To the Editor
Several cross-sectional studies have shown an association between obesity and asthma, and meta-analyses of prospective studies have concluded that obesity precedes the development of asthma and wheezing in both adults and children.1 However, a recent study of 2 longitudinal cohorts suggested that children with asthma are at increased risk of subsequent development of obesity.2 The nature and the direction of the association between the 2 conditions thus remain unclear.3 Diurnal peak flow variability (PFvar) is considered an important measurement of airway lability in the screening and diagnosis of asthma in population-based studies. We have previously found increased PFvar and response to albuterol in girls who became overweight or obese during the school years as compared with those who did not,4 suggesting a link between an abnormal regulation of the airway tone and weight gain in the school years. The aim of the current study was to determine whether airway lability in children is associated with subsequent body mass index (BMI) increase up to young adult life in the longitudinal Tucson Children’s Respiratory Study birth cohort.5
Detailed methods and definitions are reported in this article’s Online Repository at www.jacionline.org. Questionnaires on participants’ respiratory symptoms and physical activity were completed by their parents at age 11 years (Yr11: mean ± SD, 10.7 ± 0.5 years) and 16 years (Yr16: 16.6 ± 0.6 years) and by participants themselves at age 22 years (Yr22: 22.1 ± 0.9 years) and 26 years (Yr26: 26.5 ± 0.9 years). At each survey, skin prick tests were performed and BMI (kg/m2) was calculated. At a mean age of 10.8 years, peak expiratory flow data were collected for 1 week and PFvar was calculated as previously reported6 (see this article’s Online Repository at www.jacionline.org). BMI and PFvar values were logarithmically transformed (base 10) to obtain a normal distribution. The potential confounding effects of parental education, ethnicity, metabolic equivalents of task, and smoke exposure were also tested. This study was approved by the Human Subjects Committee at the University of Arizona, and informed consent was obtained from the parents before age 18 years and, subsequently, from the participants themselves.
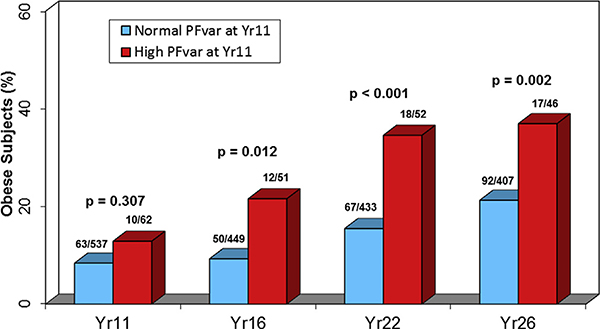
Among the 600 children with PFvar information at Yr11, the number of subjects with subsequent BMI information was 500 at Yr16, 485 at Yr22, and 453 at Yr26 (see this article’s Online Repository at www.jacionline.org). The characteristics of study subjects at Yr11 in comparison with those who were excluded because of missing information on BMI or PFvar (see Table E1 in this article’s Online Repository at www.jacionline.org) and at each subsequent survey (see Table E2 in this article’s Online Repository at www.jacionline.org) are reported in this article’s Online Repository at www.jacionline.org. At Yr11, the geometric mean (95% CI) PFvar was 7.6% (7.3–8.0), range 0.7% to 41.9%, and 62 children (10.3%) had a high PFvar. In univariate analyses, a significant linear correlation was found between log-transformed PFvar at Yr11 and log-transformed BMI at Yr11 (r = 0.10; P = .015), Yr16 (r = 0.14; P = .001), Yr22 (r = 0.14; P =.002), and Yr26 (r = 0.11; P =.019). Fig E1 (in this article’s Online Repository at www.jacionline.org) shows that, as compared with participants with normal PFvar, those with high PFvar at Yr11 were more likely to be obese at Yr16 (risk ratio [RR] [95% CI] from generalized estimating equation models, 2.00 [1.17–3.39]), Yr22 (2.31 [1.53–3.48]), and Yr26 (1.77 [1.23–2.56]), but not at Yr11 (1.38 [0.75– 2.54]). Similarly, no significant association was found between high PFvar at Yr11 and obesity earlier in childhood (see this article’s Online Repository at www.jacionline.org).
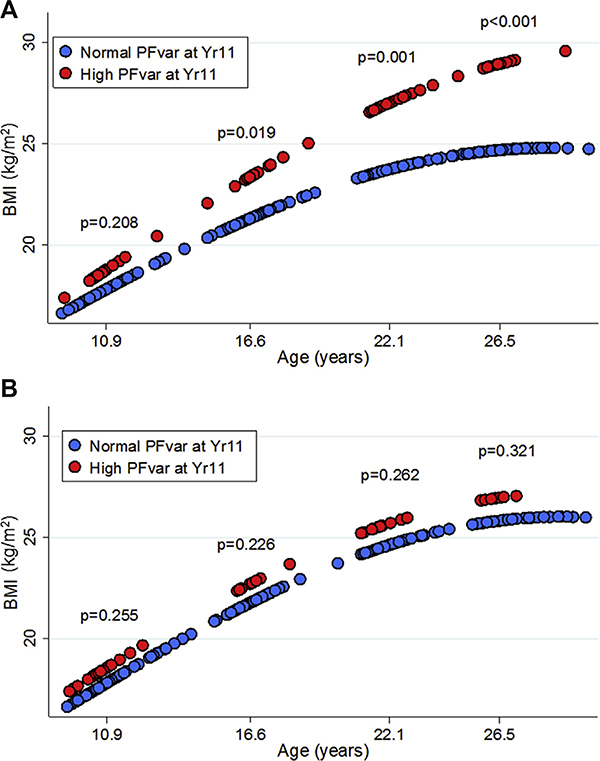
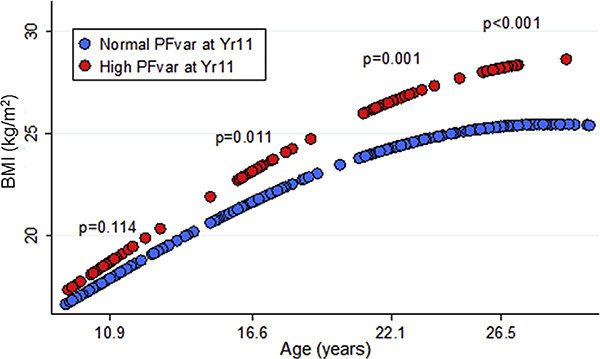
In random-effects multivariate models with log-transformed BMI as the continuous dependent variable, we found subjects with high PFvar to have only marginally increased BMI at Yr11 but significantly higher BMI at all other ages compared with subjects with normal PFvar, with the largest differences achieved by Yr26 (Fig E2). The best-fitting model (see Table E3 in this article’s Online Repository at www.jacionline.org) included both age and age-squared terms, consistent with deceleration of BMI increase from childhood to young adult life; also (Table E3), the effect of high PFvar on subsequent BMI was mainly explained by the interaction between high PFvar and age, indicating that after adjustment for covariates, high PFvar at Yr11 was associated with a steeper increase over time (or, in other words, a higher velocity of growth) in BMI. After further adjustment of the model for BMI at Yr11 and/or smoke exposure, results remained virtually unchanged (data not shown). However, when random-effects models were stratified by sex, we found notable differences in the association between PFvar and BMI increase between females and males (Table I and Fig 1). The interaction between high PFvar and age was highly significant in females (P < .001) but not in males (P = .968), showing that the effect of high PFvar on the subsequent BMI increase was present only in the former group (Table I). These effects were confirmed when repeating the analysis in subjects who were neither overweight/obese nor had asthma at Yr11 (see this article’s Online Repository at www.jacionline.org).
TABLE I.
Longitudinal analysis by random-effects models of base 10 log-transformed BMI at Yr11, Yr16, Yr22, and Yr26 as the dependent variable and potential confounders at the 4 surveys stratified by sex*
| Females (n = 308; obs = 898) |
Males (n = 276; obs = 772) |
|||||
|---|---|---|---|---|---|---|
| Independent variables | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value |
| High PFvar at Yr11 | 0.018 | −0.019 to 0.054 | .338 | 0.018 | −0.014 to 0.050 | .275 |
| Age | 0.018 | 0.016 to 0.020 | <.001 | 0.020 | 0.017 to 0.022 | <.001 |
| Age2 | −0.0005 | −0.0006 to −0.0004 | <.001 | −0.0005 | −0.0006 to −0.0004 | <.001 |
| High PFvar × Age | 0.003 | 0.001 to 0.005 | <.001 | 0.0004 | −0.002 to 0.002 | .968 |
| Concurrent asthma | 0.020 | 0.007 to 0.033 | .002 | −0.002 | −0.011 to 0.014 | .802 |
| Concurrent atopy | −0.006 | −0.016 to 0.005 | .312 | −0.003 | −0.015 to 0.009 | .649 |
| Concurrent METs† | −0.002 | −0.006 to 0.003 | .503 | −0.004 | −0.008 to 0.0009 | .114 |
| Maternal education ≤12 y | 0.020 | −0.004 to 0.047 | .095 | −0.0008 | −0.024 to 0.022 | .948 |
| Paternal education ≤12 y | 0.015 | −0.012 to 0.042 | .276 | 0.034 | 0.011 to 0.057 | .004 |
| At least 1 Hispanic parent‡ | 0.013 | −0.012 to 0.037 | .306 | 0.018 | −0.003 to 0.040 | .097 |
| Other ethnicity‡ | 0.033 | 0.003 to 0.064 | .032 | −0.013 | −0.043 to 0.017 | .398 |
MET, Metabolic equivalent of task; obs, observations.
Age was centered at the youngest age, 9.2 y, to facilitate interpretation of the main effect coefficient of PFvar.
METs were used as an ordinal variable at each survey where the first category was 0 MET h/wk, the second category was between 1 and 40 MET h/wk, and the third category was >40 MET h/wk (see text).
Reference category: both non-Hispanic white parents.
FIG 1.
Linear prediction of base 10 log-transformed BMI vs age for subjects with high (red circles) and normal (blue circles) PFvar at Yr11 after stratifying by sex (A: females; B: males). Linear predictions were calculated from random-effects models adjusted for maternal and paternal education, and ethnicity as fixed effects and current asthma, atopy, and metabolic equivalents of task at each survey as time-dependent covariates. P values related to group differences at each time point were tested using linear contrast.
In summary, we found high PFvar at Yr11 to predict a steeper subsequent BMI increase up to young adult life independent of multiple covariates, including current asthma at Yr11. Interestingly, the effects of high PFvar at Yr11 were much stronger on subsequent than on concomitant levels of BMI, suggesting that our findings are very unlikely to be explained by concomitant effects of obesity on airway lability. In other words, if the relationship between PFvar and BMI were simply explained by the fact that subjects with a higher BMI are deconditioned and have a lower lung function, one might expect a better correlation between high PFvar and elevated BMI during childhood surveys, which was not the case in our study. These findings support the possibility that the association between asthma and obesity may be bidirectional or due to shared factors that preexist both conditions3 (for previous literature and the limitations of the study, see this article’s Online Repository at www.jacionline.org ). Obesity and asthma are known to share common genetic mechanisms7,8; furthermore, developmental and early-life factors have been suggested as affecting the development of both obesity and asthma.8,9 We propose that such common factors might cause both an alteration in the regulation of airway tone and a greater tendency to gain weight during the growing years and that the asthma-related phenotype might thus precede the obesity phenotype.
Acknowledgments
We gratefully acknowledge the contributions of Dr Lynn Taussig, who started the Tucson Children’s Respiratory Study in 1980. We thank Bruce Saul for data management and our study nurses, Marilyn Lindell, Lydia de la Ossa, Nicole Pargas, and Silvia S. Lopez, for data collection and participant follow-up. We thank the CRS subjects for their continued participation in the study.
This study was supported by the National Heart, Lung, and Blood Institute (grant no. 132523).
Disclosure of potential conflict of interest: S. Guerra, A. L. Wright, D. A. Stern, W. J. Morgan, and F. D. Martinez received support for this research from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) (grant no. 132523). E. Lombardi has received fees or travel grants during the past 5 years from Angelini, Boehringer, Chiesi, Lusofarmaco, Omron, Vertex Pharmaceuticals, and Vifor. S. Guerra reports grants from NIH/NHLBI, NIH/National Institute of Allergy and Infectious Diseases (NIAID), and the Cystic Fibrosis Foundation. W. J. Morgan reports grants from NIH/NHLBI, NIH/NIAID, and the Cystic Fibrosis Foundation; personal fees from the Cystic Fibrosis Foundation; and personal fees from Genentech. F. D. Martinez reports grants from NIH/NHLBI, NIH/NIAID, NIH/National Institute of Environmental Health Sciences, NIH/Office of the Director, and Johnson & Johnson. He was also a consultant for Commense INC and Copeval. The rest of the authors declare that they have no relevant conflicts of interest.
FIG E1.
Proportions of obese children at the 4 surveys by PFvar at age 11 years. P values related to group differences at each time point were tested using linear contrast from longitudinal Generalized estimating equation univariate analyses with PFvar at Yr11 as the independent variable and obesity at Yr11, Yr16, Yr22, and Yr26 as the dependent variable.
FIG E2.
Linear prediction of base 10 log-transformed BMI vs age for subjects with high (red circles) and normal (blue circles) PFvar at Yr11. Linear predictions were calculated from random-effects models adjusted for sex, maternal and paternal education, and ethnicity as fixed effects and current asthma, atopy, and METs at each survey as time-dependent covariates in the whole study group. P values related to group differences at each time point were tested using linear contrast.
TABLE E1.
Characteristics of the study subjects at Yr11 (for proportions, the actual numbers are shown in parentheses)*
| Yr11 |
||
|---|---|---|
| Characteristic | Included (n = 600) | Not included (n = 356) |
| Age (y), mean ± SD | 10.7 ± 0.5 | 11.1 ± 0.8† |
| Sex: male, % | 48.2 (289 of 600) | 49.7 (177 of 356) |
| Current asthma, % | 17.6 (104 of 592) | 13.2 (46 of 349)‡ |
| Atopy, % | 54.7 (327 of 598) | 57.3 (63 of 110) |
| Current physical activity, % | 51.3 (307 of 599) | 57.9 (206 of 356)§ |
| MET (h/wk), % | ||
| 0 | 48.8 (292 of 598) | 42.3 (150 of 355)§ |
| 1–40 | 25.4 (152 of 598) | 23.9 (85 of 355) |
| >40 | 25.8 (154 of 598) | 33.8 (120 of 355) |
| Maternal education ≤12 y, % | 28.9 (173 of 599) | 25.6 (91 of 356) |
| Paternal education ≤12 y, % | 26.2 (154 of 588) | 23.1 (81 of 351) |
| Parental ethnicity, % | ||
| Non-Hispanic white | 60.8 (365 of 600) | 70.8 (252 of 356)§ |
| ≥1 Hispanic white | 25.8 (155 of 600) | 16.0 (57 of 356) |
| Other ethnicity | 13.3 (80 of 600) | 13.2 (47 of 356) |
| BMI (kg/m2), GM (95% CI) | 18.6 (18.3–18.9) | 18.3 (18.0–18.7) |
| Overweight/obese, % | 27.4 (164 of 599) | 24.6 (75 of 305) |
| Obese, % | 12.2 (73 of 599) | 10.2 (31 of 305) |
GM, Geometric mean.
Totals are not the same for all individual characteristics because information is missing for some subjects.
P < .001 by t test vs study subjects at same age.
.1 > P > .05 by χ2 test vs study subjects at same age.
P < .05 by χ2 test vs study subjects at same age.
TABLE E2.
Characteristics of the study subjects at Yr11, Yr16, Yr22, and Yr26 (for proportions, the actual numbers are shown in parentheses)*
| Characteristic | Yr11 (n = 600) | Yr16 (n = 500) | Yr22 (n = 485) | Yr26 (n = 453) |
|---|---|---|---|---|
| Age (y), mean ± SD | 10.7 ± 0.5 | 16.6 ± 0.6 | 22.1 ± 0.9 | 26.5 ± 0.9 |
| Sex: male, % | 48.2 (289 of 600) | 49.2 (246 of 500) | 46.6 (226 of 485) | 46.1 (209 of 453) |
| Current asthma, % | 17.6 (104 of 592) | 19.4 (95 of 489) | 18.7 (90 of 482) | 20.9 (92 of 445) |
| Atopy, % | 54.7 (327 of 598) | 72.0 (317 of 440) | 72.6 (278 of 383) | 72.9 (240 of 329) |
| Current physical activity, % | 51.3 (307 of 599) | 57.9 (287 of 496) | 64.0 (307 of 480) | 66.6 (297 of 446) |
| METs (h/wk), % | ||||
| 0 | 48.8 (292 of 598) | 40.8 (197 of 483) | 36.2 (173 of 478) | 34.5 (147 of 426) |
| 1–40 | 25.4 (152 of 598) | 17.6 (85 of 483) | 24.7 (118 of 478) | 32.4 (138 of 426) |
| >40 | 25.8 (154 of 598) | 41.6 (201 of 483) | 39.1 (187 of 478) | 33.1 (141 of 426) |
| Maternal education ≤12 y, % | 28.9 (173 of 599) | 24.6 (123 of 499) | 25.4 (123 of 485) | 25.0 (113 of 452) |
| Paternal education ≤12 y, % | 26.2 (154 of 588) | 23.4 (115 of 491) | 25.4 (121 of 477) | 23.1 (103 of 446) |
| Parental ethnicity | ||||
| Non-Hispanic white | 60.8 (365 of 600) | 62.4 (312 of 500) | 63.1 (306 of 485) | 64.7 (293 of 453) |
| ≥1 Hispanic white | 25.8 (155 of 600) | 24.6 (123 of 500) | 24.7 (120 of 485) | 23.6 (107 of 453) |
| Other ethnicity | 13.3 (80 of 600) | 13.0 (65 of 500) | 12.2 (59 of 485) | 11.7 (53 of 453) |
| BMI (kg/m2), GM (95% CI) | 18.6 (18.3–18.9) | 22.5 (22.1–22.9) | 24.9 (24.4–25.4) | 26.2 (25.7–26.8) |
| Overweight/obese, % | 27.4 (164 of 599) | 23.2 (116 of 500) | 39.2 (190 of 485) | 50.8 (230 of 453) |
| Obese, % | 12.2 (73 of 599) | 12.4 (62 of 500) | 17.5 (85 of 485) | 24.1 (109 of 453) |
GM, Geometric mean.
Totals are not the same for all individual characteristics because information is missing for some subjects.
TABLE E3.
Longitudinal analysis by random-effects model of base 10 log-transformed BMI at Yr11, Yr16, Yr22, and Yr26 as the dependent variable and potential confounders at the 4 surveys (n = 584; obs = 1670)*
| Independent variables | Coefficient | 95% CI | P value |
|---|---|---|---|
| High PFvar at Yr11 | 0.016 | −0.008 to 0.041 | .195 |
| Age | 0.019 | 0.018 to 0.021 | <.001 |
| Age2 | −0.0005 | −0.0006 to −0.0004 | <.001 |
| High PFvar × Age | 0.002 | 0.0005 to 0.003 | .008 |
| Male sex | 0.002 | −0.012 to 0.016 | .784 |
| Concurrent asthma | 0.010 | −0.0007 to 0.019 | .035 |
| Concurrent atopy | −0.005 | −0.013 to 0.003 | .220 |
| Concurrent METs† | −0.003 | −0.007 to −0.0001 | .041 |
| Maternal education ≤12 y | 0.011 | −0.006 to 0.0297 | .218 |
| Paternal education ≤12 y | 0.023 | 0.005 to 0.041 | .012 |
| ≥1 Hispanic parent‡ | 0.014 | −0.003 to 0.030 | .105 |
| Other ethnicity‡ | 0.015 | −0.007 to 0.037 | .173 |
Yr11 was centered at the youngest age, 9.2 y, to facilitate interpretation of the main effect coefficient of PFvar.
METs were used as an ordinal variable at each survey where the first category was 0 MET h/wk, the second category was between 1 and 40 MET h/wk, and the third category was >40 MET h/wk (see text).
Reference category: both non-Hispanic white parents.
REFERENCES
- 1.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med 2017;195:1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stukus DR. Obesity and asthma: the chicken or the egg? J Allergy Clin Immunol 2015;135:894–5. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 2001;163:1344–9. [DOI] [PubMed] [Google Scholar]
- 5.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study, I: design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol 1989;129: 1219–31. [DOI] [PubMed] [Google Scholar]
- 6.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax 1997;52:946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez JR, Caceres A, Esko T, Cusco I, Puig M, Esnaola M, et al. A common 16p11.2 inversion underlies the joint susceptibility to asthma and obesity. Am J Hum Genet 2014;94:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Permaul P, Kanchongkittiphon W, Phipatanakul W. Childhood asthma and obesity–what is the true link? Ann Allergy Asthma Immunol 2014;113:244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litonjua AA, Gold DR. Asthma and obesity: common early-life influences in the inception of disease. J Allergy Clin Immunol 2008;121:1075–84. [DOI] [PubMed] [Google Scholar]