INTRODUCTION
Patients fear lymphedema as an unpredictable daily reminder of breast cancer treatment. The medical community has assumed for years that the development of breast cancer–related lymphedema (BCRL) stemmed solely from the primary surgical extirpation of the axillary lymph nodes. However, contemporary data suggest that BCRL development is multifactorial, influenced by multimodality locoregional and systemic treatment strategies and perhaps by the individual patient’s ability to form collateral lymphatic pathways after injury, as well as potentially modifiable risk factors such as body mass index (BMI). Understanding the interaction between comprehensive locoregional treatment strategies and their collective impact on overall survival and long-term adverse effects such as BCRL is critical to providing patients individualized treatment recommendations. Herein, we review important factors for the development, diagnosis, prevention, and treatment of BCRL that should be considered when determining the contemporary locoregional management of breast cancer.
RISK FACTORS
Axillary Surgery
The precise incidence of BCRL is difficult and complicated to determine as a result of the prolonged period of latency from breast cancer treatment to initial BCRL signs or symptoms. There is no doubt that the extent of axillary surgery is a significant risk factor.1 Axillary lymph node dissection (ALND) results in greater lymphatic disruption than sentinel lymph node biopsy (SLNB) and can quadruple the rate of BCRL.1-4 Removal of more lymph nodes and the total number of positive lymph nodes are consistently cited as BCRL risk factors but are likely corollaries for extent of dissection or need for multimodality therapy, respectively. Currently, the progression of breast cancer clinical trial development has focused on strategic de-escalation of locoregional therapy, particularly to the axilla.2,5,6 Although primary outcomes for these trials have been survival or local recurrence related, many have included secondary aims focusing on BCRL, providing contemporary insight into incidence.
Collectively, the risk of BCRL after SLNB is between 3% and 8% based on prospective randomized trials.1,3 Single-institution series corroborate these data. Byun et al7 observed 7,617 patients for a median of 60 months, reporting BCRL in 3% of patients who underwent SLNB. Similarly, Belmonte et al8 reported BCRL in 3.4% of SLNB-negative patients. Regarding ALND, the B-32, IBCSG, Z1071, and AMAROS trials documented BCRL risk to range from 13% to 60%, with most studies using a > 10% relative volume change (RVC) as diagnostic for BCRL.1-3,6 Importantly, length of follow-up and criteria for diagnosis can sway incidence rates and mandate critical synthesis when broadly comparing BCRL incidence across studies. For example, Wetzig et al9 defined BCRL as > 15% volume change, finding BCRL in only 5% of ALND patients noting progressive swelling changes over 5 years. When their definition changed to include any swelling, 26% of ALND patients were categorized as having BCRL.9
In the prospective American College of Surgeons Oncology Group Z1071 trial, all patients proceeded to ALND and 87% had additional radiation.2 At a median follow-up of 3 years, 37.8% of patients reported symptoms of arm swelling, 58.4% had measured BCRL > 10%, and 36.9% had > 20% RVC in the ipsilateral arm. In this trial, however, BCRL was not confirmed by clinical exam, nor is it clear whether training was provided to those performing the measurements to limit interrater variability. Others have also found a relationship between ALND and regional lymph node radiation (RLNR) but document lower BCRL rates ranging from 31.2% to 38.7%. Because these BCRL rates are higher than with either ALND or RLNR alone, they too support the additive influence of multimodality regional nodal therapies.
RLNR
Positive data from MA.20 and the European Organisation for Research and Treatment of Cancer trials have increased the number of lymph node–positive and high-risk node-negative patients receiving RLNR,10,11 a significant risk factor for BCRL. Warren et al12 evaluated 1,476 patients, finding that the supraclavicular (SCV) field regardless of posterior axillary boost (PAB) significantly increased BCRL. Chandra et al13 reported that the extent of the lateral border of the nodal field, dose per fraction, energy used, and tangent types were not correlated with BCRL incidence. Conversely, Gross et al14 found higher BCRL rates when the lateral border of the nodal field encompassed more than one third of the humoral head. Interestingly, this study also showed that covering the SCV field using anterior oblique beams with and without PAB yielded similar BCRL rates to treatment with parallel opposed beams to include upper level I, II, and III axilla. Unfortunately, this study lacked preoperative baseline arm measurements and quantified BCRL using tape measurements only to completion of radiation sessions. Gross et al14 found that the axillary-lateral thoracic vessel juncture (superior to level I) dose was most associated with BCRL risk (P < .001). These results have not been prospectively validated.
Finally, Naoum et al15 prospectively observed 1,811 patients and showed that BCRL risk depends mainly on the extent of axillary surgery. The authors classified patients according to extent of axillary surgery with or without RLNR; SLNB alone, SLNB plus RLNR, ALND alone, and ALND plus RLNR yielded cumulative incidences of BCRL of 7.7%, 10.8%, 29%, and 38.7%, respectively, at 5 years. Multivariable analysis showed no significant difference in BCRL risk between SLNB groups and ALND groups regardless of use of RLNR; both ALND groups had higher BCRL risks than those who underwent SLNB. Local control rates were similar across the 4 groups. These data, together with a recent meta-analysis,4 validate the AMAROS trial data,6 suggesting the significant reduction of BCRL rates if RLNR replaces ALND in patients with 1-2 positive sentinel lymph nodes.
BMI
BMI of ≥ 30 kg/m2 at breast cancer diagnosis is an independent BCRL risk factor.16 Weight gain or loss during or after treatment and its effect on BCRL risk is evolving. In a recent randomized trial involving overweight survivors of breast cancer with BCRL, effects of weight loss were examined. Although women in the weight loss group and the combined weight loss and home-based exercise group lost −7.37% (95% CI, −8.90% to 5.84%) and −8.06% (95% CI, −9.82% to −6.29%) of their baseline weight, respectively, weight loss did not improve BCRL outcomes17 (clinical assessment, symptoms, BCRL exacerbations, cellulitis, or limb volume). The exact impact of postoperative weight fluctuation warrants more study to effectively inform patient education.
Cellulitis
Cellulitis is a well-established BCRL risk factor.18-20 Cellulitis exacerbates preexisting BCRL, leading to a recurrent cellulitis-BCRL flare cycle.20 The pathophysiologic relationship between cellulitis and BCRL remains unclear.
Low-Level Limb Volume Changes
Low-level arm volume changes after breast cancer surgery increase progression to BCRL.21,22 One study found that patients developing RVC increases of 3% to < 5% from baseline within 3 months of surgery or 5% to < 10% from baseline at any point after surgery were more likely to progress to BCRL (RVC ≥ 10%).23 Another study found similar results in patients who had ≥ 5 lymph nodes removed and arm swelling at 6 or 12 months.22
TIMING OF BCRL ONSET
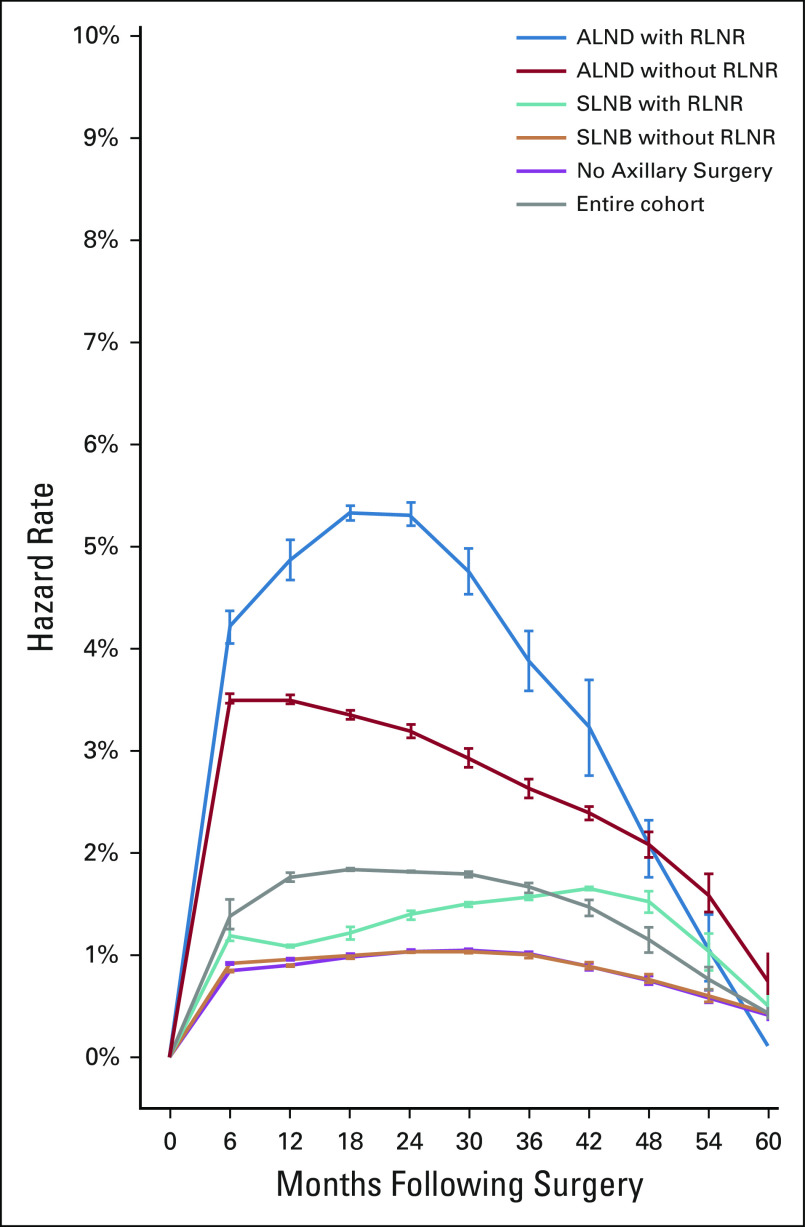
In a cohort of 2,171 prospectively screened women, BCRL onset peaked between 12 and 30 months postoperatively; however, timing of onset varied with treatment (Fig 1). Early-onset BCRL (< 12 months postoperatively) was associated with ALND (hazard ratio [HR], 4.75; P < .0001) but not RLNR (HR, 1.21; P = .55). Late-onset BCRL (> 12 months) was associated with RLNR (HR, 3.86; P < .0001) and ALND (HR, 1.86; P = .029). BCRL risk was highest at 6-12 months in the ALND group (no RLNR), at 18-24 months in the ALND plus RLNR group, and at 36-48 months in the SLNB plus RLNR group. The understanding of the onset of BCRL will inform screening practices and education.23
FIG 1.
Semiannual hazard rate for development of breast cancer–related lymphedema for the entire cohort and by axillary surgery and radiation groups. ALND, axillary lymph node dissection; RLNR, regional lymph node radiation; SLNB, sentinel lymph node biopsy. Reprinted from McDuff et al,23 with permission of Elsevier.
BCRL SCREENING
Widespread support is emerging for a prospective screening model using objective measures, symptoms, and clinical exam for early diagnosis and prevention of BCRL progression.24,25 The American Society of Breast Surgeons (ASBrS),26 the National Comprehensive Cancer Network (NCCN),27 the National Lymphedema Network (NLN),28 and the International Society of Lymphology (ISL)29 all endorse prospective screening beginning at the time of breast cancer diagnosis.
Critical Preoperative Baseline Measurement
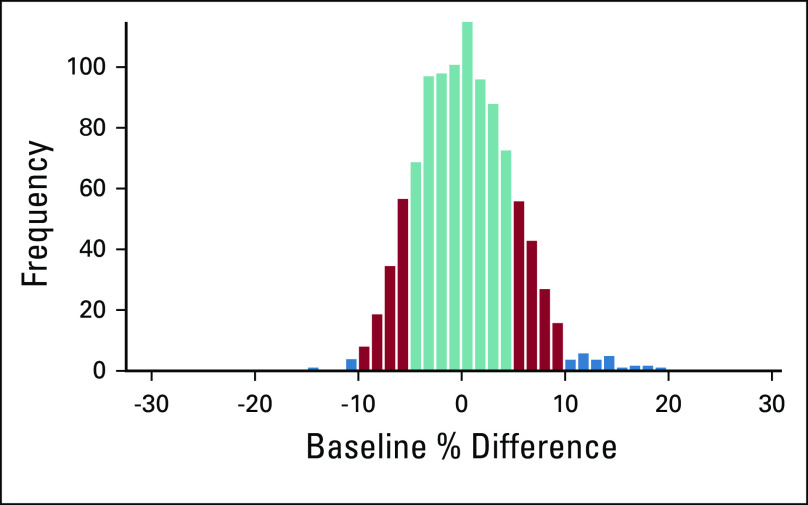
Objective limb measurements should be performed at baseline and at regular follow-up intervals for best diagnostic accuracy.24,29,30 Lack of baseline measurements results in incorrect diagnoses of BCRL because arm asymmetry naturally exists. A prospectively screened cohort of 1,028 patients demonstrated that 28.3% and 2.9% of patients had preoperative arm asymmetry of ≥ 5% and ≥ 10%, respectively (Fig 2).31 Misdiagnosis occurred in 40%-50% of patients when a postoperative pseudo-baseline was substituted for a true preoperative baseline measurement in a cohort of 1,028 patients prospectively screened for BCRL from preoperative baseline.31
FIG 2.
Histogram of baseline arm asymmetry. Magnitude of baseline asymmetry > 5% and > 10% is shaded; 28.3% and 2.9% of the study cohort have magnitude of baseline asymmetry > 5% and > 10%, respectively. Reprinted from Sun et al,31 by permission from Springer Nature.
RVC
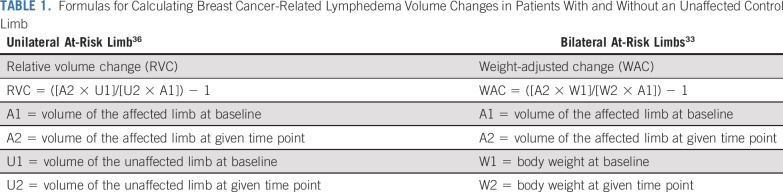
BCRL diagnosis should incorporate volume changes in the affected limb when compared with preoperative baseline, while taking into account weight fluctuations.32 Formulas for determining RVCs are listed in Table 1. For patients who have undergone unilateral surgery, the contralateral arm functions as the control. For patients who have undergone bilateral surgery and therefore lack a control arm for comparison, the weight-adjusted change (WAC) equation was developed (Table 1),33 accounting for weight fluctuations relative to baseline.
TABLE 1.
Formulas for Calculating Breast Cancer-Related Lymphedema Volume Changes in Patients With and Without an Unaffected Control Limb
BCRL Definition
BCRL is defined as RVC ≥ 10% or WAC ≥ 10% more than 3 months after breast surgery. Although RVC or WAC ≥ 10% is generally accepted as a diagnostic threshold for intervention, some studies have shown treatment effectiveness at RVC as low as 3%25; however, these studies lacked a control group not receiving treatment.
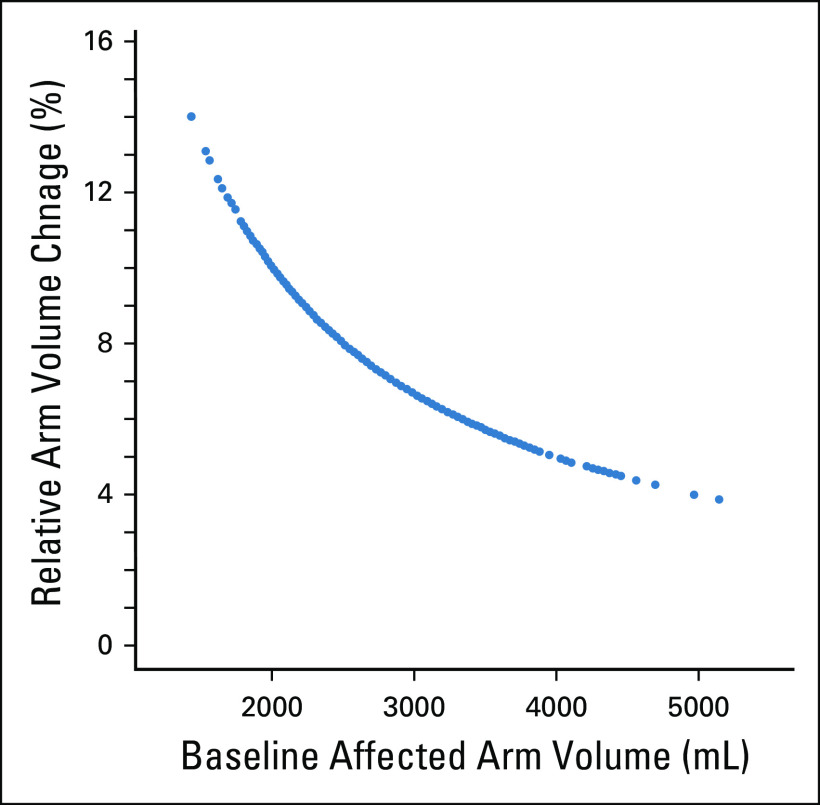
Historically, an increase in volume of 200 mL or in circumference of 2 cm in the at-risk arm32 has been used to diagnose BCRL, which is fraught with error. This does not take into account baseline arm volume or common weight fluctuations. In one study, an absolute volume increase of ≥ 200 mL corresponded to RVC from 2.9%-15.7%, depending on preoperative arm volume (Fig 3),32 whereas BCRL defined as a 2-cm increase in the affected arm relative to baseline resulted in an RVC from 6.0%-9.8%.32
FIG 3.
Relative arm volume change corresponding to arm volume increase by 200 mL in the unaffected arm of 677 patients. Reprinted from Ancukiewicz et al,32 by permission from Springer Nature.
Objective Screening Measures
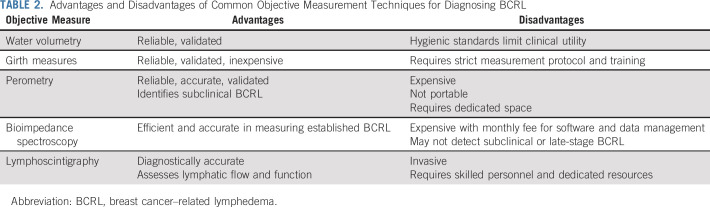
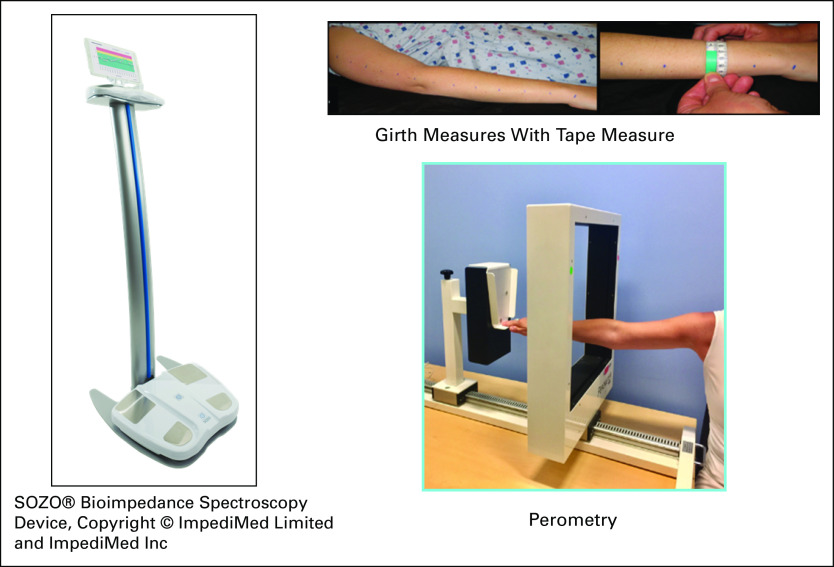
The lack of standardization in measurement has significantly hindered research in BCRL. Several methods of capturing objective data are reported, each with unique strengths and limitations (Table 2 and Fig 4). Limb circumferences taken with a tape measure at regular intervals along the arm may be used to calculate limb volume. Although 4-cm and 10-cm intervals are frequently used, a minimum of 6 anatomic landmarks may also be used.30 Commercially available calculators can aid in calculating total limb volume from girth measures, which may then be entered into the RVC or WAC equations (Table 1). Although time consuming, this method is inexpensive and the most commonly used. However, significant training and practice are required to ensure ongoing reliability of this method.34
TABLE 2.
Advantages and Disadvantages of Common Objective Measurement Techniques for Diagnosing BCRL
FIG 4.
Common objective measurement tools for breast cancer–related lymphedema screening: bioimpedance spectroscopy37 (SOZO Bioimpedance Spectroscopy Device; ImpediMed, Carlsbad, CA). Copyright © ImpediMed Limited and ImpediMed, Perometry, Girth Measures.
A perometer is a reliable, valid, and diagnostically accurate limb volume measurement system consisting of a frame containing infrared lamp-light receiver pairs. The patient sits in an upright position abducting her arm to 90 degrees while the frame is moved along the arm length. Each arm is measured 3 times, which is completed in < 3 minutes.24 Perometry identifies subclinical BCRL.25,35,36 Volumes calculated from perometry are then entered into the WAC or RVC equations (Table 1).
Bioimpedance spectroscopy (BIS) assesses tissue resistance to an electrical current and converts it into a score reflecting interstitial fluid content.37 The newest BIS technology, SOZO (ImpediMed, Carlsbad, CA), takes < 1 minute, with the patient standing on a platform without shoes, socks, or jewelry holding the machine handles.37 Although BIS is effective in detecting established BCRL,37 it may not detect early- or late-stage BCRL when tissues become fibrotic.38,39
Lymphoscintigraphy is a gold standard for BCRL diagnosis, allowing for direct visualization of lymphatic function. Radiotracer injected into the hand or wrist is taken up by the lymphatic vessels and nodes, and single-photon emission computed tomography assesses dermal backflow and lymphatic blockages. Although accurate, lymphoscintigraphy is not feasible for routine screening as a result of cost and logistics.
SYMPTOMS RELATED TO BCRL
Patients suffering from BCRL report lower quality of life than those without BCRL,40 and symptoms may be the earliest predictor of BCRL.41 Armer et al41 found that BCRL was predicted by patient report of “heaviness in past year” and “swelling now.” Fu and Rosedale42 found that, upon interview, patients reported up to 10 symptoms daily classified into the following 4 psychosocial themes: living with perpetual discomfort; confronting the unexpected; losing prelymphedema being; and feeling handicapped. The study also confirmed the nonlinear relationship between type and number of symptoms and limb volume.40
The ASBrS, NCCN, ISL, and NLN26-29 recommend incorporating longitudinal symptom assessment into BCRL screening alongside objective measurements.41 Educating at-risk patients on BCRL symptoms facilitates self-screening, even in the absence of formal screening programs.
CLINICAL EXAM
Examination should include a basic history (considering individual BCRL risk factors, swelling onset, location, inciting factors, and symptoms) and vascular exam. Other potential causes of swelling such as deep vein thrombosis should be ruled out. The Cancer-Related Lymphedema of the Upper Extremity (CLUE) tool was developed and validated to standardize clinical examinations for lymphedema, providing a single score accounting for multiple constructs.43 Subscores include obscuration of anatomic architecture, deviation from normal anatomic contour, tissue texture, and edema (pitting). Swelling in early BCRL (ISL stage 0, I, or early II)29 is pitting, because it is mostly fluid, but in chronic BCRL, swelling becomes fatty and fibrotic, and therefore, there is less pitting.29 Each CLUE subscale is scored from 0-18, with a scoring system for each subscale. This tool showed good intrarater reliability (intraclass correlation coefficient [ICC], 0.88; 95% CI 0.71 to 0.96), good interrater reliability (ICC, 0.90; 95% CI, 0.79 to 0.95), and moderately strong concurrent validity with perometry (Pearson r = 0.79) and subjective measurements (Pearson r = 0.52).43 Of note, patients with subclinical BCRL (ISL stage 0)29 may have minimal to no edema on clinical examination but report symptoms that may or may not progress to BCRL.
DEVELOPING A BCRL SCREENING STRATEGY
Optimal BCRL screening consists of both objective and subjective assessments.24 The objective screening modality used will vary by institution based on resources and workflow. Providers must understand advantages and disadvantages of each potential modality and ensure a strict measurement protocol is consistently followed regardless of the modality used. Baseline measurements of both arms are critical for accurate BCRL diagnosis24; RVC should be used (if using volumetric measures) and screening should continue longitudinally every 6-12 months for a minimum of 2-3 years.
REFERRAL TO A CERTIFIED LYMPHEDEMA THERAPIST
Patients should be referred to a certified lymphedema therapist (CLT) for treatment when RVC from baseline is ≥ 10%,44 when the bioimpedance score changes 7 L-Dex units from baseline using BIS,45 or when patients at risk experience symptoms or focal edema on clinical exam. CLTs may be found through the Lymphology Association of North America,46 the NLN,47 and an international directory through the Vodder School.48
CONSERVATIVE MANAGEMENT
Conservative management consists of a reduction phase and a maintenance phase. The reduction phase aims to decrease limb volume and symptoms through complete decongestive therapy (CDT), which is a combination of manual lymphatic drainage (MLD), multiple layer compression bandaging, exercise, skin care, and patient education. The reduction phase continues for several days per week over several weeks. Once maximal volume and symptom reduction is achieved, treatment shifts to maintenance of limb volume and symptom reduction. Maintenance typically includes a transition from multiple-layer bandaging to compression garments, self-MLD, exercise, and skin care.
Compression alone may be used to prevent swelling progression in patients with subclinical BCRL, and it may reduce limb volume with or without MLD in those with BCRL.49 Advanced BCRL, however, requires CDT.
Intermittent pneumatic compression (IPC) pumps have been used to treat BCRL since the 1950s. The IPC pump and corresponding appliance are placed on the limb, inflating and deflating in pressure gradients, mimicking MLD performed by a CLT. Whether IPC improves BCRL outcomes is unclear.50 IPC may be considered for patients unable to attend clinic regularly for CDT or as an adjunct to CDT. It is not recommended for first-line or stand-alone BCRL treatment.
EXERCISE
Exercise has emerged as a crucial survivorship recommendation after breast cancer treatment.51 Schmitz et al52,53 conducted the Physical Activity and Lymphedema Trial (PAL), a 12-month, randomized controlled trial of twice-per-week weight lifting or standard care in survivors of breast cancer both at risk for and with BCRL. They found that a slowly progressive facility-based weight lifting program decreased BCRL by 35%.52 Patients with BCRL participating in the weight lifting program had fewer BCRL flare-ups and reduced symptoms compared with those in the control group.53 They concluded that an individually prescribed, initially supervised, and slowly progressed aerobic and resistance exercise program does not incite or worsen BRCL.52,53 More recently, the Women in Steady Exercise Research (WISER) trial17 evaluated 351 overweight survivors of BC with BCRL. Patients were randomly assigned to a control group, an exercise group (52 weeks; 2 sessions per week of home-based resistance training and 180 minutes walking per week), a weight loss group (20 weeks of meal replacements, 52 weeks of lifestyle modification counseling), or a combined exercise and weight loss group. The study found no significant differences in BCRL between groups in clinical values or symptoms at 12 months. The authors concluded that the home-based exercise program was not enough to elicit a physiologic effect on BCRL and that a facility-based, supervised, progressive program as in the PAL trial52,53 may be superior to a home-based program for patients with BCRL.
PREVENTION
Precautionary Measures
There are several precautionary recommendations made by the NLN intended to decrease risk of BCRL.54 These guidelines include skin care; preferential avoidance of blood pressure cuffs, venipuncture, and trauma; and wearing a compression garment during air travel.54 These guidelines were developed based on an abundance of caution; however, following these guidelines has not been shown to reduce BCRL risk.19,20,55 In 632 prospectively screened patients, Ferguson et al19 reported that blood pressure readings, blood draws, and air travel were not associated with arm volume increase. Later, the same group reported similar results from 327 patients who underwent bilateral breast cancer surgery.55 Others have reported that in women with ≥ 5 lymph nodes removed, air travel, arm trauma, medical procedures, and arm use did not increase BCRL.56 ASBrS recommendations state the following: “Within the context of an early detection/surveillance program incorporating baseline and follow-up assessments, the routine application of many risk-reducing behaviors is not supported. Use of the ipsilateral arm for IVs or blood pressures is not contraindicated.”57(p2828) This recommendation is echoed by the ISL.29 Nevertheless, at this point, there is no universal agreement on precautionary measure guidelines because this issue is evolving.
Surgery for Lymphedema Prevention
There is increased focus on prophylactic surgical techniques performed at initial axillary surgery intending to prevent BCRL. Axillary reverse mapping (ARM) hypothesizes that blue dye injected into the volar aspect of the upper arm can map upper extremity lymphatic drainage.58 At surgery, the surgeon then seeks to protect the blue lymphatics or nodes.
A systematic review of 8 studies each with at least 50 patients having ARM plus SLNB or ARM plus ALND found that BCRL occurred in 0%-6% of ARM plus SLNB and 5.9%-24% of ARM plus ALND patients.59 Concern remains over the risk of crossover nodes (node draining both breast and upper extremity), which occurs in up to 10% of patients, of whom 0%-20% had metastases in the crossover nodes. In addition, ARM nodes were unable to be preserved in 11%-18% of patients, of whom up to 19% of patients had metastases in the ARM node.59 A recent prospective trial in which the patient and assessor were blinded to the surgical intervention randomly assigned 107 patients needing ALND to ARM or no ARM.60 With 24 months of follow-up, no difference in objective BCRL existed (ALND, 32.3%; ARM plus ALND, 23.5%; P = .43); however, patients who underwent both ARM and ALND had significantly less patient-reported symptomatic BCRL than patients who underwent ALND alone (6.1% v 26.7%, respectively; P = .025).60 The Alliance for Clinical Trials has recently opened a prospective randomized trial (A221702) evaluating SLNB or ALND with and without ARM to formally evaluate ARM.
Yuan et al61 sought to build upon the principles of ARM and upper extremity nodal identification and preservation. They described the Identification and Preservation of Arm and Lymphatic (DEPART) technique. After identification of the ARM node, the ARM node was further injected intraoperatively to map and therefore protect the next echelon of lymph nodes involved in upper extremity lymphatic drainage. Proving feasibility in their technique, they subsequently randomly assigned 1,354 patients needing ALND to DEPART plus ALND or ALND alone. Overall, more ALND patients developed BCRL (defined as > 10% RVC) than those undergoing DEPART plus ALND (15.3% v 3.3%, respectively; P < .001). ARM nodes were not identified in 17% of patients and contained metastases in 6.8%. Regional recurrence rates did not differ between the groups, with both being < 1.4% (P = .39).
Another technique, the Lymphatic Microsurgical Preventative Healing Approach (LYMPHA), seeks to maintain lymphatic flow into the venous system using microsurgical techniques to “dunk” transected axillary lymphatics into a nearby vein with a competent valve. Although fewer patient series have been published affirming this procedure, limited data suggest it may be valuable. In the initial publication, Boccardo et al62 reported BCRL in 30% of patients with ALND without LYMPHA but in only 4% of patients with LYMPHA. More recently, Feldman et al63 found BCRL in substantially more standard ALND patients (50%) than LYMPHA patients (12.5%); however, those in the standard ALND cohort were ineligible for LYMPHA as a result of extensive disease or inability to identify usable lymphatics, suggesting the cohorts may not have been evenly matched. Further study on the role of LYMPHA is needed.
Breast Reconstruction
An emerging body of data indicates that breast reconstruction does not adversely affect the risk of BCRL. Unfortunately, few studies stratify breast reconstruction and BCRL rates by type or timing of reconstruction. In a prospective single-institution series, Miller et al64 analyzed 616 patients undergoing reconstruction. Multivariable analysis suggested implant-based reconstruction may reduce BCRL risk (HR, 0.35; P < .0001).64 Others find that autologous reconstruction may provide more effective BCRL risk reduction; for example, Lee et al65 found the BCRL incidence to be 4.2% after autologous reconstruction and 9.3% after implant reconstruction (HR, 0.39; 95% CI, 0.19 to 0.82; P = .012). A meta-analysis of 19 studies concluded that fewer women with breast reconstruction developed BCRL (odds ratio [OR], 0.66; 95% CI, 0.55 to 0.79; P < .001), with no difference in rates between autologous or implant-based techniques (OR, 0.92; 95% CI, 0.48 to 0.1.77).66 These data collectively support that breast reconstruction is safe and will not adversely influence BCRL risk.
SURGICAL MANAGEMENT OF BCRL
Advances in microsurgical skills and microsurgical technology have reenergized the discussions surrounding surgical intervention for symptomatic and progressive BCRL. Broadly, interventions are classified as reductive (resection of fibrotic lymphatic tissue) or reconstructive (reanastomosis of lymphatic vessels to veins, other lymphatics, or lymph node transfer). Suction-assisted protein lipectomy, the contemporary reductive technique, is effective at removing nearly 100% of excess limb volume in advanced stages of BCRL.67 However, it subsequently mandates strict adherence to lifelong compression therapy to maintain volume reductions because it does not improve lymphatic function. Reconstructive techniques aim to restore lymphatic flow and are predicated on some level of existing residual lymphatic function. As a result, they are more effective in earlier stage I or II BCRL.67 Emerging data are heterogeneous with respect to indications, benefit, follow-up, and outcomes, making standard implementation difficult. It is clear though that, when considered, these patients should be assessed by a multidisciplinary team that has an understanding of BCRL and aftercare where surgery is considered part of a multimodality treatment plan.57
THE FUTURE OF BCRL
Exciting contemporary science postulates alternative inherent risk factors for BCRL, specifically genetic predisposition and biomarker identification. Individual genetic variations may explain why only a percentage of patients undergoing the same locoregional and systemic treatments ultimately develop BCRL. Some variations may afford protection from BCRL, whereas others negatively contribute to risk.68,69 Visser et al70 recently identified 18 genes influencing BCRL risk, adding to the mechanistic theory of BCRL development. Evolving data also suggest that inflammatory,71 immunologic,72,73 and extracellular matrix modulator73 biomarkers may influence BCRL risk. In fact, pilot studies demonstrate promise for oral anti-inflammatory medications such as ketoprofen in lymphedema treatment.74 Hopefully, these data can complement existing clinicopathologic and treatment variables to better inform on risk stratification or future patient selection for therapeutic intervention.
CONCLUSION
The development of BCRL is multifactorial, and modern-day breast cancer physicians must acknowledge the contribution and synergism of individual local, regional, and systemic therapies on BCRL risk. Screening for BCRL should be standard practice, including baseline bilateral objective measurements. Patient education should start at the time of breast cancer diagnosis, and longitudinal screening programs,24 including subjective and objective measures and clinical exam, are imperative for early diagnosis and possible effective management. BCRL as a treatment adverse effect must be considered by the breast cancer community at large.
ACKNOWLEDGMENT
We acknowledge the contribution of Madison Bernstein, Clinical Research Coordinator, Lymphedema Research Program, for her outstanding effort and help in the preparation of this article for submission. We also acknowledge George Naoum, MD, Research Fellow, Department of Radiation Oncology, Massachusetts General Hospital.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
SUPPORT
Supported by Award No. R01CA139118 (A.T.) and Award No. P50CA08393 (A.T.) from the National Cancer Institute. Also supported by the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema (A.T.), the Heinz Family Foundation (A.T.), and the Olayan-Xefos Family Fund for Breast Cancer Research (A.T.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Alphonse Taghian
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Breast Cancer–Related Lymphedema: Risk Factors, Screening, Management, and the Impact of Locoregional Treatment
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Cheryl L. Brunelle
Consulting or Advisory Role: PureTech Health
Alphonse Taghian
Honoraria: UpToDate
Consulting or Advisory Role: Vision RT, Puretech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): A phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaitelman SF, Chiang Y-J, Griffin KD, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: A systematic review and network meta-analysis. Breast Cancer Res Treat. 2017;162:201–215. doi: 10.1007/s10549-016-4089-0. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byun HK, Chang JS, Im SH, et al. Risk of lymphedema following contemporary treatment for breast cancer: An analysis of 7617 consecutive patients from a multidisciplinary perspective. Ann Surg. doi: 10.1097/SLA.0000000000003491. [epub ahead of print on July 23, 2019] [DOI] [PubMed] [Google Scholar]
- 8.Belmonte R, Messaggi-Sartor M, Ferrer M, et al. Prospective study of shoulder strength, shoulder range of motion, and lymphedema in breast cancer patients from pre-surgery to 5 years after ALND or SLNB. Support Care Cancer. 2018;26:3277–3287. doi: 10.1007/s00520-018-4186-1. [DOI] [PubMed] [Google Scholar]
- 9.Wetzig N, Gill PG, Espinoza D, et al. Sentinel-lymph-node-based management or routine axillary clearance? Five-year outcomes of the RACS sentinel node biopsy versus axillary clearance (SNAC) 1 trial: Assessment and incidence of true lymphedema. Ann Surg Oncol. 2017;24:1064–1070. doi: 10.1245/s10434-016-5669-2. [DOI] [PubMed] [Google Scholar]
- 10.Poortmans P, Collette S, Struikmans H, et al. Fifteen-year results of the randomised EORTC trial 22922/10925 investigating internal mammary and medial supraclavicular (IM-MS) lymph node irradiation in stage I-III breast cancer. J Clin Oncol. 36 2018 (suppl 15; abstr 504) [Google Scholar]
- 11.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys. 2014;88:565–571. doi: 10.1016/j.ijrobp.2013.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra RA, Miller CL, Skolny MN, et al. Radiation therapy risk factors for development of lymphedema in patients treated with regional lymph node irradiation for breast cancer. Int J Radiat Oncol Biol Phys. 2015;91:760–764. doi: 10.1016/j.ijrobp.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross J, Sachdev S, Lipps D, et al. Lymphedema risk following regional nodal irradiation in breast cancer: Implications for field arrangement and treatment volume. Int J Radiat Oncol. 2017;99:S5–S6. [Google Scholar]
- 15.Naoum GE, Roberts SA, Shui AM, et al. Quantifying the impact of regional lymph node irradiation on lymphedema risk in breast cancer patients treated with SLNB or ALND: Long-term results from a prospective screening trial. Int J Radiat Oncol. 2019;105:S42. [Google Scholar]
- 16.Jammallo LS, Miller CL, Singer M, et al. Impact of body mass index and weight fluctuation on lymphedema risk in patients treated for breast cancer. Breast Cancer Res Treat. 2013;142:59–67. doi: 10.1007/s10549-013-2715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1001/jamaoncol.2019.2109. Schmitz KH, Troxel AB, Dean LT, et al: Effect of home-based exercise and weight loss programs on breast cancer-related lymphedema outcomes among overweight breast cancer survivors: The WISER survivor randomized clinical trial. JAMA Oncol 10.1001/jamaoncol.2019.2109 [epub ahead of print on August 15, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson CM, Swaroop MN, Horick N, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol. 2016;34:691–698. doi: 10.1200/JCO.2015.61.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asdourian MS, Skolny MN, Brunelle C, et al. Precautions for breast cancer-related lymphoedema: Risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016;17:e392–e405. doi: 10.1016/S1470-2045(16)30204-2. [DOI] [PubMed] [Google Scholar]
- 21.Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: What level of arm volume increase predicts progression? Breast Cancer Res Treat. 2013;140:485–494. doi: 10.1007/s10549-013-2655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilbreath SL, Lee M-J, Refshauge KM, et al. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support Care Cancer. 2013;21:2207–2215. doi: 10.1007/s00520-013-1770-2. [DOI] [PubMed] [Google Scholar]
- 23.McDuff SGR, Mina AI, Brunelle CL, et al. Timing of lymphedema after treatment for breast cancer: When are patients most at risk? Int J Radiat Oncol Biol Phys. 2019;103:62–70. doi: 10.1016/j.ijrobp.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunelle C, Skolny M, Ferguson C, et al. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the Massachusetts General Hospital: Lessons learned. J Pers Med. 2015;5:153–164. doi: 10.3390/jpm5020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin SA, Staley AC, Vicini F, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: Recommendations from a multidisciplinary expert ASBrS panel: Part 1: Definitions, assessments, education, and future directions. Ann Surg Oncol. 2017;24:2818–2826. doi: 10.1245/s10434-017-5982-4. [DOI] [PubMed] [Google Scholar]
- 27. Freedman-Cass D, McMillian N, Baker SK, et al: NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline): Version 3.2017 Survivorship. https://oncolife.com.ua/doc/nccn/Survivorship.pdf.
- 28. National Lymphedema Network: Position paper: The diagnosis and treatment of lymphedema. https://www.lymphnet.org/resources/nln-position-paper-the-diagnosis-and-treatment-of-lymphedema.
- 29. International Society of Lymphology: The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology 46:1-11, 2013. [PubMed] [Google Scholar]
- 30. National Lymphedema Network: Position statement of the National Lymphedema Network: Screening and measurement for early detection of breast cancer-related lymphedema. https://lymphnet.org/position-papers.
- 31.Sun F, Skolny MN, Swaroop MN, et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat. 2016;157:229–240. doi: 10.1007/s10549-016-3821-0. [DOI] [PubMed] [Google Scholar]
- 32.Ancukiewicz M, Miller CL, Skolny MN, et al. Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: The flaws in current studies and need for universal methodology. Breast Cancer Res Treat. 2012;135:145–152. doi: 10.1007/s10549-012-2111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CL, Specht MC, Horick N, et al. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology. 2013;46:64–74. [PubMed] [Google Scholar]
- 34.Tidhar D, Armer JM, Deutscher D, et al. Measurement issues in anthropometric measures of limb volume change in persons at risk for and living with lymphedema: A reliability study. J Pers Med. 2015;5:341–353. doi: 10.3390/jpm5040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanton AWB, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (perometer) Lymphology. 1997;30:77–97. [PubMed] [Google Scholar]
- 36.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ImpediMed: SOZO App and System instructions for use. https://www.impedimed.com.
- 38.Bundred NJ, Stockton C, Keeley V, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat. 2015;151:121–129. doi: 10.1007/s10549-015-3357-8. [DOI] [PubMed] [Google Scholar]
- 39.Seward C, Skolny M, Brunelle C, et al. A comprehensive review of bioimpedance spectroscopy as a diagnostic tool for the detection and measurement of breast cancer-related lymphedema. J Surg Oncol. 2016;114:537–542. doi: 10.1002/jso.24365. [DOI] [PubMed] [Google Scholar]
- 40.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 41.Armer JM, Radina ME, Porock D, et al. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Fu MR, Rosedale M. Breast cancer survivors’ experiences of lymphedema-related symptoms. J Pain Symptom Manage. 2009;38:849–859. doi: 10.1016/j.jpainsymman.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spinelli B, Kallan MJ, Zhang X, et al. Intra- and interrater reliability and concurrent validity of a new tool for assessment of breast cancer-related lymphedema of the upper extremity. Arch Phys Med Rehabil. 2019;100:315–326. doi: 10.1016/j.apmr.2018.08.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3:208–217. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- 45.Ridner SH, Dietrich MS, Spotanski K, et al. A prospective study of L-Dex values in breast cancer patients pretreatment and through 12 months postoperatively. Lymphat Res Biol. 2018;16:435–441. doi: 10.1089/lrb.2017.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lymphology Association of North America: LANA certified therapist listings. https://www.clt-lana.org/search/therapists/.html.
- 47. National Lymphedema Network: Membership directory search. https://ww2.eventrebels.com/er/Membership/OnlineMembershipDirectorySearch.jsp?Token=C3FEFY366SEZAZ6WHNAFM9TMDJ.
- 48. Vodder Schools International: Find a professional. https://www.vodderschool.com/contacts/therapist.
- 49.McNeely ML, Magee DJ, Lees AW, et al. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: A randomized controlled trial. Breast Cancer Res Treat. 2004;86:95–106. doi: 10.1023/B:BREA.0000032978.67677.9f. [DOI] [PubMed] [Google Scholar]
- 50.Shao Y, Qi K, Zhou Q-H, et al. Intermittent pneumatic compression pump for breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trials. Oncol Res Treat. 2014;37:170–174. doi: 10.1159/000360786. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: A randomized trial. JAMA. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 54. National Lymphedema Network: Lymphedema risk reduction practices. https://atlanticlymph.ca/en/wp-content/uploads/2012/09/nlnriskreduction.pdf.
- 55.Asdourian MS, Swaroop MN, Sayegh HE, et al. Association between precautionary behaviors and breast cancer-related lymphedema in patients undergoing bilateral surgery. J Clin Oncol. 2017;35:3934–3941. doi: 10.1200/JCO.2017.73.7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilbreath SL, Refshauge KM, Beith JM, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast. 2016;28:29–36. doi: 10.1016/j.breast.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 57.McLaughlin SA, DeSnyder SM, Klimberg S, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema, recommendations from an expert panel: Part 2: Preventive and therapeutic options. Ann Surg Oncol. 2017;24:2827–2835. doi: 10.1245/s10434-017-5964-6. [DOI] [PubMed] [Google Scholar]
- 58.Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14:1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed M, Rubio IT, Kovacs T, et al. Systematic review of axillary reverse mapping in breast cancer. Br J Surg. 2016;103:170–178. doi: 10.1002/bjs.10041. [DOI] [PubMed] [Google Scholar]
- 60.Luiten EJT, Beek MA, Rubio IT. Clinical utility of axillary reverse mapping (ARM) in an era of changing perceptions concerning axillary surgery. Eur J Surg Oncol. 2016;42:585–587. doi: 10.1016/j.ejso.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Yuan Q, Wu G, Xiao SY, et al. Identification and preservation of arm lymphatic system in axillary dissection for breast cancer to reduce arm lymphedema events: A randomized clinical trial. Ann Surg Oncol. 2019;26:3446–3454. doi: 10.1245/s10434-019-07569-4. [DOI] [PubMed] [Google Scholar]
- 62.Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011;18:2500–2505. doi: 10.1245/s10434-011-1624-4. [DOI] [PubMed] [Google Scholar]
- 63.Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol. 2015;22:3296–3301. doi: 10.1245/s10434-015-4721-y. [DOI] [PubMed] [Google Scholar]
- 64.Miller CL, Colwell AS, Horick N, et al. Immediate implant reconstruction is associated with a reduced risk of lymphedema compared to mastectomy alone: A prospective cohort study. Ann Surg. 2016;263:399–405. doi: 10.1097/SLA.0000000000001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee KT, Bang SI, Pyon JK, et al. Method of breast reconstruction and the development of lymphoedema. Br J Surg. 2017;104:230–237. doi: 10.1002/bjs.10397. [DOI] [PubMed] [Google Scholar]
- 66.Siotos C, Sebai ME, Wan EL, et al. Breast reconstruction and risk of arm lymphedema development: A meta-analysis. J Plast Reconstr Aesthet Surg. 2018;71:807–818. doi: 10.1016/j.bjps.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 67.Granzow JW. Lymphedema surgery: The current state of the art. Clin Exp Metastasis. 2018;35:553–558. doi: 10.1007/s10585-018-9897-7. [DOI] [PubMed] [Google Scholar]
- 68.Rockson SG. A population-based assessment of the problem of lymphatic disease. Lymphat Res Biol. 2008;6:1–2. doi: 10.1089/lrb.2008.6101. [DOI] [PubMed] [Google Scholar]
- 69.Rockson SG. Progress in the approach to lymphatic vascular malformation. Lymphat Res Biol. 2019;17:495. doi: 10.1089/lrb.2019.29071.sr. [DOI] [PubMed] [Google Scholar]
- 70.Visser J, van Geel M, Cornelissen AJM, et al. Breast cancer-related lymphedema and genetic predisposition: A systematic review of the literature. Lymphat Res Biol. 2019;17:288–293. doi: 10.1089/lrb.2017.0083. [DOI] [PubMed] [Google Scholar]
- 71.Zaleska MT, Olszewski WL. Serum immune proteins in limb lymphedema reflecting tissue processes caused by lymph stasis and chronic dermato-lymphangio-adenitis (cellulitis) Lymphat Res Biol. 2017;15:246–251. doi: 10.1089/lrb.2017.0003. [DOI] [PubMed] [Google Scholar]
- 72.Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herrada AA, Mejías C, Lazo-Amador R, et al. Development of new serum biomarkers for early lymphedema detection. Lymphat Res Biol. doi: 10.1089/lrb.2019.0008. 18:136-145, 2020. [DOI] [PubMed] [Google Scholar]
- 74.Rockson SG, Tian W, Jiang X, et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight. 2018;3:3. doi: 10.1172/jci.insight.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]