Abstract
Age-related declines in physical performance predict cognitive impairment, disability, chronic disease exacerbation, and mortality. We conducted a metabolome-wide association study of physical performance among Bogalusa Heart Study participants. Bonferroni corrected multivariate-adjusted linear regression was employed to examine cross-sectional associations between single metabolites and baseline gait speed (N=1,227) and grip strength (N=1,164). In a sub-sample of participants with repeated assessments of gait speed (N=282) and grip strength (N=201), significant metabolites from the cross-sectional analyses were tested for association with change in physical performance over 2.9 years of follow-up. Thirty-five and seven metabolites associated with baseline gait speed and grip strength respectively, including six metabolites that associated with both phenotypes. Three metabolites associated with preservation or improvement in gait speed over follow-up, including: sphingomyelin (40:2) (P=2.6×10-4) and behenoyl sphingomyelin (d18:1/22:0) and ergothioneine (both P<0.05). Seven metabolites associated with declines in gait speed, including: 1-carboxyethylphenylalanine (P=8.8×10-5), and N-acetylaspartate, N-formylmethionine, S-adenosylhomocysteine, N-acetylneuraminate, N2,N2-dimethylguanosine, and gamma-glutamylphenylalanine (all P<0.05). Two metabolite modules reflecting sphingolipid and bile acid metabolism associated with physical performance (minimum P=7.6×10-4). These results add to the accumulating evidence suggesting an important role of the human metabolome in physical performance and specifically implicate lipid, nucleotide, and amino acid metabolism in early physical performance decline.
Keywords: physical performance, biomarkers, gait, successful aging, grip strength
INTRODUCTION
Age related declines in physical performance are common among older adults [1] and robustly predict frailty, sarcopenia, disability, fracture, falls, cognitive impairment, reduced quality of life, comorbid chronic health conditions, and all-cause mortality [1–3]. Gait speed and hand grip strength are simple and non-invasive measures of physical performance in aging adults. Although reduced gait speed and grip strength have been established as risk factors for adverse health outcomes, their underlying biological pathways remain largely unknown. Examination of the human metabolome, which reflects endogenous and exogenous processes and their interactions [4], provides a unique opportunity to identify small molecule biomarkers of physical performance. Identified metabolites may serve as clinically relevant biomarkers and prognostic indicators of future physical performance decline.
Metabolomics has previously been used to examine aging and frailty. A previous study that compared centenarians to elderly individuals identified phospho/sphingolipids as markers of healthy aging [5]. A longitudinal analysis conducted among Framingham Heart Study participants found that some longevity related metabolomic pathways associate with risk of common causes of death [6]. Findings from patients with breast cancer included frailty-associated changes in amino acid and phospholipid metabolism [7]. Additionally, research in elderly participants identified 15 markers associated with frailty and suggested that oxidative stress could be implicated in the development of frailty [8].
Physical performance metabolomics studies have also been conducted. Previous research has identified metabolites associated with gait speed [9–12], other gait parameters [10], grip strength [11], combined muscle mass and strength outcomes [13, 14], and Short Physical Performance Battery (SPPB) score [11]. Although these findings are promising, limitations of past work include the sole use of cross sectional examination without longitudinal follow up [10, 11, 13–16], sole use of targeted metabolomics approaches which are restricted to pathways of presumed biological relevance [10–12, 14–16], small numbers of metabolites tested [10–16], small sample sizes [13–16], and lack of adjustment for multiple comparisons [10, 11, 14, 16]. Thus, there is a compelling need for research on the relationship between serum metabolites and physical performance that takes advantage of longitudinal data and utilizes agnostic metabolomic methods.
Our study was designed to fill this gap in knowledge by first examining the cross sectional relationships between serum metabolites identified through untargeted metabolomics and physical performance measures while utilizing stringent control for multiple testing. We next examined the relationships between identified metabolites and prospective declines in physical performance observed at a subsequent study visit. The Bogalusa Heart Study (BHS) was selected as the study population to increase generalizability and reproducibility of study findings through the use of a large, ethnically diverse, community-based sample and to identify metabolites that are relevant in middle age, prior to onset of age related sarcopenia or mobility disability.
RESULTS
Participant characteristics
The BHS is a community-based long-term study investigating the natural history of CVD among a multi-ancestry sample (35% black and 65% white) of residents from Bogalusa, Louisiana [17]. The current BHS population includes 1,298 participants born between 1959 and 1979 who were screened at least two times during childhood and two times during adulthood. Participant characteristics are presented in Table 1. Participants were mostly middle aged adults (mean age: 48.2) and obese [mean body mass index (BMI)>30]. Over 60% were hypertensive, while fewer than 20% had diabetes. Approximately 3% had chronic kidney disease. As was expected for the age and health status of the study participants, the cohort overall had high physical function at baseline, with a mean SPPB score of over 11 out of 12. Baseline gait speed and grip strength were modestly correlated (ρ=0.3, P<0.0001). The distributions of baseline gait speed and grip strength presented in Supplementary Figures 1, 2. Participants of the longitudinal study were similar to the entire cohort with respect to important prognostic indicators (Supplementary Table 1).
Table 1. Characteristics of BHS participants.
| Overall (N=1,239) | Female | Male | |||
| Black (n=267) | White (n=463) | Black (n=160) | White (n=349) | ||
| Age, years, mean (SD) | 48.2 (5.3) | 47.6 (5.4) | 48.2 (5.1) | 47.2 (6.0) | 49 (5.0) |
| Post-high school education, n (%) | 609 (49.2) | 106 (39.7) | 278 (60.0) | 45 (28.1) | 180 (51.6) |
| Smoking, n (%) | |||||
| Never | 633 (51.1) | 156 (58.4) | 249 (53.8) | 55 (34.4) | 173 (49.6) |
| Former | 362 (29.2) | 67 (25.1) | 138 (29.8) | 48 (30.0) | 109 (31.2) |
| Current | 244 (19.7) | 44 (16.5) | 76 (16.4) | 57 (35.6) | 67 (19.2) |
| Drinking, n (%) | |||||
| Never | 153 (12.4) | 59 (22.1) | 60 (13.0) | 19 (11.9) | 15 (4.3) |
| Former | 395 (31.9) | 81 (30.3) | 149 (32.2) | 49 (30.6) | 116 (33.2) |
| Current | 691 (55.8) | 127 (47.6) | 254 (54.9) | 92 (57.5) | 218 (62.5) |
| BMI, kg/m2, mean (SD) | 31.5 (7.8) | 34.9 (8.8) | 30.2 (7.4) | 31.2 (8.7) | 30.5 (6.0) |
| SBP, mmHg, mean (SD) | 123.3 (16.8) | 125.6 (20.9) | 117.3 (14.4) | 131.2 (15.7) | 125.8 (13.9) |
| Hypertension*, n (%) | 771 (62.3) | 196 (73.4) | 225 (48.6) | 127 (79.4) | 223 (64.1) |
| Glucose, mg/dL, mean (SD) | 107.6 (38.3) | 108.3 (42.5) | 105.2 (38.3) | 110.5 (45.1) | 109 (30.7) |
| Diabetes†, n (%) | 207 (16.8) | 47 (17.6) | 73 (15.9) | 29 (18.4) | 58 (16.8) |
| eGFR, mL/min/1.73 m², mean (SD) | 93.7 (17.0) | 101.1 (18.4) | 92 (14.2) | 95.3 (20.3) | 89.5 (15.7) |
| CKD (GFR‡<60 mL/min/1.73 m²), n (%) | 39 (3.2) | 7 (2.6) | 14 (3.0) | 6 (3.8) | 12 (3.4) |
| SPPB score | 11.1 (1.4) | 10.7 (1.6) | 11.2 (1.3) | 10.8 (1.6) | 11.2 (1.3) |
| Six-minute walk distance (m) | 424.7 (86.1) | 383.7 (72.6) | 431.4 (81.7) | 411.4 (81.5) | 451.8 (90.8) |
| Gait speed (m/s) | 1.2 (0.2) | 1.1 (0.2) | 1.2 (0.2) | 1.1 (0.2) | 1.3 (0.3) |
| Grip strength (kg) | 35.1 (11.9) | 28.6 (7.1) | 27.2 (5.8) | 44.9 (9.7) | 46.1 (9.6) |
Note. BHS=Bogalusa Heart Study, BMI=body mass index, CKD=chronic kidney disease, DBP=diastolic blood pressure, eGFR=glomerular filtration rate, SBP=systolic blood pressure, SD=standard deviation, SPPB=short physical performance battery.
· Hypertension was defined as SBP≥130 mmHg, DBP≥80 mmHg, or use of antihypertensive medication.
† Diabetes was defined as fasting plasma glucose≥126 mg/dL or use of diabetes medication.
Metabolomics
Untargeted metabolomics resulted in the detection and relative quantification of 1,466 metabolites. These included 1,073 known biochemical compounds (Metabolomics Standards Initiative [MSI] levels 1 or 2) in pathways related to amino acids (n=201), carbohydrates (n=25), cofactors and vitamins (n=35), energy (n=9), lipids (n=435), nucleotides (n=42), peptides (n=52), and xenobiotics (n=256). An additional 18 partially characterized molecules (MSI level 3) and 393 unnamed compounds (MSI level 4) were also detected. The unnamed compounds may be identified upon the eventual acquisition of a matching purified standard (or via classical structural analysis). Of the metabolites examined, 1,202 metabolites passed rigorous quality control standards.
Baseline overall analyses
Multivariable linear regression models were used to analyze associations between each metabolite and each baseline physical performance measure, after adjustment for age, gender, race, BMI, estimated glomerular filtration rate (eGFR), education, cigarette smoking, and alcohol drinking. Results of baseline multivariate adjusted linear regression models for gait speed and grip strength respectively are presented graphically as the magnitudes of P-values versus effect sizes (Supplementary Figure 3). Significant direct and inverse associations of metabolites with gait speed were identified, while significant metabolite associations with grip strength were all in the inverse direction.
Baseline gait speed metabolite associations
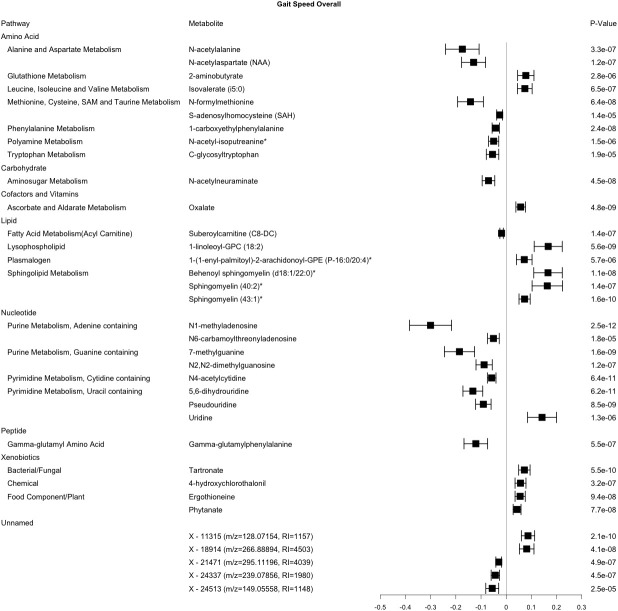
In the cross-sectional analysis, 35 metabolites were robustly associated with gait speed, including 30 from the amino acid, carbohydrate, cofactor and vitamin, lipid, nucleotide, peptide, and xenobiotic pathways, and 5 unnamed compounds (Figure 1). Metabolites associated with gait speed, that internally replicated across sex or race are shown in Supplementary Figures 4–7. Results of a sensitivity analysis with additional adjustment for clinical covariates were generally consistent with our main findings for gait speed (Supplementary Table 2).
Figure 1.
Metabolites significantly associated with gait speed. This forest plot depicts the beta estimate and 95% confidence interval for significant metabolites from both sex- and race-stratified analyses. 6 unknown metabolites are not shown. * indicates compounds with Metabolomics Standards Initiative confidence level 2.
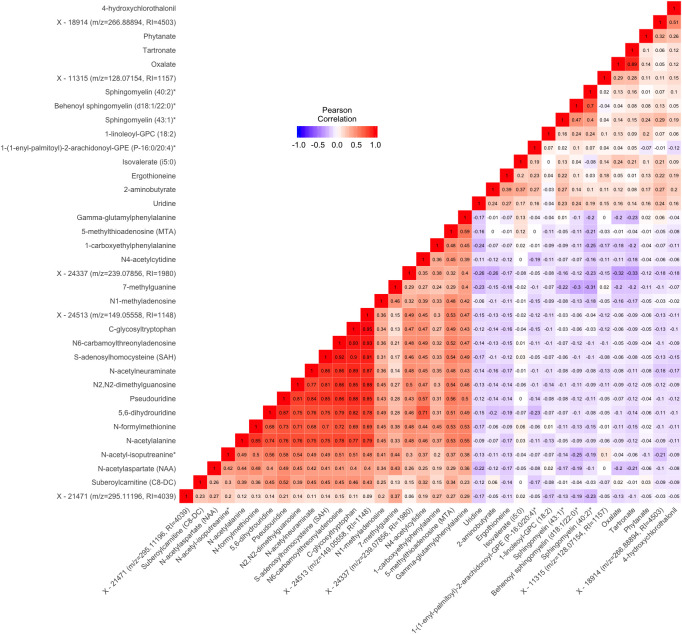
There were notable correlations between groups of metabolites associated with gait speed (Figure 2). Most of the metabolites in the lipid pathway had positive associations with gait speed. The 3 metabolite signals in the sphingolipid pathway were modestly to highly
Figure 2.
Pairwise Pearson correlations between metabolites significantly associated with either gait speed or grip strength in cross sectional analysis. Metabolites are ordered according to correlation coefficient. Correlations between each pair of metabolites are displayed in the cells of the heatmap. Cells are color coded with colors ranging from blue to red to depict correlations ranging from -1 to 1. RI=retention index. * indicates compounds with Metabolomics Standards Initiative confidence level 2.
correlated (pairwise ρ ranging from 0.4 to 0.7). Additionally, oxalate from the cofactors and vitamins pathway and tartronate from the xenobiotics pathway were highly correlated (ρ=0.89). Most of the other pairs of metabolites positively associated with gait speed had lower correlations (ρ<0.3). Among the metabolites negatively associate with gait speed, a group of 10 were highly correlated [pairwise ρ ranging from 0.68 to 0.93, N-formylmethionine, N-acetylalanine, Pseudouridine, 5,6-dihydrouridine, S-adenosylhomocysteine, N6-carbamoylthreonyladenosine, C-glycosyltryptophan, N-acetylneuraminate, N2,N2-dimethylguanosine, and X - 24513 (m/z=149.05558, RI=1148)]. With some exceptions, lipid and xenobiotic metabolites tended to display modest negative correlations with the amino acid, carbohydrate, nucleotide, and peptide metabolites.
Baseline grip strength metabolite associations
Cross-sectional study also identified 7 metabolites that were robustly associated with grip strength and included those from the amino acid, carbohydrate, and nucleotide (Figure 3). Six of these metabolites were also associated with baseline gait speed (C-glycosyltryptophan, N-acetylneuraminate, N1-methyladenosine, N4-acetylcytidine, 5,6-dihydrouridine, and Pseudouridine) and six had pairwise ρ>0.6 (C-glycosyltryptophan, N-acetylneuraminate, N1-methylinosine, N6-succinyladenosine, 5,6-dihydrouridine, and Pseudouridine) (Figure 2). Metabolites associated with grip strength, that internally replicated across sex or race are shown in Supplementary Figures 9–11. A sensitivity analysis with additional adjustment for clinical covariates generally produced results consistent with our main findings for grip strength (Supplementary Table 3).
Figure 3.
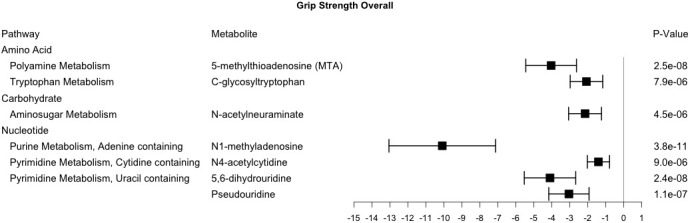
Metabolites significantly associated with grip strength. This forest plot depicts the beta estimate and 95% confidence interval for significant metabolites from both sex- and race-stratified analyses.
Longitudinal metabolite associations
After a mean (SD) follow up time of 2.9 (0.5) years, mean (SD) gait speed declined by 0.04 (0.20) meters per second. Mean (SD) grip strength declined by 0.6 (4.9) kg. Changes in gait speed and grip strength were not correlated (ρ=0.1, P=0.13). We found that 2 metabolites (out of the 35 from the baseline analysis) were associated with longitudinal change in gait speed at the Bonferroni corrected significance level (0.05/35=1.7×10-3). Despite the limited follow up time, 8 additional metabolites were nominally associated with change in gait speed (P<0.05) (Table 2), and 9 of the metabolites identified longitudinally had effect directions that were consistent with the baseline analysis (Supplementary Table 4). None of the 7 metabolites tested for association with longitudinal change in grip strength met nominal statistical significance thresholds. Even among null findings, effect directions for longitudinal change in gait speed and grip strength were generally consistent with effect directions identified in the cross-sectional analysis (Supplementary Table 5).
Table 2. Associations with longitudinal change in gait speed between baseline and follow up.
| Pathway | Metabolite | Beta (SE) | P-Value |
| Positive in Cross-Sectional Analysis | |||
| Lipid | |||
| Sphingolipid Metabolism | Behenoyl sphingomyelin (d18:1/22:0)* | 0.15 (0.06) | 0.02 |
| Sphingomyelin (40:2)* | 0.26 (0.07) | 2.6×10-4 | |
| Xenobiotics | |||
| Food Component/Plant | Ergothioneine | -0.05 (0.02) | 9.6×10-3 |
| Negative in Cross-Sectional Analysis | |||
| Amino Acid | |||
| Alanine and Aspartate Metabolism | N-acetylaspartate (NAA) | -0.13 (0.06) | 0.04 |
| Methionine, Cysteine, SAM and Taurine Metabolism | N-formylmethionine | -0.12 (0.05) | 0.01 |
| S-adenosylhomocysteine (SAH) | -0.03 (0.01) | 0.02 | |
| Phenylalanine Metabolism | 1-carboxyethylphenylalanine | -0.09 (0.02) | 8.8×10-5 |
| Carbohydrate | |||
| Aminosugar Metabolism | N-acetylneuraminate | -0.05 (0.03) | 0.04 |
| Nucleotide | |||
| Purine Metabolism, Guanine containing | N2,N2-dimethylguanosine | -0.05 (0.03) | 0.05 |
| Peptide | |||
| Gamma-glutamyl Amino Acid | Gamma-glutamylphenylalanine | -0.12 (0.05) | 0.03 |
* Indicates compounds with Metabolomics Standards Initiative confidence level 2.
2 unnamed metabolites are not shown.
Overlap with kidney function metabolites
The metabolites inversely associated with physical performance were all also associated with kidney function in our previous study in the BHS, with consistent effect direction [18]. These metabolites are presented in Supplementary Table 6, along with lookups of their associations with aging, inflammation, and mortality in previous research.
Metabolite module associations
The 9 metabolite modules identified among BHS participants are shown in Figure 4. A module, with sphingolipids as top metabolites, was positively associated with gait speed (p=7.6×10-4). Another module, with top metabolites in the primary and secondary bile metabolism pathways (top metabolite: glycochenodeoxycholate), was negatively associated with both gait speed (p=1.4×10-3) and grip strength (p=1.9×10-3).
Figure 4.
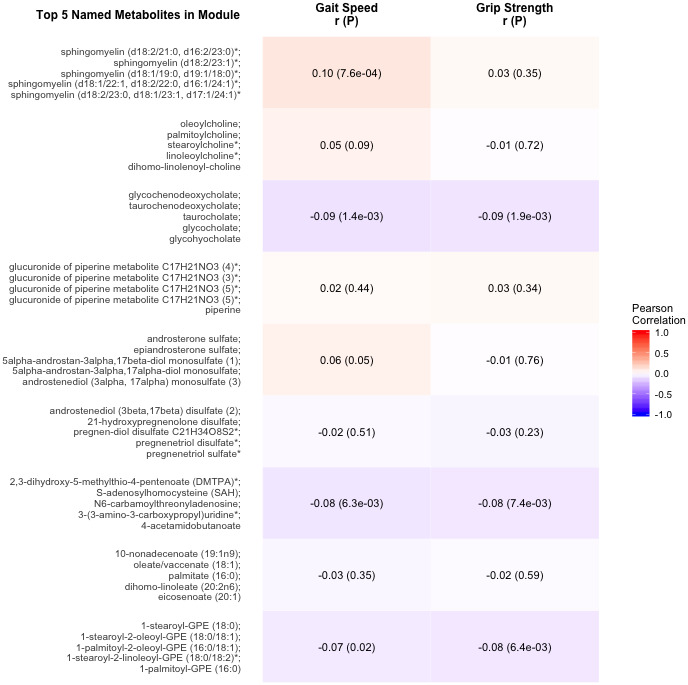
Correlations of metabolite modules with physical performance. Colors represent correlation strength, ranging from blue (-1) to red (1). * indicates compounds with Metabolomics Standards Initiative confidence level 2.
DISCUSSION
This study examined cross-sectional and longitudinal associations of untargeted serum metabolites with two measures of physical performance, gait speed and grip strength. Thirty-five and 7 metabolites robustly associated with gait speed and grip strength, respectively. Six of these metabolites associated with both phenotypes, suggesting both common and disparate biological mechanisms underlying these physical performance phenotypes. Effect directions in longitudinal analyses were generally consistent with those of the cross-sectional study, with 2 metabolites achieving significant associations with change in gait speed after adjusting for baseline measures. No metabolites achieved significant longitudinal associations with grip strength. We additionally identified two metabolite modules associated with physical performance measures. Many of the identified metabolites represent novel findings in physical performance metabolomics literature, and implicate lipids, amino acids, and nucleotides in the physiologic processes related to this complex phenotype.
Lipid metabolites
Sphingolipids, and a metabolite module with top metabolites in the sphingolipids pathway, were positively associated with gait speed in the current study. The three metabolite signals in this pathway identified in our study, behenoyl sphingomyelin (d18:1/22:0), sphingomyelin (40:2) and sphingomyelin (43:1), represent novel findings. Other sphingolipids, however, have previously been shown to associate with gait speed [10, 11] and other physical performance measures [11], adding credence to the robustness of our findings. Sphingolipids levels have also been shown to be decreased in multiple sclerosis [19], a disease characterized by demyelination, leading to axonal and neuronal loss [20] reductions in gait speed [21], and other gait abnormalities [21]. Previous research in older adults posits that sphingolipid metabolism may relate to physical performance similarly, through the insulation of nerve cell axons by myelin sheaths, thus influencing nerve conduction signals [11]. Additionally, sphingolipids have been previously associated with aging [22], neurodegeneration [23], and cognitive decline [24]. Further research is needed to elucidate whether decreased serum sphingolipid levels represent a potential novel clinical biomarker of demyelination in the general population that can be used to predict incident reductions in physical performance.
Nucleotide metabolites
We identified several modified nucleosides with known relationships to whole body RNA degradation, including N2,N2-dimethylguanosine [25], 5,6-dihydrouridine [26], 7-methylguanine [25], N4-acetylcytidine [27], and pseudouridine [25]. The higher levels of these metabolites found in participants in our study with lower physical performance mirror the elevated levels of these metabolites in serum from human patients with pulmonary arterial hypertension [28] and end stage renal disease [27], urine from those with cancer [29], AIDS [29], and recent surgical stress [30], as well as urine from tree shrews with increased social stress [31]. Modified nucleosides enter circulation during stress [32], accelerated cell proliferation [29], and rapid tissue breakdown [29]. One potential hypothesis explaining the elevated levels of these metabolites in serum of participants with lower and declining physical performance is that the metabolites and lower physical performance measures are both markers of increased stress and tissue breakdown. While the modified nucleoside, pseudouridine, was inversely associated with gait speed (at baseline and longitudinally) and grip strength, its nucleoside precursor, uridine was directly associated baseline gait speed. Similarly to a metabolomics study of esophageal adenocarcinoma that found higher levels of pseudouridine and lower levels of uridine in cases compared to controls [33], one potential explanation is that participants with slower gait speed could have a higher rate of conversion of uridine to pseudouridine than those with faster gait speed. Further study is needed to examine whether these metabolites, along with reductions in physical performance, represent early biomarkers of future declines in health status.
Kidney function and aging
Interestingly, all of the metabolites with inverse associations with physical performance measures were also inversely associated with kidney function in the BHS [18]. Many of these metabolites were also associated with kidney function in other populations [27, 34–37]. In prior study of a population with chronic kidney disease (CKD), CKD severity was associated with poor physical performance and frailty in a graded fashion [38]; however, the biological mechanisms leading to reduced physical function in CKD patients remain unknown. It is well known that small molecules accumulate in serum as kidney function declines [39]. One theory on the relation between kidney function and physical performance is that this accumulation of small molecules has a detrimental effect on other systems in the body [40], including those that govern physical performance – that declines in kidney function lead to declines in physical performance through serum metabolites. In support of this theory, two recent papers posit a relation between declines in kidney function and sarcopenia through serum metabolites [41, 42]; however, the extent to which these findings apply to other physical performance measures is unclear. An alternative theory is that declines in kidney function and declines in physical performance are both governed by a common ‘accelerated aging’ phenotype [38], and that identified common metabolites may be markers of accelerated aging. In our study, this theory is supported by the very low prevalence of CKD among the BHS population and the significance of the identified metabolites after adjustment for eGFR. This theory is additionally supported by shared risk factors between physical performance decline and CKD, such as lower socioeconomic status, lower physical activity, and increased rates of cardiovascular disease and cerebrovascular disease, chronic low-grade inflammation, and hyperglycemia [9]. In further support of this theory, several of our findings, that associated with both physical performance and kidney function, are also associated with aging [43–48], inflammation [49, 50], and mortality [51–55]. Additional research is warranted to examine the role of the identified metabolites in the relationship between reduced kidney function and declines in physical performance.
Strengths and limitations
This study has several important strengths. The longitudinal design allowed us to identify metabolites that associated with future changes in physical performance, while adjusting for baseline performance variables. Metabolites identified longitudinally are more likely to be relevant to the development of physical performance declines than metabolites identified solely through cross sectional analysis. To our knowledge, this study had a larger baseline sample size than prior physical performance metabolomics research, increasing the power to detect metabolite-phenotype associations. Additionally, metabolites were detected using an untargeted approach, thus increasing the likelihood of finding novel signals. To reduce the likelihood of false positive associations and increase the generalizability of the findings, only signals that achieved significance in the overall sample and one gender or race group, with consistency in effect direction and nominal significance in the other gender or race group, were reported here. While this method should minimize spurious signals, it also prohibits the identification of gender- or race-specific findings. One notable limitation is that metabolites measured in middle-age could reflect the result of early life influences rather than directly influencing the aging process. Our study lacked external replication, however, we replicated our findings internally across gender or race. Other limitations include the relatively short mean follow up time of less than three years, use of a subset of the baseline sample for the longitudinal analysis, and the unknown clinical significance of the unnamed metabolites identified. Although these limitations reduced our power to detect longitudinal associations, several compelling temporal relationships were detected. Due to the age and overall high functioning status of the study participants, we were unable to examine physical performance using the SPPB, however, baseline SPPB information has been collected on these participants and changes in scores may be available for future studies in this population.
CONCLUSIONS
In summary, we identified 36 metabolites cross-sectionally associated with physical performance measures, including 35 for gait speed and 7 for grip strength. Of these metabolites, 2 were longitudinally associated with declines in gait speed. These findings suggest important roles for sphingolipid metabolism and whole body RNA breakdown in declining physical performance in a middle-aged population.
MATERIALS AND METHODS
Participants
The BHS is a long-term study investigating cardiovascular health over the life-course. From 1973 to 2016, 7 surveys were conducted in children and adolescents aged 4 to 17 years, and 10 surveys were conducted among adults aged 18 to 51 years who had been examined previously as children. The BHS has been described in detail elsewhere [17]. Data and specimens collected in the recent 2013 to 2016 visit cycle were leveraged in our cross-sectional analysis and served as the baseline measures for longitudinal study of physical performance decline. Those missing baseline metabolomics (n=37), grip strength (n=17), gait speed (n=80) or covariable (n=17) data were excluded from all analyses. A total of 1,227 and 1,164 participants remained for the cross-sectional metabolomics study of grip strength and gait speed, respectively. The longitudinal study included 282 and 201 of these participants with repeated measures of grip strength and gait speed, respectively, which were collected an average of 2.9 years following the baseline examination.
Informed consent was obtained from all study participants after detailed explanation of the study. This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Tulane University Institutional Review Board.
Metabolomics
Untargeted, ultrahigh performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS) of BHS serum samples was conducted by Metabolon Inc. (Durham, NC) [56] using samples that were stored at -80°C since the 2013 to 2016 visit. Rigorous quality assurance was conducted, which included the use of blanks, blind duplicates (5% of samples), and standard biochemical compounds which were integrated into every run. Batch effects were assessed using principal components analysis, which revealed no evidence of clustering of metabolite data by run-days. A complete list of the metabolites examined and their properties is presented as Supplementary Data.
Similar to previous analyses [57], data filtering excluded 213 metabolites that were below the detection threshold in more than 80% of samples and 51 metabolites with a reliability coefficient <0.3 based on blind duplicate analysis. Among the 1,202 metabolites passing quality control, 167 metabolites were below the detection threshold in 50% to 80% of the samples, and were analyzed as ordinal variables after categorization into one of three mutually exclusive groups: 1) below-the-detection-limit; 2) below the median of measured values; or 3) greater than or equal to the median. The remaining 1,035 metabolites, which were above the detection threshold in more than 50% of samples, were analyzed as continuous variables, scaled to set the median of detected values for each metabolite equal to 1, where the minimum observed value was imputed for metabolites with below-the-detection-limit values.
Outcomes and covariables
Among BHS participants, phenotype and covariable data were collected following stringent protocols [58]. Gait speed, in meters per second, was determined by dividing six-minute walking distance by 360 seconds. Grip strength was measured using a Jamar hand held dynamometer and was averaged across both hands. Questionnaires were administered to obtain information on demographic characteristics, lifestyle risk factors, and personal medical history. Anthropometric measures were obtained by trained staff with participants in light clothing without shoes. During each visit, body weight and height were measured twice to the nearest 0.1 kg and 0.1 cm, respectively. The mean values of height and weight were used to estimate BMI, which was calculated as weight in kilograms divided by height in meters squared. BHS participants were instructed to fast for 12 hours prior to blood sample collection. Serum creatinine level was measured by Laboratory Corporation of America (LabCorp, Burlington, NC) using the kinetic Jaffe method. Estimated glomerular filtration rate (eGFR) was calculated using the 2009 CKD-EPI equation [59].
Statistical analysis
Characteristics of BHS participants were presented as means and standard deviations (SDs) for continuous variables and as percentages for categorical variables.
Association of single metabolites with physical performance phenotypes
The two physical performance measures studied were gait speed and grip strength. Multivariable linear regression models (using SAS [version 9.4; SAS Institute, Cary, NC] function PROC GLM) were employed to analyze associations between each metabolite and each untransformed baseline physical performance measure, after adjustment for age, BMI, eGFR, education, cigarette smoking, and alcohol drinking. Analyses were performed according to gender and race, and in an overall analysis after additional adjustment for gender and race. All analyses accounted for multiple testing using the Bonferroni method. To further reduce false positive findings, we relied on internal replication across gender or race. Metabolites were considered robustly significant if they were significant in the overall analysis, and significant in either gender or race with a consistent effect direction and nominal significance (p<0.05) in the other gender or race. Pairwise Pearson correlations were calculated between significant metabolites for each physical performance measure, and heatmaps were created using the ggplot2 and reshape R (version 3.4.3) packages. To account for potential confounding by clinical factors, we conducted a sensitivity analysis with adjustment for fasting glucose, systolic blood pressure, and low-density lipoprotein, in addition to the covariates from the main analysis. We additionally examined the overlap in significant findings between this study and the findings of our previous kidney function metabolomics publication in the BHS [18].
Longitudinal changes in gait speed and grip strength were calculated by subtracting baseline measures from follow up measures. Multivariable linear regression models (using SAS function PROC GLM) were also employed to analyze the associations between each metabolite that was robustly significant in overall cross-sectional analysis and change in each physical performance measure, after adjustment for the appropriate baseline physical performance measure, follow up time, age, gender, race BMI, eGFR, education, cigarette smoking, and alcohol drinking. Due to the small sample size and criteria of consistency of associations across race or gender groups in cross-sectional analyses, longitudinal analyses were not stratified by gender or race.
Association of metabolite modules with physical performance phenotypes
We used weighted correlation network analysis (WGCNA) [60] to identify networks of highly correlated metabolites. WGCNA is an unsupervised data reduction technique that allows for dependency between components, which may represent the biological pathways of identified metabolites more accurately than principal components analysis [60, 61]. The use of WGCNA and its application to metabolomics studies has been previously reported [62]. In brief, the metabolite network was constructed as an adjacency matrix based on the weighted pairwise correlations of all metabolites [63]. Modules, defined as densely interconnected metabolites, were then identified from the network using an unsupervised hierarchical clustering approach [64]. For each module, an eigenmetabolite was generated. This measure represents the module’s first principal component and can be interpreted as its weighted average metabolite value. Metabolite modules were constructed using metabolite data for the 1,202 metabolites passing quality control among all study participants.
Adjusted physical performance measures were created using the residual values generated by regressing each raw physical performance phenotype on gender, race, age, BMI, eGFR, education, cigarette smoking, and alcohol drinking. The correlations between each module (eigenmetabolite) and the adjusted physical performance phenotypes were then estimated. We employed a Bonferroni corrected alpha threshold of 5.56×10-3 (0.05/9) to account for testing 9 metabolite modules. WGCNA analysis was performed using the WGCNA R package. The figure depicting the WGCNA results was created using the ggplot2 R package.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the contribution of all staff members who were involved in conducting the BHS. We extend our sincerest gratitude to the participants of the BHS, many of whom have diligently participated since they were children.
Footnotes
CONFLICTS OF INTEREST: JMK is employed by Metabolon, Inc. He contributed to the logistics, optimization, and interpretation of the untargeted metabolomics. Metabolon, Inc. was not involved in the study design, statistical analysis, or interpretation of the results.
FUNDING: This work was supported by the National Institute on Aging at the National Institutes of Health under (grant numbers R01AG041200, R21AG051914); and partially supported by the National Institute of General Medical Sciences at the National Institutes of Health (grant number P20GM109036).
REFERENCES
- 1.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003; 58:M911–16. 10.1093/gerona/58.10.m911 [DOI] [PubMed] [Google Scholar]
- 2.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an international academy on nutrition and aging (IANA) task force. J Nutr Health Aging. 2009; 13:881–89. 10.1007/s12603-009-0246-z [DOI] [PubMed] [Google Scholar]
- 3.Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Glynn NW, Hsu FC, Walkup M, Pahor M. Health-related quality of life in older adults at risk for disability. Am J Prev Med. 2007; 33:214–18. 10.1016/j.amepre.2007.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature. 2008; 455:1054–56. 10.1038/4551054a [DOI] [PubMed] [Google Scholar]
- 5.Montoliu I, Scherer M, Beguelin F, DaSilva L, Mari D, Salvioli S, Martin FP, Capri M, Bucci L, Ostan R, Garagnani P, Monti D, Biagi E, et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging (Albany NY). 2014; 6:9–25. 10.18632/aging.100630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S, Larson MG, McCabe EL, Murabito JM, Rhee EP, Ho JE, Jacques PF, Ghorbani A, Magnusson M, Souza AL, Deik AA, Pierce KA, Bullock K, et al. Distinct metabolomic signatures are associated with longevity in humans. Nat Commun. 2015; 6:6791. 10.1038/ncomms7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corona G, Polesel J, Fratino L, Miolo G, Rizzolio F, Crivellari D, Addobbati R, Cervo S, Toffoli G. Metabolomics biomarkers of frailty in elderly breast cancer patients. J Cell Physiol. 2014; 229:898–902. 10.1002/jcp.24520 [DOI] [PubMed] [Google Scholar]
- 8.Kameda M, Teruya T, Yanagida M, Kondoh H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci USA. 2020; 117:9483–89. 10.1073/pnas.1920795117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy RA, Moore S, Playdon M, Kritchevsky S, Newman AB, Satterfield S, Ayonayon H, Clish C, Gerszten R, Harris TB. Metabolites associated with risk of developing mobility disability in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2019; 74:73–80. 10.1093/gerona/glx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wennberg AM, Schafer MJ, LeBrasseur NK, Savica R, Bui HH, Hagen CE, Hollman JH, Petersen RC, Mielke MM. Plasma sphingolipids are associated with gait parameters in the mayo clinic study of aging. J Gerontol A Biol Sci Med Sci. 2018; 73:960–65. 10.1093/gerona/glx139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li D, Misialek JR, Huang F, Windham GB, Yu F, Alonso A. Independent association of plasma hydroxysphingomyelins with physical function in the atherosclerosis risk in communities (ARIC) study. J Gerontol A Biol Sci Med Sci. 2018; 73:1103–10. 10.1093/gerona/glx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, Khadeer M, Salem N Jr, Ferrucci L, Semba RD. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2019; 74:62–67. 10.1093/gerona/gly100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Q, Shen H, Su KJ, Tian Q, Zhao LJ, Qiu C, Garrett TJ, Liu J, Kakhniashvili D, Deng HW. A joint analysis of metabolomic profiles associated with muscle mass and strength in caucasian women. Aging (Albany NY). 2018; 10:2624–35. 10.18632/aging.101574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Karagounis LG, Ng TP, Carre C, Narang V, Wong G, Tan CT, Zin Nyunt MS, Gao Q, Abel B, Poidinger M, Fulop T, Bosco N, Larbi A. Systemic and metabolic signature of sarcopenia in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2020; 75:309–17. 10.1093/gerona/glz001 [DOI] [PubMed] [Google Scholar]
- 15.Lum H, Sloane R, Huffman KM, Kraus VB, Thompson DK, Kraus WE, Bain JR, Stevens R, Pieper CF, Taylor GA, Newgard CB, Cohen HJ, Morey MC. Plasma acylcarnitines are associated with physical performance in elderly men. J Gerontol A Biol Sci Med Sci. 2011; 66:548–53. 10.1093/gerona/glr006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, Primiano A, Coelho-Junior HJ, Bossola M, Urbani A, Landi F, Bernabei R, Marzetti E. A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients. 2018; 10:1691. 10.3390/nu10111691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berenson GS, Wattigney WA, Bao W, Srinivasan SR, Radhakrishnamurthy B. Rationale to study the early natural history of heart disease: the bogalusa heart study. Am J Med Sci. 1995. (Suppl 1); 310:S22–28. 10.1097/00000441-199512000-00005 [DOI] [PubMed] [Google Scholar]
- 18.Nierenberg JL, He J, Li C, Gu X, Shi M, Razavi AC, Mi X, Li S, Bazzano LA, Anderson AH, He H, Chen W, Kinchen JM, et al. Novel associations between blood metabolites and kidney function among bogalusa heart study and multi-ethnic study of atherosclerosis participants. Metabolomics. 2019; 15:149. 10.1007/s11306-019-1613-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob S, Al-Kandari A, Alroughani R, Al-Temaimi R. Assessment of plasma biomarkers for their association with multiple sclerosis progression. J Neuroimmunol. 2017; 305:5–8. 10.1016/j.jneuroim.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 20.Nafee T, Watanabe R, Fregni F. Multiple sclerosis. Neuromethods: Elsevier Ltd; 2018; 138:263–95. 10.1007/978-1-4939-7880-9_8 [DOI] [Google Scholar]
- 21.Comber L, Galvin R, Coote S. Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. 2017; 51:25–35. 10.1016/j.gaitpost.2016.09.026 [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Covarrubias V, Beekman M, Uh HW, Dane A, Troost J, Paliukhovich I, van der Kloet FM, Houwing-Duistermaat J, Vreeken RJ, Hankemeier T, Slagboom EP. Lipidomics of familial longevity. Aging Cell. 2013; 12:426–34. 10.1111/acel.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savica R, Murray ME, Persson XM, Kantarci K, Parisi JE, Dickson DW, Petersen RC, Ferman TJ, Boeve BF, Mielke MM. Plasma sphingolipid changes with autopsy-confirmed lewy body or alzheimer’s pathology. Alzheimers Dement (Amst). 2016; 3:43–50. 10.1016/j.dadm.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010; 31:17–24. 10.1016/j.neurobiolaging.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topp H, Sander G, Heller-Schöch G, Schöch G. Determination of 7-methylguanine, N2,N2-dimethylguanosine, and pseudouridine in ultrafiltrated serum of healthy adults by high-performance liquid chromatography. Anal Biochem. 1987; 161:49–56. 10.1016/0003-2697(87)90650-6 [DOI] [PubMed] [Google Scholar]
- 26.Topp H, Duden R, Schöch G. 5,6-dihydrouridine: a marker ribonucleoside for determining whole body degradation rates of transfer RNA in man and rats. Clin Chim Acta. 1993; 218:73–82. 10.1016/0009-8981(93)90223-q [DOI] [PubMed] [Google Scholar]
- 27.Niwa T, Takeda N, Yoshizumi H. RNA metabolism in uremic patients: accumulation of modified ribonucleosides in uremic serum. Technical note. Kidney Int. 1998; 53:1801–06. 10.1046/j.1523-1755.1998.00944.x [DOI] [PubMed] [Google Scholar]
- 28.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017; 135:460–75. 10.1161/CIRCULATIONAHA.116.024602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano K, Nakao T, Schram KH, Hammargren WM, McClure TD, Katz M, Petersen E. Urinary excretion of modified nucleosides as biological marker of RNA turnover in patients with cancer and AIDS. Clin Chim Acta. 1993; 218:169–83. 10.1016/0009-8981(93)90181-3 [DOI] [PubMed] [Google Scholar]
- 30.Marway JS, Anderson GJ, Miell JP, Ross R, Grimble GK, Bonner AB, Gibbons WA, Peters TJ, Preedy VR. Application of proton NMR spectroscopy to measurement of whole-body RNA degradation rates: effects of surgical stress in human patients. Clin Chim Acta. 1996; 252:123–35. 10.1016/0009-8981(96)06300-0 [DOI] [PubMed] [Google Scholar]
- 31.Jöhren O, Topp H, Sander G, Schöch G, Fuchs E. Social stress in tree shrews increases the whole-body RNA degradation rates. Naturwissenschaften. 1991; 78:36–38. 10.1007/BF01134043 [DOI] [PubMed] [Google Scholar]
- 32.Kirchner S, Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat Rev Genet. 2015; 16:98–112. 10.1038/nrg3861 [DOI] [PubMed] [Google Scholar]
- 33.Djukovic D, Baniasadi HR, Kc R, Hammoud Z, Raftery D. Targeted serum metabolite profiling of nucleosides in esophageal adenocarcinoma. Rapid Commun Mass Spectrom. 2010; 24:3057–62. 10.1002/rcm.4739 [DOI] [PubMed] [Google Scholar]
- 34.Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 2014; 85:1214–24. 10.1038/ki.2013.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura T, Yasuda K, Yamamoto R, Soga T, Rakugi H, Hayashi T, Isaka Y. Identification of biomarkers for development of end-stage kidney disease in chronic kidney disease by metabolomic profiling. Sci Rep. 2016; 6:26138. 10.1038/srep26138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Römisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, et al. A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol. 2016; 27:1175–88. 10.1681/ASN.2014111099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab. 2016; 101:696–704. 10.1210/jc.2015-3345 [DOI] [PubMed] [Google Scholar]
- 38.Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, Baker JF, Carlow D, Sulik MJ, Lo JC, Go AS, Ky B, Mariani L, et al. , and CRIC Study Investigators. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013; 38:307–15. 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato E, Kohno M, Yamamoto M, Fujisawa T, Fujiwara K, Tanaka N. Metabolomic analysis of human plasma from haemodialysis patients. Eur J Clin Invest. 2011; 41:241–55. 10.1111/j.1365-2362.2010.02398.x [DOI] [PubMed] [Google Scholar]
- 40.Lisowska-Myjak B. Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract. 2014; 128:303–11. 10.1159/000369817 [DOI] [PubMed] [Google Scholar]
- 41.Lustgarten MS, Fielding RA. Metabolites related to renal function, immune activation, and carbamylation are associated with muscle composition in older adults. Exp Gerontol. 2017; 100:1–10. 10.1016/j.exger.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, Saigusa D, Miura D, Morikawa-Ichinose T, Saito R, Oba-Yabana I, Oe Y, Kisu K, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep. 2016; 6:36618. 10.1038/srep36618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruggieri M, Tortorella C, Ceci E, Paolicelli D, Solfrizzi V, Di Bitonto G, Pica C, Mastrapasqua M, Livrea P, Trojano M. Age-related changes of serum n-acetyl-aspartate in healthy controls. Age Ageing. 2011; 40:391–95. 10.1093/ageing/afr021 [DOI] [PubMed] [Google Scholar]
- 44.Zierer J, Kastenmüller G, Suhre K, Gieger C, Codd V, Tsai PC, Bell J, Peters A, Strauch K, Schulz H, Weidinger S, Mohney RP, Samani NJ, et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany NY). 2016; 8:77–94. 10.18632/aging.100874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menni C, Kastenmüller G, Petersen AK, Bell JT, Psatha M, Tsai PC, Gieger C, Schulz H, Erte I, John S, Brosnan MJ, Wilson SG, Tsaprouni L, et al. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol. 2013; 42:1111–19. 10.1093/ije/dyt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tritsch GL, Luch JM, Evans JT, Mittelman A. Age dependence of human urinary pseudouridine excretion. Biochem Med. 1979; 22:387–90. 10.1016/0006-2944(79)90027-9 [DOI] [PubMed] [Google Scholar]
- 47.Collino S, Montoliu I, Martin FP, Scherer M, Mari D, Salvioli S, Bucci L, Ostan R, Monti D, Biagi E, Brigidi P, Franceschi C, Rezzi S. Metabolic signatures of extreme longevity in northern italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013; 8:e56564. 10.1371/journal.pone.0056564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan BH, Bencsath FA, Gaubatz JW. Steady-state levels of 7-methylguanine increase in nuclear DNA of postmitotic mouse tissues during aging. Mutat Res. 1990; 237:229–38. 10.1016/0921-8734(90)90004-b [DOI] [PubMed] [Google Scholar]
- 49.Lustgarten MS, Fielding RA. Metabolites associated with circulating interleukin-6 in older adults. J Gerontol A Biol Sci Med Sci. 2017; 72:1277–83. 10.1093/gerona/glw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerri MA, Beltrán-Nuñez A, Bernasconi S, Dejana E, Bassi L, Bazzoni G. Inhibition of cytokine production and endothelial expression of adhesion antigens by 5’-methylthioadenosine. Eur J Pharmacol. 1993; 232:291–94. 10.1016/0014-2999(93)90787-i [DOI] [PubMed] [Google Scholar]
- 51.Mindikoglu AL, Opekun AR, Putluri N, Devaraj S, Sheikh-Hamad D, Vierling JM, Goss JA, Rana A, Sood GK, Jalal PK, Inker LA, Mohney RP, Tighiouart H, et al. Unique metabolomic signature associated with hepatorenal dysfunction and mortality in cirrhosis. Transl Res. 2018; 195:25–47. 10.1016/j.trsl.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J, Weinstein SJ, Moore SC, Derkach A, Hua X, Liao LM, Gu F, Mondul AM, Sampson JN, Albanes D. Serum metabolomic profiling of all-cause mortality: a prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort. Am J Epidemiol. 2018; 187:1721–32. 10.1093/aje/kwy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers AJ, McGeachie M, Baron RM, Gazourian L, Haspel JA, Nakahira K, Fredenburgh LE, Hunninghake GM, Raby BA, Matthay MA, Otero RM, Fowler VG, Rivers EP, et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS One. 2014; 9:e87538. 10.1371/journal.pone.0087538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu JR, Coresh J, Inker LA, Levey AS, Zheng Z, Rebholz CM, Tin A, Appel LJ, Chen J, Sarnak MJ, Grams ME. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018; 94:381–89. 10.1016/j.kint.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E. Associations between the serum metabolome and all-cause mortality among african americans in the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2016; 183:650–56. 10.1093/aje/kwv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009; 81:6656–67. 10.1021/ac901536h [DOI] [PubMed] [Google Scholar]
- 57.Zheng Y, Yu B, Alexander D, Mosley TH, Heiss G, Nettleton JA, Boerwinkle E. Metabolomics and incident hypertension among blacks: the atherosclerosis risk in communities study. Hypertension. 2013; 62:398–403. 10.1161/HYPERTENSIONAHA.113.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster TA, Berenson GS. Measurement error and reliability in four pediatric cross-sectional surveys of cardiovascular disease risk factor variables—the bogalusa heart study. J Chronic Dis. 1987; 40:13–21. 10.1016/0021-9681(87)90092-0 [DOI] [PubMed] [Google Scholar]
- 59.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008; 9:559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013; 1:92–107. 10.2174/2213235X11301010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, et al. , and MetaHIT Consortium. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016; 535:376–81. 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 63.Yip AM, Horvath S. Gene network interconnectedness and the generalized topological overlap measure. BMC Bioinformatics. 2007; 8:22. 10.1186/1471-2105-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the dynamic tree cut package for R. Bioinformatics. 2008; 24:719–20. 10.1093/bioinformatics/btm563 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.