A 10-year-old girl presented with leukocytosis (199×109/L), anemia (9 g/dL), and thrombocytopenia (34×109/L). After bone marrow (BM) study, she was diagnosed with B-lymphoblastic leukemia with t(4;11)(q21;q23) and KMT2A-AFF1 rearrangement. Following acute lymphoblastic leukemia therapy and early relapse, she participated in a clinical study with daratumumab (anti-CD38 monoclonal antibody).
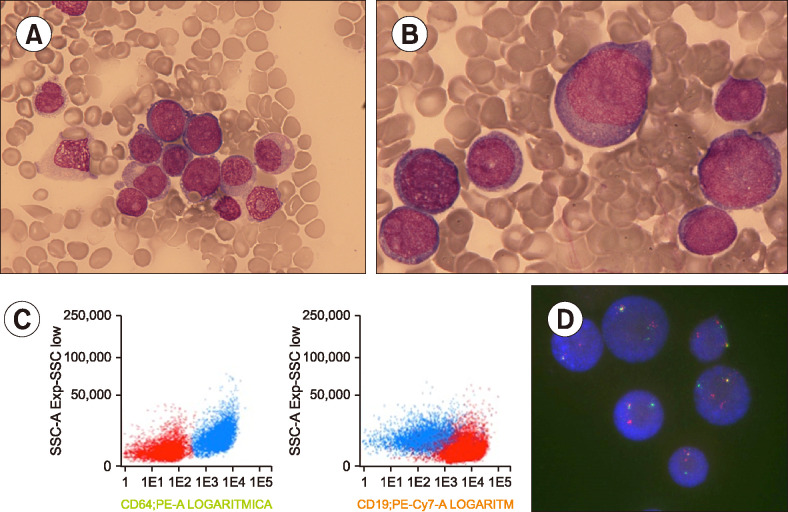
BM re-evaluation after one treatment cycle showed that 40% of total nucleated cells (TNC) comprised medium blasts with irregular-shaped nucleus, scant basophilic cytoplasm, and similar immunophenotypic characteristics as the B-lymphoblasts during initial diagnosis; however, 56% of TNCs comprised large blasts with abundant cytoplasm and round nucleus. Flow cytometry showed these were similar to monoblasts and positive for CD64, CD4, CD15, and CD33 (A, B, May Grünwald-Giemsa stain, ×600 and ×1,000, respectively; C, monoblasts in blue, lymphoblasts in red). Fluorescence in situ hybridization confirmed KMT2A rearrangement in both populations (D). She was diagnosed with mixed-phenotype acute leukemia with t(4;11)(q21;q23).
Acute leukemia lineage switch is rare and has poor outcome. KMT2A rearrangement is frequently involved because of its relationship with an immature hematopoietic precursor which can be transformed under certain circumstances. This is the first case involving daratumumab. Light microscopy is essential to detect lineage shift.