Abstract
Autophagy has been strongly linked with hormesis, however, it is only relatively recently that the mechanistic basis underlying this association has begun to emerge. Lysosomal autophagy is a group of processes that degrade proteins, protein aggregates, membranes, organelles, segregated regions of cytoplasm, and even parts of the nucleus in eukaryotic cells. These degradative processes are evolutionarily very ancient and provide a survival capability for cells that are stressed or injured. Autophagy and autophagic dysfunction have been linked with many aspects of cell physiology and pathology in disease processes; and there is now intense interest in identifying various therapeutic strategies involving its regulation. The main regulatory pathway for augmented autophagy is the mechanistic target of rapamycin (mTOR) cell signaling, although other pathways can be involved, such as 5′-adenosine monophosphate-activated protein kinase. Mechanistic target of rapamycin is a key player in the many highly interconnected intracellular signaling pathways and is responsible for the control of cell growth among other processes. Inhibition of mTOR (specifically dephosphorylation of mTOR complex 1) triggers augmented autophagy and the search is on the find inhibitors that can induce hormetic responses that may be suitable for treating many diseases, including many cancers, type 2 diabetes, and age-related neurodegenerative conditions.
Keywords: aging, AMPK, autophagy, cancers, cell signaling, hormesis, lysosomes, mTOR, neurodegenerative diseases, therapeutics
Introduction
Hormesis is a biphasic dose–response to an environmental agent or condition characterized by low dose stimulation with beneficial effect and a high dose inhibitory with harmful effect.1,2 Hormesis is induced by mild physiological stress caused by a variety of stressors including toxic metals, organic xenobiotics, phytochemicals, drugs, ionizing radiation, nutrient deprivation (ie, fasting and caloric restriction [CR]), hypoxia, and oxidative stress (Figure 1).3 This type of functional response seems to occur in all eukaryotic organisms, which include yeasts and fungi, protozoans, plants, and metazoan animals. Lysosomal autophagy (self-eating) appears to be strongly associated with the mechanism underlying hormesis4-8; and Blagosklonny9 has proposed that the mechanistic target of rapamycin (mTOR) cell signaling system is a crucial feature of hormetic responses, particularly due to its relationship with lysosomal autophagy (Figure 2).
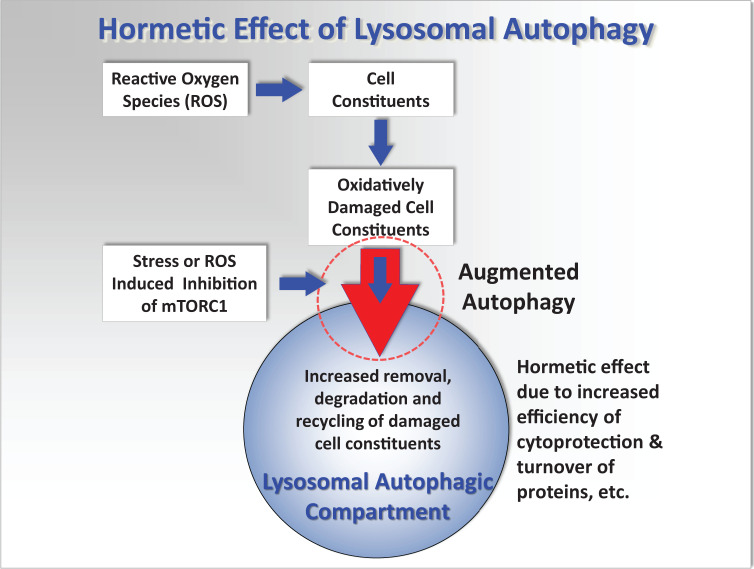
Figure 1.
Simplified conceptual mechanistic model showing the interactions of reactive oxygen species (ROS) with cellular components, lysosomal and autophagic processes, that can lead to oxidative stress, cell injury, and pathology. It is hypothesized that repeated stimulation of augmented autophagy (large red arrow circled) by various environmental factors such as fasting and exercise, or low concentrations of toxic chemicals that induce mild stress, will result in a more effective recycling of damaged cellular proteins and organelles; and before major oxidative damage occurs. This will consequently reduce lipofuscin formation that will otherwise accelerate ROS generation and cell injury and thus protect the cell from further oxidative injury. See more detailed diagram in Moore et al.10
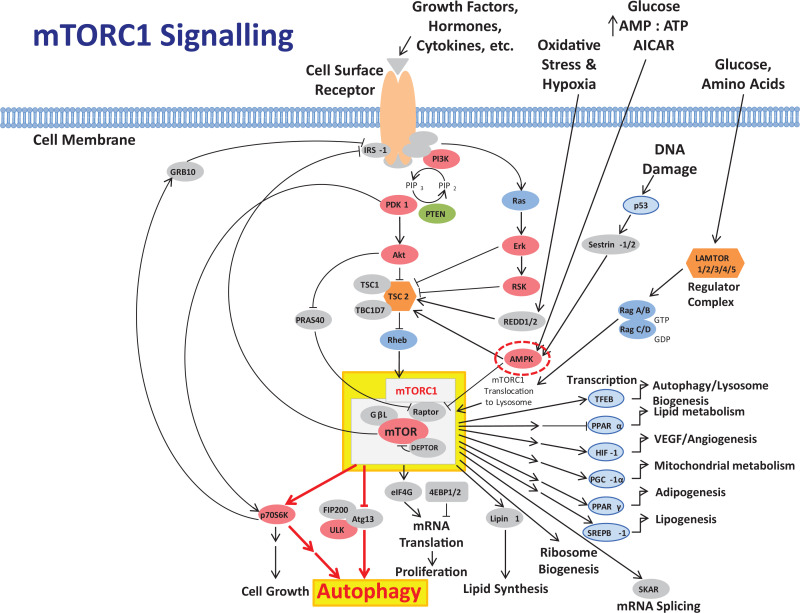
Figure 2.
Abbreviated diagrammatic representation of the PI3K, Akt, mTOR cell signaling pathway, and associated pathways, as a major regulator of cell function (see Laplante and Sabatini11 for a more extensive chart of mTOR-related cell signaling). The mTOR is an atypical serine/threonine kinase that is present in 2 distinct complexes. The first, mTORC1, is highlighted and is composed of mTOR, Raptor, GβL, and DEPTOR and is inhibited by rapamycin. It is a master growth regulator that senses and integrates diverse nutritional and environmental cues, including growth factors, energy levels, cellular stress, and amino acids. It couples these signals to the promotion of cellular growth by phosphorylating substrates that potentiate anabolic processes such as mRNA translation and lipid synthesis or limit catabolic processes such as autophagy. The pathways from mTORC1 leading to activation of autophagy (highlighted) are indicated in red (heavy arrows). Interactions with AMPK (circled) are also shown. Overactivity of mTORC1 is believed to trigger inflammatory processes which can result in pathological injury and processes leading to many cancers and degenerative diseases; and aberrant mTOR signaling is involved in many disease states including cancer, cardiovascular disease, and diabetes. Adapted from Moore12 and with permission from Cell Signaling Technology, mTOR Signaling Interactive Pathway: https://www.cellsignal.com/contents/science-cst-pathways-pi3k-akt-signaling-resources/mtor-signaling-interactive-pathway/pathways-mtor-signaling. Key to major constituents and symbols: Akt indicates serine/threonine kinase or protein kinase B; AMPK, 5′-adenosine monophosphate-activated protein kinase; mRNA, messenger RNA; mTOR, mechanistic target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphatidylinositol-3 kinase; PIP3, phosphatidylinositol 3,4,5 trisphosphate; PTEN, phosphatase and tensin homolog; ROS, reactive oxygen species; activation, ↑; inhibition, T.
Lysosomes have been linked to hormetic responses for over 40 years.13 Low level toxic metal stress induced changes in the lysosomal–vacuolar system of a marine coelenterate (Campanularia flexuosa), at concentrations of the metals that were an order of magnitude less than that required for the induction of growth hormesis. This finding was interpreted at the time as a precursor to the stimulation of growth by the metals (copper, cadmium, and mercury) that may have been associated with the augmentation of lysosomal autophagy as a survival strategy.13-15 Lysosomes are also notable for their ability to sequester and accumulate many metals, organic xenobiotics (including drugs such as chloroquine), radionuclides, microplastics, and nanomaterials.16-23 This sequestration probably has a protective function by compartmentalizing harmful materials from the rest of the cell; although it can result in lysosomal membrane destabilization (ie, permeablization) and release of intralysosomal iron with subsequent generation of reactive oxygen species (ROS) and cell injury.22,24 Furthermore, it is now apparent that autophagy is triggered by the inhibition of the mechanistic target for rapamycin complex 1 (mTORC1) cell signaling system (Figure 2).25 Mechanistic target for rapamycin complex 1 is a central focus for regulation of cell function: it acts as a nutrient sensor and is switched off by nutrient deprivation; and it is also inhibited by ROS (Figure 2).11 Hormesis now appears to be inextricably interconnected with mTORC1 and autophagic turnover of damaged cellular components including proteins and organelles.9,15,26-28
Hormesis has been simulated in a cell physiological model using a network modeling method.29 In this model, health status or homeostasis is represented by connectance: This is essentially the degree of system complexity or connectivity within the interconnected nodes representing the various molecular and cellular processes that constitute a living cell. Mild stress, such as nutritional deprivation, induced an increase in connectance, indicating an increase in health status that is consistent with hormesis. Increasing stress resulted in a decline in connectance, indicative of a loss of complexity and increasing cell injury.21,29,30 Connectivity associated with autophagy was a significant factor in the increase in connectance induced by mild stress. The outputs from this simulation have since been validated in experimental studies with molluscan hepatopancreas or digestive gland.10,21,30,31
Role of Autophagy in the Economy of the Cell
Autophagy is a cellular “garbage removal” process that is activated in response to various types of metabolic stress, including nutrient deprivation, generation of ROS, growth factor depletion, and hypoxia (Figure 1).14,15,32 Molecular studies involving targeted mutations resulting in deletions of autophagy-related genes, in organisms as varied as yeast, slime molds, plants, and mice, have shown that autophagy is essential for eukaryotic life.33
As well as providing an evolutionarily primitive survival mechanism against starvation and other environmental stressors, such as toxic chemicals, lysosomally mediated autophagic processes are crucial for the normal (basal) degradation and turnover of cellular components in autolysosomes.14,15 Lysosomal autophagy is also implicated in many disease processes, cell injury, cell death, and adaptive responses.10,14,15,17,18,21,22,31,34-39 Autophagy comprises at least 3 related cellular processes (ie, macroautophagy, microautophagy, and chaperone-mediated autophagy [CMA]); and it is essential for maintenance of cell homeostasis.14,15,40 Furthermore, cell morphological evidence has long indicated that lysosomal autophagy is a highly conserved mechanism evolutionarily.14,33 Damaged cellular constituents and redundant products are removed by lysosomal autophagy, but it is also critically involved, along with other proteolytic systems (eg, proteasomes), in the continuous turnover of intracellular components.41 Autophagy is upregulated in times of stress or physiological change: by breaking down longer-lived proteins and organelles and recycling the products into protein-synthesis and energy-production pathways, the process allows cells to be temporarily self-sustaining during periods when nutrients are restricted (Figure 1).14,40
Autophagic lysosomal degradation is the principal route for intracellular protein turnover and clearance.42 Proteins and lipids targeted for degradation enter the lysosomes via macroautophagy, microautophagy, and CMA.14,15 Once transported to the autolysosomes, these materials are split into their component molecules. Macroautophagy and microautophagy nonselectively remove entire portions of cytosolic regions and whole organelles, whereas CMA solely targets specific cytosolic proteins. While macroautophagy is induced close to the onset of fasting conditions, evidence suggests that this nonselective degradation can only be maintained for a short period of time in mammalian cells (ca 10 hours43). After this, CMA is the main form of autophagy. This is thought to allow the concurrent degradation of proteins and lipids into the amino acids required for cell survival, while preventing the degradation of molecules critical for basic cell function. Thus, CMA can be designated a stress-induced pathway that is activated not only by nutrient deprivation but also by mild oxidative stress44 and exposure to toxic compounds.14,21,31 This was clearly demonstrated by Massey et al45 who impaired CMA in mouse cells. The survival rates of these cells were unaffected when maintained under optimum conditions and it was hypothesized that the cells were able to upregulate macro- and microautophagic pathways to sustain protein turnover homeostasis. However, these non-CMA autophagic pathways were unable to compensate for the elevated levels of protein damage resulting from exposure to pro-oxidants and ultraviolet as evidenced by an exponential decrease in cell viability, increase in apoptosis, and cell death.45
Role of Autophagy and Hormesis in Disease and Aging
In addition to low nutrient scenarios where the autophagic processes break down macromolecules to their constituents, which may be used for energy and growth; autophagy plays a much more important role in the overall homeostasis of the cell.14,15,29,32,33 The principal intracellular roles of autophagy are:
removal of unfolded and defective proteins and cellular organelles, as in mitophagy of mitochondria14,33;
prevention of abnormal protein aggregate accumulation15 and;
removal of intracellular bacterial and viral pathogens by xenophagy.46,47
These 3 mechanisms are implicated in many bacterial, viral, heritable, and aging-related diseases, including atherosclerosis and various cancers, as well as neurodegenerative diseases (eg, Alzheimer disease, Huntington chorea, Motor neuron disease, bovine spongiform encephalopathy (BSE), Creutzfeldt-Jakob disease, and Parkinson disease.39,48-53 (Cuervo, 2008). There is also a related autophagic process called mitophagy to cull dysfunctional mitochondria, such as occurs in fibromyalgia.54,55
With some cancers, it is generally accepted that autophagy can suppress tumor initiation and cancer growth; and since autophagy blocks growth and increases breakdown of proteins, this is quite logical.56,57 (Cuervo, 2004, 2008). Cancer cells often have much lower levels of basal autophagy than normal cells. Additionally, many oncogenes and tumor-suppressor genes are intimately associated with cellular autophagy (Figure 2).11 An example of these includes the phosphatase and tensin homolog (PTEN) tumor-suppressor gene, which blocks the phosphatidylinositol-3 kinase (PI3K)/ protein kinase B (Akt)/mTORC1 cell signaling pathway, thus activating an autophagic response in cells (Figure 2).11 However, mutations to PTEN, which are known to be very common in many cancers, can lead to lower levels of autophagy, with the subsequent elevated risk of cancer. However, there is also a downside, and the relationship between autophagy and cancer appears to be a double-edged sword,58 since as some cancers progress, autophagy may actually facilitate tumor survival, just as autophagy appears to help the survival of all cells subjected to a stressful environment.14 Some cancers, which may grow so quickly as to outstrip their own blood supply, may thus be aided by increased autophagy, as this would supply much needed energy and deal with stress.48 Consequently, autophagy can have both prometastatic and antimetastatic roles and interacts with the other programmed cell death pathways (ie, apoptosis and necroptosis) in tumor progression.59
The other area of intense research interest in hormesis and inducible autophagic processes is in the investigation of age-related neurodegenerative diseases including Alzheimer disease, Parkinson disease, and Huntington chorea.32,39,51,60-63 While all of these pathological conditions manifest differently, they all share one pathological similarity. All of these neurodegenerative diseases are characterized by excessive buildup of proteins and protein aggregates inside neurons leading to cell dysfunction and ultimately disease.51 Consequently, the failure of protein degradation pathways by autophagy may play a very important role in the etiology of these diseases. However, the exact role of autophagy in these diseases is still uncertain. Further, growing research also implicates mitochondrial dysfunction and associated oxidative stress as a key pathway in the development of neurodegenerative diseases, as mentioned above for fibromyalgia.54,55 Furthermore, Matai et al64 have shown that endoplasmic reticulum hormesis improves proteostasis and viability in a mammalian cellular model of neurodegenerative disease.
Mitophagy is the selective autophagic targeting of defective or dysfunctional mitochondria. If the mitochondria are not working effectively, then the process of mitophagy targets them for lysosomal degradation.54,55 The key regulators of this process include the tumor suppressor gene PTEN.11 5′-Adenosine monophosphate-activated protein kinase (AMPK), for example, will also stimulate mitophagy, as well as new mitochondrial growth, essentially replacing old mitochondria with new ones in a subcellular renewal process (Figure 2).11,65 Consequently, this is one of the reasons that metformin is often proposed as an antiaging compound, not so much for its antidiabetic blood-sugar level effects; but instead, because of its effect on AMPK and augmented autophagy (Figure 2).11,25,65
As already mentioned, many antiaging interventions may show hormetic features, suggesting that preconditioning might have a preventive medical character (Figure 3 and Table 1).8,34,66-72 Hence, mild dietary stress (ie, CR without malnutrition or glucose restriction) achieved through various fasting regimes may exert its beneficial effects on life- and health span, at least in part, through hormetic mechanisms.10,30,34,73,74 Glucose restriction has been shown to cause an increase in mitochondrial generation of ROS in a nematode model.74 This mitochondrial ROS may in turn trigger an autophagic response via inhibition of mTORC1 (Figure 2).75-78 Furthermore, various repurposed approved drugs have been found to increase life span and health span in rotifers (Table 1).71 Similarly, physical exercise may counteract aging by virtue of a hormetic dose–response relationship. Consequently, both lack of physical activity and overexercise are harmful, while regular but moderate exercise is beneficial, probably mediated by exercise generated ROS inducing mild oxidative stress leading to preconditioning (Figure 3).8,66-68
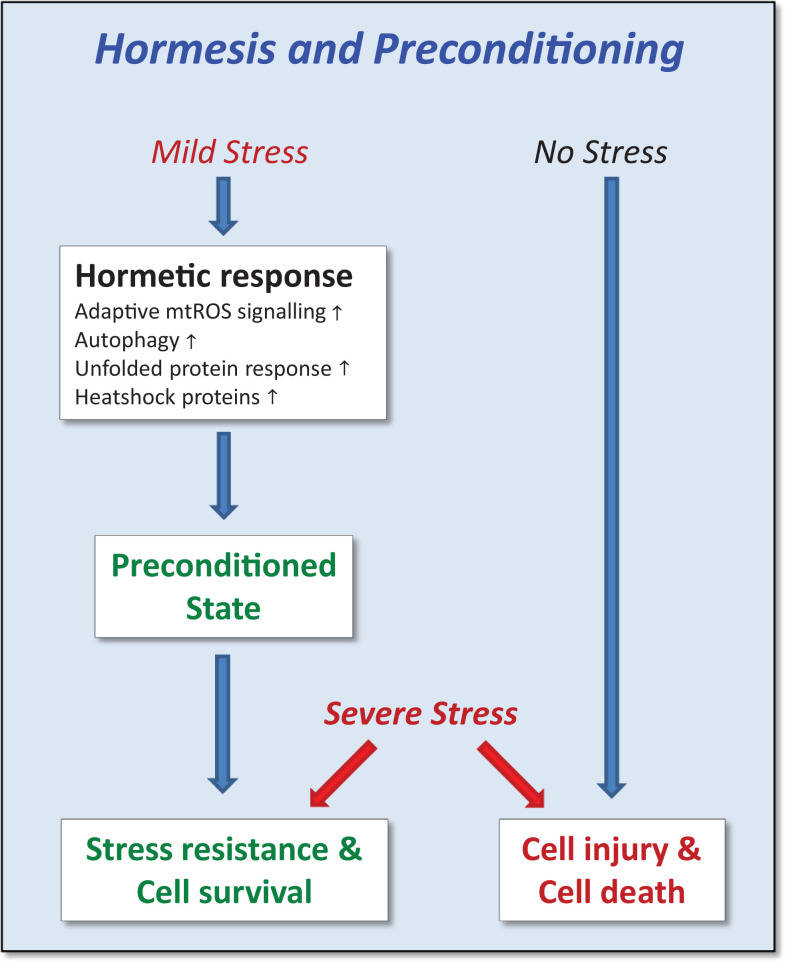
Figure 3.
When exposed to mild stress, cells/organisms respond by a variety of pleiotropic adaptive cellular programs that procure a preconditioned state.66,67 When an severe stress is applied subsequently, preconditioned but not naive cells/organisms exhibit stress resistance, hormesis, and eventually improved survival. mtROS indicates mitochondrial reactive oxygen species. Adapted from Zimmermann et al.8
Table 1.
Summary List of Chemical Compounds With Life-Span and Health-Span Extending Properties.a
| Compound (chemical class) | Medication/supplement name(s) | Effects on organism | Cell targets |
|---|---|---|---|
| Aspirin (NSAID) | Ecotrin Aspir 81 Aspir-low Aspirin low strength |
Anti-inflammation Anticancer Antistress |
COX-1, COX-2, PTGS2, AMPK, NF-κB pathway, PI3/Akt/mTOR pathway |
| Capecitabine (fluoropyrimidine) | Xeloda | Antimetabolite | Thymidylate synthase |
| Curcumin (polyphenol) | Theracurmin Meriva Longvida BCM-95 |
Anti-inflammation Anticancer Antiatherogenic Antidiabetic Antidepressant Neuroprotective Antistress |
NF-κB, COX-1, COX-2, TNF-α, p53, PPARγ, TR, Nrf2, FAK, PI3K/AKT/mTOR pathway, LOX, AMPK, Src, GSK3, AP1, |
| Dihydroresveratrol (dihydrostilbenoid) | Dihydroresveratrol | Resveralogue Antifibrotic Anti-inflammation |
Moderation of splicing factor levels, PI3K/AKT/mTOR pathway, NF-κB pathway |
| Epigallocatechin gallate (catechin) | Green tea extract | Anti-inflammation Anticancer Antiamyloid Antiatherogenic Antiobesity Antidiabetic Neuroprotective Antistress |
Bcl2, NOS2, LamR, EGFR, telomerase, topoisomerase II, DNMT1, PI3K/Akt/mTOR pathway |
| Erythromycin (antibiotic) | Erythrocin Ery-Tab EryPed E-Mycin |
Antibacterial Gastroparetic |
Aminoacyl translocation |
| Fisetin (flavonoid) | Fisetin | Anti-inflammation Anticancer Antiatherogenic Antiobesity Antidiabetic Antioxidant CR mimetic Antistress |
Akt, Cdk6, mTOR, ERK, PI3K/Akt/mTOR pathway |
| Ivermectin (macrocyclic lactone) | Heartgard Sklice Stromectol Ivomec Mectizan Ivexterm |
Antiparasitic | Glutamate-gated chloride channels |
| Melatonin (biogenic amine) | Melatonin Circadin Clocktonin |
Neuroprotective Antistress Antimigraine Sedation Sleep quality Antidepressive Antistress |
MT1, MT2, MT3, GPR50 |
| Metformin (biguanide) | Act Metformin Bio-metformin Fortamet Glucophage Glumetza Metformin Riomet |
Anti-inflammation Anticancer Antiatherogenic Antidiabetic Antidepressant Neuroprotective Cardioprotective CR mimetic |
AMPK, PI3K/Akt/mTOR pathway |
| Quercetin (flavonoid) | Quercetin Rutin |
Antiatherogenic Anti-inflammation Cardioprotective Antioxidant |
SIRT1, PLA2, PI3K, pp60src Phosphotransferase Protein kinases Cyclic GMP phospho-diesterases, PI3K/Akt/mTOR pathway |
| Rapamycin (antibiotic) | Rapamycin Sirolimus Rapamun |
Anti-inflammation Anticancer Antiamyloid Antiatherogenic Neuroprotective Cardioprotective CR mimetic Antistress |
mTOR |
| Resveratrol (stilbenoid) | Resveratrol | Anti-inflammation Anticancer Antiatherogenic Antiobesity Neuroprotective Cardioprotective CR mimetic Antistress |
Sirt2, p53 AMPK, PGC1-α, PI3K/Akt/mTOR pathway, ERK pathway |
| Statins | Atorvastatin Fluvastatin Lovastatin Pitavastatin Pravastatin Rosuvastatin Simvastatin |
Antihyperlipidemic Cardioprotective Antidiabetic Antiatherogenic Anti-inflammation Anti-Alzheimer |
Hydroxy-methyl-glutaryl-CoA reductase |
Abbreviations: Akt, serine/threonine kinase or protein kinase B; AMPK, 5′-adenosine monophosphate-activated protein kinase; AP1, activation protein 1; COX-1, cyclooxygenase 1; DNMT1, DNA methyltransferase 1; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GMP, guanosine monophosphate; GSK3, glycogen synthase kinase-3; LOX, lysyl oxidase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB; NOS, nitric oxide synthase; Nrf2, nuclear factor E2-related factor 2; PGC1-α, peroxisome proliferator-activated receptor-γ coactivator 1-α; PI3, phosphatidylinositol-3 kinase; PLA2, phospholipases A2; PPARγ, peroxisome proliferator-activated receptor γ; PTGS-2, prostaglandin-endoperoxide synthase 2; Scr, homeotic gene sex combs reduced; SIRT1, NAD-dependent deacetylase sirtuin-1; Sirt2, gene coding for SIRT2 - an NAD-dependent deacetylase sirtuin-2; TR, thioredoxin reductase TNF-α, tumor necrosis factor α
Hormesis appears to be executed by a variety of physiological cellular processes, including autophagy that cooperatively interact and converge on enhanced stress resistance and longevity (Figure 3).8,32,69,81,82 Calabrese et al68 and Zimmermann et al8 have postulated that cells and organisms, preconditioned by exposure to mild stress, are protected against more harmful levels of stress and that the relevance of hormesis for both human pathophysiology and specific disease treatment is being increasingly recognized.83 These authors have argued that this evolutionarily conserved process, hormesis, is directly linked to the capability to cope with pathological conditions, including aging and age-related diseases. They have also identified lysosomally mediated autophagy as a key component of this cytoprotective process.8
Central Role of mTORC1 and Related Cell Signaling Pathways
The mTORC1 cell signaling system is primarily a nutrient sensor, however, it also interacts with ROS (Figure 2). The PI3K/Akt/mTORC1-related intracellular signaling pathways are essential for eukaryotic cells, since they are the link between nutrient status and growth (Figure 2). Mechanistic target for rapamycin complex 1 is the major cellular nutrient sensor to influence autophagic processes14 and is inhibited by dephosphorylation.11 Mechanistic target for rapamycin integrates signals from insulin, nutrients (amino acids or dietary protein), and the fuel gauge of the cell AMPK (all energy including fats) to determine whether the cell should divide and grow or become dormant.11,65 Excess nutrients may stimulate the mTORC1 system, turning off autophagy and putting the body into a growth mode. This encourages growth of cells, which is generally not beneficial in most adult higher animals.15 The mTORC1 pathway is also inhibited by ROS and vitamin D (Figure 2).11,84
Diseases such as obesity, type 2 diabetes, Alzheimer disease, many cancers, atherosclerosis (heart attacks and strokes), polycystic ovarian syndrome, polycystic kidney disease, and fatty liver disease, among others, may be amenable to dietary intervention and mTORC1 inhibitors.85,86 When the nutrient sensing systems detect low nutrient availability, they signal cells to stop growing and start breaking down unnecessary parts—this is the “self-cleansing” pathway of autophagy.15,87 Consequently, diseases that involve excessive growth may be countered by reducing positive growth signaling through blocking these nutrient sensors.
Investigations of growth inhibition in humans are problematical because of the multiple interacting cell signaling pathways (Figure 2; Laplante and Sabatini, 2012).88 However, the clearest evidence usually comes from drugs that target single pathways that can be altered one at a time. The mTORC1 inhibitors (eg, rapamycin, everolimus, various phytochemicals) activate autophagy by blocking mTORC1 (Table 1).89 Some of these drugs are mainly used for their immune-suppressing effects in organ transplant medicine. However, most immune suppressants increase the risk of cancer, whereas rapamycin does not. In certain cancers, mTORC1 inhibitors have demonstrated anticancer effects (Table 1).56
Metformin, a drug widely used in type 2 diabetes, also activates autophagy but not directly through the mTORC1 system (Table 1).72 This drug increases AMPK, a protein molecule that signals the energy status of the cell (Figure 2).90 High AMPK signals that the cell has insufficient energy and consequently augments basal autophagy.65 The AMPK senses the ADP/ATP ratio, thus determining the cellular energy status. High AMPK levels directly activate augmented autophagy but also indirectly activate mitochondrial production.65
Dephosphorylation of mTOR (inhibitory) and the consequent enhancement of autophagic activity are not per se sufficient to explain positive reactions, such as increased growth of the organism, to stressors. However, inhibition of mTOR (specifically mTORC1) does seem to represent a necessary step in order to eliminate the negative effects (ie, molecular and cell injury) of the stressor but the partial dephosphorylation of mTOR must in some way be coupled with a second reaction, perhaps involving AMPK and mitochondrial function, that is able to positively stimulate cell metabolism and physiology.91 Mitochondrial hormesis (ie, mitohormesis) involving restoration of mitochondrial function and superoxide production via activation of AMPK has now been associated with improvement in markers of renal, cardiovascular, and neuronal dysfunction with diabetes. Consequently, approaches that stimulate AMPK and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α) via exercise, CR, and medications result in stimulation of mitochondrial oxidative phosphorylation activity, restore physiological mitochondrial superoxide production, and promote organ healing.91 The expression of PGC1α is highly inducible by physiological stressors, including exercise, cold, and fasting92; and a central function of PGC1α is its intimate link to mitochondrial biogenesis and the detoxification of ROS.93
Hormesis and Autophagy in Animal and Human Evolution
Over the geological time course of biological evolution, exposures to low concentrations of foreign or xenobiotic chemicals, both natural and pyrogenic (eg, from forest fires, volcanism, and cooked food), will have undoubtedly acted as selective pressures on the cytoprotective defences of biological systems (see review by Moore).12 It is probably not unreasonable to propose that hormesis arose as a result of these selective pressures on the cellular interactions of toxic chemicals, their metabolites, and frequently generated reactive oxygen and nitrogen species with cell constituents. Such interactions will have included cell signaling networks that regulate xenobiotic biotransformation (phase I and II, esterases, etc) mitochondrial and lysosomal function, autophagy, and programmed cell death (see review by Moore).29 Consequently, these interactions have resulted in the integrated toolbox of evolutionarily highly conserved physiological responses that is now recognized as comprising the phenomenon of hormesis.
Natural products can have both harmful and beneficial effects; and many of our dietary constituents contain micronutrients, some of which are considered to be nutraceuticals, such as many phenolic, polyphenolic, and isothiocyanate phytochemicals, which are also often biogenic pesticides (see reviews by Moore).12,94 Many phenolic and polyphenolic phytochemicals and their metabolic derivatives, such as curcumin, resveratrol, dihydroresveratrol, and quercetin, are potent inhibitors of the PI3K/AKT/mTORC1 signaling pathway and inducers of autophagy (Table 1 and Figure 2).12,80,94-98
Modification of splicing factor expression by resveralogues (analogues of resveratrol), including dihydroresveratrol, a major gut microbiome metabolite of resveratrol that is readily absorbed,99 was associated with the rescue of multiple features of senescence, making senescent cells not only look physically younger but start to behave more like young cells and start dividing (Table 1).79 This hormetic antiaging effect is the first demonstration that moderation of splicing factor levels is associated with reversal of cellular senescence in human fibroblasts and suggests that small molecule modifiers of splicing factor expression could represent promising novel antidegenerative therapies.
Moore94 hypothesized that inhalation and ingestion (with upper respiratory tract mucus) of certain natural products, such as aerosolized harmful algal toxins (phycotoxins), may have direct effects on the body’s molecular regulatory systems resulting in health benefits (eg, anti-inflammatory, anticancer, antiaging).100 Recent support for this phytohormetic hypothesis has come from work by Asselman et al,101 demonstrating that some phycotoxins in natural marine aerosols can inhibit mTOR and induce autophagy in cultured lung cells. Growth inhibition and apoptosis, both linked to mTOR pathway activity, may explain these effects, as yessotoxins were shown to downregulate this pathway.102 Fungal toxins, such as aflatoxins and ochratoxins, can also induce autophagy and apoptosis and interact via cell signaling pathways; and there appears to be some evidence for hormetic effects.103
Evidently, hormesis and phytohormesis resulting from exposure to small amounts of toxic pollutants or some naturally occurring biogenic chemicals (ie, from both prokaryotes and eukaryotes) can have a stimulatory effect on the various cellular or cytoprotective processes including autophagy (Table 1).81,94,104-107 Currently, this is an area of very active research, particularly in the context of the therapeutic potential for phytochemicals to behave as nutraceuticals and have anti-inflammatory, antidementias, antiaging, and anticancer properties (see reviews by Moore).12,94
Many toxic materials are natural components of the environment and will have interacted with human biological systems over the time span of human and hominid evolution. Human hormetic cytoprotective systems will probably have benefitted from evolutionary changes in the longer term from exposure to toxic metals, organic xenobiotics, and toxic biogenic products. As mentioned previously, many metals and organic xenobiotics accumulate within lysosomes, where they can cause permeabilization of the lysosomal membrane resulting in the release of intralysosomal iron into the cytoplasm.17,22,24,26,108 This released lysosomal iron generates ROS that can cause oxidative damage to cellular components22,24,108; however, the ROS will also inhibit the PI3K/AKT/mTORC1 pathway thus inducing augmented autophagy.21,31 Many of the phytochemicals that are considered to be potentially beneficial at low concentrations are in fact toxic at high concentrations: They are chemical defensive products of the evolution of protective mechanisms in plants to counter pathogens, parasites, and consumption by herbivores.109-112 Exposure to these biogenic products, particularly in foodstuffs, will probably have beneficially influenced the evolution of human cellular processes, particularly anti-inflammatory cell signaling mechanisms.12,25
Exposure to toxins and natural biogenic products, such as phytochemicals, phycotoxins, mycotoxins, and bacterial toxins, has probably had a beneficial role as an evolutionary driver for cellular hormetic cytoprotective systems in humans and many other species (Table 1).107,112-117 The phase I and phase II drug metabolizing enzymes that biotransform many of the organic xenobiotics that enter the body, either by ingestion in food and cooked and burnt food (eg, pyrogenic generation of polycyclic aromatic hydrocarbons and arylamines) or by inhalation, have been selected over the course of hominid evolution.112-114,117-119 These selective processes will probably have driven the human capability to detoxify many of the organic chemical pollutants (xenobiotics) encountered during recent industrial history as well as conferring the ability to activate the vast majority of pharmaceuticals used in current therapeutic applications. Antioxidant protection against reactive derivatives of toxic metal and xenobiotic exposure has also been subject to the same type of evolutionary pressures; and this has endowed humans with the remarkable defensive capacity that is a necessary requirement for surviving in a “sea of poisons.”107,120,121 Perhaps low-level hormesis is actually the real physiological status of most organisms that live in the real environment, including humans and, consequently, are exposed to low levels of a myriad of stressors, rather than under laboratory controlled conditions.2,94
Novel industrial chemical and nanomaterial products are being produced constantly, and some of these will undoubtedly present hazards for human and animal health, as the drug metabolizing system and other cytoprotective processes, such as autophagy and hormesis, will be confronted with completely new molecular and supramolecular structures, not previously encountered during the course of human and earlier evolution.16,122-124 However, recent research has indicated that metalliferous nanoparticles can be generated naturally in deep groundwater from geological metal ore deposits,125 so natural exposure to these types of materials has probably been a factor in the evolution of cytoprotection. Risks to health as a result of exposure to nanomaterials, other than combustion particulates, are still largely unknown, although considerable concern has been expressed as to the safety of engineered nanomaterials.31,39,83,126 Furthermore, there is now some evidence that engineered nanoparticles (eg, C60 fullerene and glass nanofibres) can inhibit mTORC1, induce augmented autophagy, and some may even induce pathological dysfunctional autophagy21,24,31,108; and these materials are finding their way into the environment and may impact on animal and human food networks and possibly on human health.127
Finally, breakdown of various plastic products can produce both nanoplastic and microplastic contamination of the natural environment; and there are increasing environmental health concerns regarding these materials.128-130 Microplastics have been shown to cause cell injury to the lysosomal system in marine animals (mussels) and also can bind potentially harmful persistent organic pollutants that will enter the human food chain with the microplastic.23
Conclusion
Hormesis is intimately linked with autophagic responses as part of the overall repertoire of lysosomal function. Initiation of augmented autophagy via mTORC1 inhibition or AMPK provides the cell with essential nutrients in stressful scenarios; and these recycled nutrients facilitate survival and growth by supplying energy and building blocks for cellular repair during mild stress. Autophagic removal of unwanted damaged or misfolded proteins, harmful protein aggregates, damaged membranes, and even damaged parts of the genome will protect the cell and/or organism from further damage and aid recovery from environmental insults.14,15,33,40,131
Autophagy and its regulation by mTOR cell signaling is evolutionarily very ancient and the genes involved are highly conserved from yeasts to humans.33 This indicates that the interconnectivity between autophagy and hormesis probably emerged very early in the evolution of the eukaryotic lineages.
Finally, agents that can induce autophagy and hormetic type responses, such as fasting, exercise, aspirin, metformin, and phytochemicals as well as mimetics of metformin and rapamycin (rapalogs and CR-mimetics),65,90,132,133 have considerable potential for significant impact in antiaging, cancer and neurodegenerative disease therapeutics.
Acknowledgments
This article is dedicated to the late Dr Tony Stebbing who introduced me to the concept of hormesis in 1973 and with whom I was privileged to work, during his seminal investigations of hormesis in coelenterates, induced by low concentrations of toxic metals. Credit is also due to my late colleague and friend, Dr Andreas Bubel, who first introduced me to the pathophysiology of lysosomes induced by environmental pollutants 50 years ago: without his keen insight, I would probably not have pursued this area of research for much of my research career. I also want to thank my colleagues of many years, Professor Aldo Viarengo (University of Genoa, Genoa, and Mario Negri Institute for Pharmacological Research—IRCCS, Milan, Italy) and Professor Dr Angela Köhler (Alfred Wegener Institute—AWI, Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany) for critically reviewing this manuscript. This work was supported by Plymouth Marine Laboratory, the University of Exeter Medical School—European Centre for Environment and Human Health, and the University of Plymouth—School of Biological & Marine Sciences.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The author(s) received support in kind but no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michael N. Moore  https://orcid.org/0000-0003-2181-2916
https://orcid.org/0000-0003-2181-2916
References
- 1. Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13 doi:10.1038/s41514-017-0013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421(6924):691–692. [DOI] [PubMed] [Google Scholar]
- 4. Jia K, Levine B. Autophagy is required for dietary restriction-mediated life span extension in C. elegans. Autophagy. 2007;3(6):597–599. [DOI] [PubMed] [Google Scholar]
- 5. Kozlowski L, Garvis S, Bedet C, et al. The Caenorhabditis elegans HP1 family protein HPL-2 maintains ER homeostasis through the UPR and hormesis. Proc Natl Acad Sci USA. 2014;111(16):5956–5961. doi:10.1073/pnas.1321698111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumsta C, Chang JT, Schmalz J, et al. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat Commun. 2017;8:14337 doi:10.1038/ncomms14337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morselli E, Maiuri MC, Markaki M, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10 doi:10.1038/cddis.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmermann A, Bauer MA, Kroemer G, et al. When less is more: hormesis against stress and disease. Microb Cell. 2014;1(5):150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging. 2011;3(11):1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore MN, Shaw JP, Pascoe C, et al. Anti-oxidative hormetic effects of cellular autophagy induced by nutrient deprivation in a molluscan animal model. Mar Environ Res. 2020;156 doi:10.1016/j.marenvres.2020.104903 [DOI] [PubMed] [Google Scholar]
- 11. Laplante M, Sabatini DM. . mTOR signaling in growth control and disease. Cell. 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore MN. Environmental health impacts of natural and man-made chemicals In: Oxford Research Encyclopedia of Environmental Science. Oxford University Press; 2019. doi:10.1093/acrefore/9780199389414.013.343 [Google Scholar]
- 13. Moore MN, Stebbing ARD. The quantitative cytochemical effects of three metal ions on a lysosomal hydrolase of a hydroid. J Mar Biol Assoc UK. 1976;56(4):995–1005. [Google Scholar]
- 14. Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14(2):70–77. [DOI] [PubMed] [Google Scholar]
- 15. Cuervo AM. Calorie restriction and aging: the ultimate “cleansing diet”. J Gerontol A Biol Sci Med Sci. 2008;63(6):547–549. [DOI] [PubMed] [Google Scholar]
- 16. Moore MN, Depledge MH, Readman JW, et al. An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat Res. 2004;552(1-2):247–268. [DOI] [PubMed] [Google Scholar]
- 17. Moore MN, Allen JI, McVeigh A, et al. Lysosomal and autophagic reactions as diagnostic and predictive indicators of environmental pollutant toxicity in aquatic animals. Autophagy. 2006;2(3):217–220. [DOI] [PubMed] [Google Scholar]
- 18. Moore MN, Viarengo A, Donkin P, et al. Autophagic and lysosomal reactions to stress in the hepatopancreas of blue mussels. Aquat Toxicol. 2007;84(1):80–91. [DOI] [PubMed] [Google Scholar]
- 19. Moore MN, Koehler A, Lowe D, et al. Lysosomes and autophagy in aquatic animals In: Klionsky D, ed. Methods in Enzymology. Academic Press/Elsevier; 2008:451:582–620. [DOI] [PubMed] [Google Scholar]
- 20. Rashid F, Horobin RW, Williams MA. Predicting the behaviour and selectivity of fluorescent probes for lysosomes and related structures by means of structure-activity models. Histochem J. 1991;23(10):450–459. [DOI] [PubMed] [Google Scholar]
- 21. Sforzini S, Moore MN, Oliveri C, et al. Probable role of mTOR in autophagic and lysosomal reactions to environmental stressors in molluscs. Aquat Toxicol. 2018;195:114–128. [DOI] [PubMed] [Google Scholar]
- 22. Shaw JP, Moore MN, Readman JW, et al. Oxidative stress, lysosomal damage damage and dysfunctional autophagy in molluscan hepatopancreas (digestive gland) induced by chemical contaminants. Mar Environ Res. 2019. doi:10.1016/j.marenvres.2019. 104825 [DOI] [PubMed] [Google Scholar]
- 23. Von Moos N, Burkhardt-Holm P, K, ö, hler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46(20):11327–11335. [DOI] [PubMed] [Google Scholar]
- 24. Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20 doi:10.1186/1743-8977-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saxton RA, Sabatini DA. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33(5):611–619. [DOI] [PubMed] [Google Scholar]
- 27. Grune T, Jung T, Merker K, et al. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and “aggresomes” during oxidative stress, ageing and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. [DOI] [PubMed] [Google Scholar]
- 28. Höhn A, Grune T. Lipofuscin: formation, effects and role of macroautophagy. Redox Biol. 2013;1(1):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore MN. Is toxicological pathology characterised by a loss of system complexity? Mar Environ Res. 2010;69(suppl):S37–S41. [DOI] [PubMed] [Google Scholar]
- 30. Moore MN, Shaw JP, Ferrar Adams DR, et al. Anti-oxidative cellular protection effect of fasting-induced autophagy as a mechanism for hormesis. Mar Environ Res. 2015;107:35–44. [DOI] [PubMed] [Google Scholar]
- 31. Sforzini S, Oliveri C, Barranger A, et al. Effects of fullerene C60 in blue mussels: role of mTOR in autophagy related cellular/tissue alterations. Chemosphere. 2020. doi:10.1016/j.chemosphere. 2019.125707 [DOI] [PubMed] [Google Scholar]
- 32. Yen W-L, Klionsky DJ. How to live long and prosper: autophagy, mitochondria, and aging. Physiology. 2008;23:248–262. [DOI] [PubMed] [Google Scholar]
- 33. Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937. [DOI] [PubMed] [Google Scholar]
- 34. Escobar KA, Cole NH, Mermier CM, et al. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18(1):e12876 doi:10.1111/acel.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore MN. Diet restriction induced autophagy: a lysosomal protective system against oxidative- and pollutant-stress and cell injury. Mar Environ Res. 2004;58(2-5):603–607. [DOI] [PubMed] [Google Scholar]
- 38. Moore MN. Autophagy as a second level protective process in conferring resistance to environmentally-induced oxidative stress. Autophagy. 2008;4(2):254–256. [DOI] [PubMed] [Google Scholar]
- 39. Numan MS, Brown JP, Michou L. Impact of air pollutants on oxidative stress in common autophagy-mediated aging diseases. Int J Environ Res Public Health. 2015;12(2):2289–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19(6):365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park Y, Park J, Kim YK. Crosstalk between translation and the aggresome-autophagy pathway. Autophagy. 2018;14(6):1079–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keller JN, Dimayuga E, Chen Q, et al. Autophagy, proteasomes, lipofuscin and oxidative stress in the aging brain. Int J Biochem Cell Biol. 2004;36(12):2376–2391. [DOI] [PubMed] [Google Scholar]
- 43. Wing S, Chiang HL, Goldberg AL, et al. Proteins containing peptide sequences related to Lys-Phe-Glu-Arg-Gln are selectively depleted in liver and heart, but not skeletal muscle, of fasted rats. Biochem J. 1991;275(Pt 1):165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiffin R, Christian C, Knecht E, et al. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Massey AC, Kaushik S, Sovak G, et al. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5905–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wileman T. Autophagy as a defence against intracellular pathogens. Essays Biochem. 2013;55:153–163. [DOI] [PubMed] [Google Scholar]
- 47. Sharma V, Verma S, Seranova E, et al. Selective autophagy and xenophagy in infection and disease. Front Cell Dev Biol. 2018;6:147 doi:10.3389/fcell.2018.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bao Y, Wang W, Zhou Z, et al. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS One. 2014;9(12):e114764 doi:10.1371/journal.pone.0114764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol. 2019;9:790 doi:10.3389/fendo.2018.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hou X, Watzlawik JO, Fiesel FC, et al. Autophagy in Parkinson’s disease. J Mol Biol. 2020. doi:10.1016/j.jmb.2020.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menzies F, Fleming A, Rubinsztein D. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345–357. doi:10.1038/nrn3961 [DOI] [PubMed] [Google Scholar]
- 52. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. [DOI] [PubMed] [Google Scholar]
- 53. Shirakabe A, Ikeda Y, Sciarretta S, et al. Aging and autophagy in the heart. Circ Res. 2016;118(10):1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cotán D, Cordero MD, Garrido-Maraver J, et al. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. 2011;25(8):2669–2687. [DOI] [PubMed] [Google Scholar]
- 55. Miranda-Díaz AG, Rodríguez-Lara SQ. The role of oxidants/antioxidants, mitochondrial dysfunction, and autophagy in fibromyalgia In: Discussions of Unusual Topics in Fibromyalgia. IntechOpen; 2017. doi:10.5772/intechopen.70695 [Google Scholar]
- 56. Agostini D, Natalucci V, Baldelli G, et al. New insights into the role of exercise in inhibiting mTOR signaling in triple-negative breast cancer. Oxid Med Cell Longev; 2018. Article ID 5896786, 19 pages doi:10.1155/2018/5896786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jiang J, Chen S, Li K, et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATPAMPK-mTOR axis for hepatocellular carcinoma. Int J Hyperthermia. 2019;36(1):499–510. [DOI] [PubMed] [Google Scholar]
- 58. Lleonart ME, Abad E, Graifer D, et al. Reactive oxygen species-mediated autophagy defines the fate of cancer stem cells. Antioxid Redox Signal. 2018;28(11):1066–1079. [DOI] [PubMed] [Google Scholar]
- 59. Su Z, Yang Z, Xu Y, et al. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48 doi:10.1186/s12943-015-0321-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mane NR, Gajare KA, Deshmukh AA. Mild heat stress induces hormetic effects in protecting the primary culture of mouse prefrontal cerebrocortical neurons from neuropathological alterations. IBRO Rep. 2018;5:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mao L, Franke J. Hormesis in aging and neurodegeneration—a prodigy awaiting dissection. Int J Mol Sci. 2013;14(7):13109–13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matus S, Castillo K, Hetz C. Hormesis. Autophagy. 2012;8(6):997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang G. Hormesis, cell death, and regenerative medicine for neurodegenerative diseases. Dose Response. 2013;11(2):238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matai L, Sarkar GC, Chamoli M, et al. Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc Natl Acad Sci USA. 2019;116(35):17383–17392. doi:10.1073/pnas.1900055116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Y, Chen Y. AMPK and autophagy. In: Qin ZH, ed. Autophagy: Biology and Diseases. Advances in Experimental Medicine and Biology, vol 1206 Springer; 2019;1206:85–108. [DOI] [PubMed] [Google Scholar]
- 66. Calabrese EJ. Preconditioning is hormesis part I: documentation, dose-response features and mechanistic foundations. Pharmacol Res. 2018;110:242–264. [DOI] [PubMed] [Google Scholar]
- 67. Calabrese EJ. Preconditioning is hormesis part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacoll Res. 2018;110:265–275. [DOI] [PubMed] [Google Scholar]
- 68. Calabrese EJ, Dhawan G, Kapoor R, et al. Hormesis: a fundamental concept with widespread biological and biomedical applications. Gerontology. 2016;62(5):530–553. [DOI] [PubMed] [Google Scholar]
- 69. Demirovic D, Rattan SIS. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013;48(1):94–98. [DOI] [PubMed] [Google Scholar]
- 70. Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging. 2011;3(9):821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Snell TW, Johnston RK, Matthews AB, et al. Repurposed FDA-approved drugs targeting genes influencing aging can extend lifespan and healthspan in rotifers. Biogerontology. 2018;19:145–157. doi:10.1007/s10522-018-9745-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vaiserman A, Lushchak O. Implementation of longevity-promoting supplements and medications in public health practice: achievements, challenges and future perspectives. J Transl Med. 2017;15(1):160 doi:10.1186/s12967-017-1259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pifferi F, Terrien J, Perret M, et al. Promoting healthspan and lifespan with caloric restriction in primates. Commun Biol. 2019;2:107 doi:10.1038/s42003-019-0348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zarse K, Schulz TJ, Birringer M, et al. Impaired respiration is positively correlated with decreased life span in Caenorhabditis elegans models of Friedreich Ataxia. FASEB J. 2007;21(4):1271–1275. [DOI] [PubMed] [Google Scholar]
- 75. Juarez-Flores DL, Gonzalez-Casacuberta I, Garrabou G. Mitohormesis and autophagic balance in Parkinson disease. Aging. 2019;11(2):301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2015;98:123–130. doi:10.1016/j.freeradbiomed.2015.11.032 [DOI] [PubMed] [Google Scholar]
- 77. Musci RV, Hamilton KL, Linden MA. Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension. Sports. 2019;7:170 doi:10.3390/sports7070170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pinto S, Sato VN, De-Souza EA, et al. Enoxacin extends lifespan of C. elegans by inhibiting miR-34-5p and promoting mitohormesis. Redox Biol. 2018;18:84–92. doi:10.1016/j.redox.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Latorre E, Birar VC, Sheerin AN, et al. Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biol. 2017;18(1):31 doi:10.1186/s12860-017-0147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsang SW, Guan YF, Wang J, et al. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: implication for treatment of acute pancreatitis. Sci Rep. 2016;6:22859 doi:10.1038/srep22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gezer C. Stress response of dietary phytochemicals in a hormetic manner for health and longevity In: Uchiumi F, ed. Gene expression and Regulation in Mammalian Cells - Transcription Toward the Establishment of Novel Therapeutics. 2018. doi:10.5772/intechopen.71867 [Google Scholar]
- 82. Madeo F, Zimmermann A, Maiuri MC, et al. Essential role for autophagy in life span extension. J Clin Invest. 2015;125(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barranger A, Langan LM, Sharma V, et al. Antagonistic interactions between benzo[a]pyrene and fullerene (C60) in toxicological response of marine mussels. Nanomaterials (Basel). 2019;9(7):987 doi:10.3390/nano9070987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011;10(12):1888–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hua H, Kong Q, Zhang H, et al. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71 doi:10.1186/s13045-019-0754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274‐293. doi:10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wengrod JC, Gardner LB. Cellular adaptation to nutrient deprivation: crosstalk between the mTORC1 and eIF2α signaling pathways and implications for autophagy. Cell Cycle. 2015;14(16):2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bendavit G, Aboulkassim T, Hilmi K, et al. Nrf2 transcription factor can directly regulate mTOR: linking cytoprotective gene expression to a major metabolic regulator that generates redox activity. J Biol Chem. 2016;291(49):25476–25488. doi:10.1074/jbc.M116.760249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aliper A, Jellen L, Cortese F, et al. Towards natural mimetics of metformin and rapamycin. Aging. 2017;9(11):2245–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64(3):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Handschin C, Kobayashi YM, Chin S, et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397-408. [DOI] [PubMed] [Google Scholar]
- 94. Moore MN. Do airborne biogenic chemicals interact with the PI3K/Akt/mTOR cell signalling pathway to benefit human health and wellbeing in rural and coastal environments? Environ Res. 2015;140:65–75. [DOI] [PubMed] [Google Scholar]
- 95. Avila-Rojas SH, Lira-León A, Aparicio-Trejo OE, et al. Role of autophagy on heavy metal-induced renal damage and the protective effects of curcumin in autophagy and kidney preservation. Medicina. 2019;55:360 doi:10.3390/medicina55070360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kalache A, de Hoogh AI, Howlett SE, et al. Nutrition interventions for healthy ageing across the lifespan: a conference report. Eur J Nutr. 2019;58(suppl 1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Niso-Santano M, González-Polo RA, Paredes-Barquero M, et al. Natural products in the promotion of healthspan and longevity. Clin Pharmacol Transl Med. 2019;3(1):149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rainey N, Motte L, Aggarwal BB, et al. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis. 2015;6(12):e2003 doi:10.1038/cddis.2015.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10(11):1651 doi:10.3390/nu10111651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hooyberg A, Roose H, Grellier J, et al. General health and residential proximity to the coast in Belgium: Results from a cross-sectional health survey. Environ Res. 2020. doi:10.1016/j.envres.2020.109225 [DOI] [PubMed] [Google Scholar]
- 101. Asselman J, Van Acker E, De Rijcke M, et al. Marine biogenics in sea spray aerosols interact with the mTOR signaling pathway. Sci Rep. 2019;9(1):675 doi:10.1038/s41598-018-36866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Van Acker E, De Rijcke M, Asselman J, et al. Aerosolizable marine phycotoxins and human health effects: in vitro support for the biogenics hypothesis. Mar Drugs. 2020;18(1):46 doi:10.3390/md18010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baldi A, Fusi E, Sangalli L, et al. In vitro evaluation of cytotoxicity and oxidative damage induced by ochratoxin A and aflatoxin B1: protective role of antioxidants. Ital J Anim Sci. 2003;2:S231–S233. [Google Scholar]
- 104. Duke SO. Phytochemical phytotoxins and hormesis—a commentary. Dose Response. 2010;9(1):76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee HJ, Yoon YS, Lee SJ. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis. 2019;9:712 doi:10.1038/s41419-018-0749-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Menendez JA, Joven J, Aragonès G, et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: a new family of gerosuppressant agents. Cell Cycle. 2013;12(4):555–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Suter S, Lucock M. Xenohormesis: applying evolutionary principles to contemporary health issues. Explor Res Hypothesis in Med. 2017;2(4):79–85. [Google Scholar]
- 108. Koehler A, Marx U, Broeg K, et al. Effects of nanoparticles in Mytilus edulis gills and hepatopancreas—a new threat to marine life? Mar Environ Res. 2008;66(1):12–14. [DOI] [PubMed] [Google Scholar]
- 109. Miresmailli S, Isman MB. Botanical insecticides inspired by plant–herbivore chemical interactions. Trends Plant Sci. 2014;19(1):29–35. [DOI] [PubMed] [Google Scholar]
- 110. Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121(2):325–332. [PMC free article] [PubMed] [Google Scholar]
- 111. Stegelmeier BL, Edgar JA, Colegate SM, et al. Pyrrolizidine alkaloid plants, metabolism and toxicity. J Nat Toxins. 1999;8(1):95–116. [PubMed] [Google Scholar]
- 112. Wöll S, Kim SH, Greten HJ, et al. Animal plant warfare and secondary metabolite evolution. Nat Prod Bioprospect. 2013;3(1):1–7. [Google Scholar]
- 113. Glenn AE, Davis CB, Gao M, et al. Horizontally transferred xenobiotic resistance gene clusters associated with detoxification of benzoxazolinones by Fusarium species. PLoS One. 2016;11(1):e0147486 doi:10.1371/journal. pone.0147486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gurley BJ. Pharmacokinetic herb-drug interactions (part 1): origins, mechanisms, and the impact of botanical dietary supplements. Planta Med. 2012;78(13):1478–1489. [DOI] [PubMed] [Google Scholar]
- 115. Koppel N, Rekdal VM, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356(6344):eaag2770 doi:10.1126/science. aag2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lila MA, Raskin I. Health-related interactions of phytochemicals. J Food Sci. 2005;70(1):20–27. [Google Scholar]
- 117. Sullivan RJ, Hagen EH, Hammerstein P. Revealing the paradox of drug reward in human evolution. Proc Biol Sci. 2008;275(1640):1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fiehn O, Barupal DK, Kind T. Extending biochemical databases by metabolomic surveys. J Biol Chem. 2011;286(27):23637–23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oz E. Effects of smoke flavoring using different wood chips and barbecuing on the formation of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in salmon fillets. PLoS One. 2020;15(1):e0227508 doi:10.1371/journal.pone.0227508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bjørklund G, Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. [DOI] [PubMed] [Google Scholar]
- 121. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cronin MTD, Jaworska JS, Walker JD, et al. Use of QSARs in international decision-making frameworks to predict health effects of chemical substances. Environ Health Perspect. 2003;111(10):1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gil PR, Oberdörster G, Elder A, et al. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano. 2014;4(10):5527–5531. [DOI] [PubMed] [Google Scholar]
- 124. Keller AA, Lazareva A. Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett. 2014;1(1):65–70. [Google Scholar]
- 125. Yi Z, Cao J, Jiang T, et al. Characterization of metal-bearing particles in groundwater from the Weilasituo Zn-Cu-Ag deposit, Inner Mongolia, China: implications for mineral exploration. Ore Geol Rev. 2020;117 doi:10.1016/j.oregeorev.2019.103270 [Google Scholar]
- 126. Aschberger K, Micheletti C, Sokull-Kl, ü, ttgen B, et al. Analysis of currently available data for characterising the risk of engineered nanomaterials to the environment and human health: lessons learned from four case studies. Environ Int. 2011;37(6):1143–1156. [DOI] [PubMed] [Google Scholar]
- 127. Martirosyan A, Schneider Y-J. Engineered nanomaterials in food: implications for food safety and consumer health. Int J Environ Res Public Health. 2014;11(6):5720–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Thompson RC, Olsen Y, Mitchell RP, et al. Lost at sea: where is all the plastic? Science. 2004;304(5672):838. [DOI] [PubMed] [Google Scholar]
- 129. Galloway TS, Lewis CN. Marine microplastics spell big problems for future generations. Proc Natl Acad Sci USA. 2016;113(9):2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Galloway T, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. 2017;1(5). doi:10.1038/ s41559-017-0116 [DOI] [PubMed] [Google Scholar]
- 131. Mijaljica D, Devenish RJ. Nucleophagy at a glance. J Cell Sci. 2013;126(Pt 19):4325–4330. [DOI] [PubMed] [Google Scholar]
- 132. De P, Miskimins K, Dey N, et al. Promise of rapalogues versus mTOR kinase inhibitors in subset specific breast cancer: old targets new hope. Cancer Treat Rev. 2013;39(5):403–412. doi:10.1016/j.ctrv.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 133. Sohal R, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273(5271):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]