Abstract
Background
In animal studies early life antibiotic exposure causes metabolic abnormalities including obesity through microbiota disruption, but evidence from human studies is scarce. We examined involvement of gut microbiota in the associations between infant antibiotic exposure and childhood adiposity.
Methods
Infant antibiotic exposure in the first year of life was ascertained using parental reports during interviewer-administered questionnaires. Primary outcomes were childhood obesity [body mass index (BMI) z-score>95th percentile] and adiposity [abdominal circumference (AC) and skinfold (triceps+subscapular (SST)) measurements] determined from ages 15-60 months. At age 24 months, when gut microbiota are more stable, stool samples (n=392) were collected for gut microbiota profiling using co-abundancy networks. Associations of antibiotic exposure with obesity and adiposity (n=1016) were assessed using multiple logistic and linear mixed effects regressions. Key bacteria associated with antibiotics exposure were identified by partial redundancy analysis and multivariate association with linear models.
Results
Antibiotic exposure was reported in 38% of study infants. In a fully adjusted model, a higher odds of obesity from 15-60 months of age was observed for any antibiotic exposure [OR(95% CI)=1.45(1.001, 2.14)] and exposure to ≥3 courses of antibiotics [2.78(1.12, 6.87)]. For continuous adiposity indicators, any antibiotic exposure was associated with higher BMI z-score in boys [β=0.15(0.01, 0.28)] but not girls [β=-0.04(-0.19, 0.11)] (P-interaction=0.026). Similarly, exposure to ≥3 courses of antibiotics was associated with higher AC in boys [1.15(0.05, 2.26)cm] but not girls [0.57(-1.32, 2.45)cm] (P-interaction not significant). Repeated exposure to antibiotics was associated with a significant reduction (FDR-corrected P-values<0.05) in a microbial co-abundant group (CAG) represented by Eubacterium hallii, whose proportion was negatively correlated with higher child adiposity. Meanwhile, a CAG represented by Tyzzerella 4 was positively correlated with the repeated use of antibiotics and childhood adiposity.
Conclusions
Infant antibiotic exposure was associated with disruption of gut microbiota and higher risks of childhood obesity and increased adiposity.
Introduction
Childhood obesity, a growing global epidemic, is associated with a broad spectrum of adverse health outcomes in adulthood such as type 2 diabetes1. Identifying modifiable determinants of childhood obesity is thus a pressing research need. Some human observational studies have reported associations between antibiotic exposure during infancy and higher childhood adiposity and obesity risk. Most of these studies observed stronger associations in boys2–4 and with earlier timing2,5–8 and higher frequency2,5,6,9 of antibiotic exposure. However, no associations between infant antibiotic exposure and childhood weight gain10 and obesity11 were observed in two recent studies. Differences in population characteristics, antibiotic exposure pattern, and study design are likely explanations for the discordance in results.
Accumulating evidence indicates that colonization of the gut microbiota at an early age plays a pivotal role in the weight gain and development of obesity in the later life12–14. At early ages antibiotics are a major factor disrupting the normal colonization and development of infant gut microbiota, which may consequently influence child weight gain and obesity risk. For example, early life exposure to antibiotics in mice led to metabolic changes (increased total fat mass and higher abdominal, visceral, and liver adiposity) that persisted even after restoration of normal microbiota population structures upon cessation of antibiotics15,16. The effect is greater with earlier exposure (pre-weaning), indicating that infancy may be a critical period for host-microbe metabolic interaction16. Nonetheless, to our knowledge, no human study to date has evaluated the potential implication of gut microbiota on the association between early life antibiotic exposure and childhood obesity.
We hypothesized that antibiotic exposure in infancy is associated with higher childhood obesity risk and adiposity, and that gut microbiota is a potential contributor to this association.
Methods
Study population
We used data from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) mother-offspring cohort study, described in detail elsewhere17. 1247 women were recruited into the study. Pregnant women who were bearing twins (n=10) or dropped out before delivery (n=65) were excluded from the present analysis, resulting in 1172 singleton deliveries. The study was granted ethical approval by the institutional review boards of the respective hospitals and written informed consent was collected from all women at recruitment. The current study is a prospective analysis linking infancy antibiotic exposure with later childhood adiposity.
Exposure: Infant antibiotic exposure
Offspring exposure to antibiotics was ascertained through parental report of interviewer-administered questionnaires at ages 3, 6, 9, and 12 months. We focused on the binary response (yes/no) and recalled age at antibiotic exposure. Since approximately half of the parents did not recall and report the name of the antibiotic, potential differential influences of different antibiotic types were not investigated. From the binary response, total number of antibiotic courses exposed was derived by adding the reported antibiotic exposure for all questionnaires in the first year of life. Age of first antibiotic exposure was generated as the earliest reported child’s age of antibiotic exposure for all the questionnaires. Of the 1172 singleton births, 1060 children had information on antibiotic exposure. Baseline descriptive information was thus generated for this subset. The participant flow is detailed in Supplementary Figure 1.
Outcomes: Child adiposity
The offspring’s anthropometry (weight, length/height, abdominal circumference (AC)) was measured at ages 15, 18, 24, 36, 48, 54, and 60 months. Measurements of weight (SECA weighing scale) and length/height (mobile mat/stadiometer) have been described in detail previously18. BMI was calculated as weight (kg)/length2 (m2), and transformed into age-and-sex-specific z-score using a local Singapore reference19. AC was measured using an inelastic measuring tape (Butterfly brand, China) to the nearest 1 mm. All measurements were taken based on standardized protocols in duplicates (and subsequently averaged)20.
At ages 18, 24, 36, 48, 54, and 60 months, triceps (TS) and subscapular (SS) skinfolds were measured on the right side of the body to the nearest 0.2 mm using Holtain skinfold calipers (Holtain Ltd, Crymych, UK). Three measurements were taken with the two closest values averaged. Sum of skinfold thickness (SST) was derived by adding TS and SS. The anthropometric measurements were measured by a team of trained clinical coordinators who were blinded to the child antibiotic exposure history.
Covariates
Maternal characteristics
Data on maternal ethnicity, age, educational attainment, regular cigarette smoking, and self-reported pre-pregnancy weight were collected from participants at recruitment. Maternal weights (SECA model 803, SECA Corp., Hamburg, Germany) and height during pregnancy (SECA stadiometer model 213) were measured. Total weight gains were calculated by subtracting self-reported pregnancy weight from the last measured weight before delivery. Pre-pregnancy BMI was calculated as weight(kg)/height2 (m2). Gestational diabetes mellitus (GDM) was defined based on the 1999 World Health Organization (WHO) criteria21,22.
Child characteristics
Information on gestational age at birth, birth weight, infant sex, mode of delivery, and birth order was abstracted from obstetric records. Gestational age was determined with a dating ultrasound scan in the first trimester. Low birth weight was defined as birth weight <2500 g and preterm birth as delivery of live birth at <37 weeks of gestation. Duration of any breastfeeding was calculated from parental report of infant milk feeding through interviewer-administered questionnaires at ages 3, 6, 9, and 12 months.
Gut microbiota
DNA extraction and V4 region of 16S rRNA gene sequencing
Since the gut microbiota becomes more stabilized around age 1-3 years23, we evaluated the gut microbiota at 24 months. Stool samples (n=392) were collected and stored at -80°C until further use. To ensure sufficient statistical power for gut microbiota analyses, these stool samples were collected only from the main study ethnic group, i.e. Chinese participants. Total microbial DNA was extracted by MoBio PowerFecal DNA kit (Qiagen, Carlsbad, CA) following the Earth Microbiome Project protocols (www.earthmicrobiome.org)24. The V4 region of 16S rRNA gene was amplified by polymerase chain reaction with modified 515F and 806R primers. The amplicon sequencing was performed on the MiSeq platform (Illumina, San Diego, CA) based on the standard protocol to generate 250 bp paired-end reads24. Quality control and raw sequencing data were processed with the same method as described in the previous study (see SupplementaryText 1)25. Altogether, 312 operational taxonomic units (OTUs) were obtained for the later analysis.
Statistical analysis
Maternal and child characteristics were first summarized [mean ± SD or n (%)]; independent sample t-tests (for continuous variables) and chi-square tests (for categorical variables) were used to test differences in characteristics according to childhood antibiotic exposure and obesity status.
Logistic regression was first used to assess the associations between antibiotic exposure in the first year of life and risk of any obesity between 15 to 60 months, defined as age-and-sex-specific BMI >95th percentile (using local reference) at any time point. The analysis was first adjusted for potential confounders (characteristics related to both antibiotic exposure and childhood obesity in this study): (categorically) ethnicity (Chinese/Malay/Indian), regular cigarette smoking (yes/no); (continuously) pre-pregnancy BMI. The full model was further adjusted for other determinants of childhood adiposity and other conceptually important factors in the literature: (categorically) birth order (first-born/non-first-born), mode of delivery (vaginal/non-vaginal), preterm birth (yes/no), low birth weight (yes/no), duration of any breastfeeding (<1 month, 1 to <3 months, 3 to <6 months, 6 to <12 months, and ≥12 months), gestational diabetes (yes/no); (continuously) maternal age at recruitment, height, and gestational weight gain. Because previous studies have reported a greater influence of antibiotic exposure on obesity risk in boys, we also stratified our analyses by sex. We also tested potential interaction of delivery mode and infancy antibiotic exposure on childhood adiposity and conducted corresponding stratified analyses. In sensitivity analyses, alternative longitudinal analysis methods using generalized estimating equations and Cox proportional hazard regression were performed. Furthermore, analyses were also repeated using BMI z-score derived using the WHO growth reference26. We have also used more stringent obesity outcomes, defined as 1) more persistent childhood obesity outcome (classified as obese at ≥2 time points, which represents roughly 8% of the study population) and 2) any obesity between 24-60 months.
The longitudinal associations of early life antibiotic exposure with offspring adiposity (BMI z-scores, AC, and SST as continuous variables) from 15 through 60 months were examined using linear mixed effects (LME) models with an unstructured covariance matrix for random effects variables (intercept and slope) and maximum likelihood estimation method27. A random intercept and random linear slope for age were also included in addition to the fixed effect of age (linear, quadratic, and cubic terms). The LME analysis was adjusted for the same factors in the binary outcome analyses.
For epidemiological analyses involving clinical outcomes, we consider childhood obesity as the primary outcome. For subgroup analysis, sex-stratified analyses were determined a priori and thus P <0.05 would also be a reasonable statistical threshold. Other associations should be considered exploratory and hypothesis generating that should be confirmed in future studies.
Gut microbiota data analysis
For microbiota analysis we utilized co-abundant network analysis constructed by SparCC algorithm and PERMANOVA test as stated in the Supplementary Text 125,28. The 312 OTUs clustered into 34 co-abundant groups (CAGs). Network of 29 CAGs with the absolute correlation coefficient value > 0.3 was visualized by using Cytoscape v3.5.1 (http://www.cytoscape.org/). The relative abundance of each CAG was derived by summing the abundance of OTUs present within them. The OTU with the highest relative abundance in each CAG was selected as the representative OTU.
Partial redundancy analysis (partial RDA) was used to identify CAGs associated with antibiotic exposure after adjusting for covariates in CANOCO 5.0 (Microcomputer Power, Ithaca, USA). Antibiotic exposure was used as the exposure variable while covariates included FUT2 secretor status (determined by polymorphisms of the FUT2 gene, which affected child gut microbiota (unpublished data)), delivery mode, duration of breastfeeding and birth weight (see Supplemental Text 2 for further details). Monte-Carlo permutation (999 permutations under the unrestricted model) was performed to evaluate the influence of antibiotics on the child gut microbiota during partial RDA. Further, we used multivariate associations with linear models (MaAsLin) to investigate each key CAG’s significance and association with the antibiotic exposure after adjusting for covariates29. The same analysis strategy is employed to the OTU-level analysis; α-diversity and β-diversity of gut microbiota were assessed using strategies stated in Supplementary Text 1. Heatmaps and scatter plots were plotted using MATLAB 2016b (The MathWorks, MA, USA). Relative abundances of CAGs were plotted using GraphPad Prism version 7 for Windows (GraphPad Software, CA, USA). Spearman correlation was used to evaluate the relationship between CAG/OTU and adiposity.
Results
The characteristics of GUSTO participants according to antibiotic exposure in the first year of life are shown in Table 1. Compared with non-exposed children, children exposed to antibiotics in the first year were more likely to be boys, born to mothers who were older, smoked cigarettes regularly, and who had a higher pre-pregnancy BMI. Chinese children were less likely to be exposed to antibiotics. Similar results were observed when participants were summarized according to total number of antibiotic courses exposed (Supplementary Table 1). When characteristics were summarized according to childhood obesity status (Supplementary Table 2), we found that obese children were more likely to be Malay or Indian, born low birthweight and to mothers with lower educational attainment and a higher pre-pregnancy BMI. The percentages of children meeting criteria for obesity at each time point and occasions classified as obese were shown in Supplemental Table 3.
Table 1. Characteristics of included GUSTO participants according to antibiotic exposure in the first year of life.
| Total (n= 1060) | Not exposed (n= 660) | Exposed (n= 400) | P-value1 | |
|---|---|---|---|---|
| Sex | 0.024 | |||
| Male | 557 (53%) | 329 (50%) | 228 (57%) | |
| Female | 503 (47%) | 331 (50%) | 172 (43%) | |
| Ethnicity | 0.06 | |||
| Chinese | 607 (57%) | 396 (60%) | 211 (53%) | |
| Malay | 266 (25%) | 153 (23%) | 113 (28%) | |
| Indian | 187 (18%) | 111 (17%) | 76 (19%) | |
| Birth order | 0.90 | |||
| First-born | 477 (45%) | 298 (45%) | 179 (45%) | |
| Non-first-born | 583 (55%) | 362 (55%) | 221 (55%) | |
| Education status | 0.28 | |||
| Primary or secondary | 309 (29%) | 191 (29%) | 118 (30%) | |
| Post-secondary | 382 (36%) | 228 (35%) | 154 (39%) | |
| University | 369 (35%) | 241 (37%) | 128 (32%) | |
| Maternal gestational diabetes | 0.28 | |||
| No | 876 (83%) | 539 (82%) | 337 (84%) | |
| Yes | 184 (17%) | 121 (18%) | 63 (16%) | |
| Preterm birth | 0.39 | |||
| No | 992 (94%) | 621 (94%) | 371 (93%) | |
| Yes | 68 (6%) | 39 (6%) | 29 (7%) | |
| Low birthweight | 0.36 | |||
| No | 980 (92%) | 614 (93%) | 366 (92%) | |
| Yes | 80 (8%) | 46 (7%) | 34 (9%) | |
| Mode of delivery | 0.95 | |||
| Vaginal | 738 (70%) | 460 (70%) | 278 (70%) | |
| Non-vaginal | 322 (30%) | 200 (30%) | 122 (30%) | |
| Maternal pre-pregnancy regular smoking | 0.004 | |||
| No | 925 (87%) | 591 (90%) | 334 (84%) | |
| Yes | 135 (13%) | 69 (10%) | 66 (17%) | |
| Breastfeeding duration | 0.68 | |||
| <1 month | 301 (28%) | 192 (29%) | 109 (27%) | |
| 1 to <3 months | 198 (19%) | 125 (19%) | 73 (18%) | |
| 3 to <6 months | 169 (16%) | 106 (16%) | 63 (16%) | |
| 6 to <12 months | 178 (17%) | 102 (15%) | 76 (19%) | |
| ≥12 months | 214 (20%) | 135 (20%) | 79 (20%) | |
| Maternal age, y | 30.8 ± 5.1 | 31.1 ± 5.1 | 30.5 ± 5.1 | 0.05 |
| Maternal height, cm | 158.3 ± 5.6 | 158.4 ± 5.7 | 158.2 ± 5.5 | 0.44 |
| Maternal pre-pregnancy BMI, kg/m2 | 22.7 ± 4.4 | 22.4 ± 4.3 | 23.2 ± 4.5 | 0.005 |
| Maternal pregnancy weight gain, kg | 13.5 ± 5.4 | 13.4 ± 5.1 | 13.5 ± 5.8 | 0.67 |
From independent sample t-test (continuous variables) and chi-square test (categorical variables)
In the fully adjusted logistic regression analyses, a higher odds of obesity age 15-60 months was observed for any antibiotic exposure in the first year of life (OR=1.45; 95% CI: 1.001, 2.11), particularly for exposure to ≥3 courses of antibiotics (OR=2.78; 95% CI: 1.12, 6.87) and for those exposed between ages 6 to 12 months (OR=1.57; 95% CI: 1.01, 2.42), as compared with children who were not exposed to antibiotics (Table 2). When the more parsimonious adjustment model (Model 1) was applied for sex-stratified analyses, the results were largely the same. These associations were also observed in unadjusted analyses and in a simpler model adjusting for only confounders. These results were more prominent in boys than in girls, although the interaction term was not statistically significant. Results were similar when BMI z-score was derived using the WHO (instead of local) growth reference (Supplementary Table 4) or when Cox proportional hazard regression and generalized estimating equation (instead of logistic regression) were used (Supplementary Table 5 and Supplementary Table 6, respectively). The patterns of associations, including potential sex-interaction, between antibiotic exposure and later obesity outcomes remained similar using persistent obesity and any obesity between 24-60 months as the outcomes, although general attenuation and wider confidence intervals were observed (Supplemental Table 7 & 8).
Table 2. Associations between early life antibiotic exposure with later childhood obesity (age- and sex- specific BMI > 95th percentile at any time point between ages 15-60 months)1.
| Total population (n= 1016) | Boys (n= 535) | Girls (n= 481) | Boys (n= 535) | Girls (n= 481) | ||||
|---|---|---|---|---|---|---|---|---|
| Obese | Unadjusted OR | Model 12 | Model 23 | Model 12 | Model 12 | Model 23 | Model 23 | |
| Any antibiotic in first year | P-interaction= 0.40 | P-interaction= 0.40 | ||||||
| Not exposed | 73 (12%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Exposed | 69 (18%) | 1.60 (1.12, 2.28)* | 1.47 (1.02, 2.11)* | 1.45 (1.001, 2.11)* | 1.63 (0.998, 2.67) | 1.24 (0.71, 2.16) | 1.59 (0.95, 2.64) | 1.16 (0.64, 2.10) |
| Number of antibiotic courses | P-interaction= 0.41 | P-interaction= 0.57 | ||||||
| Not exposed | 73 (12%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 44 (17%) | 1.49 (0.99, 2.24) | 1.38 (0.91, 2.09) | 1.37 (0.89, 2.09) | 1.54 (0.88, 2.71) | 1.18 (0.63, 2.20) | 1.50 (0.84, 2.67) | 1.05 (0.53, 2.05) |
| 2 | 14 (15%) | 1.37 (0.74, 2.54) | 1.16 (0.61, 2.18) | 1.12 (0.58, 2.17) | 0.88 (0.36, 2.16) | 1.27 (0.52, 3.10) | 0.88 (0.35, 2.22) | 1.27 (0.48, 3.37) |
| ≥3 | 8 (27%) | 2.73 (1.17, 6.37)* | 2.77 (1.16, 6.62)* | 2.78 (1.12, 6.87)* | 4.36 (1.60, 11.88)** | - | 3.67 (1.29, 10.44)* | - |
| Age at first antibiotic prescription | P-interaction= 0.39 | P-interaction= 0.40 | ||||||
| Not exposed | 73 (12%) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| <3 months | 8 (12%) | 1.06 (0.48, 2.30) | 0.96 (0.43, 2.11) | 0.86 (0.38, 1.93) | 0.92 (0.32, 2.61) | 0.95 (0.27, 3.32) | 0.74 (0.25, 2.22) | 0.92 (0.25, 3.43) |
| 3 to <6 months | 16 (17%) | 1.54 (0.85, 2.79) | 1.48 (0.81, 2.70) | 1.40 (0.75, 2.62) | 1.35 (0.60, 3.03) | 1.81 (0.72, 4.54) | 1.34 (0.58, 3.06) | 1.62 (0.57, 4.59) |
| 6 to 12 months | 42 (18%) | 1.70 (1.12, 2.57)* | 1.52 (0.995, 2.33) | 1.57 (1.01, 2.42)* | 1.88 (1.06, 3.32)* | 1.08 (0.55, 2.12) | 1.89 (1.05, 3.41)* | 1.01 (0.50, 2.05) |
Values are odds ratio (95% CI) from logistic regressions. *P< 0.05; **P <0.01
Model 1: adjusted for ethnicity and pre-pregnancy BMI
Model 2: adjusted for ethnicity, maternal age, education status, height, pre-pregnancy BMI, weight gain during pregnancy, gestational diabetes, regular smoking, mode of delivery, child birth order, preterm birth, low birth weight, duration of any breastfeeding
Because estimate for ≥3 courses of antibiotic exposure was not estimable for girls, we collapsed the exposure categories into “not exposed (ref.)”, “exposed to 1 course”, and “exposed to ≥2 courses” for stratified analysis in girls.
When adiposity measures (BMI z-score, SST, and AC) of the children were analyzed continuously, no statistically significant associations were observed for the overall population (Table 3). However, in sex-stratified analyses, antibiotic exposure (binary, exposure to 1 or ≥3 courses, and exposure at ages 6 to 12 months) was associated with higher BMI z-score (P-interactions≤0.08 for all) and AC in boys. In girls, exposure to antibiotics at age 3 to <6 months specifically was associated with higher AC (P-interaction=0.013), but not for BMI z-score and SST or for all other antibiotic exposure classifications.
Table 3. Associations between antibiotic exposure in the first year of life and adiposity measures between ages 15-60 months1,2.
| BMI z-score, SD | Sum of skinfolds, mm | Abdominal circumference, cm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n= 1016) | Boys (n= 535) | Girls (n= 481) | Total (n= 996) | Boys (n= 524) | Girls (n= 472) | Total (n= 1016) | Boys (n= 535) | Girls (n= 481) | |
| Any antibiotic in first year | P-interaction= 0.026 | P-interaction= 0.50 | P-interaction= 0.16 | ||||||
| Not exposed | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Exposed | 0.07 (-0.03, 0.17) | 0.15 (0.01, 0.28)* | -0.04 (-0.19, 0.11) | 0.05 (-0.30, 0.41) | 0.13 (-0.35, 0.62) | -0.06 (-0.58, 0.47) | 0.25 (-0.10, 0.59) | 0.37 (-0.08, 0.82) | 0.02 (-0.49, 0.53) |
| Number of antibiotic courses | P-interaction= 0.08 | P-interaction= 0.14 | P-interaction= 0.42 | ||||||
| Not exposed | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 0.06 (-0.06, 0.17) | 0.16 (0.01, 0.31)* | -0.08 (-0.24, 0.08) | 0.26 (-0.14, 0.66) | 0.41 (-0.13, 0.96) | 0.07 (-0.52, 0.66) | 0.24 (-0.15, 0.62) | 0.42 (-0.10, 0.93) | -0.08 (-0.64, 0.49) |
| 2 | 0.05 (-0.12, 0.23) | 0.07 (-0.15, 0.30) | 0.05 (-0.22, 0.31) | -0.57 (-1.19, 0.04) | -0.79 (-1.60, 0.02) | -0.29 (-1.24, 0.67) | 0.02 (-0.58, 0.61) | 0.08 (-0.68, 0.83) | -0.07 (-1.01, 0.87) |
| ≥3 | 0.12 (-0.17, 0.40) | 0.23 (-0.10, 0.56) | -0.11 (-0.64, 0.43) | 0.27 (-0.72, 1.26) | 0.81 (-0.34, 1.96) | -1.22 (-3.15, 0.71) | 0.93 (-0.05, 1.91) | 1.15 (0.05, 2.26)* | 0.57 (-1.32, 2.45) |
| Age at first antibiotic prescription | P-interaction= 0.037 | P-interaction= 0.17 | P-interaction= 0.013 | ||||||
| Not exposed | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| <3 months | -0.06 (-0.25, 0.14) | 0.01 (-0.26, 0.27) | -0.09 (-0.39, 0.20) | -0.32 (-1.03, 0.39) | -0.03 (-1.01, 0.96) | -0.59 (-1.64, 0.46) | -0.39 (-1.08, 0.30) | 0.18 (-0.72, 1.09) | -0.99 (-2.02, 0.03) |
| 3 to <6 months | 0.07 (-0.10, 0.24) | 0.11 (-0.10, 0.32) | 0.11 (-0.17, 0.39) | 0.33 (-0.27, 0.94) | 0.07 (-0.68, 0.83) | 1.02 (-0.02, 2.07) | 0.53 (-0.05, 1.12) | 0.31 (-0.39, 1.02) | 1.31 (0.33, 2.28)** |
| 6 to 12 months | 0.09 (-0.03, 0.21) | 0.21 (0.04, 0.37)* | -0.09 (-0.26, 0.08) | 0.07 (-0.35, 0.50) | 0.29 (-0.30, 0.87) | -0.22 (-0.83, 0.39) | 0.30 (-0.11, 0.71) | 0.53 (-0.02, 1.08) | -0.18 (-0.77, 0.41) |
Values are beta coefficients (95% CI) from linear mixed effects models. *P< 0.05, **P< 0.01
Estimates were adjusted for ethnicity, maternal age, education station, height, pre-pregnancy BMI, weight gain during pregnancy, gestational diabetes, regular smoking, mode of delivery, child exact age at measurement, sex (except for BMI z-score and in sex-stratified analyses), birth order, preterm birth, low birth weight, duration of any breastfeeding
There were no statistically significant interactions between delivery mode and infancy antibiotic exposure on childhood obesity risk and adiposity (all P-interactions > 0.15) (Supplemental Table 9 and 10). However, in stratified analyses, exposure to ≥3 courses of antibiotic during infancy was associated with a higher risk of obesity only among children delivered through vagina, whereas later antibiotic exposure (between 6 to 12 months) was only associated with higher later obesity risk among non-vaginal delivered children (Supplemental Table 9). These associations of sub-groups should be confirmed in bigger studies with sufficient number of cases in both modes of delivery.
Antibiotic exposure is associated with the child gut microbial community
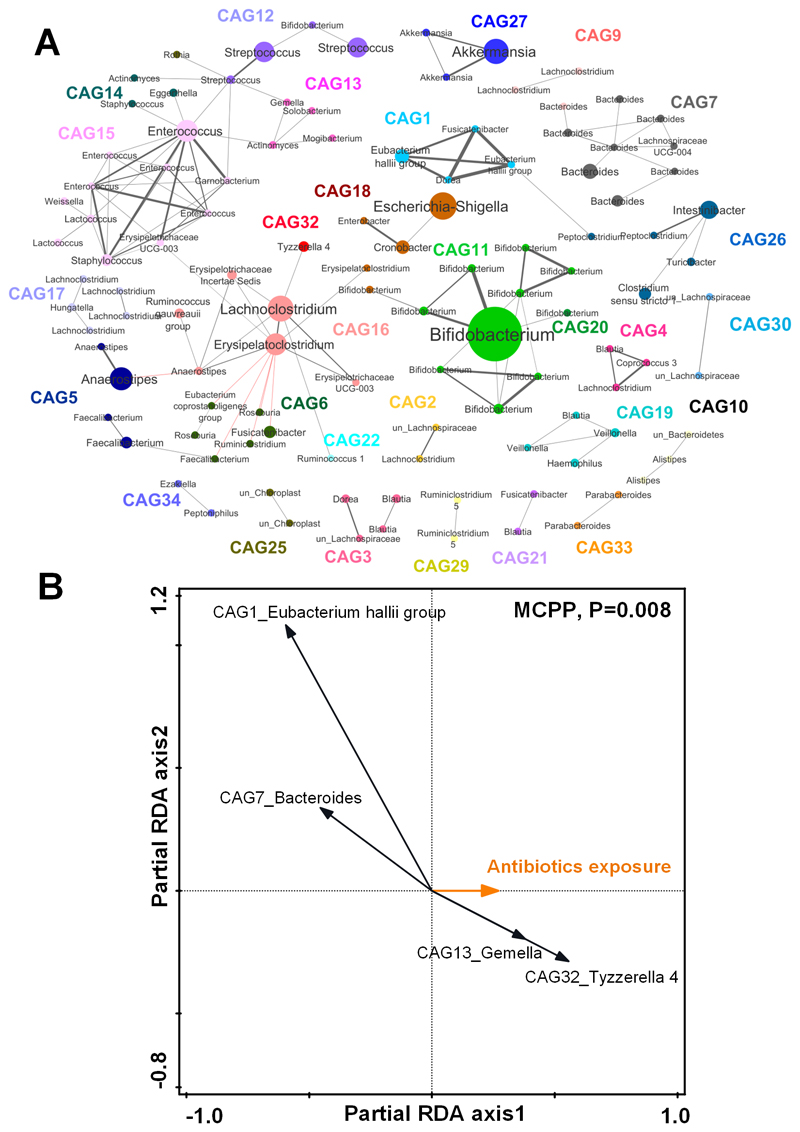
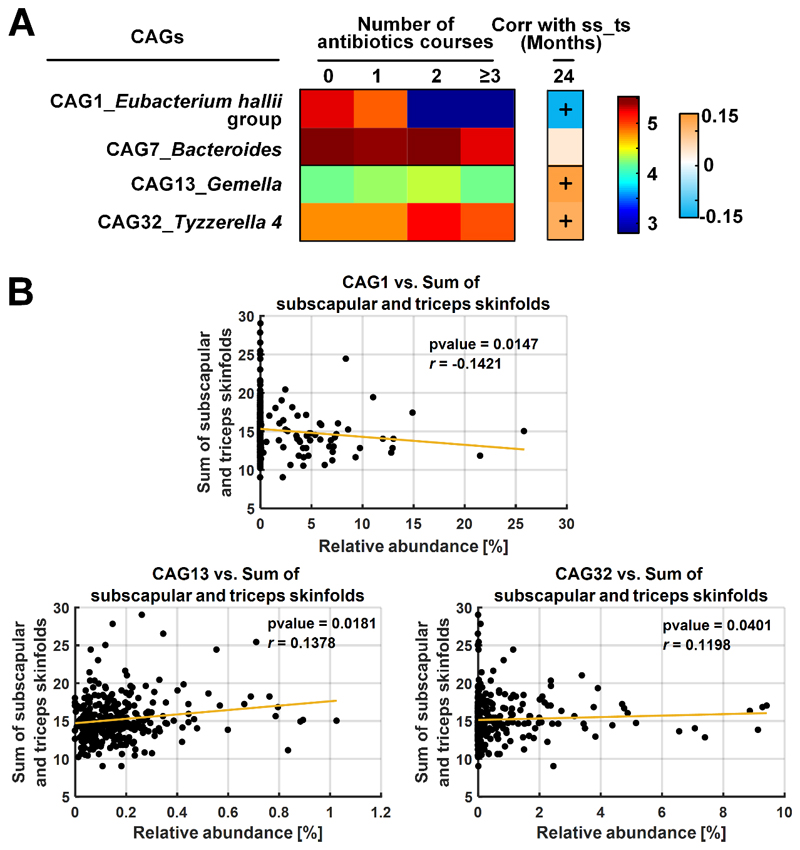
The co-abundant network of gut microbiota is shown in Figure 1A. The OTUs clustered into 29 CAGs and were used in the partial RDA to assess the influence of antibiotic exposure on the child’s overall gut microbiota. After accounting for covariates, antibiotic exposure in the first 12 months affected gut microbiota (P<0.01) (Figure 1B). We identified 4 key CAGs affected by the antibiotic exposure (Figure 2A). Among them, the relative abundances of 2 CAGs decreased, while the other two increased, with increasing antibiotic exposure. In particular, antibiotic exposure inhibited CAG1 represented by the Eubacterium hallii group (FDR-P-value<0.001). The mean relative abundance of CAG1 was reduced from 1.78% to 0.01% if children received at least 3 courses of antibiotic treatment compared with non-treatment (Supplementary Figure 2A and Supplementary Table 11). Moreover, CAG1 in turn showed an inverse association with SST (Figure 2B). In contrast, CAG13 and CAG32 represented by Gemella (FDR-P-value=0.02) and Tyzzerella 4 (FDR-P-value<0.001, Supplementary Figure 2B and Supplementary Table 11) were enriched with increasing antibiotic exposure and showed positive association with SST (Figure 2B).
Figure 1. Co-abundant network analysis of the child gut microbiota at 24 months and key co-abundant groups associated with the antibiotic exposure by partial redundancy analysis (partial RDA).
A) Co-abundant network highlighting relationships between OTUs of the child gut microbiota at 24 months. Each node represents one OTU. Nodes with the same color are from the same co-abundant group (CAG). Node size indicates the average abundance of each OTU in this cohort. The taxonomy of each OTU is labeled. The OTU with the highest relative abundance in each CAG was selected as the representative OTU. Grey and red lines show significant positive and negative correlations between two OTUs with an absolute coefficient value greater than 0.3. B) Partial RDA biplots illustrating the key CAGs associated with the effects of antibiotic exposure after adjusting for the covariates. The number of antibiotic courses infants received in the first year of life was used as the exposure variable. Secreting status, delivery mode, duration of breastfeeding and birth weight were all used as covariates. CAGs that explained ≥ 1% of the variability of samples on the first axis are indicated by arrows. The significance of the effects of the antibiotic exposure was tested using the Monte-Carlo Permutation Procedure (MCPP).
Figure 2. Heatmap of key co-abundant groups (CAGs) associated with antibiotic exposure identified by the partial RDA and Spearman’s correlation between identified CAGs and the accumulation of adiposity at 24 months.
A) Heatmap of key CAGs identified by partial RDA. The panel on extreme left represents the mean relative abundance (log-transformed) of the CAGs in each antibiotic exposure group. Color scale used for representing the CAG abundance is provided in the third panel from left, where brown represents the highest and dark blue represents the lowest abundance. The second panel from left represents R-value of Spearman’s correlation between the CAG and the sum of subscapular and triceps skinfolds (ss-ts) at 24 months. ‘+’ in the boxes indicate the associations that passed a significant of P <0.05. Color scale for spearman correlation is provided in the extreme right panel. B) Scatter plots of relative abundances of key CAGs against the sum of subscapular and triceps skinfolds at 24 months.
Similar results were observed at the OTU level, reinforcing the notion that first-year antibiotic exposure is associated with child gut microbial composition at age 24 months. Principal coordinate analysis based on Bray-Curtis distance suggested that antibiotic exposure altered child’s gut microbiota structure (Supplementary Figure 3). By using partial RDA, we subsequently identified 14 OTUs associated with the repeated antibiotic exposure from 312 OTUs. Five OTUs were reduced by the cumulative antibiotic exposure in the first year, while 9 were enriched (Supplementary Figure 4-6, Supplementary Table 12). Among the 5 reduced OTUs, 2 OTUs from Eubacterium hallii group and 1 from Bacteroides correlated negatively with BMI z-score and SST at 24 months (Supplementary Figure 5 and Supplementary Figure 7). Among 9 enriched OTUs, OTU1466 from Tyzzerella 4 correlated positively with SST at age 24 months (Supplementary Figure 5 and Supplementary Figure 12). Antibiotic exposure did not change the α-diversity of gut microbiota. Sex-specific gut microbiota analyses were not feasible due to lower number of subjects with available microbiota data.
Discussion
In this multi-ethnic Asian mother-offspring cohort, we found that antibiotic exposure in the first year of life was associated with higher adiposity and higher risk of obesity between ages 15-60 months, especially in boys and with repeated exposure. To our knowledge, we showed for the first time in human pediatric population that antibiotic-use related alterations in the gut microbiota composition can be a potential mechanism linking the observed antibiotics-obesity associations.
Previous studies investigating associations between infant antibiotic exposure and childhood obesity mainly utilized data from prospective birth cohort studies or retrospective cohorts using electronic health databases. These studies in general reported that antibiotic exposure in infancy was associated with higher obesity risk or higher adiposity in childhood, with a stronger association observed in boys2–4, with earlier exposure timing2,5–8, and with repeated exposure2,5,6,9. When compared with the only study that included Asian participants3, concordance in results was also noted. Nonetheless, two studies reported no associations between infant antibiotic exposure and childhood weight gain10 and obesity11 in their primary analyses. The latter study did not examine influence of repeated exposure to antibiotics and both studies did not investigate potential sex interactions. Our study confirmed that the association of antibiotic exposure with higher childhood obesity risk is stronger in boys and with repeated exposure to antibiotics. The sex differences observed may be due to differential intestinal adaptation in response to antibiotics in males and females as shown in animal studies30, though exact mechanisms should be further explored. We, however, did not observe that earlier exposure (<3 months old) in the context of periods within the first year of life was associated with higher risk of obesity. At the time of study subjects recruitment, the duration of maternity leave after childbirth in Singapore was 12 weeks 31, after which infants may be sent to infant care centers while mothers return to work. During this transitional period, the infants are exposed to more diverse microbes in the care center and also through interaction with other infants, and less breastfeeding/breastmilk consumption, which in turn could have exacerbated the influence of antibiotic exposure. This explanation is, however, speculative. A previous study, albeit focusing on antibiotics exposure from birth to age 18, reported that both recent and cumulative doses of antibiotic exposure were associated with higher childhood BMI, and that some of these impacts strengthened with age9. Taken together, it may be important to exercise continued judicious use of antibiotics throughout childhood. Alternatively, our results based on timing of antibiotic exposure could have been affected by chance findings and should be further examined in studies with a higher number of participants.
The associations between antibiotic exposure attenuated with more stringent outcome definition (classified as obese at ≥2 time points or any obesity between 24-60 months). This may suggest that infancy antibiotic exposure is correlated more strongly with more transient or immediate obesity outcomes after the exposure. However, our study might have also been underpowered to detect such associations, thus these observations will need to be confirmed in larger study.
Gut microbiota is one of the key factors maintaining host health and development of metabolic diseases25,32–34. Disruption in gut microbial colonization by repeated antibiotic exposure in early life may be the mechanism underlying our observations. Particularly in mice models, antibiotic-induced variations in gut microbiota during early life have been associated with weight gain and adiposity accumulation through changing host metabolism and immunological development 15,16. In a human pediatric population we showed that microbial variations induced by cumulative antibiotic exposure in the first year were associated with adiposity accumulation at 24 months of age.
We observed that repeated antibiotic exposure in the first year significantly reduced CAG1 represented by the OTU from Eubacterium hallii, which was negatively correlated with BMI z-score and SST at 24 months. Eubacterium hallii is a member of the butyrate-producers35,36. Following fecal transplantation from lean donors, Eubacterium hallii was enriched in small intestinal biopsies of 9 obese and insulin resistant subjects; this increase was associated with improved metabolic syndrome measures37. In contrast, repeated antibiotic exposure significantly enriched CAG32 represented by the OTU from Tyzzerella 4, which was positively correlated with SST at 24 months. Tyzzerella 4 might contain potentially unfavorable bacteria, since this taxa has been reported to be enriched in individuals with high risk of cardiovascular disease38. Additionally, obese mice fed on a high-fat-diet (compared with normal diet) tended to harbor more Tyzzerella 4 in the intestine39.
A strength of our study is the high resolution of outcome variables through frequent measurement of anthropometry and adiposity indicators in the study children; these measurements were obtained by trained research personnel, thus reducing measurement (recall) errors associated with self-reports as in some previous studies. Furthermore, we adjusted for a comprehensive list of confounders, including prenatal and antenatal maternal factors, which may not have been collected in previous studies based on electronic databases. Despite our best efforts to account for confounding from the available data, unmeasured confounder(s) can remain a concern. For example, prenatal antibiotic exposure has been associated with both offspring gut microbiota and obesity40 but not adjusted in our analyses. We thus assessed sensitivity of our estimates to unmeasured confounding via the E-value proposed by VanderWeele and Ding41. The higher the E-value, the stronger the confounder associations must be to explain an effect. For main antibiotics-childhood obesity estimates, our observed odds ratios of 1.45, 2.78, 1.57 (for binary antibiotics, ≥3 antibiotic courses, and exposure at 6 to 12 months, respectively) yielded E-values of 2.26, 5.00, and 2.52, respectively. This means that the main estimates could be explained by an unmeasured confounder that was associated with both the exposure and outcome by an odds ratio of 2.26, 5.00, and 2.52-fold, respectively, above and beyond the measured confounders, but weaker confounding could not do so. For lower confidence intervals, a relatively weak unmeasured confounder associated with both early life antibiotic exposure and childhood obesity by an odds ratio of 1.03, 1.49, and 1.11-fold could explain the lower confidence limit.
Our data on child’s antibiotic exposure was self-reported by the parents, which may have increased misclassification. However, since obesity and adiposity outcomes happened after the administration of questionnaires concerning antibiotic exposure, the probability of differential misclassification bias was likely reduced, resulting in more conservative observed estimates. We were also limited by a modest sample size (cf. studies using electronic health databases). Due to our moderate study size, we are underpowered to study the association between antibiotic exposure and more severe childhood obesity outcomes (e.g. BMI z-score >99 percentile); these should be further investigated in future studies with a sufficient sample size. Furthermore, because many statistical tests were conducted for the current study, multiple comparisons are a potential concern, and associations other than childhood obesity measure and sex-stratified analyses should be considered hypothesis generating. Selection bias could also arise due to losses of potential participants during follow-up. Compared to followed-up singleton births included in the current epidemiological investigation, children who were not included were less likely to be preterm or classified as low birthweight babies at birth; their mothers were older at recruitment, had a higher educational attainment, and tended to breastfeed for a longer duration (Supplemental Table 13). Similar differences were noted comparing children included for gut microbiota analysis vs. those not included (Supplemental Table 14). These observations suggest that our results may be more generalizable to more educated, health-conscious mothers and healthier children in Singapore, and our effect estimates could have been underestimations for more vulnerable populations, though this should be further investigated. Our findings may also have less generalizability outside of Singapore. In this study, we used 16S rRNA gene sequencing for the profiling of gut microbiota, which can be limiting in the taxonomic resolution and identification of functional genes related with the antibiotics treatment. A future analysis utilizing metagenomics sequencing would help build more granularity in the findings. Last, as with any observational study causality cannot be claimed.
Conclusions
Antibiotic exposure during the first year of life, especially among boys or with repeated exposure, was associated with higher risk of childhood obesity and increased adiposity from ages 15-60 months. The antibiotic-use associated alterations in the gut microbiota and their link with child adiposity sheds light on the potential mechanism through which administration of antibiotics can lay the foundation for metabolic adversities of future. The findings of this study add to calls for careful consideration of the benefits vs. risks of administrating antibiotics and the frequency of their use in early life.
Supplementary Material
Acknowledgements
The authors thank the GUSTO study group, which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Carolina Un Lam, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Xiu Ling Loo, Fabian Yap, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Izzuddin Bin Mohd Aris, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira., Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Peter D. Gluckman, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Victor Samuel Rajadurai, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan, Yung Seng Lee and Zhongwei Huang.
Funding
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore. Study sponsors were not involved in the design of the study, statistical analysis and results interpretation. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group and NIHR Southampton Biomedical Research Centre) and by the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP).
List of Abbreviation
- AC
abdominal circumference
- BMI
body mass index
- CAG
co-abundant group
- GDM
gestational diabetes mellitus
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- LME
linear mixed effects
- OTU
operational taxonomic units
- RDA
redundancy analysis
- SS
subscapular skinfold
- SST
sum of skinfold thickness
- TS
triceps skinfold
- WHO
World Health Organization
Footnotes
Competing interests
KMG, Y-SC, and YSL have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. NK, KMG and Y-SC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no financial or personal conflict of interest to declare.
Authors' contributions
L-WC conducted statistical analysis, interpreted the data, and wrote the first draft of the paper. JX conducted the microbiota analysis, interpreted the data, and co-wrote the first draft of the paper. SES acquired antibiotic exposure data. NK and JAG generated the microbiota data and contributed to the microbiota analysis. L-WC, JX, SES, IMA, and MT-T contributed to data collection, cleaning, and analysis. PDG, KHT, LP-CS, Y-SC, FY, KMG, and YSL designed the GUSTO study. All authors critically revised and approved the final manuscript. YSL had primary responsibility for the final content.
References
- 1.The Lancet. Managing the tide of childhood obesity. Lancet. 2015;385:2434. doi: 10.1016/S0140-6736(15)61122-9. [DOI] [PubMed] [Google Scholar]
- 2.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic Exposure in Infancy and Risk of Being Overweight in the First 24 Months of Life. Pediatrics. 2015;135:617–626. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 3.Murphy R, Stewart AW, Braithwaite I, Beasley R, Hancox RJ, Mitchell EA. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obes. 2014;38:1115–1119. doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 4.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes. 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 5.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016;151:120–129.e5. doi: 10.1053/j.gastro.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbakwa CA, Scheres L, Penders J, Mommers M, Thijs C, Arts ICW. Early Life Antibiotic Exposure and Weight Development in Children. J Pediatr. 2016;176:105–113.e2. doi: 10.1016/j.jpeds.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Ajslev TA, Andersen CS, Gamborg M, Sørensen TIA, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 8.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz BS, Pollak J, Bailey-Davis L, Hirsch AG, Cosgrove SE, Nau C, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes. 2016;40:615–621. doi: 10.1038/ijo.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, et al. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA. 315:1258–65. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 11.Li D-K, Chen H, Ferber J, Odouli R, Ha C, Lam Y, et al. Infection and antibiotic use in infancy and risk of childhood obesity: a longitudinal birth cohort study. Lancet Diabetes Endocrinol. 2016;0:16498–16517. doi: 10.1016/S2213-8587(16)30281-9. [DOI] [PubMed] [Google Scholar]
- 12.Isolauri E, Salminen S, Rautava S. Early Microbe Contact and Obesity Risk: Evidence Of Causality? J Pediatr Gastroenterol Nutr. 2016;63(Suppl 1):S3–5. doi: 10.1097/MPG.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 13.Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev. 2015;73 doi: 10.1093/nutrit/nuv039. [DOI] [PubMed] [Google Scholar]
- 15.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2013 doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 18.Chen L-W, Aris I, Bernard J, Tint M-T, Chia A, Colega M, et al. Associations of Maternal Dietary Patterns during Pregnancy with Offspring Adiposity from Birth Until 54 Months of Age. Nutrients. 2016;9:2. doi: 10.3390/nu9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Healthcare Group Polyclinics. Age and gender-specific national BMI cut-offs (Singapore) 2010 [Google Scholar]
- 20.Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–60. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Chong Y-S, Cai S, Lin H, Soh SE, Lee Y-S, Leow MK-S, et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth. 2014;14:345. doi: 10.1186/1471-2393-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22:713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 24.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: a Multicenter, Randomized, Open Label Clinical Trial. MBio. 2018;9 doi: 10.1128/mBio.02392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 27.Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. Epidemiol Perspect Innov. 2007;4:8. doi: 10.1186/1742-5573-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman J, Alm EJ. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput Biol. 2012;8:e1002687. doi: 10.1371/journal.pcbi.1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miles RD, Butcher GD, Henry PR, Littell RC. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult Sci. 2006;85:476–485. doi: 10.1093/ps/85.3.476. [DOI] [PubMed] [Google Scholar]
- 31.Ong SF, Chan W-CS, Shorey S, Chong YS, Klainin-Yobas P, He H-G. Postnatal experiences and support needs of first-time mothers in Singapore: A descriptive qualitative study. Midwifery. 2014;30:772–778. doi: 10.1016/j.midw.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine. 2015;2:968–984. doi: 10.1016/j.ebiom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science (80-) 2013;341 doi: 10.1126/science.1241214. 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9:552–562. doi: 10.1038/ismej.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan SH, Louis P, Flint HJ. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty SA, Ritari J, Paulin L, Smidt H, De Vos WM. Complete Genome Sequence of Eubacterium hallii Strain L2-7. Genome Announc. 2017;5 doi: 10.1128/genomeA.01167-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-Bacterial Mutualism in the Human Intestine. Science (80-) 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y, Li Y, Du Y, Guo L, Chen M, Huang X, et al. Konjaku flour reduces obesity in mice by modulating the composition of the gut microbiota. Int J Obes. 2018 doi: 10.1038/s41366-018-0187-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Differding MK, Benjamin-Neelon SE, Østbye T, Hoyo C, Mueller NT. Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome. Ann Clin Microbiol Antimicrob. 2019;18:18. doi: 10.1186/s12941-019-0318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-Value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.