Abstract
We explored the roles of adenylyl cyclases (ADCYs) in acute myeloid leukemia (AML). Expression ADCYs in AML and their effect on prognosis was analyzed using data from Oncomine, GEPIA and cBioPortal databases. Frequently altered neighbor genes (FANGs) of ADCYs were detected using the 3D Genome Browser, after which the functions of these FANGs were predicted using Metascape tools. Cell viability and apoptosis were assessed using CCK-8 and Annexin V-FITC/PI kits. Expression levels of ADCYs were higher in AML cells lines and in bone marrow-derived mononuclear cells from AML patients than in control cells, and were predictive of a poor prognosis. A total of 58 ADCY FANGs were identified from the topologically associating domains on the basis of the Hi-C data. Functional analysis of these FANGs revealed abnormal activation of the MAPK signaling pathway. Drug sensitivity tests showed that fasudil plus trametinib or sapanisertib had a synergistic effect suppressing AML cell viability and increasing apoptosis. These findings suggest that dysregulation of ADCY expression leads to altered signaling in the MAPK pathway in AML and that the ADCY expression profile may be predictive of prognosis in AML patients.
Keywords: prognostic value, AML, ADCYs factors, MAPK signaling pathway, 3D genome
INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy [1, 2]. Three types of gene mutations are thought to play major roles in the pathogenesis of classical AML. Types I and II mutations are related to cellular proliferation and differentiation, while type III mutations affect genes encoding epigenetic factors involved in the pathogenesis and progression of AML [3].
Adenylyl cyclases (ADCYs) have been attracting increased attention in recent years [4]. These enzymes, which catalyze the generation of cAMP from ATP [5, 6], differ in their responses to upstream regulatory pathways and their distribution, and play essential roles in learning, synaptic plasticity, cardiovascular responses and tumorigenesis [7–10]. The nine members of the ADCY family (ADCY1-ADCY9) exhibit distinct responses to G protein coupled receptors and have been grouped into three subgroups based on their functional activities and sequence homology. Group 1 consists of ADCY1, ADCY3 and ADCY8, which are mainly distributed in neuronal tissues and stimulated by Ca+2/calmodulin [10]. Group 2 contain ADCY2, ADCY4 and ADCY7, which are Ca+2- independent and are stimulated by G proteins [11]. Group 3 includes ADCY5 and ADCY6, which are mainly expressed in heart and brain and are suppressed by G proteins [12]. In addition, there is ADCY9, which exhibits limited expression and is distinct from the other isoforms in that it is not activated by forskolin [13].
Aberrant expression of these isoforms can lead to changes in receptor-mediated activation of ADCYs, as well as alterations in the downstream signaling pathways [14]. However, the clinical impact of abnormal ADCY expression and its prognostic value has rarely been explored [15]. Dysregulated expression of ADCYs has been identified in colorectal cancer (CRC), hepatocellular carcinoma (HCC), prostate cancer, pancreatic cancer and cervical cancer [11, 16–18]. However, the mechanism underlying the abnormal ADCY expression seen in hematopoietic malignancies and their functional analysis has not yet been fully elucidated.
In the present study, we used bioinformatics analysis with online public data to explore possible functions of ADCYs and their unique prognostic value in AML. We also examined the regulation of ADCY-related pathways as potential targets for therapeutic intervention.
RESULTS
Transcription of ADCYs in AML
We initially used Oncomine databases to compare ADCY expression in various tumors and in controls (Figure 1). We found that ADCYs were highly expressed in cases of kidney tumor and leukemia. In Stegmaier’s dataset, a 3.002-fold increase in ADCY1 expression was detected in AML as compared to control tissues. Similar increases in ADCY1 expression in AML were also seen in Haferlach’s dataset (4.766-fold), Valk’a Leukemia Statistics (1.503-fold), and Andersson’s Leukemia Statistics (1.298-fold) (Table 1). Elevated ADCY2 expression (1.034-fold) was also detected in AML patients vs. control samples. Consistent with that finding, Valk et al.’s data showed that ADCY2 was upregulated 1.823-fold in AML, while Stegmaier et al. showed it to be upregulated 1.8331-fold. In addition, ADCY3 expression was upregulated 1.312-fold in AML vs. control, and ADCY5 and ADCY6 expression was upregulated 1.025-fold and 1.105-fold, respectively. In Haferlach’s dataset, ADCY9 expression increased 1.292-fold in AML, while Valk Leukemia Statistics showed a 1.137-fold increase (Table 1).
Figure 1.
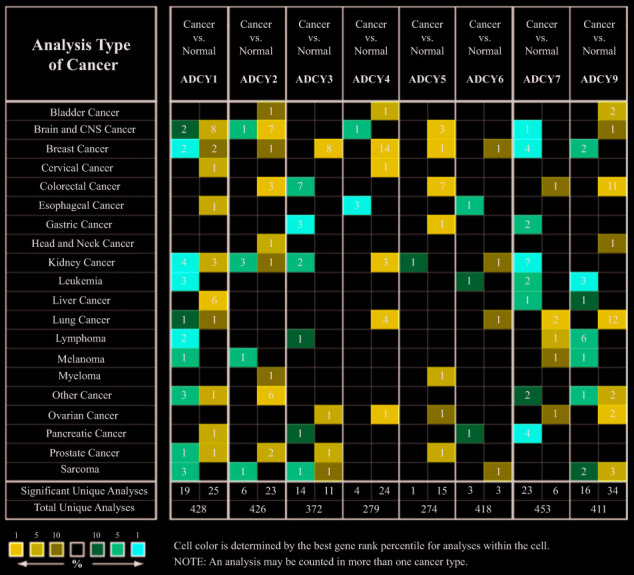
Transcription of ADCYs within tumors (Oncomine).
Table 1. The significant transcriptional changes of ADCYs in AML(Oncomine).
| Fold Change | p Value | t Test | Source and/or Reference | |
| ADCY1 | 3.002 | 0.001 | 3.917 | Stegmaier Leukemia Statistics |
| 1.503 | 0.029 | 2.243 | Valk Leukemia Statistics | |
| 4.766 | 0.00000244 | 1.05 | Haferlach Leukemia Statistics | |
| 1.298 | 0.048 | 1.806 | Andersson Leukemia Statistics | |
| ADCY2 | 1.034 | 0.009 | 2.522 | Haferlach Leukemia Statistics |
| 1.823 | 0.009 | 2.894 | Valk Leukemia Statistics | |
| 1.831 | 0.046 | 1.664 | Stegmaier Leukemia Statistics | |
| ADCY3 | 1.312 | 2.73E-27 | 12.339 | Haferlach Leukemia Statistics |
| 1.223 | 0.036 | 2.085 | Valk Leukemia Statistics | |
| ADCY4 | NA | NA | NA | NA |
| ADCY5 | 1.025 | 0.000799 | 3.24 | Haferlach Leukemia Statistics |
| ADCY6 | 1.105 | 0.00018 | 3.678 | Haferlach Leukemia Statistics |
| ADCY7 | NA | NA | NA | NA |
| ADCY9 | 1.292 | 0.0000000000917 | 6.758 | Haferlach Leukemia Statistics |
| 1.137 | 0.022 | 2.27 | Valk Leukemia Statistics |
NA, not available.
Prognosis analysis of ADCYs in AML
Expression levels of ADCYs between AML and matched control data from The Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx) databases were compared on the basis of the Gene Expression Profiling Interactive Analysis (GEPIA) dataset (http://gepia.cancer-pku.cn/). These results showed that the expression of genes for all 9 ADCY isoforms was higher in AML samples than in control samples (Figure 2A, 2B). In addition, the association between ADCY expression and AML patient survival was explored using the LinkedOmics website (http://www.linkedomics.org/login.php). Using Kaplan-Meier analysis, we found that increased mRNA expression of ADCY2 (P = 0.03994), ADCY3 (P = 0.01924), ADCY4 (P = 0.02211), and ADCY7 (P = 0.01772) were significantly associated with poor overall survival (OS) in AML patients (Figure 3A). In addition, decreased levels of ADCY9 mRNA tended to indicate a poorer prognosis (P=0.078), though the effect was not statistically significant. Analysis of the GEPIA dataset revealed that median OS was shorter in AML patients showing higher expression of ADCY2, 3, 4, and 7 (Figure 3B).
Figure 2.
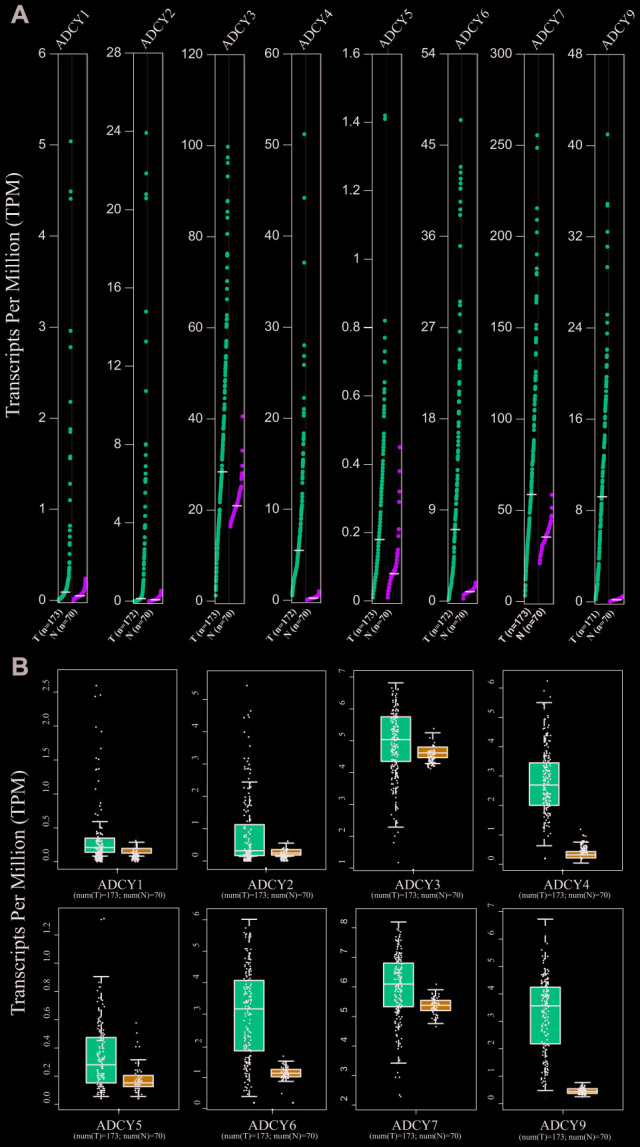
Expression of ADCYs in AML and normal samples (GEPIA). (A) The expression levels of ADCYs in AML compared with normal samples. (B) The TPM values of ADCYs in AML and normal samples. T represents AML samples and N represents normal samples.
Figure 3.
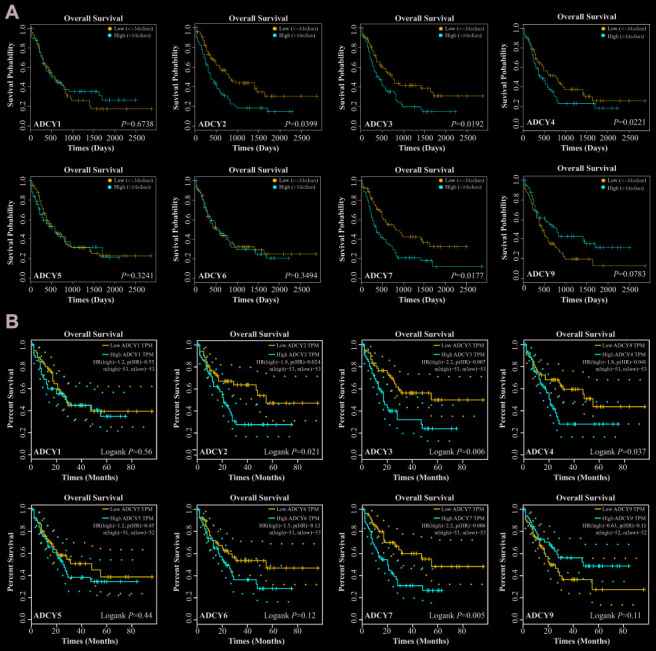
Significance of ADCYs in predicting the prognosis for AML patients. (A) The prognostic values of ADCYs in LinkedOmics datasets. (B) The prognostic values of ADCYs in GEPIA.
We next used TCGA datasets to analyze the relationship between the common genetic and epigenetic mutations and ADCY expression. The samples were divided into two groups based on their mutations in AXSL1, CEBPA, FLT3, IDH1, IDH2, KIT, MLL, NPM1, RAS, TET2 and WT1 and then compared ADCY expression levels between the wild type (WT) and mutation (MUT) groups (Supplementary Table 2). We detected greater ADCY1 expression in the CEBPA WT group than the MUT group (p=0.005), whereas expression of ADCY9 was higher in the MUT group (p<0.001). For NPM1, expression levels of ADCY1 (p=0.02), ADCY2 (p<0.001) and ADCY4 (p=0.009) were much higher in MUT than WT group, while expression of ADCY3 (p<0.001) and ADCY6 (p=0.006) was higher in WT group. There were also scattered differences among these mutations. For example, expression of ADCY2 was higher in the FLT3 MUT group (p<0.001), and ADCY4 was more highly expressed in the IDH1 and IDH2 MUT groups (p=0.019 and p<0.001 respectively). Analysis of the relationships between the three subgroups of ADCY isoforms and these common mutations revealed no significant correlation with gene mutations (Supplementary Table 2). We therefore suggest that there is little correlation between these gene mutations in AML.
Enrichment analysis of the frequently altered neighbor genes (FANGs) of ADCYs in AML
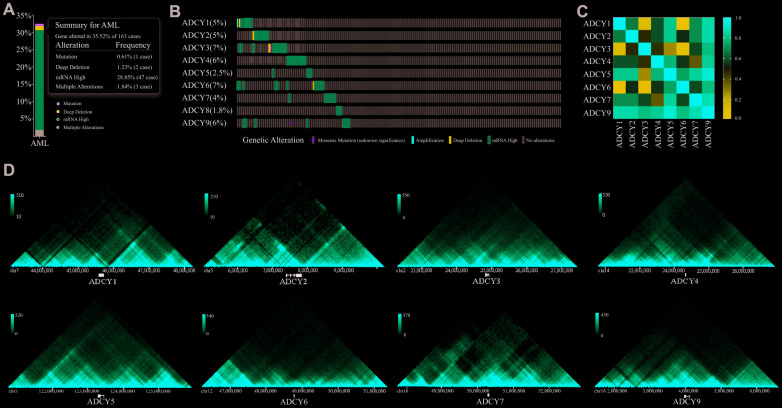
We investigated ADCY alterations, gene relevance, and the interaction networks using the cBioPortal database for AML (TCGA Provisional; http://www.linkedomics.org/admin.php). ADCY alterations were detected in 32.52% of 163 AML patients (Figure 4A, 4B). In addition, exploration of the relevance of ADCYs showed the potential for positive gene relevance among the ADCY family (Figure 4C).
Figure 4.
Visual summary of ADCY alterations. (A) Summary of ADCY alterations in AML (cBioPortal). (B) Details of ADCY alterations in AML (cBioPortal). (C) Gene relevance analysis among the ADCY family. (D) 3D genome of ADCYs in THP1 cells.
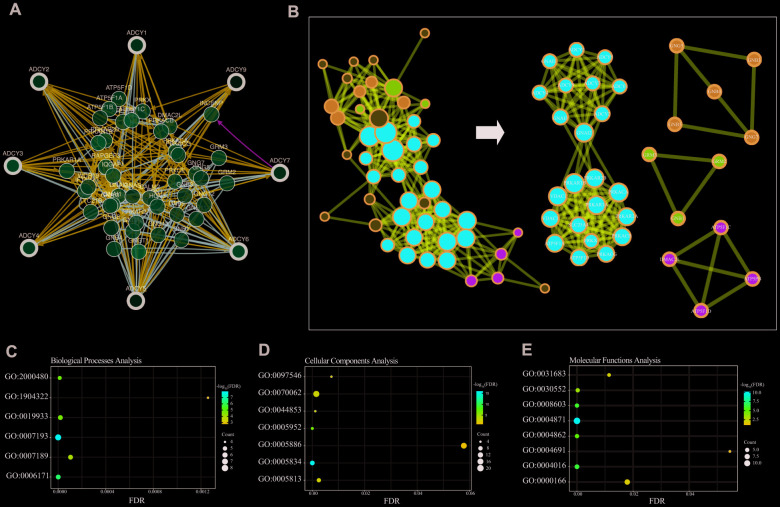
We then constructed a network for ADCYs using the 3D Genome Browser (http://3dgenome.org). This enabled us to simultaneously examine the gene regulatory events and the 3D genomic organization. Topologically associating domains (TADs) of ADCYs were identified through analysis of Hi-C data from THP1 cells, which enables us to predict the potential target genes (Figure 4D). The top 58 FANGs were screened in combination with the related ADCY genes reported in the cBioPortal dataset (Figure 5A). The results showed that genes involved in energy metabolism, including ATP5F1A, ATP5F1B, CMPK1, GNAI1 and GNAI2, were closely associated with ADCYs (Supplementary Table 1). In addition, Figure 5B shows the protein-protein interactome network determined using Metascape (http://metascape.org/gp/index.html). The neighborhood protein network, where proteins were densely connected, was identified using the MCODE algorithm.
Figure 5.
Enrichment analysis of ADCY FANGs in AML patients. (A) Network for ADCYs and the top 58 FANGs (cBioPortal). (B) Detailed net-structure of ADCY proteins in AML (Metascape). Bubble diagrams showing the top 58 FANGs in AML. (C) Biological processes. (D) Cellular components. (E) Molecular functions.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/summary.jsp) was used to predict potential functional pathways of ADCYs as well as their associated genes. Biological processes analysis showed that the cAMP-mediated signaling (GO: 0019933), the cAMP biosynthetic process (GO: 0006171), and the adenylate cyclase – activating/inhibiting G-protein coupled receptor signaling pathway (GO: 0007193 and 0007189) were significantly regulated by ADCYs in AML (Figure 5C). Using molecular functions analysis, we also found that cAMP-dependent protein kinase inhibitor/regulator activity (GO: 0008603, 0004862 and 0004691), cAMP binding (GO:0030552), and G-protein beta/gamma-subunit complex binding (GO: 0031683) were highly enriched in the ADCYs in AML (Figure 5E). In addition, cellular components analysis showed that plasma membrane/membrane raft (GO: 0005886 and 0044853), heterotrimeric G-protein complex (GO: 00058334), and cAMP-dependent protein kinase complex (GO: 0005952) were also significantly regulated by ADCYs (Figure 5D). Most of these were genes known to be associated with energy metabolism.
Predicted therapeutic targets for ADCYs in AML
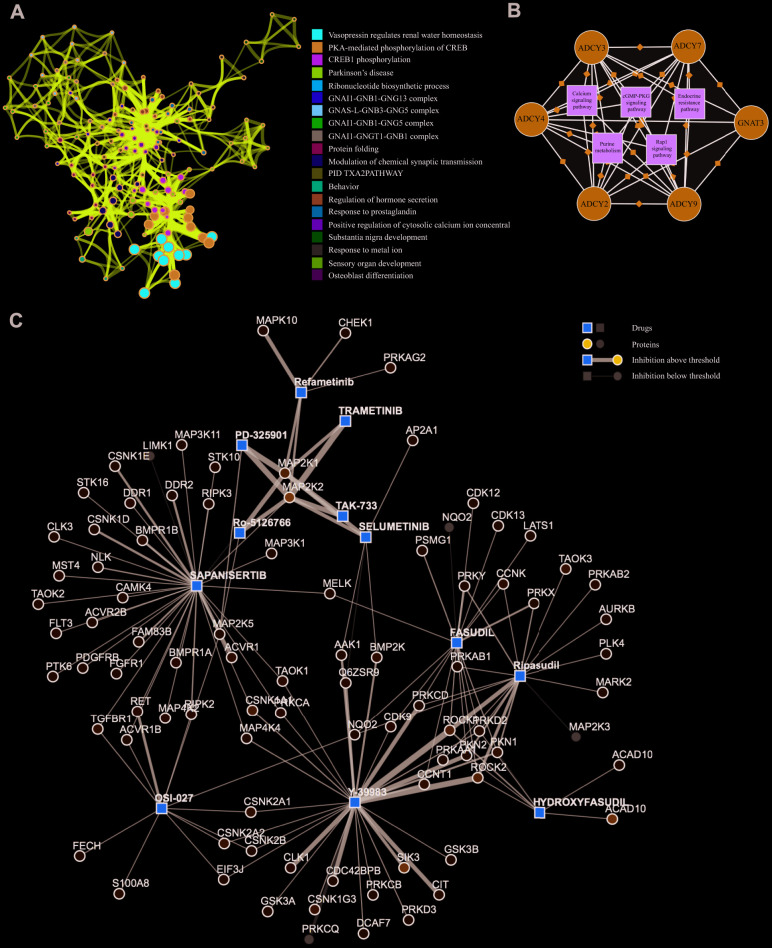
Significant terms among the gene membership profiles were identified using the Metascape tools and then hierarchically divided into a tree based on Kappa-statistical resemblance (Figure 6A). Next, Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were carried out to examine the pathways related to ADCY and FANG functions using the proteomicsdb dataset (https://www.proteomicsdb.org/). We found these genes were enriched in six important pathways related to AML leukemogenesis: endocrine resistance pathway, purine metabolism pathway, the Calcium signal transduction pathway, the MAPK signaling pathway, the cGMP-PKG signal transduction pathway, and the Rap1 signal transduction pathway (Figure 6B). Among those, the MAPK signaling pathway was found to be critical. The protein-drug interaction map suggested that MAPK signaling pathway inhibitors, including ROCK inhibitors (Fasudil, Y-39983 and Ripasudil), MEK inhibitors (Trametinib, TAK-733, Selumetinib, Ro-5126766, Refametinib, Pimasertib and PD-325901), and mTOR inhibitors (Sapanisertib, OSI-027) were associated with a tightly connected network that could potentially mediate therapeutic effects in AML (Figure 6C).
Figure 6.
KEGG Enrichment and therapeutic targets of ADCYs in AML. (A) Significant terms among the gene memberships (Metascape). (B) KEGG analysis of FANGs in AML. (C) Protein-drug interaction map for inhibitors of the MAPK pathway in AML.
Sensitivity effect and synergistic effect of inhibitors in AML
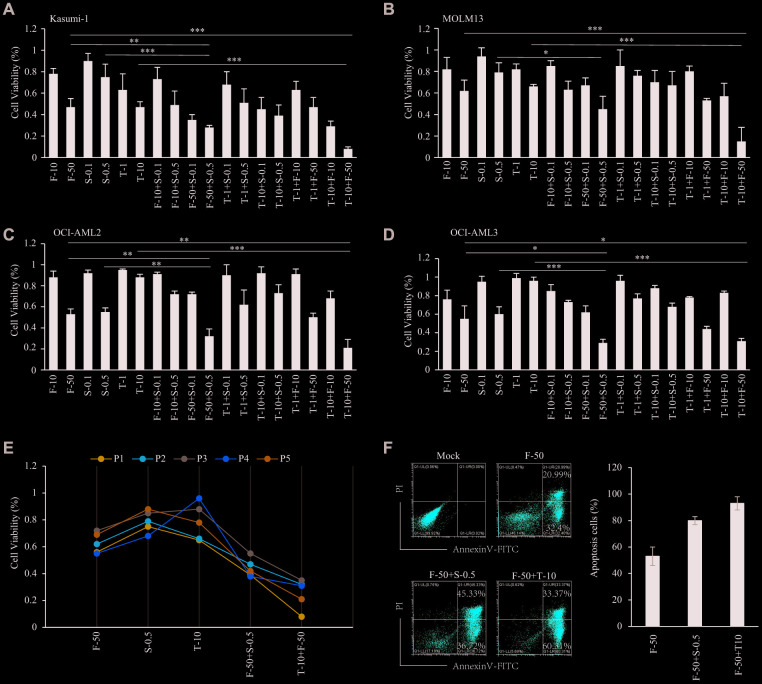
From among the abovementioned drugs, we chose three inhibitors that are currently in clinical trials and assessed their effects on the viability of cells from four AML cell lines. Using fasudil (ROCK inhibitor), trametinib (MEK inhibitor) and sapanisertib (mTOR inhibitor) with Kasumi-1, MOLM13, OCI-AML3 and OCI-AML2 cells, we found that all cells were sensitive to 50 μM fasudil, with about 50-60% of cells remaining viable after treatment for 48 h. Kasumi-1 cells were the most sensitive to trametinib (10 nM), with 50% of cells viable after 48 h (Figure 7A), and MOLM13 cells also showed sensitivity, with 60-70% remaining viable after 48 h (Figure 7B). By contrast, these drugs elicited no reduction in OCI-AML3 and OCI AML2 cell viability. However, both OCI-AML2 and OCI-AML3 cells were sensitive to 500 nM sapanisertib, with only 50-60% of cells remaining viable after 48 h (Figure 7C, 7D), which was consistent with previous reports [19]. Kasumi-1 and MOLM13 cells were less sensitive to 500 nM sapanisertib, with about 80% of cells remaining viable after 48 h. The IC50 of inhibitors in these AML cell lines were displayed in Table 2. Notably, AML cell viabilities could be significantly decreased by combining fasudil with trametinib or sapanisertib. The synergistic effect of fasudil plus trametinib was somewhat greater than fasudil plus sapanisertib. On the other hand, no synergistic effect on AML cell viability was seen with trametinib plus sapanisertib (Table 2).
Figure 7.
Sensitivity effect of inhibitors in AML. (A–D) Sensitivity effect of fasudil, trametinib and sapanisertib in AML cell lines of Kasumi-1 (A), MOLM13 (B), OCI-AML2 (C) and OCI-AML3 (D). (E) Sensitivity effect of inhibitors in AML patient samples. (F) Apoptosis induced by inhibitors among AML patient cells.
Table 2. IC50 and combination index values in AML cell lines.
| Treatment | Kasumi-1 | MOLM13 | OCI-AML2 | OCI-AML3 |
| Fasudil (IC50) | 37.8μM | 53.1μM | 47.9μM | 52.2μM |
| Sapanisertib (IC50) | 2.1μM | 1.3μM | 0.4μM | 0.6μM |
| Trametinib (IC50) | 3.8nM | 8.5nM | 19.7nM | 14.2nM |
| F-50μM + S-0.5μM (Combination index) | 0.65 | 0.81 | 0.71 | 0.68 |
| T-10nM + S-0.5μM (Combination index) | 0.86 | 0.93 | 1.06 | 1.03 |
| T-10nM + F-50μM (Combination index) | 0.48 | 0.34 | 0.27 | 0.22 |
Given the ability of fasudil, trametinib and sapanisertib to reduce the viability in AML cell lines, we next sought to validate the effects of these inhibitors using mononuclear cells isolated from the bone marrow of primary AML patients. Consistent with the results from cell lines, patient cells treated with fasudil and trametinib or sapanisertib were less viable than cells treated with a single inhibitor. The synergistic efficiency of fasudil plus trametinib was greater than that of fasudil plus sapanisertib (Figure 7E). Consistent with those results, annexin V and PI staining showed that single inhibitors induced apoptosis among AML patient cells and that this effect was enhanced by treatment with fasudil plus trametinib or sapanisertib (Figure 7F).
DISCUSSION
Dysregulation of ADCY expression has been reported in various solid cancers [11, 13, 15, 17], but the role of ADCYs in the development and progression of those cancers remains unclear, and bioinformatics analysis in AML is still lacking [20]. To explore the potential functions of ADCYs and their regulatory network in AML, we used the data published online to gain further insight into AML. Examination of both the Oncomine and TCGA datasets revealed that levels of ADCY expression are higher in AML patients than in control subjects. Additionally, we found that ADCYs may have prognostic values in AML, and that among the ADCY, high expression of group 1 (ADCY3, not ADCY1) and group 2 (ADCY2, ADCY4 and ADCY7) was associated with a poorer prognosis for all AML samples. Among the ADCY gene family members, ADCY1 is the most extensively investigated in solid cancers, where it is highly expressed in cancer tissues and related to a poor prognosis [10]. By contrast, ADCY1 expression did not affect the prognosis of AML patients, suggesting the actions of group 2 adenylyl cyclases (ADCY2, ADCY4 and ADCY7) in AML differ from those in other cancers.
Functional analysis suggested that cAMP may affect extracellular signaling in several tumors [12]. In the present study, the 3D genome organization of the THP1 leukemic cell line was explored using Hi-C technology. A total of 58 FANGs that were the most frequently altered in hematopoietic malignances were selected for further cluster analysis. Our results suggest that the MAPK signaling pathway is central among the six pathways involved in the leukemogenesis of AML. Furthermore, construction of a protein-drug interaction map revealed possible therapeutic strategies for treatment of AML using ROCK, MEK and mTOR inhibition to alter signaling in the MAPK pathway.
Smoking is thought to be a major risk factor for AML in older adults and childhood leukemia [21, 22]. We therefore sought to investigate the relationship between smoking and ADCY expression in AML [23]. However, information about smoking status is not available in either TCGA or the beatAML database [24]. We did find a case-control study that mentions the relationship between clonal hemopoiesis, therapy related myeloid malignancies, and smoking status [25]; unfortunately, those investigators did not perform RNA-seq or gene array analyses with these patients. Nonetheless, our findings are insufficient to shed light on the relationship between the ADCY expression profile and prognosis in patients with AML.
CONCLUSIONS
Our findings suggest dysregulation of ADCY expression leads to altered signaling in the MAPK pathway in AML, and that the ADCY expression profile may be predictive of prognosis in AML patients.
MATERIALS AND METHODS
Patient samples
Between 2019 and 2020, a total of 5 AML patients newly diagnosed at the Sun Yat-sen University Cancer Center were enrolled in this study. All participants provided written informed consent in accordance with the regulations of the Institutional Review Boards of the Hospitals in agreement with the Declaration of Helsinki.
Reagents
Inhibitors fasudil (S1573), trametinib (S2673) and sapanisertib (S2811) were obtained from Selleck Chemicals LLC (Houston, TX).
Cell viability and apoptosis assay
AML cell lines (Kasumi-1, OCI-AML3, OCI-AML2 and MOLM13) were obtained from Da-Wei Wang (Ruijin hospital) and Da-Jun Yang’s lab (Sun Yat-sen University Cancer Center) and cultured in RPMI-1640 (Gibco, NY) supplemented with 10% fetal bovine serum (Biochrom AG, Berlin, Germany). Cells were seeded into 96-well plates at a density of 105 cells/well. To determine the cytotoxicity of the inhibitors tested, cells were separately incubated with appropriate concentrations of each inhibitor. After 48 h, 10 μL of reagent from a Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) were added to each well. The samples were then incubated for an additional 4 to 6 h, at 37°C and the absorbance at 450 nm was measured using a spectrophotometer. An Annexin V FITC/PI staining kit (FA111-02, Transgen Biotech) was used to detect apoptotic cells induced by the inhibitors.
Oncomine analysis
The Oncomine website (https://www.oncomine.org/re-source/login.html) was utilized to obtain ADCY gene expression data from cancer and control samples. ADCY expression was compared between clinical cancer specimens and paired normal controls using Student’s t test.
GEPIA dataset
Thousands of normal and tumor specimens collected from the GTEx and TCGA were enrolled in the GEPIA dataset. Functional analyses, such as survival analysis and gene correlation analysis, were also carried out using this dataset.
The c-BioPortal analysis
The cBioPortal (http://cbioportal.org) is an open-access website that contains over 225 tumor genomics datasets. ADCY alterations in the LAML samples were analyzed using this website. Genetic information, including mutations, gene splicing and copy number variations (CNVs), was also found in this dataset. The neighboring genes were excluded unless the frequencies were > 20%.
Metascape analysis and the LinkeOmics dataset
Metascape provides a comprehensive gene function analysis. In this dataset, the MCODE algorithm was applied to identify the interacting network components and densely-related complexes, while Cytoscape is used to generate visualizations of these networks. LinkedOmics is also a publicly available portal that includes TCGA Cancer types. The relevance of differentially expressed genes among ADCYs was investigated in this dataset.
3D genome browser analysis
The Hi-C data from THP1 cells were analyzed using the 3D Genome Browser. In addition, TADs were identified to screen for potentially interacting genes in THP1 cells.
The proteomicsDB dataset
The potential drug network was analyzed using the ProteomicsDB dataset. Selected from this website were MAPK pathway inhibitors, which exhibited the networks between the FANGs of ADCYs factors and the potential MAPK signaling pathway inhibitors.
Combination index analysis
CompuSyn software (version 1.0; ComboSyn, Inc.Paramus, NJ, USA) were used to calculate the combination indexes according to the average fraction of viable cells in the cytotoxicity assays [26, 27]. The Combinatorial effects were classified into 4 parts: strong synergism for CI= 0.1–0.3; distinct synergism for CI = 0.3–0.7, mild synergism for CI = 0.7–0.9; additive for CI = 0.9–1.1.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of our colleagues at the Sun Yat-Sen University Cancer Center for constructive discussions and technical help.
Abbreviations
- AML
Acute Myeloid Leukemia
- ADCYs
Adenylyl Cyclases
- FANGs
Frequently Altered Neighbor Genes
- TADs
Topologically Associating Domains
- CRC
Colorectal Cancer
- HCC
Hepatocellular Carcinoma
- TCGA
The Cancer Genome Atlas
- GTEx
Genotype-Tissue Expression
- GEPIA
Gene Expression Profiling Interactive Analysis
- OS
Overall Survival
- DEGS
Differentially Expressed Genes
- KEGG
Kyoto Encyclopedia of Genes and Genomes
Footnotes
AUTHOR CONTRIBUTIONS: Si-Liang Chen, Fang Hu and Da-Wei Wang performed research,; Yu-Jun Dai, Fang Hu, Ze-Yuan Qin and Si-Liang Chen performed bioinformatics analyses,; Yu-Jun Dai, and Yang Liang designed the overall research and wrote the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FUNDING: This work was supported by Cancer Center (Contracted Research Professor, One Hundred Talents Recruitment Program) Leaders in Disciplines with Talent Shortage, No. 201603; Contract for the Introduction of Innovation and Entrepreneurship Team in “Pearl River Talent Plan” of Guangdong Province 2017ZT07S096; National Natural Science Foundation of China (NSFC Grant No. 81873428 and 81660682).
REFERENCES
- 1.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013; 381:484–95. 10.1016/S0140-6736(12)61727-9 [DOI] [PubMed] [Google Scholar]
- 2.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, McLeod JL, Doedens M, Medeiros JJ, et al. , and HALT Pan-Leukemia Gene Panel Consortium. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014; 506:328–33. 10.1038/nature13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012; 366:1079–89. 10.1056/NEJMoa1112304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel TB, Du Z, Pierre S, Cartin L, Scholich K. Molecular biological approaches to unravel adenylyl cyclase signaling and function. Gene. 2001; 269:13–25. 10.1016/s0378-1119(01)00448-6 [DOI] [PubMed] [Google Scholar]
- 5.Ludwig MG, Seuwen K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct Res. 2002; 22:79–110. 10.1081/rrs-120014589 [DOI] [PubMed] [Google Scholar]
- 6.Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F, Riteau N, Couillin I. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015; 6:e1629. 10.1038/cddis.2014.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodges GJ, Gros R, Hegele RA, Van Uum S, Shoemaker JK, Feldman RD. Increased blood pressure and hyperdynamic cardiovascular responses in carriers of a common hyperfunctional variant of adenylyl cyclase 6. J Pharmacol Exp Ther. 2010; 335:451–57. 10.1124/jpet.110.172700 [DOI] [PubMed] [Google Scholar]
- 8.Caputo L, Souza LF, Alloisio S, Cornara L, De Feo V. Coriandrum sativum and lavandula angustifolia essential oils: chemical composition and activity on central nervous system. Int J Mol Sci. 2016; 17:1999. 10.3390/ijms17121999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy. 2013; 9:2033–45. 10.4161/auto.26336 [DOI] [PubMed] [Google Scholar]
- 10.Sethna F, Feng W, Ding Q, Robison AJ, Feng Y, Wang H. Enhanced expression of ADCY1 underlies aberrant neuronal signalling and behaviour in a syndromic autism model. Nat Commun. 2017; 8:14359. 10.1038/ncomms14359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masugi Y, Tanese K, Emoto K, Yamazaki K, Effendi K, Funakoshi T, Mori M, Sakamoto M. Overexpression of adenylate cyclase-associated protein 2 is a novel prognostic marker in Malignant melanoma. Pathol Int. 2015; 65:627–34. 10.1111/pin.12351 [DOI] [PubMed] [Google Scholar]
- 12.Motiani RK, Tanwar J, Raja DA, Vashisht A, Khanna S, Sharma S, Srivastava S, Sivasubbu S, Natarajan VT, Gokhale RS. STIM1 activation of adenylyl cyclase 6 connects ca2+ and cAMP signaling during melanogenesis. EMBO J. 2018; 37:e97597. 10.15252/embj.201797597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rautureau Y, Deschambault V, Higgins MÈ, Rivas D, Mecteau M, Geoffroy P, Miquel G, Uy K, Sanchez R, Lavoie V, Brand G, Nault A, Williams PM, et al. ADCY9 (adenylate cyclase type 9) inactivation protects from atherosclerosis only in the absence of CETP (cholesteryl ester transfer protein). Circulation. 2018; 138:1677–92. 10.1161/CIRCULATIONAHA.117.031134 [DOI] [PubMed] [Google Scholar]
- 14.Rossi ÁD, Faucz FR, Melo A, Pezzuto P, de Azevedo GS, Schamber-Reis BL, Tavares JS, Mattapallil JJ, Tanuri A, Aguiar RS, Cardoso CC, Stratakis CA. Variations in maternal adenylate cyclase genes are associated with congenital zika syndrome in a cohort from northeast, Brazil. J Intern Med. 2019; 285:215–22. 10.1111/joim.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Xie J, Lu Z, Chen C, Yin Y, Zhan R, Fang Y, Hu X, Zhang CC. ADCY7 supports development of acute myeloid leukemia. Biochem Biophys Res Commun. 2015; 465:47–52. 10.1016/j.bbrc.2015.07.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pernicová Z, Slabáková E, Fedr R, Šimečková Š, Jaroš J, Suchánková T, Bouchal J, Kharaishvili G, Král M, Kozubík A, Souček K. The role of high cell density in the promotion of neuroendocrine transdifferentiation of prostate cancer cells. Mol Cancer. 2014; 13:113. 10.1186/1476-4598-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y, Zheng S. SPARCL1, Shp2, MSH2, e-cadherin, p53, ADCY-2 and MAPK are prognosis-related in colorectal cancer. World J Gastroenterol. 2011; 17:2028–36. 10.3748/wjg.v17.i15.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, Piano S, Fasolato S, Rosi S, Gatta A, Angeli P. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013; 57:266–76. 10.1002/hep.26021 [DOI] [PubMed] [Google Scholar]
- 19.Dai YJ, Wang YY, Huang JY, Xia L, Shi XD, Xu J, Lu J, Su XB, Yang Y, Zhang WN, Wang PP, Wu SF, Huang T, et al. Conditional knockin of Dnmt3a R878H initiates acute myeloid leukemia with mTOR pathway involvement. Proc Natl Acad Sci USA. 2017; 114:5237–42. 10.1073/pnas.1703476114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun CC, Li SJ, Hu W, Zhang J, Zhou Q, Liu C, Li LL, Songyang YY, Zhang F, Chen ZL, Li G, Bi ZY, Bi YY, et al. Comprehensive analysis of the expression and prognosis for E2Fs in human breast cancer. Mol Ther. 2019; 27:1153–65. 10.1016/j.ymthe.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chelghoum Y, Danaïla C, Belhabri A, Charrin C, Le QH, Michallet M, Fiere D, Thomas X. Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002; 13:1621–27. 10.1093/annonc/mdf269 [DOI] [PubMed] [Google Scholar]
- 22.Fircanis S, Merriam P, Khan N, Castillo JJ. The relation between cigarette smoking and risk of acute myeloid leukemia: an updated meta-analysis of epidemiological studies. Am J Hematol. 2014; 89:E125–32. 10.1002/ajh.23744 [DOI] [PubMed] [Google Scholar]
- 23.Hope BT, Nagarkar D, Leonard S, Wise RA. Long-term upregulation of protein kinase a and adenylate cyclase levels in human smokers. J Neurosci. 2007; 27:1964–72. 10.1523/JNEUROSCI.3661-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, Long N, Schultz AR, Traer E, Abel M, Agarwal A, Blucher A, Borate U, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018; 562:526–31. 10.1038/s41586-018-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillis NK, Ball M, Zhang Q, Ma Z, Zhao Y, Yoder SJ, Balasis ME, Mesa TE, Sallman DA, Lancet JE, Komrokji RS, List AF, McLeod HL, et al. Clonal haemopoiesis and therapy-related myeloid Malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol. 2017; 18:112–21. 10.1016/S1470-2045(16)30627-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008; 49:2059–80. 10.1080/10428190802353591 [DOI] [PubMed] [Google Scholar]
- 27.Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res. 2010; 70:440–46. 10.1158/0008-5472.CAN-09-1947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.