. 2018;8(1):11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akram M, Nawaz A. Effects of medicinal plants on Alzheimer's disease and memory deficits. Neural Regen Res. 2017;12(4):660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nawaz A, Nazar H, Usmanghani K, Sheikh ZA, Chishti MA, Ahmad I. Ways to manage hepatitis c without cirrhosis: treatment by comparison of coded eastern medicine hepcinal with interferon alpha 2b and ribavirin. Pak J Pharm Sci. 2016;29(3):919–927. [PubMed] [Google Scholar]
- 14. Daniyal M, Akram M, Hamid A, et al. Review: comprehensive review on treatment of HIV. Pak J Pharm Sci. 2016;29(4):1331–1338. [PubMed] [Google Scholar]
- 15. Nawaz A, Zaidi SF, Usmanghani K, Ahmad I. Concise review on the insight of hepatitis C. J Taibah University Med Sci. 2015;10(2):132–139. [Google Scholar]
- 16. Nawaz A, Sheikh ZA, Feroz M, Alam K, Nazar H, Usmanghani K. Clinical efficacy of polyherbal formulation Eezpain spray for muscular pain relief. Pak J Pharm Sci. 2015;28(1):43–47. [PubMed] [Google Scholar]
- 17. Chishti MA, Mohi-Ud-Din E, Usmanghani K, Nawaz A, Nazar H, Ahmad I. Comparative clinical efficacy and safety of coded herbal medicine Dermovix in the management of patients with atopic dermatitis versus allopathic medicine. Pak J Pharm Sci. 2015;28(5):1655–1663. [PubMed] [Google Scholar]
- 18. Gupta PD, De A. Diabetes Mellitus and its herbal treatment. Int J Res Pharm Biomed Sci. 2012;3(2):706–721. [Google Scholar]
- 19. Shinde V, Borkar A, Badwaik R. Evaluation and comparative study of hypoglycemic activity of aloe barbadensis Miller with oral hypoglycemic drugs (glibenclamide and metformin) in rats. Int J Medical Pharm Sci. 2014;4(6):31–36. [Google Scholar]
- 20. Kuroda M, Mimaki Y, Nishiyama T, et al. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bull. 2005;28(5):937–939. [DOI] [PubMed] [Google Scholar]
- 21. Bamosa AO, Kaatabi H, Lebdaa FM, Elq A, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344–354. [PubMed] [Google Scholar]
- 22. Naziroğlu M, Butterworth PJ. Protective effects of moderate exercise with dietary vitamin C and E on blood antioxidative defense mechanism in rats with streptozotocin-induced diabetes. Can J Appl Physiol. 2005;30(2):172–185. [DOI] [PubMed] [Google Scholar]
- 23. Alarcon-Aguilara F, Roman-Ramos R, Perez-Gutierrez S, Aguilar-Contreras A, Contreras-Weber C, Flores-Saenz J. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J Ethnopharmacol. 1998;61(2):101–110. [DOI] [PubMed] [Google Scholar]
- 24. Afrisham R, Aberomand M, Ghaffari MA, Siahpoosh A, Jamalan M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of alpha-amylase. J Botany. 2015;2015:8. [Google Scholar]
- 25. He CN, Wang CL, Guo SX, Yang JS, Xiao PG. Study on chemical constituents in herbs of Anoectochilus roxburghii II. Zhongguo Zhong Yao Za Zhi. 2005;30(10):761–763. [PubMed] [Google Scholar]
- 26. Jung M, Park M, Lee HC, Kang Y-H, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13(10):1203–1218. [DOI] [PubMed] [Google Scholar]
- 27. Badgujar S, Mahajan R. Characterization of 31-kDa, Tubulin alpha-1 chain like protein of Euphorbia nivulia Buch.-Ham. latex. Biochem An Indian J. 2011;5(4):258–262. [Google Scholar]
- 28. Mahajan R, Badgujar S. Ethnomedicinal values of laticiferous plants used by tribal people of North Maharashtra, India. Res Link. 2008;55:20–25. [Google Scholar]
- 29. Savithramma N, Sulochana C, Rao K. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J Ethnopharmacol. 2007;113(1):54–61. [DOI] [PubMed] [Google Scholar]
- 30. Kumar S, Malhotra R, Kumar D. Antidiabetic and free radicals scavenging potential of Euphorbia hirta flower extract. Indian J Pharm Sci. 2010;72(4):533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravikanth V, Reddy VLN, Reddy AV, et al. Three new ingol diterpenes from Euphorbia nivulia: evaluation of cytotoxic activity. Chem Pharm Bull. 2003;51(4):431–434. [DOI] [PubMed] [Google Scholar]
- 32. Brain KR, Turner TD. The Practical Evaluation of Phytopharmaceuticals. Vol 1: Wright-Scientechnica Bristol; 1975. [Google Scholar]
- 33. Chlopicka J, Pasko P, Gorinstein S, Jedryas A, Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci Technol. 2012;46(2):548–555. [Google Scholar]
- 34. Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–614. [DOI] [PubMed] [Google Scholar]
- 35. Pak-Dek MS, Osman A, Sahib NG, et al. Effects of extraction techniques on phenolic components and antioxidant activity of Mengkudu (Morinda citrifolia L.) leaf extracts. J Med Plants Res. 2011;5(20):5050–5057. [Google Scholar]
- 36. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40(6):945–948. [Google Scholar]
- 37. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. [DOI] [PubMed] [Google Scholar]
- 38. Chapdelaine P, Tremblay RR, Dube J. P-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin Chem. 1978;24(2):208–211. [PubMed] [Google Scholar]
- 39. Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of emblica officinalis gaertn. Leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142(1):65–71. [DOI] [PubMed] [Google Scholar]
- 40. Kiran K, Saleem F, Awan S, et al. Anti-Inflammatory and Anticancer Activity of Pteris cretica Whole Plant Extracts. Pak Veterinary J. 2018;38(3):225–230. [Google Scholar]
- 41. Bilal M, Ahmad S, Rehman T, et al. Development of herbal formulation of medicinal plants and determination of its antihyperuricemic potential in vitro and in vivo rat’s model. Pak J Pharm Sci. March 2020;33(2):641–649. [PubMed] [Google Scholar]
- 42. Siddiqui MS, Saeed A, Nawaz A, Naveed S, Usmanghani K. The effects of new polyherbal Unani formulation AJMAL06 on serum creatinine level in chronic renal failure. Pak J Pharm Sci. 2016;29(2 Suppl):657–661. [PubMed] [Google Scholar]
- 43. Senda S, Inoue A, Mahmood A, et al. Calorie restriction-mediated restoration of hypothalamic signal transducer and activator of transcription 3 (STAT3) phosphorylation is not effective for lowering the body weight set point in IRS-2 knockout obese mice. Diabetol Int. 2015;6(4):321–335. [Google Scholar]
- 44. Sheikh ZS, Usmanghani K, Nazar H, Nawaz A, Jahanzeb LR, Ahmad I. Comparative clinical study on the efficacy of biocor plus compared with simvastatin for the management of hypercholesterolemia. Pak J Pharm Sci. 2015;28(6 Suppl):2291–2295. [PubMed] [Google Scholar]
- 45. Toseef MU, Saeed A, Ud-Din EM, et al. Comparative clinical evaluation on herbal formulation pepsil, safoof-e-katira and omeprazole in gastro esophageal reflux disease. Pak J Pharm Sci. May 2015;28(3):863–870. [PubMed] [Google Scholar]
- 46. Nishida Y, Nawaz A, Kado T, et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J Cachexia Sarcopenia Muscle. 2020;11(1):241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharif A, Akhtar MF, Akhtar B, et al. Genotoxic and cytotoxic potential of whole plant extracts of Kalanchoe laciniata by Ames and MTT assay. EXCLI J. 2017;16:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McRae BH, Beier P. Circuit theory predicts gene flow in plant and animal populations. Proceed Natl Acad Scis. 2007;104(50):19885–19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swanson T. Intellectual property rights and biodiversity conservation: an interdisciplinary analysis of the values of medicinal plants Cambridge University Press; 1998. [Google Scholar]
- 50. Alinezhad H, Azimi R, Zare M, et al. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int J Food Properties. 2013;16(5):1169–1178. [Google Scholar]
- 51. Xu Y, Chen J, Yu X, et al. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res. 2010;59(10):871–877. [DOI] [PubMed] [Google Scholar]
- 52. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. [DOI] [PubMed] [Google Scholar]
- 53. Bilgin HM, Atmaca M, Obay BD, Özekinci S, Taşdemir E, Ketani A. Protective effects of coumarin and coumarin derivatives against carbon tetrachloride-induced acute hepatotoxicity in rats. Exp Toxicol Pathol. 2011;63(4):325–330. [DOI] [PubMed] [Google Scholar]
- 54. Javed H, Khan M, Ahmad A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–352. [DOI] [PubMed] [Google Scholar]
- 55. Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138(4):2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fatima H, Khan K, Zia M, Ur-Rehman T, Mirza B, Haq IU. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: an in vitro biological and phytochemical investigation. BMC Complement Altern Med. 2015;15(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ahmad M, Alamgeer ST. A potential adjunct to insulin: Berberis lycium Royle. Diabetol Croat. 2009;38(1):13–18. [Google Scholar]
- 58. Gulfraz M, Mehmood S, Ahmad A, Fatima N, Praveen Z, Williamson E. Comparison of the antidiabetic activity of Berberis lyceum root extract and berberine in alloxan-induced diabetic rats. Phytother Res. 2008;22(9):1208–1212. [DOI] [PubMed] [Google Scholar]
- 59. Zhang J, Zhao S, Yin P, et al. α-Glucosidase inhibitory activity of polyphenols from the burs of Castanea mollissima Blume. Molecules. 2014;19(6):8373–8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Elya B, Basah K, Mun’im A, Yuliastuti W, Bangun A, Septiana EK. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J Biomed Biotechnol. 2012;2012:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao BT, Le DD, Nguyen PH, et al. PTP1B, α-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem Biol Interact. 2016;253:27–37. [DOI] [PubMed] [Google Scholar]
- 62. Nabavi SF, Nabavi SM, Habtemariam S, et al. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind Crops Prod. 2013;44(3):50–55. [Google Scholar]