Capsule Summary:
Combined elevated sputum eosinophils+neutrophils in asthma associated with lowest lung function, greater healthcare utilization, and longitudinally, further spirometric loss, implicating cell-cell interactions or overlapping inflammatory pathways while increased eosinophils or neutrophils alone show less effect.
Keywords: eosinophils, neutrophils, baseline sputum inflammation, longitudinal outcomes, healthcare utilization
To the Editor:
Cellular analysis of induced sputum allows noninvasive assessment of airway inflammation in comprehensively phenotyped subjects with different levels of asthma severity. Sputum analysis in a subset of subjects in the cross-sectional SARP1+2, revealed combined higher eosinophil and neutrophil percentages associated with more severe asthma phenotypes including lower lung function and greater healthcare utilization3. Cluster analysis incorporating sputum and blood inflammatory cells with clinical parameters showed that sputum neutrophils are an important variable associated with more severe asthma5. However, longitudinal observations are required to understand the impact of airway inflammation on progression of more severe asthma. Reports of longitudinal airway inflammation in asthma differ regarding whether inflammation is associated with accelerated decline in lung function or other important clinical characteristics; however, most study intervals are a year or less1, 4, 7, 8, 9. Therefore, utilizing Severe Asthma Research Program3 data with longitudinal assessment over 3 years for 526 adult subjects, we investigated whether baseline categorization of subjects by combined eosinophils (Eos) and neutrophils (Neu) identified a more severe asthma subgroup, and provided information on longitudinal changes in lung function and healthcare utilization.
Subjects and Assessments:
Adult subjects recruited at 7 clinical sites signed informed consent approved by site IRB and by NHLBI DSMB (ClinicalTrials.gov), underwent extensive clinical assessment at baseline, including induced phenotype (single, intramuscular 40mg triamcinolone6), and annual visits for 3 years (details in online supplement).
Analyses and Statistics:
Subjects were stratified into 4 groups by baseline sputum cellularity (Low Eos+Low Neu: <2%Eos+<50%Neu; Low Eos+High Neu: <2%Eos+>50% Neu; High Eos+Low Neu: >2%Eos+<50%Neu; or High Eos+High Neu: >2%Eos+>50%Neu; similar to previous cross-sectional study3,5) and retained in these same groups for longitudinal analyses. Subjects without acceptable baseline sputum, or treated with biologic therapy during the study were excluded. Clinical characteristics for baseline, years 1, 2 and 3 data were analyzed by standard statistical tests.
Baseline Characteristics:
Baseline characteristics of the 4 sputum groups are in Table 1. Those groups with High Neu at baseline were older (p=0.0006) with greater length of time since diagnosis (p=0.0107), but did not differ for gender, or former smoker %. Those with High Eos at baseline had higher blood eosinophils (p<0.0001) and FeNO (p=0.0004). Total serum IgE was highest in High Eos groups (p=0.0246), but the number of positive specific IgEs, and frequency of >1 positive IgE did not differ. Controller medications did not differ between baseline groups (online supplement).
Table 1.
Demographic and clinical characteristics for subjects stratified by baseline sputum differential groups at enrollment (Baseline) and over the following 3 years.
| Label | Low Eos+Low Neu | Low Eos+High Neu | High Eos+Low Neu | High Eos+High Neu | P value across 4 Eos+Neu groups |
|---|---|---|---|---|---|
| N Baseline | 101 | 130 | 46 | 52 | |
| N year 1 | 95 | 120 | 44 | 47 | |
| N year 2 | 88 | 112 | 43 | 40 | |
| N year 3 | 83 | 103 | 38 | 39 | |
| Age at Baseline | 42.3 ± 13.8 | 49.1 ± 14.3 | 45.6 ± 13.0 | 50.9 ± 13.4 | 0.0006a,f |
| Years since diagnosis of asthma | 23.3 ± 14.0 | 29.7 ± 16.1 | 24.8 ± 13.0 | 30.0 ± 16.5 | 0.0107a,f |
| BMI - Baseline | 34.1 ± 8.7 | 32.5 ± 9.6 | 31.4 ± 8.8 | 31.8 ± 8.9 | 0.0625d,f |
| -year 1 | 34.3 ± 8.9 | 32.3 ± 9.3 | 31.2 ± 8.3 | 30.4 ± 6.7 | 0.0358a,d,f |
| -year 2 | 33.7 ± 8.0 | 32.5 ± 9.5 | 30.5 ± 7.0 | 30.6 ± 7.2 | 0.0686a,d,f |
| -year 3 | 33.7 ± 8.0 | 32.6 ± 9.4 | 30.4 ± 7.5 | 30.6 ± 7.0 | 0.0767a,d |
| Male sex N (%) | 35 (34.7%) | 48 (36.9%) | 12 (26.1%) | 25 (48.1%) | 0.1509 |
| Race %White/%Black/%Other | 63.4/25.7/10.9 | 70/23.1/6.9 | 63/21.7/15.2 | 53.8/25/21.2 | 0.2235/0.9433/0.0451b |
| Severe N (%)(by ATS criteria) Baseline | 51 (50.5%) | 60 (46.2%) | 27 (58.7%) | 31 (59.6%) | 0.2794 |
| -year 1 | 48 (50.5%) | 45 (37.5%) | 18 (40.9%) | 26 (55.3%) | 0.1024 |
| -year 2 | 46 (52.3%) | 38 (33.9%) | 19 (44.2%) | 20 (50%) | 0.0550 |
| -year 3 | 33 (39.8%) | 36 (35%) | 16 (42.1%) | 16 (41%) | 0.8222 |
| Ever smoked N (%) | 24 (23.8%) | 32 (24.6%) | 14 (30.4%) | 20 (38.5%) | 0.2052 |
| PreBD FEV1 %Predicted - baseline | 75.8 ± 18.5 | 76.1 ± 18.5 | 74.8 ± 17.7 | 68.9 ± 19.2 | 0.0721a,b |
| -year 1 | 77.3 ± 17.4 | 76.7 ± 19.9 | 73.7 ± 19.1 | 68.6 ± 21.8 | 0.0394a,b |
| -year 2 | 78.3 ± 18.8 | 76.2 ± 20.5 | 75.3 ± 21.2 | 66.9 ± 20.5 | 0.0194a,b |
| -year 3 | 77.8 ± 17.5 | 76.4 ± 19.2 | 75.2 ± 19.9 | 69.8 ± 21.5 | 0.1335a |
| PostBD FEV1 %Predicted - baseline | 87.6 ± 16.8 | 85.6 ± 17.9 | 88.4 ± 16.9 | 81.1 ± 18.8 | 0.0807a,c |
| -year 1 | 86.9 ± 16.0 | 84.0 ± 19.5 | 87.2 ± 18.4 | 80.3 ± 19.9 | 0.1741a |
| -year 2 | 87.6 ± 16.8 | 84.3 ± 19.4 | 89.8 ± 17.4 | 78.1 ± 19.4 | 0.0123a,b,c |
| -year 3 | 87.6 ± 16.4 | 84.4 ± 18.5 | 89.6 ± 18.8 | 79.5 ± 21.3 | 0.0593a,c |
| PreBD FEV1/FVC Baseline | 0.71 ± 0.10 | 0.71 ± 0.10 | 0.68 ± 0.08 | 0.65 ± 0.10 | 0.0001a,b,e |
| -year 1 | 0.71 ± 0.09 | 0.71 ± 0.10 | 0.68 ± 0.08 | 0.64 ± 0.10 | 0.0001a,b,c |
| -year 2 | 0.71 ± 0.10 | 0.71 ± 0.11 | 0.68 ± 0.10 | 0.64 ± 0.10 | 0.0008a,b,e |
| -year 3 | 0.71 ± 0.09 | 0.71 ± 0.10 | 0.67 ± 0.09 | 0.65 ± 0.11 | 0.0019a,b,d,e |
| Triamcinolone response: Absolute change in Pre-BD FEV %predicted at baseline | 2.1 ± 8.9 | 1.8 ± 6.3 | 3.7 ± 10.4 | 5.0 ± 8.9 | 0.0790b |
| FeNO - Baseline | 20.0 (12.0, 34.0) | 19.0 (13.0, 29.5) | 35.0 (20.0, 53.0) | 26.5 (16.0, 43.5) | 0.0004b,d,e |
| -year 1 | 19.0 (14.0, 33.0) | 19.0 (13.0, 29.0) | 28.0 (19.0, 49.0) | 38.0 (17.0, 55.0) | 0.0002a,b,d,e |
| -year 2 | 21.0 (14.0, 32.0) | 19.5 (12.0, 33.0) | 45.5 (26.0, 73.0) | 30.5 (17.0, 57.5) | <0.0001a,b,d,e |
| Sputum Eosinophils (percent) Baseline | 0.3 (0.0, 0.7) | 0.3 (0.0, 0.9) | 5.6 (2.6, 16.7) | 5.6 (3.6, 8.6) | <0.0001a,b,d,e |
| -year 1 | 0.5 (0.0, 1.1) | 0.4 (0.0, 1.3) | 2.6 (0.6, 17.6) | 4.7 (2.0, 12.0) | <0.0001a,b,d,e |
| -year 2 | 0.4 (0.0, 1.4) | 0.5 (0.0, 1.7) | 5.1 (0.8, 16.7) | 2.7 (1.2, 14.7) | <0.0001a,b,d,e |
| -year 3 | 0.2 (0.0, 0.9) | 0.2 (0.0, 0.9) | 2.7 (1.0, 11.6) | 3.1 (0.2, 11.5) | <0.0001a,b,d,e |
| Sputum Neutrophils (percent) Baseline | 28.6 (16.2, 39.3) | 74.5 (61.9, 87.5) | 35.2 (24.3, 42.6) | 70.8 (60.0, 78.6) | <0.0001a,c,d,e,f |
| -year 1 | 38.3 (21.2, 62.6) | 66.3 (46.5, 80.5) | 49.3 (29.0, 58.3) | 64.8 (49.3, 80.1) | <0.0001a,c,e,f |
| -year 2 | 49.2 (27.8, 59.5) | 69.5 (50.0, 85.1) | 44.8 (29.9, 54.9) | 57.5 (48.9, 69.5) | <0.0001a,c,e,f |
| -year 3 | 53.4 (38.9, 70.3) | 66.3 (55.6, 81.9) | 51.8 (40.9, 69.5) | 67.6 (53.8, 83.0) | 0.0012a,c,e,f |
| Blood Eosinophils (count) Baseline | 168 (105, 255) | 168 (108, 281) | 343 (222, 544) | 344 (224, 489) | <0.0001a,b,d,e |
| Blood Neutrophils (count) Baseline | 4,064 (3,045, 5,188) | 3,949 (3,100, 4,988) | 4,011 (3,180, 5,317) | 3,996 (3,025, 5,857) | 0.9394 |
| Total IgE Baseline Geometric means | 129.3 ± 3.7 | 87.2 ± 5.7 | 199.4 ± 3.8 | 166.8 ± 4.8 | 0.0246b,e |
| At Least One + Specific IgE | 84 (84%) | 103 (80.5%) | 36 (78.3%) | 38 (73.1%) | 0.4472 |
| Number of + Specific IgE tests (of 15) | 4.6 ± 3.7 | 4.1 ± 3.8 | 5.1 ± 4.6 | 4.0 ± 4.0 | 0.3855 |
High Eos+High Neu vs Low Eos+Low Neu, p<0.05
High Eos+High Neu vs Low Eos+High Neu, p<0.05
High Eos+High Neu vs High Eos+Low Neu, p<0.05
High Eos+Low Neu vs Low Eos+Low Neu, p<0.05
High Eos+Low Neu vs Low Eos+High Neu, p<0.05
Low Eos+High Neu vs Low Eos+Low Neu, p<0.05
Pre- and post-bronchodilator (BD) FEV1%predicted were lower for combined High Eos+High Neu group than the other three groups, significant for High Eos+High Neu versus Low Eos+Low Neu groups for pre-BD and post-BD FEV1%predicted (both p=0.0200), and for High Eos+High Neu versus Low Eos+High Neu for pre-BD FEV1%predicted (p=0.0124). The change in absolute pre-BD FEV1%predicted following triamcinolone tended to be higher in the High Eos+High Neu group but not significant. Higher FEV1 response to albuterol was observed for the High Eos+Low Neu group (p=0.0001). Pre-bronchodilator FEV1/FVC was lowest in the group with combined High Eos+High Neu (p=0.0001)(Table 1).
Baseline healthcare utilization for the proportion of subjects reporting emergency department (ED) visits, unscheduled or ED visits, and number of exacerbations in the previous 12 months/year, were higher for combined High Eos+High Neu, but statistically significant for ED visits only (p=0.0213)(Supplement Table S3).
Longitudinal Characteristics:
Baseline sputum groups annually reassessed over 3 years (Years 1, 2 and 3, Table 1 and supplement Tables) showed reductions for %subjects classified as ‘severe’ in all groups over years 1, 2, and 3, but did not differ across 4 groups. Sputum eosinophil% in each baseline Eos+Neu group declined but remained significantly higher in High Eos groups (Table 1). High sputum eosinophil groups with or without High Neu had higher FeNO levels than Low Eos groups, (years 1 and 2, p=0.0002 and <0.0001, respectively).
Subjects in the High Eos+High Neu group continued to have the lowest pre-BD FEV1/FVC (years 1, 2, and 3: p=0.0001, 0.0008 and 0.0019, respectively). The High Eos+Low Neu group had the highest post-BD FEV1%predicted (Figure 1A), significantly differing from High Eos+High Neu at years 2 and 3 (p=0.0040, and p=0.0319, respectively). Absolute change in pre- and post-BD FEV1%predicted from baseline remained small and did not differ across groups, although post-BD FEV1% predicted was consistently negative in High Eos+High Neu throughout 3 years compared to little change or small improvements in the other groups (Figure 1B).
Figure 1. (A).
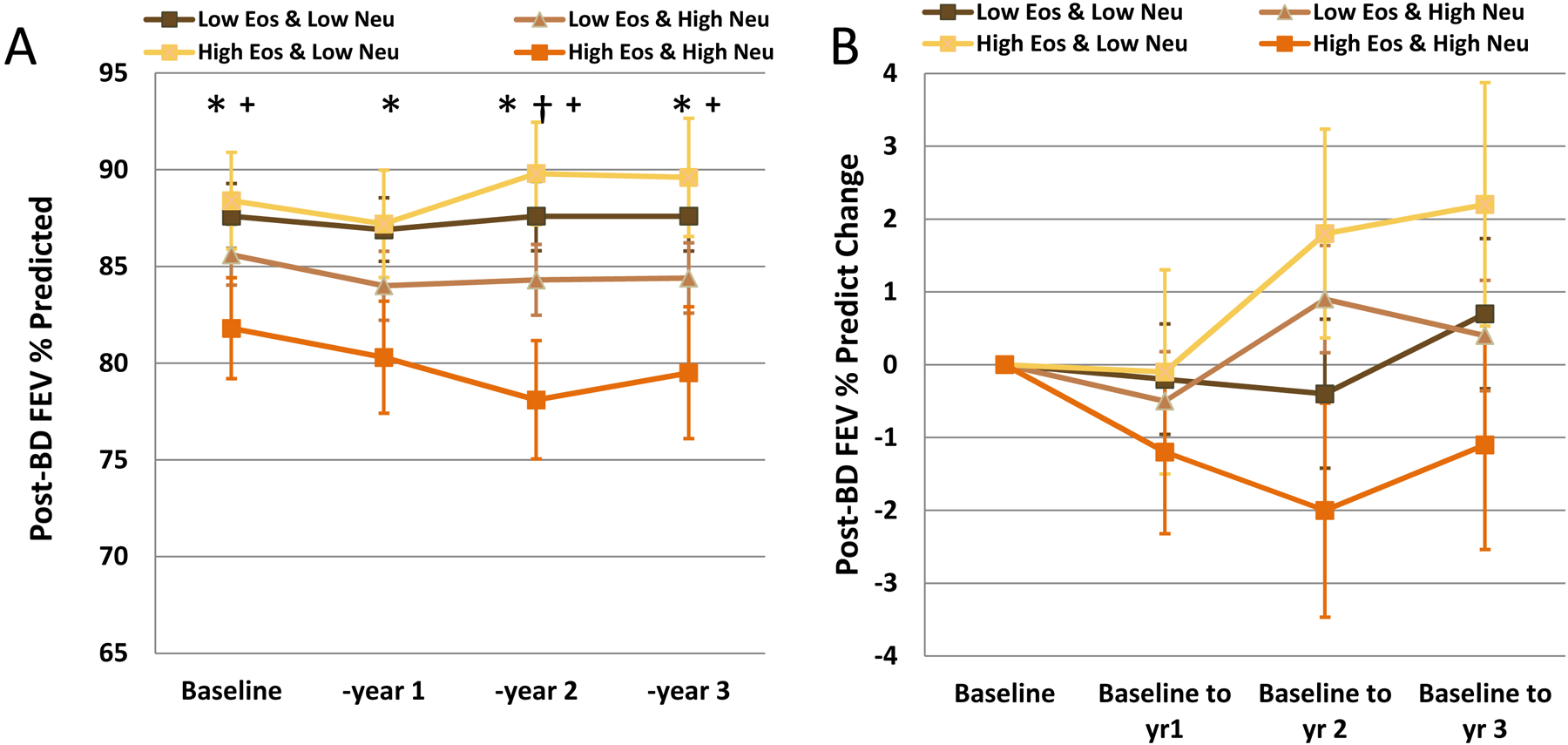
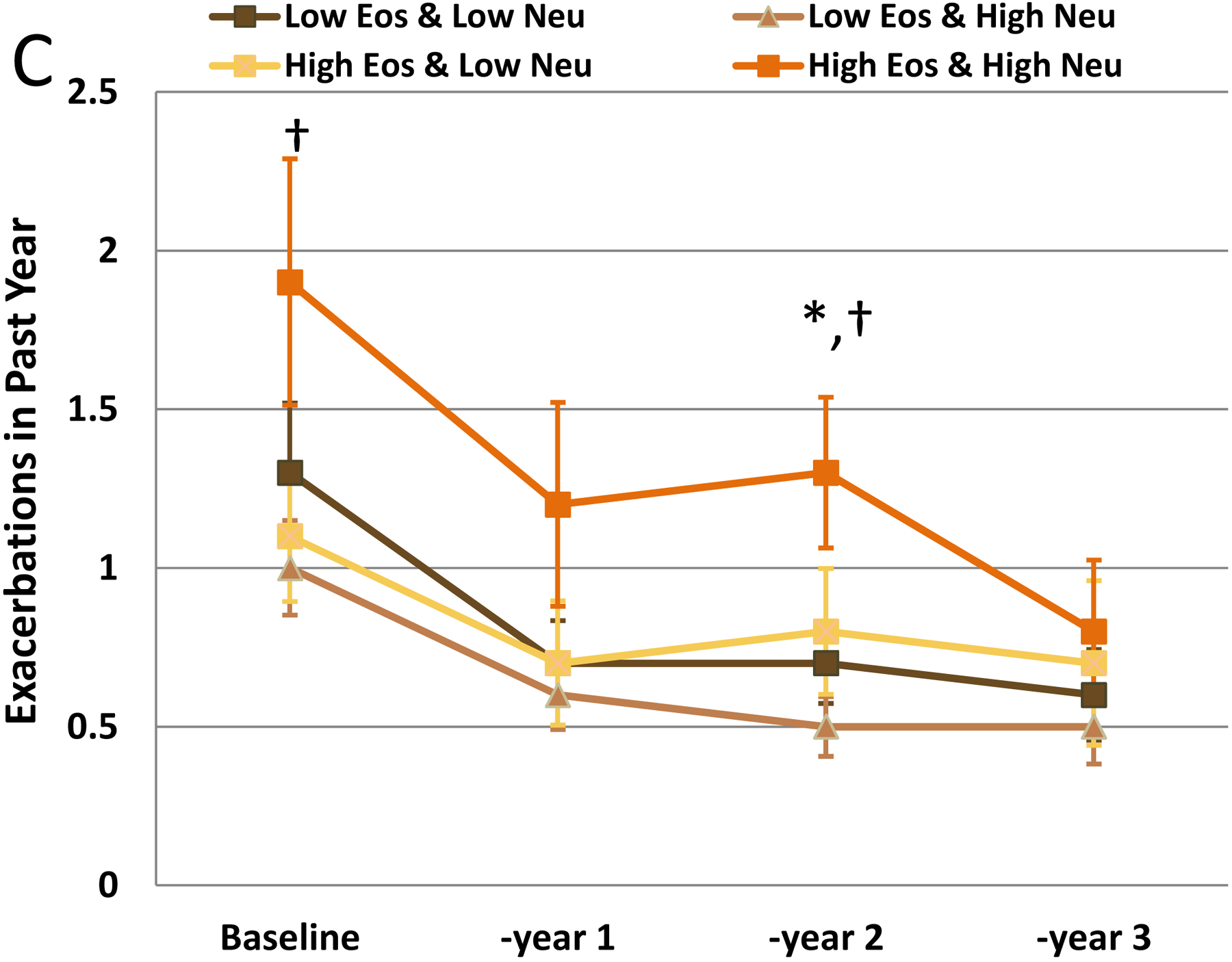
Post-bronchodilator FEV1%predicted (B) Absolute Change in Post-Bronchodilator FEV1%predicted and (C) Exacerbations at baseline and each annual visit for subjects stratified by sputum Eos + Neu differential categories determined at baseline. *High Eos+High Neu vs Low Eos+Low Neu, p<0.05; †High Eos+High Neu vs Low Eos+High Neu, p<0.05; + High Eos+High Neu vs High Eos+Low Neu, p<0.05
Healthcare utilization generally declined in all groups from baseline reported levels. ED visits decreased across groups, but the Low Eos+Low Neu group had higher %subjects reporting these visits (year 1: p=0.0337; year 2: p=0.0317, supplement). Exacerbations were lower for all groups after baseline, although the High Eos+High Neu group had a significantly higher rate for year 2 (p=0.0163) than other Eos+Neu groups (Figure 1C).
Summary:
Baseline sputum High Eos+High Neu was associated with lowest lung function and greater healthcare utilization, as we reported earlier for a different, smaller cohort3. Longitudinally over the three years from baseline, all subject groups showed declines in %’severe’ asthma, in % healthcare requirements and exacerbations. Despite these changes, the High Eos+High Neu group had consistently reduced post-BD FEV1%predicted and greater exacerbations compared to the other Eos+Neu groups with little change or even small improvements.
Loss of subjects at baseline, or dropout during study reduced numbers for total and sputum subgroups. Nevertheless, 80% of subjects remained from baseline groups in the large SARP3 cohort by year 3, compared to 31% unobtainable or missing in another recent report9. Retaining subjects in baseline groups for longitudinal assessment provides observation of groups’ clinical changes over time, but individuals may have changes in inflammation. However, High Eos groups had significantly elevated Eos throughout compared to low Eos groups. Low Neu groups had increasing %Neu over time, but remained lower than High Neu groups.
Older age for High Neu groups may contribute not only to higher Neu, but also to a lower lung function2. However, lung function for High Eos+High Neu was significantly lower than for Low Eos+High Neu, indicating High Neu were not the only factor influencing lung function. Exacerbations dropped from baseline to year 1, and leveled off afterwards for all Eos+Neu groups. Exacerbations at baseline depended on subject recall, but in years 1–3 were captured more frequently (6 month phone calls and annual visits). Improved adherence or other factors related to study participation may have contributed to the observed decrease in “severe” classification and healthcare utilization in all groups over the 3 years. The only intervention for all SARP3 subjects was short-term induced phenotype response to triamcinolone within 1st month after enrollment6. The largest pre-BD FEV1% predicted response to triamcinolone was observed in the High Eos+High Neu group, but with continued higher exacerbations longitudinally.
In conclusion, baseline subjects with combined increased sputum eosinophils and neutrophils had lowest lung function and greater healthcare requirements. Longitudinally, this High Eos+High Neu group showed further loss in lung function compared to other Eos+Neu groups, while healthcare requirements generally declined for all groups. These observations were not attributable to High Eos or High Neu alone, but suggest cell-cell interaction or overlapping inflammatory pathways.
Supplementary Material
Acknowledgements:
The authors acknowledge the contributions of the study coordinators (Allison Crosby-Thompson, Carrie Nettles, Angeles Cinelli & Meghan Le, Joy Lawrence, Donna Liu, Jenelle Mock, Danica Klaus & Gina Crisafi, Regina Smith & Jeff Krings, Rachel Weaver), laboratory staff (Daniel Nguyen & Kristin McIntire, Sara Baicker-McKee, Annabelle Charbit, John Trudeau, Heather Floerke, Susan Foster & Brian Rector, Huiqing Yin-Declue) at each of the clinical centers, and the Data Coordinating Center; in addition to all the study participants who are integral to the success of the Severe Asthma Research Program. The authors appreciate the support of the Scientific Program Officers at the National Heart, Lung and Blood Institute (Dr. Patricia Noel, Dr. Tom Croxton, and Dr. Robert Smith) and input from the members of the Data Safety and Monitoring Board.
Funding Sources: The research by the principal and co-principal investigators was funded by National Institutes of Health/National Heart Lung Blood Institute (NIH/NHLBI) Severe Asthma Research Program: ERB (PI, U10 HL109164), MC (PI, U10 HL109257), JVF (PI, U10 HL109146), EI and BL (Co-PI’s, U10 HL109172), BMG, SCE, WGT (PI and Co-PIs, U10 HL109250), NNJ (PI, U10 HL109168), SEW (PI, U10 HL109152), DTM (PI, U10 HL109086). Industry Partnerships also provided additional support: AstraZeneca, Boehringer-Ingelheim, Genetech, GlaxoSmithKline, MedImmune, Novartis, Regeneron, Sanofi and TEVA. Spirometers used in SARP III were provided by nSpire Health (Longmont, Colo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Declarations: Dr. Hastie reports grants from NIH, Genentech and GSK during the conduct of the study; Dr. Mauger reports grant support from NIH, AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA; Dr. Denlinger has grants from NIH/NHLBI and has consulted with AstraZeneca and Sanofi-Regeneron during the conduct of the study; the extension of the longitudinal phase of the SARP cohort has also been supported by AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA; Dr. Coverstone has nothing to disclose; Dr. Castro receives University Grant Funding from NIH, American Lung Association, PCORI, Pharmaceutical Grant Funding from AstraZeneca, Chiesi, Novartis, GSK, Sanofi-Aventis; consultant fees for Genentech, Theravance, VIDA, Teva, Sanofi-Aventis, is a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, & Teva, and receives Royalties from Elsevier; Dr. Erzurum reports grants from National Institutes of Health (NIH), during the conduct of the study; and Chair of the ABIM Pulmonary Disease Board; Dr. Jarjour has grants from NIH/NHLBI and has consulted with AstraZeneca and Boehringer Ingelheim, and during the extension of the longitudinal phase of the SARP cohort has also been supported, in part, by AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA; Dr. Levy reports grants from NIH during the conduct of the study; other from Nocion Therapeutics, from Entrinsic Health, grants and personal fees from Sanofi, personal fees from Pieris Pharmaceuticals, Novartis, AstraZeneca, Corbus Pharmaceuticals, Gossamer Bio, Metera Pharmaceuticals, and Teva, and grants from Samsung Research America outside the submitted work; Dr. Meyers has nothing to disclose; Dr. Moore reports grants from NIH/NHLBI, grants from AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi-Genzyme-Regeneron, and Teva during the conduct of the study; grants and personal fees from AstraZeneca, and Sanofi Regeneron, grants from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Gossamer, and Cumberland Pharmaceuticals outside the submitted work; Dr. Phillips reports grants from National Institutes of Health, grants from Boehringer-Ingelheim, TEVA, AstraZeneca, GlaxoSmithKline, Sanofi, and Genentech during the conduct of the study; Dr. Wenzel reports grants from NIH and personal fees from AstraZeneca, grants and personal fees from GSK during the conduct of the study; grants and personal fees from Sanofi-Regeneron, grants from Boehringer Ingelheim ,Novartis, and TEVA, and personal fees from Pieris outside the submitted work; Dr. Fahy reports grants from NIH/NHLBI, grants from Boehringer Ingelheim during the conduct of the study; personal fees from Boehringer Ingelheim, Pieris, Arrowhead Pharmaceuticals, and Gossamer outside the submitted work, in addition, Dr. Fahy has a patent US20110123530A1 - “Compositions and methods for treating and diagnosing asthma” issued, a patent WO2014153009A2 -Thiosaccharide mucolytic agents. issued, and a patent WO2017197360 - “CT Mucus Score” - A new scoring system that quantifies airway mucus impaction using CT lung scans; Dr. Israel reports personal fees from AstraZeneca, Biometry, Entrinsic Health Solutions, Equillium, Genentech, GlaxoSmithKline, Merck, Novartis, 4D Pharma, Pneuma Respiratory, Regeneron Pharmaceuticals, Sanofi Genzyme, Sienna Biopharmaceutical, TEVA Specialty Pharmaceuticals, and Vitaeris, Inc; grants from AstraZeneca, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Merck, Novartis, Sanofi, TEVA and Vifor-Pharma; non-financial support from Circassia, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Merck, TEVA Specialty Pharmaceuticals and Vifor-Pharma; other from Vorso Corp, outside the submitted work; Dr. Bleecker reports other from NIH grant, clinical trials through his employer, Wake Forest School of Medicine and University of Arizona for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Johnson and Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme, personal fees also serving as a paid consultant for AztraZeneca, MedImmune, Boehringer Ingelheim, Glaxo Smith Kline, Novartis, Regeneron, and Sanofi Genzyme outside the submitted work.
REFERENCES:
- 1).Al-Samri MT, Benedetti A, Prefontaine D, Olivenstein R, Lemiere C, Nair P, et al. Variability of sputum inflammatory cells in asthmatic patients receiving corticosteroid therapy: a prospective study using multiple samples. J Allergy Clin Immunol 2010;125:1161–1163. [DOI] [PubMed] [Google Scholar]
- 2).Ducharme ME, Prince P, Hassan N, Nair P, Boulet LP. Expiratory flows and airway inflammation in elderly asthmatic patients. Respir Med. 2011;105:1284–9. [DOI] [PubMed] [Google Scholar]
- 3).Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010; 125:1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. for the Asthma Clinical Research Network of the NHLBI. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med 2012;185:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol 2013;133:1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. and the Severe Asthma Research Program. Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma. Am J Respir Crit Care Med. 2016. December 14 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Silkoff PE, Laviolette M, Singh D, FitzGerald JM, Kelsen S, Backer V, et al. for the ADEPT Investigators. Longitudinal stability of asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) study. Respiratory Research 2016;17:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Van Veen IH, ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5 year follow-up study. J Allergy Clin Immunol 2009;124:615–617. [DOI] [PubMed] [Google Scholar]
- 9).Walsh CJ, Zaihra T, Benedetti A, Fugere C, Olivenstein R, Lemiere C, et al. Exacerbation risk in severe asthma is stratified by inflammatory phenotype using longitudinal measures of sputum eosinophils. Clin Exper Allergy 2016;46:1291–1302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


