Abstract
The metabolic capacity of a muscle is one of the determinants of muscle function. Muscle fiber type characteristics give an indication about this metabolic capacity. Therefore it might be expected that the lumbar multifidus (MF) as a local stabilizer contains higher proportions of slow type I fibers, compared to the erector spinae (ES) as a global mobilizer. The aim of this study is to determine the muscle fiber characteristics of the ES and MF to provide insight into their structural and metabolic characteristics, and thereby the functional capacity of both muscles. Muscle fiber type characteristics in the ES and MF were investigated with an immunofluorescence staining of the myosin heavy chain isoforms. In both the ES and MF, type I muscle fibers are predominantly present. The cross-sectional area (CSA) of type I muscle fibers is significantly larger in the lumbar MF compared to the ES. However, the mean muscle fiber type percentage for type I was not significantly different, which resulted in an insignificant difference in relative cross-sectional area (RCSA) for type I. No significant differences were found for all other muscle fiber types. This may indicate that the MF displays muscle fiber type characteristics that tend to be more appropriate to maintain stability of the spine. However, because we could not demonstrate significant differences in RCSA between ES and MF, we cannot firmly state that there are functional differences between the ES an MF based only on structural characteristics.
Keywords: Paraspinal muscles, Skeletal muscle fibers
Introduction
The human lumbar muscular system plays an important role in both stabilizing and mobilizing the lumbar spine. Therefore, it is important to classify these muscles based on their function [1]. The latest functional classification includes local stabilizers, global stabilizers and global mobilizers [2]. Local stabilizers increase muscle stiffness to control segmental movement and have a crucial role in maintaining segmental stability of the lumbar spine. These muscles are continuously active, while the global stabilizers and the global mobilizers are not. Global stabilizers consequently produce movement while preserving stability, while global mobilizers generate great torque to produce large ranges of movement [2]. The lumbar multifidus (MF) is considered a local stabilizing muscle because of its close relation with the vertebral column and its short length [3]. This muscle consists of different fasciculi, which lie immediately next to the spinous processes over the full length of the spine [4-6]. The lumbar erector spinae (ES) consists of two muscles: longissimus thoracis and iliocostalis lumborum. Both muscles link the thoracic vertebrae to the pelvis and are considered global mobilizers based on their fascicle length [4, 7, 8]. This classification is based on mechanical properties and morphological features.
Muscle function is also related to the contractile and metabolic capacity of the muscle. Muscle fiber type distribution gives an indication of this metabolic and contractile profile and thereby functional capacity of a muscle. Human skeletal muscles consist of different fiber types, characterized by their specific myosin heavy chain (MHC) isoform, which determines contractile speed and metabolic capacity. In humans, three major fiber types can be identified, based on their MHC expression: type I, type IIa and type IIx [9, 10]. However, human muscle fibers can also co-express two different adjoining MHC isoforms. These muscle fibers were classified as hybrid fibers: type I/IIa and type IIax [11]. Muscle fibers have different contractile and metabolic characteristics. Type I fibers are characterized by a slow contracting speed, an oxidative metabolism and are fatigue-resistant. Type IIx fibers are fast-contracting fibers with a glycolytic metabolism and are susceptible to fatigue. Type IIa fibers are intermediate fibers that show characteristics of both type I and type IIx fibers. These muscle fibers have a fast-contracting speed and a combined oxidative and glycolytic metabolism [9-11]. The ability of a muscle to respond to different functional demands is due to its heterogeneous fiber type composition. The link between contractile/metabolic capacity of a muscle and the functional classification of the paraspinal muscles suggests that local stabilizers, like the MF, contain high proportions of slow, fatigue-resistant type I fibers to provide continuous activity needed to maintain stability of the spine [2]. Global stabilizers and mobilizers, like the ES, might contain higher proportions of fast-contracting fibers to counterbalance forces acting on the body by quick responses or to generate great torque to produce movement. However, previous studies were not able to find differences in muscle fiber type composition between ES and MF in healthy persons [12-14].
To our knowledge, the information about muscle fiber type characteristics of the ES and MF is scarce in healthy subjects. The primary aim of the present study was to determine the muscle fiber type composition and the cross-sectional area (CSA) of different fiber types in the ES and MF in order to gain insight into their structural and metabolic characteristics and to link them to the functional capacity of these muscles. The secondary aim is to determine the inter-rater reliability of the immunofluorescence analysis of MHC isoforms to measure the muscle fiber type characteristics.
Materials and Methods
Subjects
Eighteen healthy Caucasian male and female subjects between 25 and 65 years old were recruited by means of local advertisement. All interested subjects were informed about all the aspects of the study and were included in the study after providing their informed consent. Subjects were included in the study if they had no chronic (>3 months) or acute low back pain (visual analogue scale >8/10 in the last 24 hours). Subjects who underwent rehabilitation or exercise therapy for an acute condition within the last three months were excluded. This cross sectional study was part of a larger study, and was approved by the Ethical Committee of Hasselt University, Jessa Hospital Hasselt (15.142/REVA15.14) and complies with the Declaration of Helsinki.
Biopsy procedure
Muscle samples were taken from the right ES and MF at the level of spinous process of vertebra L4 according to the procedure of Agten et al. [15]. After local anaesthesia, a small incision of 2 mm was made through the skin at the puncture site, predetermined by ultrasound. A coaxial needle was inserted perpendicularly through the incision, piercing the thoracolumbar fascia. A biopsy needle was inserted through the coaxial needle to obtain a muscle sample from the ES and the MF. Muscle samples were removed from the biopsy needle, placed and oriented on a piece of cork. These samples were covered with optimum-cutting temperature compound (Tissue-Tek; Leica Microsystem Belgium, Diegem, Belgium) and immediately frozen in isopentane, precooled in liquid nitrogen. Frozen samples were stored at –80°C until further analysis. All biopsy samples were given a unique identification number.
Immunohistochemistry
Serial transverse sections (10 µm) were cut with a microtome (CM1900 Cryostat; Leica Microsystem Belgium). To identify MHC isoforms, immunofluorescent staining was performed, based on the protocol of Bloemberg and Quadrilatero [16]. Sections were air dried for 20 minutes at room temperature. Autofluorescence was blocked using 10% of normal goat serum for one hour. The sections were incubated with primary antibodies specific to laminin (ab11575:1/500 Abcam), MHC I (BA-F8:1/50), MHC IIa (SC-71:1/500), and MHC IIx (6H1:1/50) (Development Studies Hybridoma Bank, Iowa City, IA, USA) for two hours at room temperature. After washing with 1× PBS, the sections were incubated with the appropriately conjugated secondary antibodies (Alexa fluor 532:1/500, Alexa Fluor 350:1/500, Alexa Fluor 488:1/500, Alexa Fluor 555:1/500; Life Technologies Inc., Ulm, Germany) for one hour at room temperature. After primary and secondary incubation, sections were washed in 1× PBS, and coverslips were mounted using PoLong Gold antifade reagent (Life Technologies Inc., Carlsbad, CA, USA).
Muscle fiber typing and morphometry
Stained sections were viewed with a fluorescent microscope (EL6000; Leica) and photographed at a 10× magnification. The images were analyzed with AxioVision from Zeiss (Oberkochen, Germany). Muscle fiber size (µm2) and fiber type (I, IIa, and IIx) were determined for each individual muscle fiber by measuring the CSA and counting the number of muscle fibers. These measurements were performed blinded by the first two authors to determine inter-rater reliability. Relative cross-sectional area (RCSA) was calculated based on the CSA and percentage of a muscle fiber type. RCSA is an important structural characteristic that defines the functional capacity of a skeletal muscle [17]. Fibers expressing only BA-F8 were classified as MHC I, fibers expressing only SC-71 were classified as MHC IIa, and fibers expressing strong intensities for 6H1 were classified as MHC IIx. When fibers were expressing intermediate/strong intensities for SC-71 and intermediate intensities for 6H1, they were classified as MHC IIax hybrid fibers. When fibers were expressing intermediate intensities for both BA-F8 and SC-71 they were classified as MHC I/IIa hybrid fibers. To analyze a representative sample of the entire muscle, on average, 155±35 muscle fibers were counted in the biopsy samples collected from the ES and 151±32 in the biopsy samples collected from the MF [18].
Data analysis
A sample size calculation was performed (80% power, α=0.05) based on preliminary data. Given the calculated estimates, n=18 was needed in each group to provide a power of 0.80. inter-rater reliability for measurements of CSA and muscle fiber type composition was evaluated by an analysis of the first 10 samples. These samples were blindly evaluated by the first two authors in both the ES and MF muscle. Intraclass correlation coefficients (ICC) were analyzed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Co., Armonk, NY, USA). A two-way mixed model and absolute agreement was used for the first 10 subjects. ICC’s were estimated for muscle fiber type CSA and percentage in both the MF and ES muscle. From the SD and ICC, the standard error of measurement (SEM) was calculated using the formula . The ICC gives an indication of the inter-rater reliability with an inter-rater reliability being poor for ICC values of less than 0.40, fair for values between 0.40 and 0.59, good for values between 0.60 and 0.74, and excellent for values between 0.75 and 1.0 [19].
Statistical analysis for the differences between the ES and MF muscles were performed with JMP Pro 14.1.0 software (SAS Institute Inc., Cary, NC, USA; 1989–2007). A repeated measure ANOVA was performed with fiber type and muscle as within subject factors. Normality of the data was checked using normal quartile plots calculated from the conditional residuals. A square root transformation was used in case of a non-normal distribution whenever needed. Significance was set at the 5% point with a confidence interval of 95%. When a significant interaction was found, a post-hoc Tukey honestly significant difference was used. A Kruskall-Wallis test was performed to analyze frequency distribution.
Results
In total 18 healthy subjects were included in the study. Anthropometric characteristics are presented in Table 1. Hybrid fibers co-expressing MHC I and MHC IIa were not found within the muscle samples. Therefore, the analysis was done only for fiber type I, IIa, IIx, and the hybrid fiber type IIax (expressing both MHC IIa and MHC IIx) in both the lumbar ES and MF. Detailed results on repeated measurements ANOVA and inter-rater reliability are displayed in Appendix Tables 1–3.
Table 1.
Sociodemographic characteristics (n=18)
| Characteristics | Value |
|---|---|
| Age (yr) | 40.00±7.91 |
| Sex (male:female) | 9:9 |
| Weight (kg) | 78.22±13.08 |
| Length (m) | 1.76±0.08 |
| Body mass index (kg/m2) | 25.13±2.93 |
Values are presented as mean±SD or number only.
Inter-rater reliability
For the measurements of CSA, the ICC’s ranged from 0.862 (type IIx) to 0.983 (type I) in the ES and from 0.911 (type IIax) to 0.996 (type I) in the MF. For the measurements of fiber type percentage, the ICC’s percentage ranged from 0.909 (type IIax) to 0.976 (type I) in the ES and from 0.591 (type IIax) to 0.966 (type IIx) in the MF.
Fiber type size (measured by cross-sectional area)
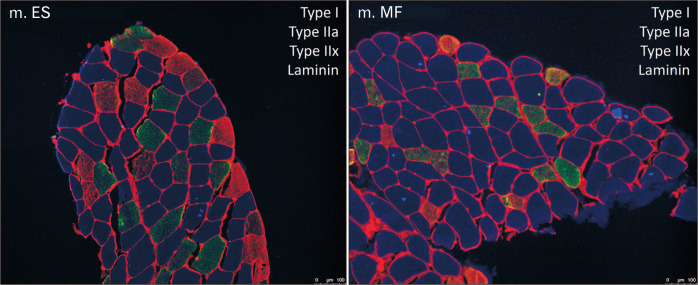
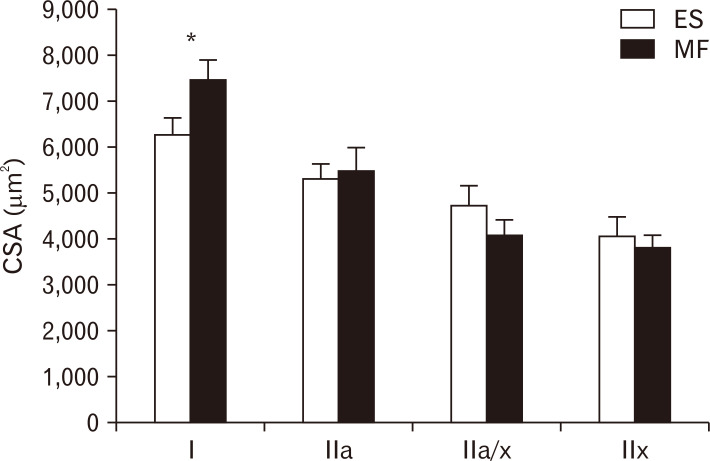
A representative example of an image from the lumbar ES and MF muscle within one subject was shown in Fig. 1. Mean CSA of type I muscle fibers was 7,439.31 µm2 for the MF, compared to 6,279.48 µm2 for the ES. The mean CSA of type I muscle fibers was 18% higher in the MF, compared to the lumbar ES (P=0.0053). The mean CSA for type IIa, IIax, and IIx were similar between the lumbar ES and MF muscle (Fig. 2).
Fig. 1.
Representative immunofluore scence image of the lumbar ES and MF muscle. Muscle cross-sections were in
cubated with primary antibody against laminin (red), MHC I (blue), MHC IIa (green) and MHC IIx (red). ES, erector spinae; MF, multifidus; MHC, myosin heavy chain.
Fig. 2.
CSA of the different muscle fibre types. CSA of type I, type IIa, type IIa/x and type IIx in the lumbar ES (white) and MF (black) muscle. Values are presented as mean±SE. CSA, cross-sectional area; ES, erector spinae; MF, multifidus; SE, standard error. *P=0.0053 ES vs. MF.
Fiber type percentage (measured by %)
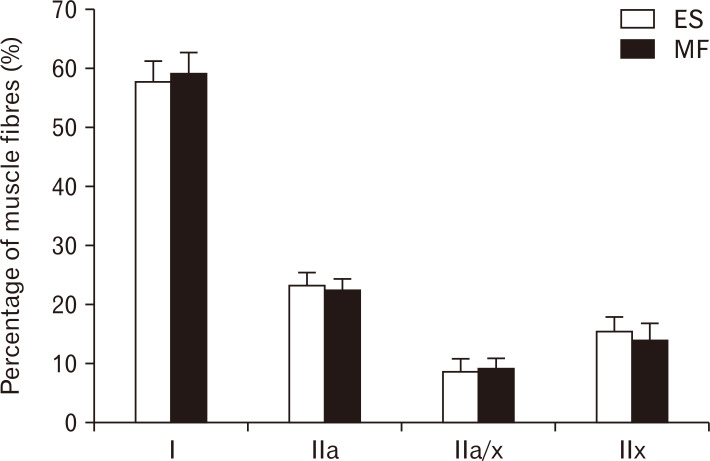
The mean percentage of fibers present within a muscle cross-section was highest for type I fibers in both muscles, 57.74% and 59.07% for the ES and MF respectively. For the ES, the mean percentage of the other muscle fiber types were 23.32%, 8.65%, and 15.43% for type IIa, type IIax, and type IIx respectively. For the MF, the mean percentage of the other muscle fiber types were 22.45%, 9.21%, and 13.89% for type IIa, type IIax, and type IIx respectively. These mean muscle fiber type percentages were not significantly different between the lumbar ES and MF (Fig. 3).
Fig. 3.
Number of the different muscle fibres. Number of type I, type IIa, type IIa/x and type IIx in the lumbar ES (white) and MF (black) muscle. Values are presented as mean±SE. ES, erector spinae; MF, multifidus; SE, standard error.Number of the different muscle fibres. Number of type I, type IIa, type IIa/x and type IIx in the lumbar ES (white) and MF (black) muscle. Values are presented as mean±SE. ES, erector spinae; MF, multifidus; SE, standard error.
Relative fiber type area (measured by relative cross-sectional area)
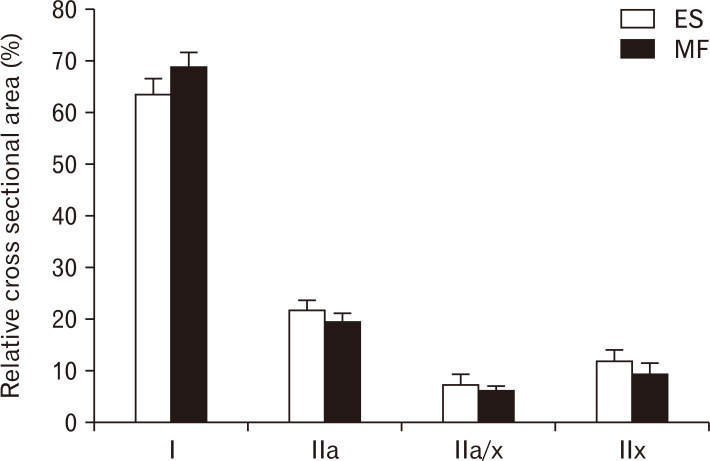
Type I muscle fibers are predominantly present in both ES and MF muscle, respectively 63.54% and 68.8%. For the ES, the mean relative area for the other muscle fiber types were 21.60%, 7.23%, and 11.8% for type IIa, type IIax and type IIx respectively. For the MF, the mean percentage of the other muscle fiber types were 19.17%, 5.93%, and 9.14% for type IIa, type IIax, and type IIx respectively. These RCSA’s for all muscle fiber types were not significantly different between the lumbar ES and MF (Fig. 4).
Fig. 4.
Relative RCSA of the different muscle fibres. RCSA of type I, type IIa, type IIa/x and type IIx in the lumbar ES (white) and MF (black) muscle. Values are presented as mean±SE. ES, erector spinae; MF, multifidus; RCSA, relative cross-sectional area; SE, standard error.
Frequency distribution
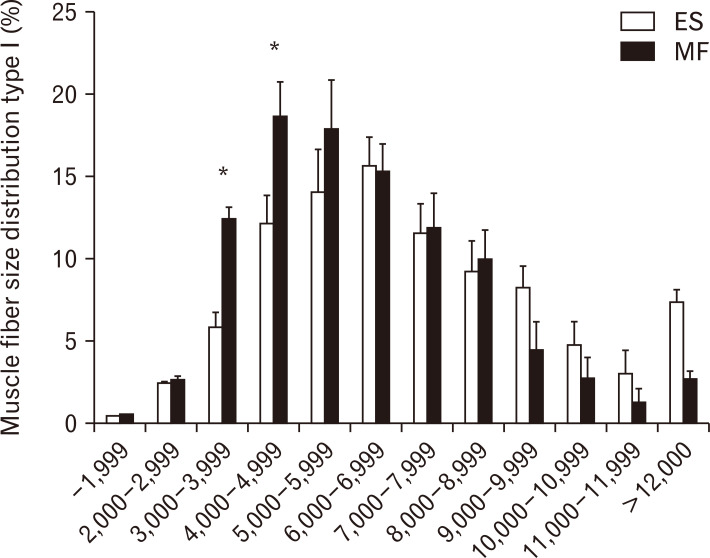
Substantial differences in the frequency distribution of muscle fiber type I were observed (Fig. 5).
Fig. 5.
Muscle fibre size distribution (in percentage) for type I muscle fibres in the MF (white) and ES (black). Values are presented as mean±SE. ES, erector spinae; MF, multifidus; SE, standard error. *P<0.05 ES vs. MF.
Small type I muscle fibers from 3,000–3,999 µm2 and from 4,000–4,999 µm2 were more prevalent in the ES than in the MF (P=0.0291 and P=0.0458, respectively). A shift in muscle fiber size distribution was observed for type I muscle fibers, with a higher percentage of small fibers in the ES compared to the MF. In the ES, 34% of the muscle fibers were smaller than 5,000 µm2, compared to 21% in the MF.
Discussion
Based on muscle fiber type characteristics, the present study reveals that the MF and the ES can both be considered postural muscles that provide stability in the lumbar vertebral column, because of their predominance in type I muscle fibers. Based on our results, the MF seems to display muscle fiber type characteristics that tend to be more appropriate to maintain stability of the spine compared to the ES, due to the fact that the MF is characterized by significant larger type I muscle fibers. However, there were no differences in fiber type percentage between both muscles. This resulted in non-significant differences in the RCSA of type I between the ES and the MF. Because we did not demonstrate significant differences in RCSA between ES and MF, we cannot firmly state that there are functional differences between these two muscles based only on muscle fiber type characteristics.
To our knowledge, there are only two studies that have examined differences in muscle fiber type characteristics between ES and MF in healthy subjects using muscle biopsy samples [12, 14]. Thorstensson and Carlson [14] biopsied 16 healthy persons (age 20–30 years), Jørgensen et al. [12] biopsied 10 healthy males (age 21–29 years) both at the level of the L3 vertebra. In contrast to our results, both studies found no significant differences in muscle fiber type characteristics between both muscles, using ATPase staining. In order to investigate muscle fiber type composition, we used immunofluorescence analysis of MHC isoforms, which is a more sensitive and reliable method compared to the traditionally used technique in which myosin ATPase activity is determined. With ATPase staining it is difficult to identify hybrid fibers [16]. Our study showed a good inter-rater reliability for the measurement of CSA and fiber type percentages. Although ICC’s varied from fair to excellent, in much of the outcomes, the SEM was high compared to the mean values. This indicates that the between subject variability was high in both the ES and MF muscle, mainly for the percentage and CSA of type IIax, despite having a fair to excellent inter-rater reliability. This indicates muscle fiber type composition is highly variable between different subjects.
In our study, the muscle biopsies were taken at the level of L4, while the muscle samples in the study of Thorstensson and Carlson [14] and the study of Jørgensen et al. [12] were obtained at the level of L3. This could possibly explain the differences in our results. The MF is best developed in the lower lumbar region, were the volume of fiber bundles is greatest [6]. The differences in biopsy level could contribute to different muscle fiber characteristics.
Other studies investigated paraspinal muscle fiber characteristics in the lumbar ES and MF muscle in cadaveric specimens [12, 13, 20, 21]. However, these samples cannot be considered as healthy tissue, due to the fact that these samples have been collected post-mortem in which protein degradation caused by cellular breakdown and autolytic activity, and structural alterations of muscle tissue cannot be excluded [22]. Rantanen et al. [13] and Hesse et al. [20] found no significant differences in muscle fiber type characteristics between both muscles, while Jørgensen et al. [12] found significantly smaller IIx muscle fibers in the lumbar MF compared to the longissimus and iliocostalis. Moreover, they showed that the number of type I muscle fibers was significantly higher in the longissimus compared to the MF and the iliocostalis, while the number of type IIx muscle fibers was significantly lower in the longissimus [12]. In contrast to these studies, our study showed significantly larger type I muscle fibers in the MF compared to the ES. It could be possible that there are differences between deep and superficial paraspinal muscles, as indicated by Sirca and Kostevcs [21].
In both the ES and MF, type I muscle fibers are predominantly present. These results are in line with the findings of MacDonald et al. [23], who confirmed the postural role of both muscles, indicated by the presence of a large RCSA of type I muscle fibers. However, muscle fiber characteristics are not the only determinant of muscle function: other mechanisms could also play an important role. Mechanical properties, such as pennation angle, fascicle length and proportion of fleshy to tendinous fascicle parts have a major impact on muscle function [24, 25]. Functional diversification could also be influenced by neural control, in which the size of the motor unit, the amount of muscle spindles, and the control by the motor cortex are all possible contributors [26-29].
This is the first study comparing muscle fiber type characteristics between the ES and the MF in healthy subjects and using a multicolour immunofluoresecent staining technique to visualise MHC, which is a much more reliable technique compared to the ATPase staining [16]. In contrast to previous studies [12, 14], we found significantly larger type I muscle fibers in the lumbar MF compared to the ES. We did not demonstrate clear differences in RCSA between the ES and MF, which suggests there are no or only small differences in muscle function based on muscle fiber type characteristics. Future studies should focus on observing paraspinal muscle fiber type characteristics in different low back pain populations to unravel possible structural alteration that can contribute to clinical symptoms.
Supplemental Materials
Acknowledgements
Our gratitude goes to Dr. Geert Souverijns (Jessa Hospital, Department of Radiology, Belgium) who gave us the opportunity to work with a physician in his department. We also want to thank the department of Pneumology (KULeuven) for the help with the immunofluorescence staining.
Footnotes
Author Contributions
Conceptualization: AA, SS, JV, BOE, AT, FV. Data acquisition: AA, SS, JV, FV. Data analysis or interpretation: AA, SS, FV. Drafting of the manuscript: AA, SS. Critical revision of the manuscript: AA, SS, JV, BOE, AT, FV. Approval of the final version of the manuscript: all authors.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Gibbons SGT, Comerford MJ. Strength versus stability part I: concept and terms. Orthopaedic Division Review. 2001;43:21–7. [Google Scholar]
- 2.Comerford MJ, Mottram SL. Movement and stability dysfunction--contemporary developments. Man Ther. 2001;6:15–26. doi: 10.1054/math.2000.0388. [DOI] [PubMed] [Google Scholar]
- 3.Goel VK, Kong W, Han JS, Weinstein JN, Gilbertson LG. A combined finite element and optimization investigation of lumbar spine mechanics with and without muscles. Spine (Phila Pa 1976) 1993;18:1531–41. doi: 10.1097/00007632-199318110-00019. [DOI] [PubMed] [Google Scholar]
- 4.Macintosh JE, Valencia F, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clin Biomech (Bristol, Avon) 1986;1:196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- 5.Hansen L, de Zee M, Rasmussen J, Andersen TB, Wong C, Simonsen EB. Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine (Phila Pa 1976) 2006;31:1888–99. doi: 10.1097/01.brs.0000229232.66090.58. [DOI] [PubMed] [Google Scholar]
- 6.Rosatelli AL, Ravichandiran K, Agur AM. Three-dimensional study of the musculotendinous architecture of lumbar multifidus and its functional implications. Clin Anat. 2008;21:539–46. doi: 10.1002/ca.20659. [DOI] [PubMed] [Google Scholar]
- 7.Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980;131(Pt 3):525–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Bustami FM. A new description of the lumbar erector spinae muscle in man. J Anat. 1986;144:81–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 10.Caiozzo VJ. Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve. 2002;26:740–68. doi: 10.1002/mus.10271. [DOI] [PubMed] [Google Scholar]
- 11.Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–6. doi: 10.1093/ptj/81.11.1810. [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Significance for isometric endurance. Spine (Phila Pa 1976) 1993;18:1439–50. doi: 10.1097/00007632-199318110-00007. [DOI] [PubMed] [Google Scholar]
- 13.Rantanen J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J. 1994;3:331–5. doi: 10.1007/BF02200146. [DOI] [PubMed] [Google Scholar]
- 14.Thorstensson A, Carlson H. Fibre types in human lumbar back muscles. Acta Physiol Scand. 1987;131:195–202. doi: 10.1111/j.1748-1716.1987.tb08226.x. [DOI] [PubMed] [Google Scholar]
- 15.Agten A, Verbrugghe J, Stevens S, Boomgaert L, O Eijnde B, Timmermans A, Vandenabeele F. Feasibility, accuracy and safety of a percutaneous fine-needle biopsy technique to obtain qualitative muscle samples of the lumbar multifidus and erector spinae muscle in persons with low back pain. J Anat. 2018;233:542–51. doi: 10.1111/joa.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 2012;7:e35273. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 1997;190(Pt 4):505–13. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceglia L, Niramitmahapanya S, Price LL, Harris SS, Fielding RA, Dawson-Hughes B. An evaluation of the reliability of muscle fiber cross-sectional area and fiber number measurements in rat skeletal muscle. Biol Proced Online. 2013;15:6. doi: 10.1186/1480-9222-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–90. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- 20.Hesse B, Fröber R, Fischer MS, Schilling N. Functional differentiation of the human lumbar perivertebral musculature revisited by means of muscle fibre type composition. Ann Anat. 2013;195:570–80. doi: 10.1016/j.aanat.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Sirca A, Kostevc V. The fibre type composition of thoracic and lumbar paravertebral muscles in man. J Anat. 1985;141:131–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenfellner B, Zissler A, Steinbacher P, Monticelli FC, Pittner S. Are animal models predictive for human postmortem muscle protein degradation? Int J Legal Med. 2017;131:1615–21. doi: 10.1007/s00414-017-1643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11:254–63. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ward SR, Tomiya A, Regev GJ, Thacker BE, Benzl RC, Kim CW, Lieber RL. Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J Biomech. 2009;42:1384–9. doi: 10.1016/j.jbiomech.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stark H, Fröber R, Schilling N. Intramuscular architecture of the autochthonous back muscles in humans. J Anat. 2013;222:214–22. doi: 10.1111/joa.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeb EP, Giszter SF, Saltiel P, Bizzi E, Mussa-Ivaldi FA. Output units of motor behavior: an experimental and modeling study. J Cogn Neurosci. 2000;12:78–97. doi: 10.1162/08989290051137611. [DOI] [PubMed] [Google Scholar]
- 27.Amonoo-Kuofi HS. The density of muscle spindles in the medial, intermediate and lateral columns of human intrinsic postvertebral muscles. J Anat. 1983;136(Pt 3):509–19. [PMC free article] [PubMed] [Google Scholar]
- 28.Botterman BR, Binder MD, Stuart DG. Functional anatomy of the association between motor units and muscle receptors. Am Zool. 1978;18:135–52. doi: 10.1093/icb/18.1.135. [DOI] [Google Scholar]
- 29.Tsao H, Danneels L, Hodges PW. Individual fascicles of the paraspinal muscles are activated by discrete cortical networks in humans. Clin Neurophysiol. 2011;122:1580–7. doi: 10.1016/j.clinph.2011.01.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.