Abstract
Background
in women without diabetes, little is known about the consequences of hyperglycaemia during pregnancy for the offspring cardiovascular structure and function.
Objective
To investigate the association of maternal glycaemia during pregnancy with cardiovascular risk markers in their children in GUSTO, a Singaporean birth cohort study.
Methods
Around 26 weeks’ gestation, a 75g oral glucose tolerance test was performed and fasting (FPG) and 2-hr postprandial plasma glucose (2-hr PPPG) concentrations were measured. Gestational diabetes mellitus (GDM) was defined using WHO 1999 diagnostic criteria. At age 6 years, we measured the child’s carotid intima-media thickness (cIMT), carotid-femoral pulse wave velocity (cfPWV), aortic augmentation index (AIx) and blood pressure (BP). Association of maternal glycaemia during pregnancy with cardiovascular risk markers in their children were analysed using multiple linear and logistic regressions.
Results
Analysis were performed on 479 mother-child dyads. Higher maternal FPG was associated with higher cIMT and in male, higher cfPWV in the offspring (adjusted β [CI 95%], cIMT: 0.08 per 10mm increase [0.02; 0.15], cfPWV: 0.36 m/s [0.01; 0.70]). Higher 2-hr PPPG was associated with higher cfPWV and AIx. GDM was associated with higher AIx. No association was found between maternal glycaemia and their offspring blood pressure.
Conclusions
among mothers without pre-existing diabetes, higher glycaemia during pregnancy was associated with mild structural and functional vascular changes in their children at age 6 years across a continuum. These results support the necessity to monitor maternal glycaemia during pregnancy even in absence of pre-existing diabetes or diagnosed gestational diabetes.
Keywords: maternal glycaemia, pregnancy, children, cIMT, PWV, AIx
Introduction
Gestational diabetes mellitus (GDM) is a state of glucose intolerance occurring for the first time during pregnancy and is associated with an increased risk of later type 2 diabetes mellitus (DM)1. There is scant evidence of the the adverse impact of GDM on the offspring’s cardiovascular (CV) health2 as most research has focused on type 1 and type 2 DM. Maternal hyperglycaemia during pregnancy is associated with an elevated risk of congenital cardiac (and non-cardiac) malformations and cardiac hypertrophy among pre-existing diabetic mothers3,4. Based on the developmental origin of health and disease paradigm, maternal hyperglycaemia during pregnancy may play a role in “fetal programming” and lead to detrimental later-life health outcomes5.
During pregnancy, a hyperglycaemic intrauterine environment is associated with fetal hyperinsulinemia which itself can cause cardiovascular structural and functional changes3,4. Growing evidence suggests that even in the absence of manifest diabetes, increasing maternal glycaemia across a continuum during pregnancy is associated with adverse perinatal outcomes such as perinatal death, bone fracture, nerve palsy, large-for-gestational age or neonatal hypoglycaemia6–8. Yet little is known about the influence of maternal hyperglycaemia on child CV health at later ages. Markers of subclinical CV disease such as carotid intima media thickness (cIMT), carotid-femoral pulse wave velocity (cfPWV), and augmentation index (AIx) can now be measured non-invasively. These markers provide surrogate information on early CV structure and function and are important predictors of future CV disease events9,10 but they have been rarely examined in children
Using data from a Singaporean multi-ethnic birth cohort study, we prospectively investigated the association of maternal glycaemia during pregnancy with cIMT, cfPWV, AIx and blood pressure (BP) in their children aged 6 years.
Methods
Study population
GUSTO (Growing Up in Singapore Towards healthy Outcomes) is a multi-ethnic mother-child cohort study. The detailed study description has been published11. Between 2009 and 2010, 1247 pregnant women aged ≥18 years who attended their first-trimester ultrasound scan at Singapore’s two major public maternity units: National University Hospital (NUH) or KK Women’s and Children’s hospital (KKH). Inclusion criteria included Singaporean citizenship or permanent resident status, Chinese, Malay or Indian ethnicity, intention to deliver either at NUH or KKH, intention to remain in Singapore for five years, and willingness to donate birth tissues at delivery. Mothers receiving chemotherapy or psychotropic drugs or who had a diagnosis of type 1 DM were excluded. Informed written consent was obtained from the women and their children when they reached age 6 years. The study was approved by both the National Healthcare Group Domain Specific Review Board (reference D/09/21) and SingHealth Centralized Institutional Review Board (reference 2009/280/D).
Maternal glycaemia
After an overnight fast during the clinic visit around 26 weeks’ gestation, a 75g oral glucose tolerance test (OGTT) was performed only in mothers without pre-existing DM and not known GDM. Venous blood samples were collected in fluoride-containing tubes, with fasting (FPG) and 2-hr postprandial (2-hr PPPG) plasma glucose concentrations measured by colorimetry [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics) or Beckman LX20 Pro analyzer (Beckman Coulter)]. GDM was defined as FPG ≥7.0 mmol/L and/or 2-h PPPG ≥7.8 mmol/L (WHO diagnostic criteria 1999). Following their OGTT in GUSTO, mothers diagnosed as having GDM were either diet-treated only or diet- and insulin-treated.
Child cIMT, cfPWV, and BP
At age 6 years, all participants were invited for a non-invasive vascular assessment performed by trained sonographers using a standard protocol. CIMT was assessed by high resolution B-mode ultrasound using a high-frequency linear transducer and commercially available ultrasound systems (Philips CX-50 xMatrix at KKH and Aloka Prosound Alpha-10 at NUH ) in accordance with the recommendations of the American Society of Echocardiography12. The right common carotid artery was scanned in the lateral plane and cIMT measured at the far wall, 1 cm proximal to the carotid bulb, in an area devoid of plaque. Triplicate cIMT measurements were averaged and used for analysis. Observer variability for cIMT assessed in 88 subjects suggested moderate rater reliability. For intra-observer variability (n=32), the intraclass correlation coefficient (ICC) based on a mean-rating (k = 2), absolute-agreement, 2-way mixed-effects model was 0.66 (95% CI, 0.30 – 0.84). The ICC for inter-observer variability (n=56) was 0.70 (95% CI, 0.54 – 0.81).
CfPWV was measured in the supine position using applanation tonometry (SphygmoCorVx, AtCor Medical, West Ryde, NSW, Australia). The carotid-femoral path length was obtained by subtracting right common carotid-suprasternal notch distance measured with a tape ruler from suprasternal notch-femoral distance. Carotid-femoral transit time was obtained by subtracting the time between onset of the electrocardiographic R-wave and the foot of the carotid pulse and the time between the R-wave and the femoral pulse, each averaged from 8 to 10 sequential waveforms. CfPWV was calculated as the carotid-femoral path length divided by the transit time. In a previous study of 96 subjects conducted at NUH, the intra-observer ICC assessed as per cIMT above was 0.94 (95% CI, 0.92 – 0.97), indicating excellent reliability.
Pulse wave analysis: from about 10 seconds of sequential radial artery waveforms obtained by the high-fidelity tonometer, the aortic pressure waveform was reconstructed by the SphygmoCorPx System using a transfer function13. This waveform depends on left ventricular ejection, as well as the timing and amount of wave reflection from branch points or areas of impedance mismatch which are determined by aortic stiffness and arteriolar tone14. From the aortic pressure waveform, central systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse pressure were derived. Central or aortic augmentation index (AIx) was calculated as the increment in pressure from the first systolic shoulder of the ascending aortic pressure wave to the peak of the second, late systolic shoulder, expressed as a percentage of the pulse pressure. Because AIx is influenced by heart rate, it was normalized to a heart rate of 75 beats/min (AIx@75) to facilitate comparison.
During the 6-year clinic visit, BP was measured using Dynamap CARESCAPETM V100 (GE Healthcare, Milwaukee, WI), with an appropriate child cuff, by trained research staff. The measurement was taken in a quiet room from the right upper arm in a seated position, with legs uncrossed and the arm resting at chest level. The child was asked to rest for 5 minutes before the measurement. Two measurements were recorded. If the second SBP or DBP differed from the first by >10 mm Hg, a third measurement was taken. The highest BP was discarded to account for child anxiety and the two lowest BP readings averaged.
Covariates
Maternal BP before 20 weeks’ gestation, child’s sex and gestational age were extracted from the maternity hospital records. Mothers were classified as normal BP (SBP<120 and DBP<80 mmHg), elevated BP (120≤SBP≤129 and DBP<80 mmHg), stage 1 hypertension (130≤SBP≤139 or 80≤DBP≤89 mmHg) and stage 2 hypertension (140>SBP or 90>DBP mmHg)15. Owing to the low percentage of stage 2 hypertensive mother (2.4 %), stage 1 and 2 hypertensive status were combined as one group. At the recruitment visit, educational attainment, ethnicity, and pre-pregnancy weight were collected through interviewer-administered questionnaires. Around 26 weeks’ gestation, maternal plasma cotinine level (ng/mL) was analysed; her current and early pregnancy active smoking status and environmental tobacco exposure at home and work were ascertained through interviewer-administered questionnaire. We defined maternal active smoking exposure as having a cotinine level ≥3.0 ng/mL16, or as having an active smoking status as reported by the subjects.
At the 26-28-week clinic visit, maternal height was measured twice to the nearest 0.1 cm using a stadiometer (SECA 213, Hamburg, Germany). If the two measurements differed by >1.0 cm, a third measurement was taken. The two closest measurements were then averaged. Maternal pre-pregnancy weight status, derived from the self-reported pre-pregnancy weight and the height at 26-28 weeks’ gestation, was defined using body mass index and the WHO classification for Asian population. At 24 or 36 months, paternal height was measured following the same protocol as for the maternal height.
At age 6 years, the child’s weight and height were measured in duplicate. A third measurement was taken if the first two differed by >0.2 kg and >1.0 cm for weight and height, respectively. The two closest measurements were averaged. Age and sex-specific BMI z-scores were derived using WHO reference17.Children were classified as overweight or obese when their BMI z-scores exceeded +2SD.
Analytic sample
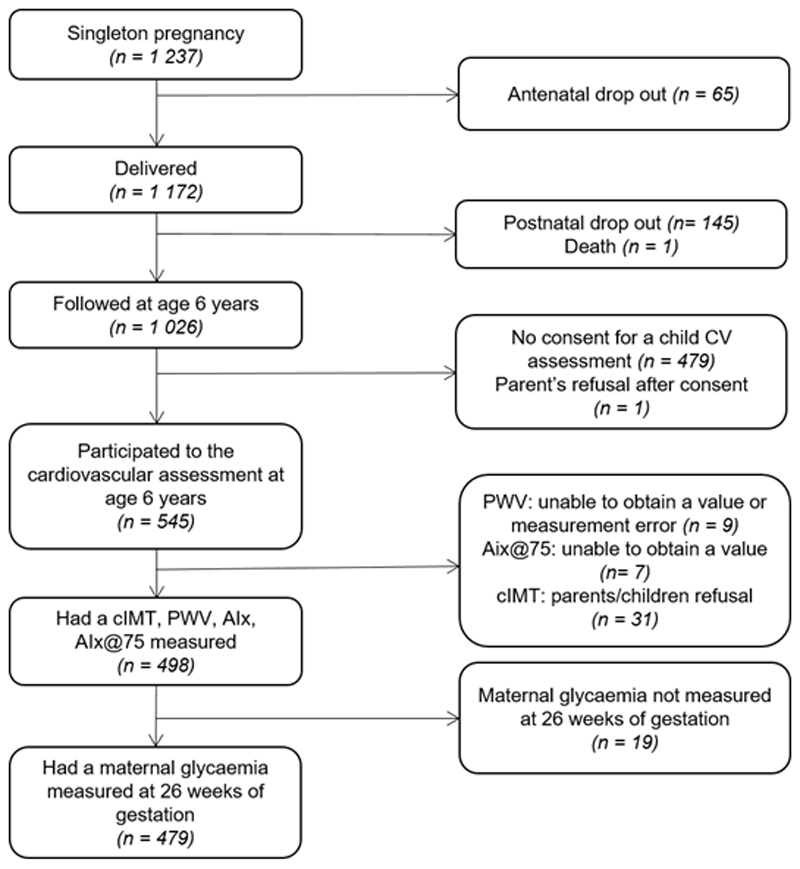
A study flow chart is shown in Figure 1. Multiple pregnancies were excluded (n=10). From the 1237 singleton pregnancies, 1172 mother-child dyads were followed-up after delivery. At age 6 years, 1026 children remained in follow-up, of whom 545 consented to cardiovascular assessment. Due to either parent or child refusal to be measured or technical problem on the day of measurement, 498 children had cIMT, cfPWV, AIx and AIx@75 measurements. Finally, only mother-child dyads with data on maternal glycaemia during pregnancy (n=479) were retained in the analysis.
Figure 1. Study flow chart.
Statistical analysis
Student’s t-tests and chi-square tests were used to compare characteristics of included and non-included participants. Unadjusted associations of FPG, 2-hr PPPG at 26 weeks’ gestation, GDM status with cIMT, cfPWV, AIx and AIx@75 were analysed using simple linear and logistic regression. Multiple linear and logistic regression models adjusted for study center, child’s sex and the following maternal characteristics: age at delivery, ethnicity, educational attainment, pre-pregnancy BMI, smoking status weeks’ gestation and environmental tobacco exposure during pregnancy, and BP category before 20 weeks’ gestation were performed. Since few mothers reported consuming alcohol (1%), models were not adjusted for this covariate. In a subsample of children with BP measurements (n=452), we examined associations of FPG, 2-hr PPPG, GDM with SBP and DBP. Interactions with child’s sex and maternal ethnicity were tested; when p-value significance was reached, an interaction term was added in the model and subgroups estimates were calculated. As a sensitivity analysis, models were run after excluding preterm infants (gestational age <37 weeks’ gestation, n=30). Models were also run among non-GDM mothers to study the influence of lower levels of maternal glycaemia and of receiving no treatment on child functional CV markers. As it has been proposed that negative AIx values be disregarded when studying wave reflection magnitude, we omitted these (n=20) in a subanalysis18. To provide insight into potential causal pathways, we performed mediation analysis to explore if the observed associations between maternal glycaemia and child cardiovascular risk markers were explained by gestational age and sex-specific birthweight z-scores, child age- and sex-specific BMI z-scores at age 5 and 6 years and child blood lipids (total cholesterol and triglycerides)19. In a post hoc analysis, we analysed the association between GDM defined by WHO 2013 criteria20 and child CV outcomes. Missing values for confounders, which were assumed to be missing at random, were handled using multiple imputations. Twenty independent datasets were generated using the Markov Chain Monte Carlo method, and pooled effect estimates were calculated. We imputed 20 datasets based on Graham et al recommendations for 10 to 30% of missing information21. All analyses were performed with SAS software (version 9.4; SAS Institute, Cary, NC, USA). Significance was set at P<0.05 except for interaction test (P<0.10) and hypothesis tests were 2-sided.
Results
Characteristics of the included and non-included participants are compared in Table 1. Of the mothers studied, 61 % were Chinese, 35 % had a university diploma, 23 % were obese and 18 % had GDM. Among mothers diagnosed with GDM, 95% were treated either by diet alone (90%) or with insulin as well (6%). Of the mothers, 95% had a FPG<5.1 mmol/L (normal range according to WHO 2013 GDM diagnosis criteria) and 82% had a 2-hr PPPG<7.8 mmol/L at 26 weeks’ gestation. Compared with non-included mothers, those included were less likely to being classified as having hypertension (13% vs 20%, overall P=.001 for BP category) and more likely to be Chinese (61% vs 53%, overall P=.03 for maternal ethnicity). No other significant differences were observed.
Table 1. Characteristics of included and non-included participantsa.
| n | Included | n | Non-included | P-value | |
|---|---|---|---|---|---|
| Child characteristics | |||||
| Male sex | 479 | 251 (52) | 758 | 368 (49) | 0.19 |
| Birth weight, kg | 479 | 3.1 (0.4) | 692 | 3.0 (0.5) | 0.74 |
| Gestational age, weeks | 479 | 38.4 (1.5) | 692 | 38.2 (1.6) | 0.13 |
| At year 6 | |||||
| cIMT, mm | 479 | 0.42 (0.29) | 34 | 0.42 (0.24) | 0.37 |
| cfPWV, m/s | 479 | 4.8 (1.2) | 63 | 5.3 (5.2) | 0.47 |
| AIx, % | 479 | 17.9 (10.1) | 66 | 16.3 (11.5) | 0.21 |
| AIx@75, % | 479 | 21.9 (10.1) | 59 | 21.8 (9.3) | 0.94 |
| SBP, mmHg | 452 | 98.8 (8.1) | 241 | 99.1 (8.4) | 0.61 |
| DBP, mmHg | 452 | 59.1 (6) | 241 | 59.6 (6.3) | 0.35 |
| BMI, kg/m2 | 478 | 15.5 (2.4) | 358 | 15.5 (2.2) | 0.92 |
| BMI z-score, SD | 478 | -0.03 (1.40) | 358 | -0.02 (1.32) | 0.81 |
| Overweight (obesity included)b | 478 | 89 (19) | 358 | 65 (18) | 0.96 |
| Maternal characteristics | |||||
| Age at delivery, years | 479 | 31.4 (5) | 692 | 31 (5.2) | 0.18 |
| Primiparous, % | 479 | 216 (45) | 692 | 319 (46) | 0.73 |
| Ethnicity, % | 479 | 758 | 0.03 | ||
| Chinese | 290 (61) | 401 (53) | |||
| Malay | 111 (23) | 211 (28) | |||
| Indian | 78 (16) | 146 (19) | |||
| Educational attainment, % | 476 | 744 | 0.69 | ||
| No formal education/primary/secondary | 145 (30) | 237 (32) | |||
| Postsecondary | 165 (35) | 265 (36) | |||
| University | 166 (35) | 242 (33) | |||
| BMI before pregnancy, kg/m2 | 437 | 639 | 0.27 | ||
| <18.5 | 45 (10) | 79 (12) | |||
| 18.5-22.9 | 230 (53) | 311 (49) | |||
| 23.0-24.9 | 60 (14) | 76 (12) | |||
| ≥25.0 | 102 (23) | 173 (27) | |||
| Glycaemia at 26 weeks’ gestation | |||||
| FPG, mmol/L | 479 | 4.3 (0.5) | 649 | 4.4 (0.5) | 0.19 |
| 2-hr PPPG, mmol/L | 479 | 6.5 (1.4) | 649 | 6.6 (1.5) | 0.13 |
| GDM using WHO 2013 criteria, % | 479 | 62 (13) | 649 | 80 (12) | 0.76 |
| GDM using WHO 1999 criteria, % | 479 | 84 (18) | 649 | 127 (20) | 0.39 |
| Diet-treated only | 76 (90) | 107 (84) | |||
| Diet and insulin-treated | 5 (6) | 12 (9) | |||
| Not treated | 3 (4) | 6 (5) | |||
| Unknown | 0 (0) | 2 (2) | |||
| Pre-pregnancy or early gestational (<20 weeks’ gestation) BP categoryc, % | 464 | 676 | 0.001 | ||
| Normotensive | 314 (68) | 399 (59) | |||
| Elevated BP | 92 (20) | 139 (21) | |||
| Hypertensive | 58 (13) | 138 (20) | |||
| Active smoking at 26 weeks’ gestation % | 429 | 24 (6) | 549 | 29 (5) | 0.83 |
| Exposure to tobacco at home and/or work in early pregnancy and at 26 weeks, % | 458 | 173 (38) | 671 | 255 (38) | 0.94 |
Values are mean ± SD or n (%). Values before multiple imputation.
Using WHO 2006 definition.
Using ACC/AHA 2017 definition15.
Abbreviations: cIMT: carotid intima media thickness, cfPWV: carotid femoral pulse wave velocity, AIx: augmentation index, AIx@75: augmentation index normalized to a heart rate of 75 beats/min, SBP: systolic blood pressure, DBP: diastolic blood pressure, BP: blood pressure, BMI: body mass index, GDM: gestational diabetes mellitus, FPG: fasting plasma glucose, PPPG: postprandial plasma glucose.
CIMT, cfPWV and AIx
The associations of maternal FPG, 2-hr PPPG at 26 weeks’ gestation, and GDM status with their offspring cIMT, cfPWV, AIx, and AIx@75 at age 6 years are shown in Table 2. FPG was not associated with AIx or AIx@75. Higher FPG was associated with higher cIMT and in male only (adjusted β [CI 95%], cfPWV: 0.35 m/s [0.01; 0.71] in male vs -0.06 [-0.39; 0.26] in female), with higher cfPWV (interaction test P=.09). No further interactions were observed with child’s sex and maternal ethnicity. Higher 2-hr PPPG was associated with higher AIx, AIx@75, with higher cfPWV even if borderline significant (P=.058) but not with cIMT. GDM status was associated with higher AIx and AIx@75 but not with cIMT and cfPWV. No interactions were found of child’s sex and maternal ethnicity on the association of 2-hr PPPG and GDM with any child CV risk markers. After omitting the preterm births from the study sample, the findings were mostly similar and the trends remained (Table 3). Associations with CV markers were non-significant when only non-GDM mothers were analysed, yet similar but diminished trends were observed (Table 3). After excluding negative AIx (Table 4), associations between AIx with 2-hr PPPG and GDM were reduced and not significant while the magnitude of the associations between AIx@75 with 2-hr PPPG and GDM were only reduced; all associations remained non-significant with FPG. In our mediation analysis, we found that the associations between FPG with cIMT was 24.2 % mediated by birthweight (indirect effect estimate, adjusted β [CI 95%]: 0.02 [-0.00; 0.02]. However, the association between FPG and cfPWV was not mediated by birthweight. Similarly, the associations between PPPG with cfPWV, AIx and AIx@75 and between GDM with AIx and AIx@75 were not mediated by birthweight. None of our studied associations were mediated by child BMI z-scores at age 5 and 6 years or child triglycerides and total cholesterol levels at age 6 years. In a post-hoc analysis, applying WHO 2013 criteria instead of WHO 1999 criteria to define GDM did not change the associations between GDM with cIMT and cfPWV. However, the magnitude of the association between GDM and AIx was attenuated (adjusted β [CI 95%]: 0.89 [-1.83; 3.62] for AIx and 0.95 [-1.79; 3.69] for AIx@75) and became non-significant.
Table 2. Associations of FPG, 2-hr PPPG, GDM status at 26 weeks’ gestation with cIMT, cfPWV, AIx AIx@75 at age 6 years.
| cIMT, per 10 mm n = 479 |
cfPWV, m/s n = 479 |
AIx, % n = 479 |
AIx@75, % n = 479 |
|||||
|---|---|---|---|---|---|---|---|---|
| β [CI 95%] | β [CI 95%] | β [CI 95%] | β [CI 95%] | |||||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| FPG, mmol/L | 0.06 [0.00; 0.12] | 0.08 [0.02; 0.15]b | 0.29 [0.06; 0.52]b | 0.16 [-0.09; 0.40] | -0.15 [-2.14; 1.84] | -0.04 [-2.19; 2.11] | 0.25 [-1.74; 2.24] | 0.09 [-2.07; 2.25] |
| 2-hr PPPG, mmol/L | 0.01 [-0.01; 0.03] | 0.01 [-0.01; 0.03] | 0.05 [-0.02; 0.13] | 0.08 [0.00; 0.15] | 0.51 [-0.12; 1.13] | 0.77 [0.10; 1.45]b | 0.75 [0.13; 1.38]b | 0.89 [0.22; 1.57]b |
| GDMe | 0.02 [0.01; 0.04]d | 0.01 [-0.07; 0.08] | 0.22 [0.16; 0.28]e | 0.23 [-0.05; 0.51] | 2.17 [1.64; 2.70]e | 2.69 [0.28; 5.10]b | 2.84 [2.31; 3.36]e | 3.07 [0.65; 5.50]b |
Multiple linear and logistic regressions on multiple imputed datasets (n=20) also adjusted for study center, child’s sex, paternal height, maternal characteristics (ethnicity, age at delivery, height, pre-pregnancy BMI, educational attainment, BP category before 20 weeks’ gestation, active smoking status at 26 weeks’ gestation, exposure to tobacco at home and/or work in early pregnancy and at 26 weeks).
P-value<0.05.
GDM defined using WHO 1999 criteria.
P-value<0.01.
P-value<0.0001.
Abbreviations: cIMT: carotid intima media thickness, cfPWV: carotid femoral pulse wave velocity, AIx: augmentation index, AIx@75: augmentation index normalized to a heart rate of 75 beats/min, SBP: systolic blood pressure, DBP: diastolic blood pressure, FPG: fasting plasma glucose, PPPG: postprandial plasma glucose, GDM: gestational diabetes mellitus.
Table 3.
Sensitivity analysis: adjusted associations of FPG, 2-hr PPPG, GDM status at 26 weeks’ gestation with cIMT, cfPWV, AIx, AIx@75 SBP and DBP at age 6 years excluding preterm children or mothers with GDM or children with negative AIxa
| cIMT, per 10 mm | cfPWV, m/s | AIx, % | AIx@75, % | SBP, mmHg | DBP, mmHg | |
|---|---|---|---|---|---|---|
| β [CI 95%] | β [CI 95%] | β [CI 95%] | β [CI 95%] | β [CI 95%] | β [CI 95%] | |
| Excluding preterm children | n = 447 | n = 447 | n = 447 | n = 447 | n = 421 | n = 421 |
| FPG, mmol/L | 0.09 [0.02; 0.15]b | 0.10 [-0.15; 0.36] | 0.04 [-2.09; 2.17] | 0.00 [-2.13; 2.14] | 1.45 [-0.34; 3.24] | 0.07 [-1.30; 1.44] |
| 2-hr PPPG, mmol/L | 0.01 [-0.01; 0.03] | 0.09 [0.00; 0.17]b | 0.75 [0.06; 1.43]b | 0.94 [0.26; 1.63]b | 0.03 [-0.56; 0.62] | -0.31 [-0.76; 0.13] |
| GDMc | 0.02 [-0.06; 0.10] | 0.20 [-0.09; 0.49] | 2.37 [-0.06; 4.81] | 2.39 [0.49; 5.36]b | 0.89 [-1.22; 3.01] | 0.16 [-1.45; 1.77] |
| Excluding mothers with GDM | n = 395 | n = 395 | n = 395 | n = 395 | n = 373 | n = 373 |
| FPG, mmol/L | 0.09 [0.01; 0.17] | 0.12 [-0.18; 0.42] | -0.41 [-3.18; 2.36] | -0.57 [-3.37; 2.22] | 1.75 [-0.45; 3.95] | 0.49 [-1.18; 2.16] |
| 2-hr PPPG, mmol/L | 0.02 [-0.01; 0.05] | 0.04 [-0.07; 0.16] | 0.71 [-0.34; 1.76] | 0.79 [-0.27; 1.84] | 0.07 [-0.76; 0.90] | -0.41 [-1.04; 0.22] |
| Excluding negative AIx | n = 459 | n = 459 | n = 459 | n = 459 | n = 434 | n = 434 |
| FPG, mmol/L | 0.07 [0.00; 0.14] b | 0.12 [-0.14; 0.38] | -0.95 [-2.84; 0.95] | -0.76 [-2.67; 1.15] | 1.55 [-0.26; 3.37] | 0.22 [-1.16; 1.60] |
| 2-hr PPPG, mmol/L | 0.01 [-0.01; 0.03] | 0.06 [-0.02; 0.14] | 0.49 [-0.10; 1.08] | 0.62 [0.03; 1.21]b | -0.05 [-0.62; 0.53] | -0.40 [-0.83; 0.03] |
| GDMc | 0.02 [-0.06; 0.09] | 0.20 [-0.08; 0.49] | 1.63 [-0.46; 3.72] | 2.09 [-0.02; 4.19]b | 0.53 [-1.53; 2.59] | 0.08 [-1.49; 1.64] |
Multiple linear and logistic regressions on multiple imputed datasets (n=20) also adjusted for study center, child’s sex, paternal height, maternal characteristics (ethnicity, age at delivery, height, pre-pregnancy BMI, educational attainment, BP category before 20 weeks’ gestation, active smoking status at 26 weeks’ gestation, exposure to tobacco at home and/or work in early pregnancy and at 26 weeks).
P-value<0.05.
GDM defined using WHO 1999 criteria.
Abbreviations: cIMT: carotid intima media thickness, cfPWV: carotid femoral pulse wave velocity, AIx: augmentation index, AIx@75: augmentation index normalized to a heart rate of 75 beats/min, SBP: systolic blood pressure, DBP: diastolic blood pressure, FPG: fasting plasma glucose, PPPG: postprandial plasma glucose, GDM: gestational diabetes mellitus.
Table 4.
Sensitivity analysis: adjusted associations of FPG, 2-hr PPPG, GDM status (according to WHO 1999 definition) at 26 weeks’ gestation with cIMT, cfPWV, AIx, AIx@75 SBP and DBP at age 6 years excluding preterm children or mothers with GDM or children with negative AIxa
| SBP, mmHg n = 452 |
DBP, mmHg n = 452 |
|||
|---|---|---|---|---|
| β [CI 95%] | β [CI 95%] | |||
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| FPG, mmol/L | 1.75 [0.13; 3.37]b | 1.52 [-0.23; 3.26] | 0.09 [-1.12; 1.30] | 0.17 [-1.16; 1.50] |
| 2-hr PPPG, mmol/L | -0.15 [-0.66; 0.37] | -0.02 [-0.58; 0.54] | -0.41[-0.79; -0.03]b | -0.36 [-0.78; 0.07] |
| GDMc | 0.48 [0.04 ; 0.92]b | 0.66 [-1.36; 2.69] | -0.10 [-0.42 ; 0.23] | 0.20 [-1.33; 1.73] |
Multiple linear and logistic regressions on multiple imputed datasets (n=20) also adjusted for study center, child’s sex, paternal height, maternal characteristics (ethnicity, age at delivery, height, pre-pregnancy BMI, educational attainment, BP category before 20 weeks of gestation, active smoking status at 26 weeks of gestation, exposure to tobacco at home and/or work in early pregnancy and at 26 weeks).
P-value<0.05.
GDM defined using WHO 1999 criteria.
Abbreviations: cIMT: carotid intima media thickness, cfPWV: carotid femoral pulse wave velocity, AIx: augmentation index, AIx@75: augmentation index normalized to a heart rate of 75 beats/min, SBP: systolic blood pressure, DBP: diastolic blood pressure, FPG: fasting plasma glucose, PPPG: postprandial plasma glucose, GDM: gestational diabetes mellitus.
Blood pressure
As shown in Table 4, maternal FPG, 2-hr PPPG and GDM were not associated with SBP or DBP in their offspring aged 6 years. After excluding the preterm births and GDM mothers, all associations remained unchanged (Table 4). Interactions of child’s sex or maternal ethnicity were non-significant. Associations between GDM with child SBP and DBP remained similar when applying either WHO 2013 or 1999 criteria to define GDM.
Discussion
To our knowledge, this is the first study to investigate the link between maternal glycaemia during pregnancy, among women without pre-existing DM, and vascular structure and function in their offspring during mid-childhood. We found that among mothers without pre-existing diabetes, higher FPG at 26 weeks’ gestation was associated with putative CV risk markers in their offspring aged 6 years, i.e. higher cIMT, and in male, higher cfPWV. Higher maternal 2-hr PPPG was associated with higher cfPWV, AIx and AIx@75 but not with cIMT. GDM status was associated with higher AIx but not with other CV risk markers. No associations were observed between maternal glycaemia and offspring SBP or DBP.
GDM can cause oxidant stress which itself can lead to altered placental function and hence, impacts fetal growth and epigenetic programming that are associated with higher cardiometabolic risk in later ages22. In both the ACHOIS trial and in the HAPO cohort study, maternal hyperglycaemia was associated with increasing risk of perinatal morbidity and mortality in a continuum, but to a lesser degree than manifest DM6–8, and GDM treatment was beneficial in reducing the risk6. From previous studies in pre-existing diabetic mothers, macrosomic neonates are known to have an increased left ventricular mass and a larger aortic intima-media thickness compared with their normal-sized counterparts3. Similarly, children born macrosomic from mothers with either pre-existing type 1 DM or GDM had higher aortic intima media thickness23.
In line with previous studies results, we found that higher FPG at 26 weeks’ gestation among GUSTO mothers was related to higher cIMT in their offspring at age 6 years, and in male to greater conduit arterial stiffness (increase in cfPWV of 0.36 m/s). Higher maternal 2-hr PPPG was associated with higher cfPWV (increase of 0.08 m/s) and AIx. GDM status was only associated with offspring’s AIx but not with cfPWV and cIMT. As a gauge of the clinical significance of our findings, an increase in cfPWV of 0.18 m/s and 0.11 m/s were associated with obesity and higher HOMA-IR, respectively, in children aged 8 years24. We acknowledge that the magnitude of our associations was small and further investigation are needed to confirm our findings. In our study, only a small number of GDM mothers were treated with insulin (6%) while the vast majority (90%) were controlled with diet alone. Since almost all GDM mothers were treated following diagnosis and strict glycaemic control enforced, this could have masked an association of GDM with cfPWV and cIMT. Evidence from randomized trials has shown that treatment of GDM led to a reduced risk of fetal overgrowth and other adverse perinatal outcomes25. Furthermore, the association between maternal glycaemia and the risk of neonatal adverse outcomes has been shown to occur across a continuum, inferring that any diagnostic threshold indicative for GDM is arbitrary8. Indeed, among non-GDM mothers, we were able to discern parallel albeit non-significant trends between maternal glycaemia with child’s vascular metrics. This lack of significance might partly be explained by the low levels of maternal glycaemia in our study. Even if some outcomes did not attain statistical significance, it is remarkable that the associations between child vascular measures and glycaemic status measured at a single time-point in pregnancy were directionally consistent. This consistency suggests common pathophysiologic mechanisms linking hyperglycaemia to arterial disease, including inflammation, endothelial dysfunction, and extracellular matrix alterations26. Besides expedient treatment of mothers with higher levels of glycaemia, another explanation of the differences observed across the different exposures could be that FPG and PPPG may influence outcomes through different causal pathways. It has been suggested that PPPG levels could be more predictive than FPG for macrosomia and hypoglycaemia in diabetic mothers or insulin treated GDM mothers27–29. While both FPG and PPPG have been associated with greater arterial stiffness, several studies have now shown a unique ability of postprandial hyperglycaemia to acutely increase arterial stiffness30. In one large Taiwanese study of nearly 5000 subjects, impaired glucose tolerance but not impaired fasting glucose was associated with greater arterial stiffness, possibly because the more pronounced degree of insulin resistance in the former leads to higher serum and tissue levels of advanced glycation end-products31.
Most previous studies of child CV outcomes have focused on BP. In a Chinese population, children aged 3-10 years of GDM mothers had higher sex- and height-specific BP z-scores for SBP and higher rates of hypertension than those of non-GDM mothers32 while in Project Viva, higher SBP at age 3 years was associated with maternal GDM33. By contrast, we found no association between maternal glycaemia and SBP or DBP of their 6-year-old offspring. We used a single stage GDM screening while the two referenced studies32,33 used a two stage GDM screening. As our results are consistent with studies that have used a single stage GDM screening34–36, the discrepant findings could be explained by different degrees of hyperglycaemia or GDM across studies.
We found that higher maternal FPG at 26 weeks’ gestation was associated with higher cfPWV only in males. Several mechanisms of sexual dimorphism in developmental programming of cardiovascular disease have been stated, but mainly from animal studies37. Human studies suggested that males may be more responsive to GDM treatment than females, as reflected in lower neonatal fat mass and lower birthweight centiles38. The risk of developing hypertension in the offspring, associated with maternal GDM, also appears more pronounced in male children32.
We acknowledge that the generalized transfer function used by the SphygmoCor device to convolve radial to aortic pressure was derived in adults. However, even when the standard adult transfer function is applied to young children, underestimation of aortic systolic BP was small (mean, 5-6 mmHg) and >90% of them had values within 10% of “reference” aortic pressure39. Irrespective of the algorithm used, any over- or under-estimation of central pressure appears to be systematic, without bias for any specific individual or subgroup39.
Our study findings may not be generalizable to the Singapore population. In the GUSTO study, only 54% of women who initially volunteered met the eligibility criteria11, and of these, half of the GUSTO families at age 6 years agreed to participate in CV assessment. We found differences in maternal ethnicity and hypertensive status between included and non-included participants. However, we believe that potential reasons of non-participation to the CV assessment should not have a strong influence on our findings although they may limit their generalizability. In GUSTO, WHO 1999 criteria was used to diagnose GDM. This limits comparisons with other studies which use WHO 2013 criteria. WHO 1999 criteria may over-diagnose GDM on the basis of 2hr-PPPG and underdiagnose GDM on the basis of FPG. However, our post hoc analysis applying WHO 2013 criteria to define GDM showed mostly similar association, except for AIx. This difference may be explained by the lower prevalence of GDM diagnosed using WHO 2013 criteria. Our results are strengthened by the prospective design of GUSTO study, the comprehensive information collected on the participants and the relatively large sample size compared to previous studies.
We found that higher maternal FPG and 2-hr PPPG at 26 weeks’ gestation was associated with offspring mild structural and functional CV changes, but not BP, in children aged 6 years. Given the magnitude of the associations found in our study and the absence of longitudinal data linking subclinical CV changes in early childhood to CV events in adult life, we believe that it would be premature to recommend public health interventions. Further follow-up of our cohort will reveal whether these risk markers predict higher risk of CV events in adulthood. If these alterations do indeed persist into adulthood and portend adverse outcomes, the important implication of our study is that CV changes due to maternal hyperglycemia can be detected as young as 6 years of age. The corollary is that at risk individuals should be more carefully monitored and targeted for health education or intervention.
Précis.
In the present study, using data from GUSTO, a Singaporean birth cohort study, we analyzed the link between maternal glycaemia measured around 26 weeks’ gestation in non-diabetic mothers and cardiovascular risk markers measured in their offspring at age 6 years (carotid intima-media thickness, carotid-femoral pulse wave velocity, aortic augmentation index, blood pressure). Higher maternal fasting plasma glucose was associated with higher carotid intima media thickness in their offspring and in male, higher carotid femoral pulse wave velocity. Higher 2-hr postprandial plasma glucose was associated with higher carotid femoral pulse wave velocity and augmentation index. Higher maternal glycaemia during pregnancy was associated with changes in their offspring vascular structure and function in mid-childhood across a continuum.
Acknowledgments
The authors’ responsibilities were as follows: Y-SC, YSL, FY, KHT, LPS, PDG, JGE, S-YC designed and coordinated GUSTO cohort study. WLY designed and conducted research. JTLC and LLH provided essential materials. JTLC, LLH and JL coordinated the data collection. WLY performed the statistical analysis. WLY wrote the paper. MK, JGE, S-YC, LLH, KMG and JTLC critically reviewed all sections of the text for important intellectual content. All authors had primary responsibility for final content. All authors read and approved the final manuscript.
Source(s) of Funding
This study is under Translational Clinical Research (TCR) Flagship Programme on Developmental Pathways to Metabolic Disease, NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014 funded by the National Research Foundation (NRF) and administered by the National Medical Research Council (NMRC), Singapore. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NF-SI-0515-10042) and Programme Early Nutrition eAcademy Southeast Asia-(573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP).
Footnotes
Conflict(s) of Interest/Disclosure(s)
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors declare no conflict of interest.
References
- 1.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 2.Nolan CJ. Controversies in gestational diabetes. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):37–49. doi: 10.1016/j.bpobgyn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Akcakus M, Koklu E, Baykan A, et al. Macrosomic newborns of diabetic mothers are associated with increased aortic intima-media thickness and lipid concentrations. Horm Res. 2007;67(6):277–283. doi: 10.1159/000098157. [DOI] [PubMed] [Google Scholar]
- 4.Loffredo CA, Wilson PD, Ferencz C. Maternal diabetes: an independent risk factor for major cardiovascular malformations with increased mortality of affected infants. Teratology. 2001;64(2):98–106. doi: 10.1002/tera.1051. [DOI] [PubMed] [Google Scholar]
- 5.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94(4):1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 7.Wendland EM, Duncan BB, Mengue SS, Schmidt MI. Lesser than diabetes hyperglycemia in pregnancy is related to perinatal mortality: a cohort study in Brazil. BMC Pregnancy Childbirth. 2011;11:92. doi: 10.1186/1471-2393-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group HSCR. Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 11.Soh SE, Tint MT, Gluckman PD, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 12.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95(7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 14.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62(1):105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 17.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes AD, Park C, Davies J, et al. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One. 2013;8(3):e59371. doi: 10.1371/journal.pone.0059371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103(3):341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Graham JW, Olchowski AE, Gilreath TD. How Many Imputations are Really Needed? Some Practical Clarifications of Multiple Imputation Theory. Prevention Science. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 22.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113(1):1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 23.Koklu E, Akcakus M, Kurtoglu S, et al. Aortic intima-media thickness and lipid profile in macrosomic newborns. Eur J Pediatr. 2007;166(4):333–338. doi: 10.1007/s00431-006-0243-8. [DOI] [PubMed] [Google Scholar]
- 24.Correia-Costa A, Correia-Costa L, Caldas Afonso A, et al. Determinants of carotid-femoral pulse wave velocity in prepubertal children. Int J Cardiol. 2016;218:37–42. doi: 10.1016/j.ijcard.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 25.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozakova M, Palombo C. Diabetes Mellitus, Arterial Wall, and Cardiovascular Risk Assessment. International journal of environmental research and public health. 2016;13(2):201. doi: 10.3390/ijerph13020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development--Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164(1 Pt 1):103–111. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 28.Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992;15(10):1251–1257. doi: 10.2337/diacare.15.10.1251. [DOI] [PubMed] [Google Scholar]
- 29.de Veciana M, Major CA, Morgan MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 30.Gordin D, Saraheimo M, Tuomikangas J, et al. Influence of Postprandial Hyperglycemic Conditions on Arterial Stiffness in Patients With Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2016;101(3):1134–1143. doi: 10.1210/jc.2015-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CH, Wu JS, Yang YC, Shih CC, Lu FH, Chang CJ. Increased arterial stiffness in subjects with impaired glucose tolerance and newly diagnosed diabetes but not isolated impaired fasting glucose. The Journal of clinical endocrinology and metabolism. 2012;97(4):E658–662. doi: 10.1210/jc.2011-2595. [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Zhang S, Li W, et al. Maternal gestational diabetes is associated with offspring's hypertension. Am J Hypertens. 2019 doi: 10.1093/ajh/hpz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22(2):215–220. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaarasmaki M, Pouta A, Elliot P, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol. 2009;169(10):1209–1215. doi: 10.1093/aje/kwp020. [DOI] [PubMed] [Google Scholar]
- 35.Patel S, Fraser A, Davey Smith G, et al. Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care. 2012;35(1):63–71. doi: 10.2337/dc11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antikainen L, Jaaskelainen J, Nordman H, Voutilainen R, Huopio H. Prepubertal Children Exposed to Maternal Gestational Diabetes Have Latent Low-Grade Inflammation. Horm Res Paediatr. 2018;90(2):109–115. doi: 10.1159/000491938. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1941–1952. doi: 10.1152/ajpregu.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahado-Singh RO, Mele L, Landon MB, et al. Fetal male gender and the benefits of treatment of mild gestational diabetes mellitus. Am J Obstet Gynecol. 2012;206(5):422 e421–425. doi: 10.1016/j.ajog.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai TY, Qasem A, Ayer JG, et al. Central blood pressure in children and adolescents: non-invasive development and testing of novel transfer functions. Journal of human hypertension. 2017;31(12):831–837. doi: 10.1038/jhh.2017.59. [DOI] [PubMed] [Google Scholar]