Abstract
Background
This study attempted to identify novel prognostic factors in patients with newly diagnosed primary central nervous system lymphoma (PCNSL) using magnetic resonance imaging (MRI).
Methods
We retrospectively evaluated 67 patients diagnosed with central nervous system (CNS) tumors. The enrollment criteria were as follows: i) pathologic diagnosis of CNS lymphoma, ii) no evidence of systemic involvement, iii) no evidence of human immunodeficiency virus-1 infection or other immunodeficiencies, and iv) MRI scans available at diagnosis. Fifty-two patients met these criteria and were enrolled.
Results
The 3-year overall survival (OS) and failure-free survival rates were 69.7% and 45.6%, respectively, with a median follow-up duration of 36.2 months. OS of patients with low apparent diffusion coefficient (ADC) was lower than those with higher ADC. Multivariate analysis revealed that old age (>60 yr) [hazard ratio (HR), 20.372; P=0.001], Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 (HR, 10.429; P<0.001), higher lactate dehydrogenase (LDH) levels (HR, 7.408; P=0.001), and low ADC (HR, 0.273; P=0.009) were associated with lower OS. We modified the conventional prognostic scoring system using low ADC, old age (>60 yr), ECOG PS ≥2, and higher LDH. The risk of death was categorized as high (score 3-4), intermediate-2 (score 2), intermediate-1 (score 1), and low (score 0), with three-year OS rates of 33.5%, 55.4%, 88.9%, and 100%, respectively.
Conclusion
ADC demonstrated significant prognostic value for long-term survival in patients with newly diagnosed PCNSL. Low ADC was an independent unfavorable prognostic factor, suggesting that ADC obtained from MRI can improve the current prognostic scoring system.
Keywords: Lymphoma, Central nervous system, Prognosis, Magnetic resonance imaging
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is a rare subtype of extranodal non-Hodgkin lymphoma (NHL), which accounts for approximately 1–2% of NHLs and 1–4% of central nervous system (CNS) tumors [1-3]. The treatment modalities for PCNSL include methotrexate (MTX)-based chemotherapy and whole-brain radiotherapy (WBRT) [4, 5]. Although combination chemotherapies containing methotrexate and WBRT have been considered to be effective therapeutic modalities for PCNSL, approximately 50% of patients who initially achieved a complete response experience relapse [6]. Performing WBRT after chemotherapy yields a better survival rate than chemotherapy alone. However, the incidence of radiotherapy-induced neurotoxicity increases in patients who have undergone combined therapy [7-9]. Therefore, precise prognostic factors are needed to develop improved risk-adapted treatment strategies, such as high-dose chemotherapy with autologous stem cell transplantation (auto-SCT) or chemotherapy, followed by WBRT in high-risk patients with PCNSL.
Efforts have been made to identify prognostic factors for PCNSL. The International Extranodal Lymphoma Study Group (IELSG) proposed a prognostic scoring system that is comprising age, Eastern Cooperative Oncology Group performance status (ECOG PS), serum lactate dehydrogenase (LDH) levels, cerebrospinal fluid (CSF) protein concentration, and involvement of deep brain structures [10, 11]. However, the IELSG scoring system is not available for several patients due to a lack of CSF examination, which is inconvenient, invasive, and is associated with complications. The advent of imaging modalities has led to the need to consider modifying the conventional prognostic scoring system for patients with newly diagnosed PCNSL. Magnetic resonance imaging (MRI) is a standard diagnostic modality for brain tumors, and several studies have reported the typical MRI features of PCNSL [12, 13]. For instance, a low signal on T2-weighted MRI and restricted diffusion with diffusion-weighted imaging (DWI) are pathognomonic characteristics of PCNSL [14, 15]. Moreover, earlier data has shown that specific MRI findings are associated with survival outcomes [16, 17]. However, the current knowledge of MRI findings is insufficient for clinical use in determining the prognosis of PCNSL.
Therefore, the objective of the present study was to identify novel prognostic factors by retrospective analyzing the conventional MRI data obtained from patients with newly diagnosed PCNSL. Moreover, we proposed a modified prognostic scoring system based on IELSG to predict the long-term clinical outcomes of PCNSL.
MATERIALS AND METHODS
Patients
This study retrospectively reviewed 67 patients who were newly diagnosed with CNS tumors between January 2011 and May 2018 at Kyungpook National University Hospital (KNUH). The participants were enrolled on the basis of the following criteria: i) pathologic diagnosis of CNS lymphoma, ii) no evidence of systemic involvement, iii) no evidence of human immunodeficiency virus-1 infection or other immunodeficiencies, and iv) MRI scans available at diagnosis. Fifty-two patients met these criteria and were included in the study. We evaluated the enrolled patients according to the International PCNSL Collaborative Group recommendations. Positron emission tomography/computed tomography scans and bone marrow biopsies were conducted to detect any non-CNS involvement. Patients’ medical records were reviewed for medical history, age, sex, pathological results, treatment method, response, and survival. The study was approved by the Institutional Review Board of KNUH.
Brain MRI
Brain MRI was performed with a 1.5-T (Signa Excite, GE Healthcare, WI, USA) and two 3-T MRI devices (Signa Excite or Signa HDxt, GE, USA). Unenhanced axial T1-weighted and T2-weighted images were obtained with various settings. T1-weighted spin-echo images were acquired with the following parameters: repetition time (TR), 550–630; echo time (TE), 11–14; flip angle, 60–70°; section thickness, 6 mm; interslice gap, 0.7–2 mm; matrix size, 352×192–1,024×192; field-of-view (FOV), 174×200–220×220; and number of signals acquired, 1–2. T2-weighted spin-echo images were acquired with the following parameters: TR, 3,588–4,400; TE, 99–125; flip angle, 90 or 180°; section thickness, 6 mm; interslice gap, 0.7–2 mm; matrix size, 512×224; and FOV, 180×200. MRI included conventional contrast-enhanced and DWI sequences. DWI-derived apparent diffusion coefficients (ADC) were measured based on the diffusion imaging sets. Patients were classified into high and low-ADC groups according to the median value, with reference to previous studies [16, 17].
Treatment and response evaluation
First-line therapy consisted of a high-dose MTX (HD-MTX) based regimen for all patients. Induction chemotherapy was administered with a combination of HD-MTX, vincristine, and procarbazine (MVP) every 2 weeks for 5 cycles as follows: HD-MTX (3.5 g/m2) and vincristine (1.4 mg/m2) were administered on day 1 with procarbazine (100 mg/m2, odd cycles only) from day 1 to day 7. The rituximab, HD-MTX, vincristine, and procarbazine (R-MVP) regimen was administered every 2 weeks for 5 cycles as follows: rituximab (500 mg/m2) was administered on day 1, HD-MTX (3.5 g/m2) and vincristine (1.4 mg/m2) on day 2 with procarbazine (100 mg/m2, odd cycles only) from days 1 to 7. Patients received consolidation chemotherapy with high-dose chemotherapy with auto-SCT as follows: thiotepa (250 mg/m2) was administered on days 1 and 2, and busulfan (3.2 mg/kg) was administered on days 3–5, followed by stem-cell rescue. WBRT (45 Gy) was performed as salvage treatment for relapsed/refractory disease after first-line chemotherapy. Responses were assessed using the International PCNSL Collaborative Group criteria, based on imaging.
Statistical analyses
Descriptive statistics were reported as proportions and medians. Categorical variables were evaluated using the chi-squared and Fisher’s exact tests, as appropriate. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or the date of the last follow-up. Failure-free survival (FFS) was calculated from the date of diagnosis to the date of relapse, progression of disease, death from any cause, or last follow-up. The Kaplan-Meier method was used to analyze the OS and FFS. The survival curves were compared using a log-rank test. Multivariate survival analyses were conducted using the Cox proportional hazard regression model. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for each factor. A P-value of less than 0.05 was considered statistically significant. SPSS version 21.0 (SPSS Inc, Chicago, IL, USA) was used to perform statistical analyses.
RESULTS
Patient characteristics
The patient characteristics are summarized in Table 1. The median age was 56 years (range, 42–82 yr) at the time of diagnosis, and 30 (57.7%) patients were men. Six (11.5%) patients had an ECOG PS >1, and 16 (30.8%) patients showed increased LDH levels. All patients were diagnosed with diffuse large B cell lymphoma (DLBCL), based on histopathological examination. Forty-four (84.6%) patients received first-line treatment with MVP, while 8 (15.4%) were treated with R-MVP. Ten (19.2%) patients underwent consolidation treatment with auto-SCT.
Table 1.
Patient characteristics.
| Variable | N (%) |
|---|---|
| N | 52 |
| Age, median yr (range) | 56 (42–82) |
| Age>60 yr | 24 (46.2) |
| Sex | |
| Male | 30 (57.7) |
| Female | 22 (42.3) |
| ECOG performance status | |
| ≤1 | 46 (88.5) |
| >1 | 6 (11.5) |
| Raised LDH | 16 (30.8) |
| Elevated CSF protein | 7 (13.5) |
| Involvement of deep structuresa) | 34 (65.4) |
| Lymphoma subtype | |
| DLBCL | 52 (100) |
| First-line treatment | |
| MVP | 44 (84.6) |
| R-MVP | 8 (15.4) |
| Response to first-line therapy | |
| CR | 40 (76.9) |
| PR | 8 (15.4) |
| Refractory | 4 (7.7) |
| Auto-SCT | 10 (19.2) |
| Relapse | 26 (50.0) |
| Death | 20 (34.6) |
a)Involvement of deep structures, basal ganglia and/or corpus callosum and/or brain stem and/or cerebellum.
Abbreviations: Auto-SCT, autologous stem cell transplantation; CR, complete response; CSF, cerebrospinal fluid; DLBCL, diffuse large B cell lymphoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MVP, methotrexate, vincristine, procarbazine; PR, partial response; R-MVP, rituximab, methotrexate, vincristine, procarbazine.
MRI findings at diagnosis
Thirty-eight (73.1%) lesions were located at the supratentorial level, while 14 lesions (26.9%) involved the infratentorial region. The corpus callosum and basal ganglia were involved in 18 (34.6%) and 30 (57.7%) patients, respectively. Homogenous enhancement of the lesion was observed in 30 (57.7%) patients, and necrosis was observed in 22. Low ADC values were identified in 36 (69.2%) patients. A majority of the patients (90.4%) had lesions measuring less than 5.0 cm, and 36 (69.2%) showed the mass effect. Hypointense signals were observed on T1-weighted imaging in 34 (65.4%) patients, while hyperintense signals were observed on T2-weighted imaging in 26 (50.0%) patients. Detailed characteristics of the lesions are presented in Table 2.
Table 2.
MRI features of the 52 patients.
| Variable | N (%) |
|---|---|
| Location | |
| Supratentorial | 38 (73.1) |
| Infratentorial | 2 (3.8) |
| Both | 12 (23.1) |
| N of lesions | |
| One | 20 (38.5) |
| Two or more | 32 (61.5) |
| Corpus callosum involvement | 18 (34.6) |
| Basal ganglia involvement | 30 (57.7) |
| Cerebral cortex involvement | 22 (42.3) |
| Enhancement | |
| Homogenous | 30 (57.7) |
| Heterogenous | 22 (42.3) |
| Necrosis | 22 (42.3) |
| Ependymal infiltration | 26 (50.0) |
| Low ADC values | 36 (69.2) |
| Mass effect | 36 (69.2) |
| Size | |
| ≥5.0 cm | 5 (9.6) |
| <5.0 cm | 47 (90.4) |
| Signal T1 | |
| Isointense | 18 (34.6) |
| Hypointense | 34 (65.4) |
| Signal T2 | |
| Isointense | 26 (50.0) |
| Hyperintense | 26 (50.0) |
Abbreviations: ADC, apparent diffusion coefficient; MRI, magnetic resonance imaging.
Response to induction chemotherapy and survival outcomes
Forty (76.9%) patients achieved complete response (CR), 8 (15.4%) achieved a partial response (PR), and 4 (7.7%) showed primary refractory illness after 5 cycles of chemotherapy. The objective response rate (ORR) was 92.3% (48 of 52).
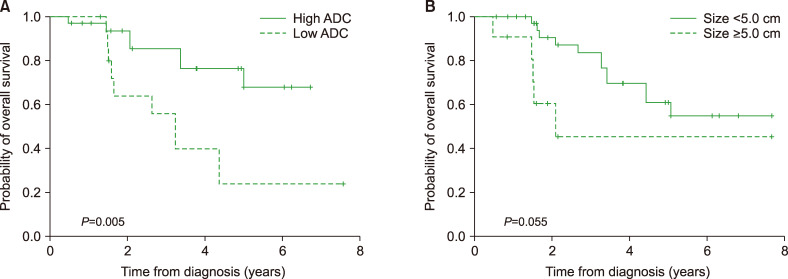
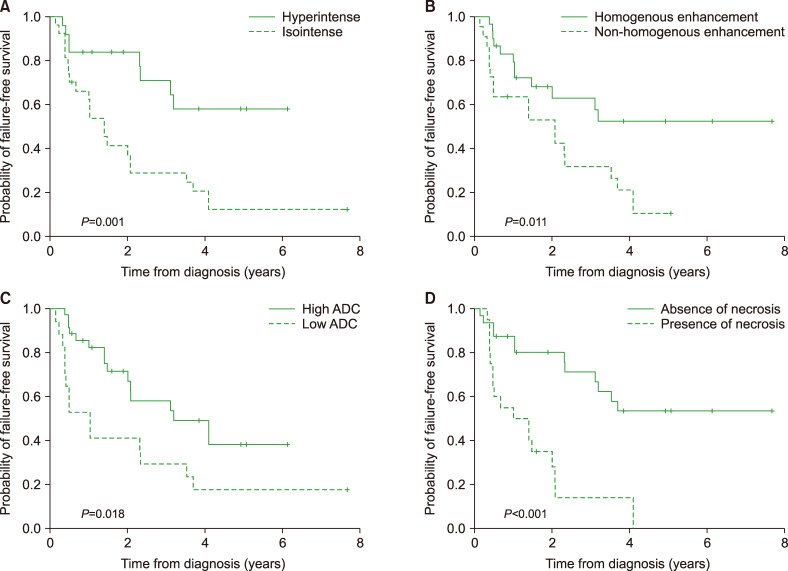
The OS and FFS rates at 3 years were 69.7±7.4% and 45.6±7.7%, respectively, with a median follow-up duration of 36.2 months (range, 5.8–93.4). Among the 52 patients, 26 (50.0%) experienced relapse and 20 (34.6%) died. According to the MRI assessment, patients with high ADC had better OS (OS rate at 3 yr, 76.5±8.6% vs. 56.0±3.7%; P=0.05) compared to those with low ADC. Tumors measuring less than 5 cm exhibited a tendency toward higher OS (OS rate at 3 yr, 83.7±6.7% vs. 45.5±7.5%; P=0.055) (Fig. 1). Patients with hyperintense signals on T2-weighted imaging (FFS rate at 3 yr, 64.6±1.3% vs. 29.0±9.1%; P=0.001) and homogenous enhancement (FFS rate at 3 yr, 57.7±1.1% vs. 31.8±1.5%; P=0.011) exhibited better FFS, while patients with low ADC (FFS rate at 3 yr, 53.6±9.9% vs. 29.4±11.1%; P=0.018) and necrosis exhibited poor FFS (FFS rate at 3 yr, 66.8±9.3% vs. 14.0±8.8%; P<0.001) (Fig. 2).
Fig. 1.
Kaplan-Meier curves for overall survival (OS). Patients with low ADC had lower OS (P=0.005) (A), while patients with tumors measuring less than 5 cm exhibited a tendency toward better OS (P=0.055) (B).
Abbreviation: ADC, apparent diffusion coefficient.
Fig. 2.
Kaplan-Meier curves for failure-free survival (FFS). Patients with hyperintense signal on T2-weighted imaging (P=0.001) (A) and homogenous enhancement (P=0.011) (B) had better FFS, while patients with low ADC (P=0.018) (C) and necrosis had poor FFS (P<0.001) (D).
Abbreviation: ADC, apparent diffusion coefficient.
Factors affecting long-term outcomes
Univariate analyses revealed that age, ECOG PS, LDH, ADC, and tumor size were prognostic factors for OS. Multivariate survival analysis revealed that old age (>60 yr) (HR, 20.372; P=0.001), ECOG PS ≥2 (HR, 10.429; P<0.001), higher LDH levels (HR, 7.408; P=0.001), and low ADC (HR, 0.273; P=0.009) were associated with lower OS. Meanwhile, univariate analysis revealed that age, ECOG PS, LDH, enhancement on MRI, necrosis, ADC, and the presence of hyperintense signals on T2-weighted imaging influenced the FFS. Multivariate survival analysis revealed that ECOG PS ≥2 (HR, 10.319; P=0.021), presence of necrosis (HR, 6.372; P=0.008), and low ADC (HR, 0.226; P=0.020) were unfavorable prognostic factors for FFS (Table 3).
Table 3.
Factors affecting long-term clinical outcomes.
| (A) Factors affecting overall survival (OS) | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age>60 vs. ≤60 yr | 20.372 | 3.466–119.724 | 0.001 | 61.945 | 5.675–676.201 | 0.001 |
| ECOG PS>1 vs. ≤1 | 10.429 | 2.851–38.155 | <0.001 | 21.384 | 2.851–160.369 | 0.003 |
| LDH, increased vs. normal | 7.408 | 2.381–23.049 | 0.001 | 6.576 | 1.655–26.139 | 0.007 |
| High ADC vs. low ADC | 0.273 | 0.103–0.721 | 0.009 | 0.392 | 0.155–0.854 | 0.012 |
| Size≥5 cm vs. <5 cm | 2.694 | 0.931–7.794 | 0.067 | |||
| (B) Factors affecting failure-free survival (FFS) | ||||||
| Univariate | Multivariate | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age>60 vs. ≤60 yr | 4.227 | 1.597–11.192 | 0.004 | |||
| ECOG PS>1 vs. ≤1 | 4.278 | 1.305–14.017 | 0.016 | 10.319 | 1.428–74.551 | 0.021 |
| LDH, increased vs. normal | 1.839 | 0.882–3.831 | 0.099 | |||
| Enhancement, homogenous vs. non-homogenous | 0.401 | 0.193–0.834 | 0.014 | |||
| Necrosis, presence vs. absence | 4.610 | 2.151–9.876 | <0.001 | 6.372 | 1.609–25.235 | 0.008 |
| High ADC vs. low ADC | 0.432 | 0.210–0.888 | 0.022 | 0.226 | 0.065–0.792 | 0.020 |
| T2-weighted imaging, hyperintense signal vs. isointense signal | 0.289 | 0.128–0.650 | 0.003 | |||
Abbreviations: ADC, apparent diffusion coefficient; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase.
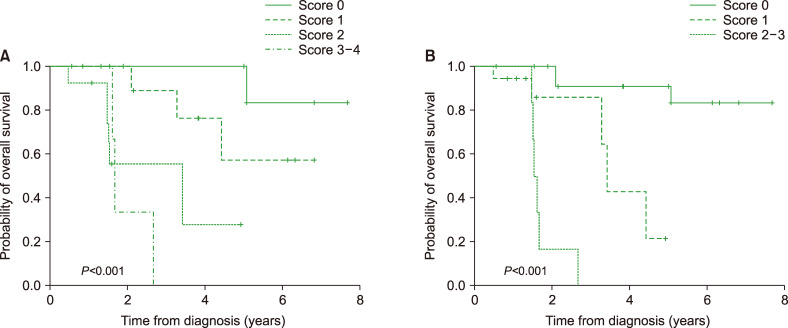
Modified prognostic scoring system with MRI findings
Our data successfully predicted the OS using old age, ECOG PS, and LDH, according to the IELSG prognostic scoring system (Fig. 3A). In this study, we attempted to modify the conventional scoring system using specific MRI findings of patients with newly diagnosed PCNSL. We modified the prognostic scoring system as follows: low ADC, old age (>60 yr), ECOG PS ≥2, and higher LDH levels. The risk of death was categorized as high (score 3–4), intermediate-2 (score 2), intermediate-1 (score 1), and low (score 0), with three-year OS rates were 33.5%, 55.4%, 88.9%, and 100%, respectively (Table 4, Fig. 3B).
Fig. 3.
Modified prognostic scoring system. The prognostic scoring system comprising old age, ECOG PS, and LDH (A) and the modified scoring system comprising old age, ECOG PS, LDH, and ADC (B).
Abbreviations: ADC, apparent diffusion coefficient; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase.
Table 4.
Modified prognostic scoring system.
| Prognostic factora) | |
|---|---|
| Age>60 yr | |
| ECOG PS>1 | |
| Raised LDH | |
| Low ADC | |
| Scores (risk) | 2-yr overall survival (%) |
| 0 (low) | 100 |
| 1 (intermediate-1) | 88.9 |
| 2 (intermediate-2) | 55.4 |
| 3–4 (high) | 33.5 |
a)Each factor was assigned a value of 1, if is present. The score was the sum of each value.
Abbreviations: ADC, apparent diffusion coefficient; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase.
DISCUSSION
PCNSL is a highly aggressive NHL. The incidence of PCNSL has increased recently, especially in individuals older than 60 years [18]. Although PCNSL typically responds favorably to chemotherapy and radiotherapy, the optimal treatment strategy for newly diagnosed PCNSL remains controversial [4, 6]. In the present study, we attempted to identify novel prognostic factors using brain MRI and modify the conventional prognostic scoring system for better risk-stratification of patients. MRI analysis revealed that ADC, age, ECOG, and LDH were promising prognostic factors for PCNSL as well as. We also modified the conventional prognostic scoring system using low ADC.
PCNSL typically appears as homogenously enhancing single lesion with a supratentorial location on brain MRI. Moreover, PCNSL preferentially involves the deep cerebral parenchyma, especially the basal ganglia, while necrotic lesions are rare. Lesions frequently appear hypointense on T1-weighted imaging, and are more likely to appear hyperintense on T2-weighted imaging [12, 19]. Our results confirm previous findings that a majority of patients with PCNSL had supratentorial homogenous lesions. The hypointense signals on T1-weighted imaging and hyperintense signals on T2-weighted imaging were also typical. However, 61.5% of patients had multiple lesions, and 42.3% of lesions showed necrosis. These differences can be partially explained by technical advances in imaging modalities, which have improved radiological detection of small lesions and necrosis.
DWI is a functional MRI technique that can estimate water molecule diffusion, thus indicating the histopathological condition of organs and tissues [20]. ADC values provide a quantitative index of diffusion characteristics, which are influenced by several factors including fibrosis, cellularity, cell membrane integrity, and other histological parameters [21]. ADC values decrease in areas with restricted diffusion, such as highly cellular tissues; thus, low ADC values are associated with poorer clinical outcomes in various cancers [22, 23]. Wang et al. [24] demonstrated that low ADC values were significantly associated with higher pathological tumor grades in pancreatic cancer. Our data also showed that low ADC was an independent unfavorable prognostic factor for FFS and OS. Previous studies proposed that PCNSL could be subclassified into high and low-density tumors, which might have prognostic implications [25, 26]. The prognosis of PCNSL could be estimated through brain MRI with ADC measurements because water diffusion is often restricted in highly cellular tumors [27, 28]. Meanwhile, the number of studies that focused on identifying prognostic factors based on specific MRI features is insufficient. Our study found that the type of enhancement, presence of necrosis, and intensity on T2-weighted imaging were associated with FFS. Therefore, efforts to identify novel prognostic factors with standard or advanced MRI should be encouraged.
Two representative scoring systems exist for PCNSL. The IELSG score consists of age, ECOG PS, LDH, CSF protein concentration, and deep brain involvement. The Memorial Sloan Kettering Cancer Center prognostic score uses age and Karnofsky performance status. In this study, CSF protein concentration and the involvement of deep brain structures failed to show a statistically significant relationship with survival. Instead, we added low ADC as an independent factor to improve the IELSG scoring system. Our modified scoring system, which stratifies patients into 4 risk groups, successfully predicted OS. Consolidation therapy with auto-SCT or WBRT should be recommended in patients with high and intermediate-2 risk based on our results, despite the risk of chemotoxicity and neurotoxicity. However, our suggestion is based on an empirical attempt with low ADC, and further evaluations using characteristic features based on advanced MRI are needed.
The 5-year OS rate of the patients included this study was 51.2%, which is similar to previous reports with 5-year survival rates of 30–50% [6, 29]. Among the 52 patients enrolled, only 10 underwent consolidation therapy with auto-SCT, which did not affect long-term clinical outcomes. Combining rituximab with HD-MTX-based chemotherapy is another aspect, which should be considered for improving survival. However, no significant survival differences were observed between the survival parameters of patients treated with MVP and R-MVP.
Although the present data identified a significant prognostic role of brain MRI in newly diagnosed PCNSL, further improvement is needed. First, a standard cut-off value for ADC has not yet been clearly established. Second, genetic profiling should also be conducted, to clarify the relationship between the molecular subgroups of PCNSL and prognosis. Finally, only 10 patients underwent auto-SCT, and 8 received rituximab-combination chemotherapy. Therefore, large-scale studies are required to validate the current results.
In conclusion, this study identified characteristic MRI findings with significant prognostic value for long-term survival. Low ADC was an independent unfavorable prognostic factor in patients with newly diagnosed PCNSL. Moreover, our results provide evidence that ADC measurements obtained from MRI scans could be used as non-invasive means for improving prognostic scoring systems.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol. 2006;8:27–37. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haldorsen IS, Krossnes BK, Aarseth JH, et al. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989-2003 : time trends in a 15-year national survey. Cancer. 2007;110:1803–14. doi: 10.1002/cncr.22989. [DOI] [PubMed] [Google Scholar]
- 3.van der Sanden GA, Schouten LJ, van Dijck JA, et al. Primary central nervous system lymphomas: incidence and survival in the Southern and Eastern Netherlands. Cancer. 2002;94:1548–56. doi: 10.1002/cncr.10357. [DOI] [PubMed] [Google Scholar]
- 4.Morris PG, Abrey LE. Therapeutic challenges in primary CNS lymphoma. Lancet Neurol. 2009;8:581–92. doi: 10.1016/S1474-4422(09)70091-2. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ, DeAngelis L, Illerhaus G, et al. Whole-brain radiotherapy in primary CNS lymphoma. Lancet Oncol. 2011;12:118–9. author reply 119–20. doi: 10.1016/S1470-2045(11)70018-3. [DOI] [PubMed] [Google Scholar]
- 6.Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35:2410–8. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niparuck P, Boonsakan P, Sutthippingkiat T, et al. Treatment outcome and prognostic factors in PCNSL. Diagn Pathol. 2019;14:56. doi: 10.1186/s13000-019-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: J Clin Oncol. 2002;20:4643–8. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Dell'Oro S, Foppoli M, et al. MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas. Neurology. 2006;66:1435–8. doi: 10.1212/01.wnl.0000210464.94122.e1. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–72. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 11.Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123:4314–24. doi: 10.1002/cncr.30965. [DOI] [PubMed] [Google Scholar]
- 12.Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12:329–40. doi: 10.1007/s003300101037. [DOI] [PubMed] [Google Scholar]
- 13.Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011;32:984–92. doi: 10.3174/ajnr.A2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabavizadeh SA, Vossough A, Hajmomenian M, Assadsangabi R, Mohan S. Neuroimaging in central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30:799–821. doi: 10.1016/j.hoc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Ahn SJ, Shin HJ, Chang JH, Lee SK. Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: comparison of three different ROI methods. PLoS One. 2014;9:e112948. doi: 10.1371/journal.pone.0112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barajas RF, Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol. 2010;31:60–6. doi: 10.3174/ajnr.A1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224:177–83. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin's lymphoma. Am J Hematol. 2013;88:997–1000. doi: 10.1002/ajh.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–6. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 20.Jiang T, Xu JH, Zou Y, et al. Diffusion-weighted imaging (DWI) of hepatocellular carcinomas: a retrospective analysis of the correlation between qualitative and quantitative DWI and tumour grade. Clin Radiol. 2017;72:465–72. doi: 10.1016/j.crad.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2010;11:102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikarmane SA, Gombos EC, Jagadeesan J, Raut C, Jagannathan JP. MRI findings of radiation-associated angiosarcoma of the breast (RAS) J Magn Reson Imaging. 2015;42:763–70. doi: 10.1002/jmri.24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada S, Morine Y, Imura S, et al. Prognostic prediction of apparent diffusion coefficient obtained by diffusion-weighted MRI in mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2020 doi: 10.1002/jhbp.732. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen ZE, Nikolaidis P, et al. Diffusion-weighted magnetic resonance imaging of pancreatic adenocarcinomas: association with histopathology and tumor grade. J Magn Reson Imaging. 2011;33:136–42. doi: 10.1002/jmri.22414. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein JL, Shen A, Batchelor TT, Kadoch C, Treseler P, Shuman MA. Differential gene expression in central nervous system lymphoma. Blood. 2009;113:266–7. author reply 267–8. doi: 10.1182/blood-2008-04-152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenstein JL, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–23. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008;18:411–7. doi: 10.1111/j.1552-6569.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder PC, Post MJ, Oschatz E, Stadler A, Bruce-Gregorios J, Thurnher MM. Analysis of the utility of diffusion-weighted MRI and apparent diffusion coefficient values in distinguishing central nervous system toxoplasmosis from lymphoma. Neuroradiology. 2006;48:715–20. doi: 10.1007/s00234-006-0123-y. [DOI] [PubMed] [Google Scholar]
- 29.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–8. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]