Abstract
Background & Aims
Colorectal cancer (CRC) incidence and mortality are increasing among persons in the United States younger than 50 years old, but risk factors associated with early-onset CRC (EOCRC) have not been widely studied.
Methods
We conducted a case–control study of United States veterans 18–49 years old who underwent colonoscopy examinations from 1999 through 2014. EOCRC cases were identified from a national cancer registry; veterans who were free of CRC at their baseline colonoscopy through 3 years of follow up were identified as controls. We collected data on age, sex, race/ethnicity, body weight, body mass index (BMI), diabetes, smoking status, and aspirin use. Multivariate-adjusted EOCRC odds were estimated for each factor, with corresponding 95% CI values.
Results
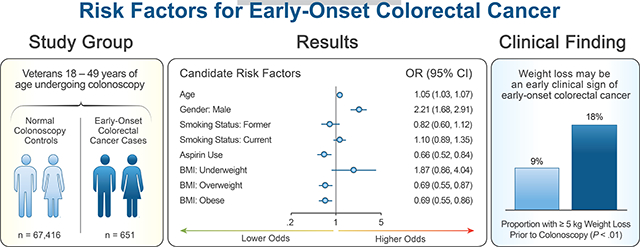
Our final analysis included 651 EOCRC cases and 67,416 controls. Median age was 45.3 years, and 82.3% were male. Higher proportions of cases were older, male, current smokers, non-aspirin users, and had lower BMIs, compared with controls (P<.05). In adjusted analyses, increasing age and male sex were significantly associated with increased risk of EOCRC, whereas aspirin use and being overweight or obese (relative to normal BMI) were significantly associated with decreased odds of EOCRC. In post-hoc analyses, weight loss of 5 kg or more within the 5-year period preceding colonoscopy was associated with higher odds of EOCRC (odds ratio, 2.23; 95% CI, 1.76–2.83).
Conclusions
In a case–control study of veterans, we found increasing age and male sex to be significantly associated with increased risk of EOCRC, and aspirin use to be significantly associated with decreased risk; these factors also affect risk for CRC onset after age 50. Weight loss may be an early clinical sign of EOCRC. More intense efforts are required to identify the factors that cause EOCRC and signs that can be used to identify individuals at highest risk.
Keywords: colon cancer, epidemiology, NSAID, weight loss
Short Summary
In an analysis of veterans, we found that weight loss is an early sign of colorectal cancer in persons younger than 50 years, and that aspirin can decrease risk.
Graphical Abstract
BACKGROUND & AIMS
Colorectal cancer (CRC) is the fourth most common cause of cancer in the United States (US) and the second leading cause of cancer death.1 Although CRC incidence and mortality trends have been declining overall across the entire population, these trends are rising in adults ages <50 years. In the US, incidence of early-onset CRC (EOCRC) has increased by 1.1 cases per 100,000 people between 2010–2014 and 2.5 cases per 100,000 people between 2000–2014.1 A majority of these new cases are left-sided cancers.2, 4–6 Current literature suggests that as many as 94% of EOCRC cases are symptomatic at diagnosis – most predominant symptoms being bleeding and abdominal/rectal pain7, 8 – and are more likely to have advanced stage at diagnosis and poorer outcomes.5, 9–11
Reasons for rising EOCRC incidence and mortality are unclear. Some have hypothesized that the rising trend may be related to common or increasingly prevalent modifiable behaviors, such as excess body weight, low physical activity, and diabetes mellitus.12–16 Additionally, non-modifiable risk factors such as race/ethnicity may be associated with EOCRC compared to later-onset CRC.17, 18 Prior studies have generally been descriptive of tumor location, stage, and histology;4, 5, 9, 19, 20 or focused only on demographic factors.18, 21–23 Few case-control studies or cohort studies of risk factors for EOCRC have been published.14, 24–26 Many prior studies have had relatively small sample sizes, or not utilized normal colonoscopy controls, which may have blunted ability to identify significant associations, and the magnitude of any associations present with precision. To address current gaps in the literature, our aim was to conduct a large case-control study to identify candidate risk factors for EOCRC.
MATERIALS AND METHODS
Study Design
We conducted a retrospective case-control study of Veterans ages <50 years and receiving care within the Veteran’s Health Administration (VHA), who underwent colonoscopy between 1999–2014.
Data Sources
The VHA is one of the largest integrated healthcare providers in the US. The VHA includes over 1,200 healthcare facilities across the US that provide health coverage to over 6 million Veterans annually.27 Since 1999, all VHA facilities have utilized a universal electronic medical record, which allows clinical data sharing. Data from the millions of clinical encounters through the VHA are collected into a Corporate Data Warehouse (CDW) and can be used for clinical research. The CDW provides access to discrete data used in this study, including demographic information, claims-based procedure and diagnostic codes, anthropometric data (e.g. heights and weights), prescriptions, and free-text procedure and pathology notes. EOCRC cases defined in our analysis were ascertained using the VA Central Cancer Registry (VACCR) and/or National Death Index (NDI) cause-specific mortality data.
Study Sample and Selection Criteria
The study base consisted of Veterans ages 18–49 years, undergoing colonoscopy between 1999–2014. A list of colonoscopy-related current procedural terminology (CPT) codes used is detailed in Appendix Table 1. From the study base, we excluded individuals with any of the following: (1) prior history of CRC based on International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes issued greater than 6 months prior to baseline colonoscopy; (2) a history of inflammatory bowel disease (IBD) based on ICD-9 codes issued 6 months before and after baseline colonoscopy.
Case Selection
EOCRC cases were ascertained by VACCR and/or NDI cause-specific mortality data within 6 months of baseline colonoscopy. Surveillance, Epidemiology, and End Results (SEER) program staging and histology information were collected for all cases. Appendix Table 2 provides highlighted information regarding selection criteria with ICD-O-3 codes. Candidate cases were excluded if missing SEER stage or if ICD-O-3 histology codes were not consistent with adenocarcinoma; Stage 0 diagnoses were included as cases. If histology code was not accessible, we included the EOCRC case as long as site, stage, and diagnosis date information were available, given that the majority of CRCs are adenocarcinomas. All cases were stratified by anatomic site based on site codes – proximal (C18.0, C18.2-C18.4), distal (C18.5-C18.7), and rectal (C19.9, C20.9).
Control Selection
Controls were defined as Veterans who had a normal baseline colonoscopy, no CRC diagnosis prior to colonoscopy, and no CRC diagnosis through 3 years follow-up. Normal colonoscopy was defined by presence of a CPT code for diagnostic colonoscopy only (45378 or G0121) and absence of a pathology report within 30 days of baseline colonoscopy. Our prior work has shown that this definition is 96.3% sensitive and 97.5% specific for normal colonoscopy, with a positive predictive value (PPV) of 97%.28, 29 Appendix Table 3 outlines the detailed study selection criteria.
Predictors
All predictor variables were collected at time of baseline colonoscopy. Candidate risk factors considered include age, sex, race/ethnicity, body mass index (BMI), diabetes, smoking status, and aspirin use. Diabetes status was ascertained using a previously validated algorithm that included inpatient encounters, outpatient encounters, and medications.30 Smoking status was determined from the VHA Health Factors domain.31 Subjects were further classified by smoking status into never, former, current and unknown subgroups. Aspirin use was characterized utilizing structured medication file data, as well as unstructured free text progress reports with a validated algorithm shown to have PPV and negative predictive value of 99.2% and 97.5%, respectfully.32 BMI and weight were derived utilizing previously developed criteria, including removal of biologically implausible values.33
Statistical Analysis
Summary statistics including means and frequencies for each candidate risk factor were used to characterize the patient population and describe the distribution of Veterans ages <50 years with EOCRC. For descriptive analyses, Chi-squared tests were was utilized to compare the distributions of categorical variables and t-tests and analysis of variance (ANOVA) tests were calculated to compare distributions of continuous variables between cases and controls. Our primary analyses included: (1) univariate analyses, examining the association of each candidate risk factor with EOCRC odds; and (2) a multivariable regression model, including multiple candidate risk factors. The multivariable logistic-regression model was derived using a forward stepwise selection of candidate risk factors. Predictors were included in the model if they had a significant association with the binary outcome (EOCRC yes vs. no) in univariate analyses at a P-value of 0.05. Model fit was assessed using the Hosmer-Lemeshow test and found to be acceptable in the final regression model described in the results. Univariate and multivariable model effects were summarized using odds ratios (OR) and corresponding 95% confidence intervals (CI).
A post-hoc analysis of weight trends (serving as a surrogate for BMI trends) over 10 years prior to baseline colonoscopy was conducted in order to characterize the overall trends of weight for EOCRC cases leading up to diagnosis. Frequency of weight loss ≥5 kg and ≥10 kg in the five-year period preceding baseline colonoscopy was computed for EOCRC cases and normal colonoscopy controls. Logistic regression analyses were conducted to determine odds of developing EOCRC give these changes in weight for cases and controls. A post-hoc analysis was also conducted to examine risk factors among the population stratified into two age groups: ages <40 and ages ≥40. In additional post-hoc analyses, we characterized indication for colonoscopy by reviewing a random sample of colonoscopy reports for 100 cases and 100 controls, and summarized indications listed as proportions (Appendix Figure 1). In this sample of 100 cases and 100 controls, we also searched for evidence of family history of colorectal cancer in clinical progress notes preceeding the index colonoscopy procedure. Statistical differences in proportions of indications within cases and controls were tested using Fisher’s exact testing.
Statistical tests were two-sided and P-values of <0.05 were considered statistically significant. Analyses were conducted using Statistical Analysis System 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Study Sample
We identified 68,067 Veterans age <50 years with colonoscopy exposure meeting inclusion criteria: 651 were EOCRC cases and 67,416 were normal colonoscopy controls. Descriptive characteristics of the sample are summarized in Table 1. Mean age of the combined sample was 43.2 years. The majority of subjects were male (82.3%) and non-Hispanic White (55.3%). A substantial majority of the combined sample was overweight or obese (72.2%), with a mean BMI of 30.0.
Table 1.
Demographic Characteristics, Study Base, Early-Onset Colorectal Cancer Cases, and Normal Colonoscopy Controls
| Variable | All Subjects n = 68,067 | EOCRC Cases n = 651 | Normal Colonoscopy Controls n = 67,416 |
|---|---|---|---|
| Age, mean (SD) | 43.2 (6.4) | 44.8 (4.9) | 43.2 (6.4) |
| Sex, n (%) | |||
| Male | 55,993 (82.3) | 594 (91.2) | 55,399 (82.2) |
| Female | 12,074 (17.7) | 57 (8.8) | 12,017 (17.8) |
| Race/Ethnicity, n (%) | |||
| White | 37,625 (55.3) | 364 (55.9) | 37,261 (55.3) |
| Black | 19,837 (29.1) | 193 (29.7) | 19,644 (29.1) |
| Asian | 1,087 (1.6) | 7 (1.1) | 1,080 (1.6) |
| American Indian | 463 (0.7) | ** | 462 (0.7) |
| Hispanic | 4,191 (6.2) | 32 (4.9) | 4,159 (6.2) |
| Other | 1,468 (2.2) | 15 (2.3) | 1,453 (2.2) |
| Unknown | 3,396 (5.0) | 39 (6.0) | 3,357 (5.0) |
| Diabetes, n (%) | |||
| Yes | 7,425 (10.9) | 68 (10.4) | 7,357 (10.9) |
| No | 60,642 (89.1) | 583 (89.6) | 60,059 (89.1) |
| Smoking Status, n (%) | |||
| Never | 21,108 (31.0) | 171 (26.3) | 20,937 (31.1) |
| Former | 7,499 (11.0) | 53 (8.1) | 7,446 (11.0) |
| Current | 18,887 (27.8) | 189 (29.0) | 18,698 (27.7) |
| Unknown | 20,573 (30.2) | 238 (36.6) | 20,335 (30.2) |
| Aspirin Use, n (%) | |||
| Yes | 10,254 (15.1) | 76 (11.7) | 10,178 (15.1) |
| No | 57,813 (84.9) | 575 (88.3) | 57,238 (84.9) |
| BMI, mean (SD) | 30.0 (5.6) | 29.5 (6.1) | 30.0 (5.6) |
| BMI Category, n (%) | |||
| Underweight | 298 (0.4) | 7 (1.1) | 291 (0.4) |
| Normal | 10,875 (16.0) | 131 (20.1) | 10,744 (15.9) |
| Overweight | 21,583 (31.7) | 189 (29.0) | 21,394 (31.7) |
| Obese | 27,549 (40.5) | 232 (35.6) | 27,317 (40.5) |
| Unknown | 7,762 (11.4) | 92 (14.1) | 7,670 (11.4) |
BMI, Body Mass Index; EOCRC, Early-Onset Colorectal Cancer; SD, Standard Deviation
Represents cell sample sizes <5
A majority of cases were rectal cancers (39.6%), followed by distal (30.3%) and proximal cancers (30.1%). Of the EOCRC cases, 575 (88%) were ages 40–49, 59 (9%) were ages 30–39, and 17 (3%) were <30 years of age (Appendix Figure 2). Of the 651 cases, there were 309 (47%) cases that were early-stage, 285 (44%) late-stage cases and 57 (9%) unknown. Among early-stage cases, 69% were overweight or obese, while 61% of late-stage cases were overweight or obese. In contrast, 72.2% of normal colonoscopy controls were overweight or obese; distribution of BMI for cases and controls, including for cases stratified by stage, is provided in Appendix Figure 3. On univariate analyses, EOCRC cases were more likely to be older, male, current smokers, non-aspirin users, and have lower BMI compared to normal colonoscopy controls on univariate analyses (Appendix Table 4; P<0.05 for all comparisons).
Adjusted Analysis of EOCRC Risk Factors
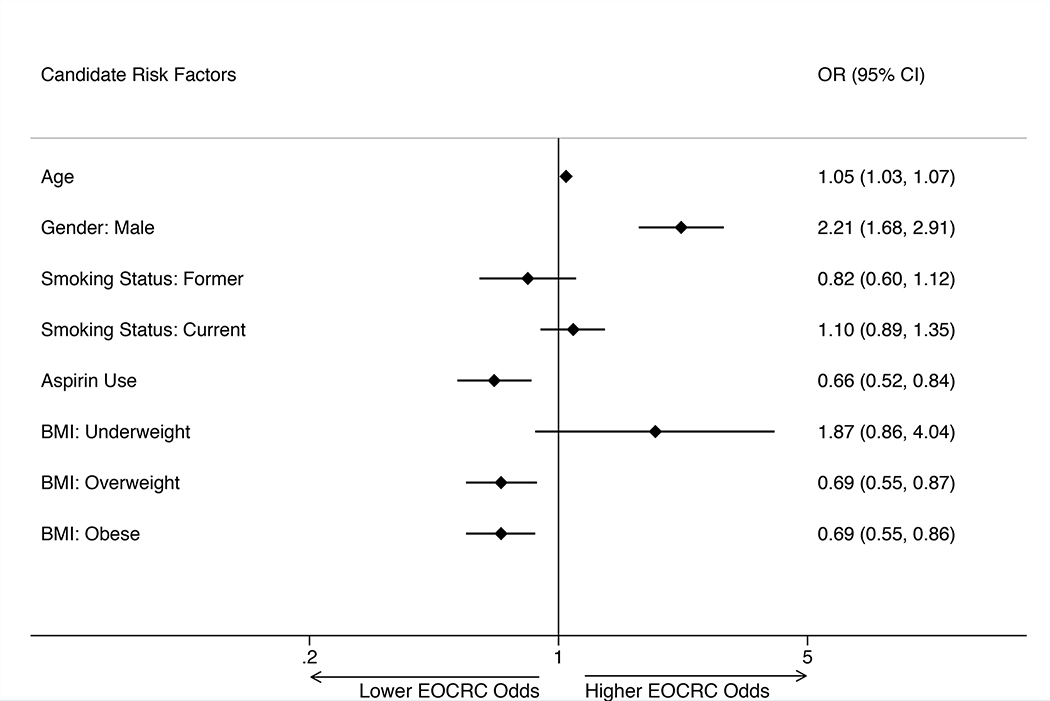
Increasing age and male sex were significantly associated with increased EOCRC odds, while aspirin use and being overweight or obese (relative to normal BMI) at time of colonoscopy were significantly associated with decreased EOCRC odds in the multivariable adjusted analysis (Figure 1). For every additional year in age, the odds of developing EOCRC increased by 5% (OR 1.05, 95% CI [1.03, 1.07]). Males had 2.2 times greater odds of developing EOCRC than females (OR 2.21, 95% CI [1.68, 2.91]). Aspirin users had a 34% reduction in odds of developing EOCRC compared to non-aspirin users (OR 0.66, 95% CI [0.52, 0.84]). Being overweight or obese at time of colonoscopy compared to normal BMI were both associated with a 31% reduction in odds of EOCRC (overweight: OR 0.69, 95% CI [0.55, 0.87]; obese: OR 0.69, 95% CI [0.55, 0.86]). Smoking status was included in the multivariable model due to significant differences seen within the “unknown” smoking status group, though we are unable to precisely interpret the findings among this group.
Figure 1. Early-Onset Colorectal Cancer Risk Factors.
Results of a multivariable model estimating odds for EOCRC with candidate risk factors is depicted. * Denotes statistical significance where p<0.05. All variables listed in the figure met forward stepwise selection criteria (p<0.05) and were included in the multivariable regression model. Variables selected out of the model include: (1) race/ethnicity and (2) diabetes. Hosmer-Lemeshow goodness-of-fit p-value = 0.6. EOCRC, early-onset colorectal cancer; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Weight Trends Prior to Colonoscopy
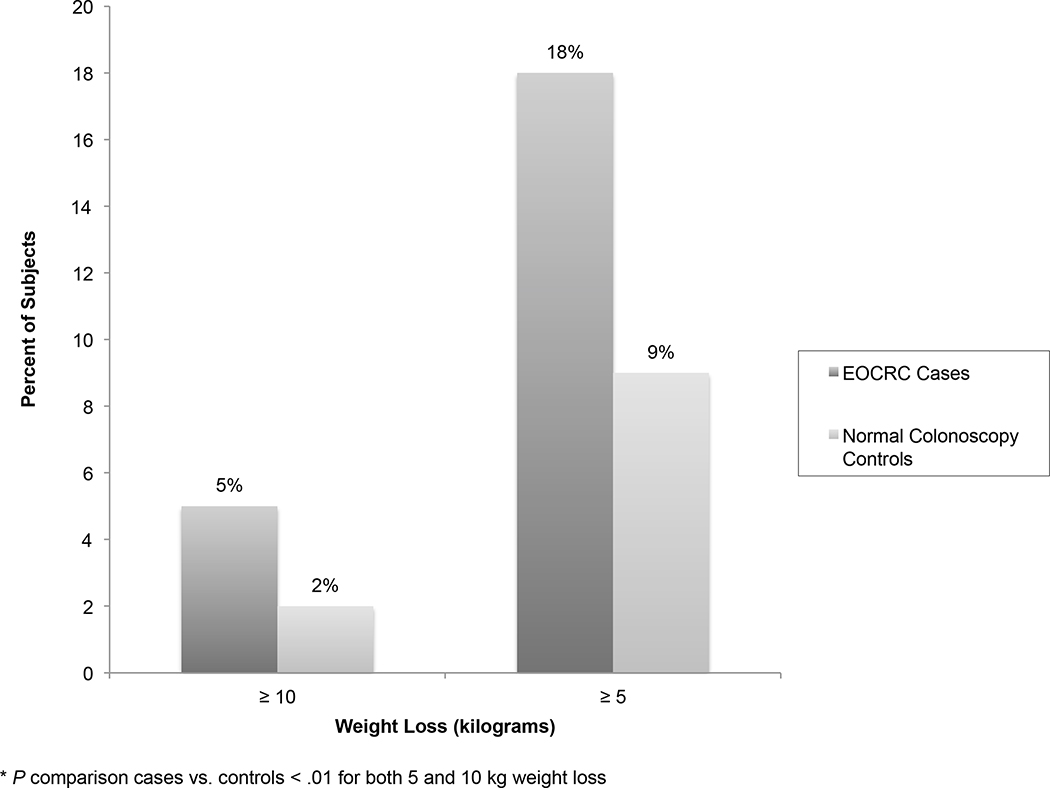
At baseline colonoscopy, average weight was significantly lower in EOCRC cases (weight=91.82 kg) as compared with normal colonoscopy controls (weight=93.79 kg; P=0.02). In a post-hoc analysis of weight-change trends prior to baseline colonoscopy, the average 10-year change in weight increased for controls, but decreased for EOCRC cases. For EOCRC cases, weight began declining five years prior to baseline colonoscopy, while weight continued to increase over this time period for normal colonoscopy controls (Appendix Figure 4). Over the five-year period, 17.5% of EOCRC cases lost ≥5 kg compared to 8.7% of controls (OR 2.23, 95% CI [1.76, 2.83]), and 5% of EOCRC cases lost ≥10 kg compared to 2.1% of controls (OR 2.50, 95% CI [1.65, 3.78]) (Figure 2).
Figure 2. Weight Trends Preceding Colonoscopy for Early-Onset Colorectal Cancer Cases and Normal Colonoscopy Controls.
Proportion of cases with ≥5 and ≥10 kg weight loss in the five year period preceding baseline colonoscopy is depicted. Differences significant at p<0.05. EOCRC, early-onset colorectal cancer; kg, kilogram.
Age-Stratified Analysis of EOCRC Risk Factors
Age-stratified unadjusted and adjusted analyses for individuals age 40 to 49 and younger than 40 years are provided in Appendix Table 5; adjusted analyses are presented herein. Overall across all age groups, and on age-stratified analyses, male sex was associated with increased risk for EOCRC. Overall, and among individuals 40 to 49 years old, aspirin was associated with reduced odds for EOCRC, but not for individuals under age 40. Overall, and among individuals 40 to 49 years old, being overweight or obese was associated with reduced odds for EOCRC. A similar, but non-statistically significant reduced odds for EOCRC was observed for individuals age <40. While being underweight was associated with a non-statistically significant 1.9-times greater odds of EOCRC overall compared to normal weight, age stratified analyses showed a statistically significant 4.5-times greater odds of EOCRC among individuals under 40 (OR 4.47, 95% CI [1.02, 19.62]), and a non-statistically significant 1.3-times increased odds of EOCRC among individuals age 40 to 49 (OR 1.29, 95% CI [0.52, 3.2]). This age-stratified finding should be interpreted with caution, as there were only 7 EOCRC cases with underweight BMI, only two of which were under age 40. Taken together, the age-stratified analyses suggest that the association of aspirin with reduced EOCRC is mainly restricted to individuals age 40 to 49, and that being underweight, while not associated with increased risk for all ages combined, was associated with increased odds of EOCRC among individuals younger than 40 years.
DISCUSSION
In a national sample of 651 EOCRC cases and 67,416 normal colonoscopy controls, we found that age and male sex are significantly associated with increased, and aspirin use and higher BMI with decreased odds for EOCRC. In a post-hoc analysis, we observed that weight began to decline in EOCRC cases five years prior to diagnosis, and that EOCRC cases compared to controls were more likely to experience clinically significant weight loss within the five years leading up to diagnosis. Our findings confirm and extend, but in some cases also contrast with prior observations of risk factors for EOCRC as well as CRC overall, providing some new insights.
Age
Increasing age was associated with increasing risk for EOCRC, which mirrors current CRC literature suggesting that annual transition rates from advanced adenomas to CRC strongly increase with age.34 You et al. found that the median age for EOCRC was 44 years with a majority (75%) of cases developing between the ages of 40–49 years as compared to less than 40 years.17 We demonstrate a similar trend with a median age for EOCRC of 46 years, and 89% of cases occurring between 40–49 years of age. Given that our work and prior reports suggest that a large proportion of EOCRC cases occur between ages 40–49, the idea that early initiation of screening at age 40 or 45 could detect the majority of early-onset cases is plausible. The American Cancer Society has issued a qualified recommendation for all average-risk adults to initiate screening at age 45 years,35 and a recent analysis has concluded that early initiation of screening could be cost-effective.36 However, the cost-effectiveness analysis suggested that investment in early initiation of screening would be less cost effective than increasing screening rates and diagnostic colonoscopy completion rates among individuals older than 50 who are at higher risk for CRC.36 The best strategy for identifying individuals younger than age 50 who might benefit from screening, in context of population prevention for CRC across the age spectrum, remains to be determined.
Sex
Consistent with prior work, we found males had a 2.2-fold increase in EOCRC odds compared to females.19, 37, 38 To date, guidelines have been consistent in suggesting the same age of initiation for screening for males and females. Future research should determine whether males with potential signs and symptoms of EOCRC such as iron deficiency anemia and hematochezia have substantially higher risk for CRC diagnosis than females.
Race/Ethnicity
Contrary to current CRC literature, we found no statistically significant difference in odds of EOCRC across racial/ethnic groups. Previous SEER data has consistently reported differences in CRC rates among race/ethnicity groups.39 For example, data have suggested a lower incidence of CRC in Hispanics compared Non-Hispanics, as well as in Asian/Pacific Islanders and American Indians compared to Whites.39 On the other hand, African Americans compared to Whites have a 13% higher overall, age-adjusted incidence of CRC.39 Combined data from the National Program of Cancer Registries and SEER from 1999–2004 initially reported a 36% increased incidence of EOCRC in African Americans compared to Whites beginning at age 40–44 years.39 More recent SEER data, however, demonstrate new trends.40 Although overall colon cancer incidence in those age 20–49 years is higher for African Americans compared to Whites (12.7 vs. 11.0 per 100,000 from 2010–2014 SEER data), incidence trends from 1992–1996 to 2010–2014 show a more dramatic rise in Whites compared to African Americans (7.5 to 11.0 per 100,000 for Whites compared to 11.7 to 12.7 per 100,000 for African Americans).40 These trends are also consistent across anatomical CRC sites, with an overall decreasing incidence rate from 1992–1996 (4.3 per 100,000) to 2010–2014 (3.9 per 100,000) for proximal colon cancer in African Americans.40
Our observation of no difference in EOCRC odds by race/ethnicity, in contrast to other published literature, may be a result of dynamic changes in incidence of EOCRC among race/ethnicity groups. Our findings may also show no difference in odds of EOCRC because we did not examine time trends or because of our focus on a Veteran population. Veterans across racial/ethnic groups have more standardized healthcare access through the VHA system which may mitigate health disparities, and, as such, their care is not representative of healthcare access in the general US population. We also used normal colonoscopy controls, a factor which could have controlled for healthcare utilization. More research is needed in order to better characterize racial/ethnic differences in EOCRC.
Diabetes
In contrast to current CRC literature, we did not observe increased risk for EOCRC among diabetics. Prior work, unrestricted by age of presentation, has demonstrated diabetes is associated with a 1.3-fold increased risk for CRC.41 The relationship between EOCRC and diabetes has not been widely reported. Our observation of no association may be related to duration of diabetes.42 La Vecchia et al. found a significant association between diabetes and CRC odds in the group with diabetes burden ≥10 years from CRC diagnosis (OR 1.6, 95% CI [1.1, 2.3]) and a non-significant association in the group with diabetes burden <10 years from CRC diagnosis (OR 1.2, 95% CI [0.8, 1.7]).25 Since diabetes incidence is highly age-specific, it is possible that EOCRC risk may not be strongly impacted by diabetes, accounting for our observation. Nonetheless, more research is needed to better characterize the association between diabetes and EOCRC risk.
Smoking
Our adjusted analyses found neither current nor former smoking to be associated with EOCRC odds compared to never smoking. This is contrary to prior analyses among CRC cases of all ages, which suggest a 1.2-fold increased risk of CRC for ever-smokers compared to never smokers.43 Prior work has also found that smoking increases CRC risk at distal,44 and rectal anatomic sites.26, 45 Our study is one of the first to evaluate the association between smoking status and EOCRC odds. While our findings indicate a non-significant association, smoking status was unknown for a substantial proportion: more research will need to be conducted to explore the relationship between smoking and EOCRC risk.
Aspirin Use
Unrestricted by age, studies have consistently shown aspirin exposure is associated with reduced risk for CRC.46–49 Our analyses extend prior work (which did not specifically report associations under age 50), by demonstrating that aspirin exposure is associated with reduced EOCRC odds. Our age-stratified subgroup analyses showed reduced risk was restricted to individuals age 40 to 49, perhaps consistent with the postulate that longitudinal exposure is required to reduce CRC risk. Aspirin might be considered as a chemopreventive strategy among young adults if our observations can be confirmed, and if a higher risk population, for whom potential benefits would outweigh potential risks, can be identified.50
BMI/Weight
In our analysis we found that increased BMI and body weight had a protective effect on the odds of EOCRC. This relationship was unexpected given that CRC has been identified as an obesity-related cancer,42 and given that several biologic correlates of being overweight or obese – particularly insulin resistance and increased circulating estrogens – are believed to increase cancer risk.42 Specific to EOCRC, the Nurses’ Health Study II found a significant association between weight gain since early childhood among women and EOCRC risk.14 Women with a BMI of 30 or above had the highest risk of EOCRC compared to women with normal BMI, with an EOCRC relative risk of CRC of 1.93 (95% CI [1.15, 3.25]).14 One potential explanation for our contrasting findings is that we used data near subjects’ baseline colonoscopy, a time in which cases were being diagnosed with EOCRC and were potentially losing weight due to presence of prevalent cancer.
Consistent with this postulate, our post-hoc analysis indicated that there was an overall decline in weight among EOCRC cases beginning at 5 years prior to baseline colonoscopy and that a loss of ≥5 kg over the 5-year period was two-fold more common in EOCRC cases compared to controls. Our findings align with a systematic review of 18 publications examining the association between CRC and weight loss around time of diagnosis that found that the likelihood of CRC was more than double for patients presenting with weight loss.7
Our findings are consistent with weight loss being one clinical sign that should trigger consideration of EOCRC on the differential diagnosis, but do not rule out a longitudinal association between increased weight, increased BMI, and risk for EOCRC.
Strengths and Limitations
Several limitations may be considered in interpreting this report. Our study group was comprised of US Veterans, which may limit generalizability to all individuals at risk for EOCRC. For example, the study sample was 82% male and included 72% of individuals who had a BMI considered overweight or obese, which is markedly higher than the 65% found among adults ages 20 and older in the general US population.51 Notably with respect to sex, the study population is approximately 18% female, which is a smaller disparity than what is seen in other studies using VHA data.52 The control group was comprised by individuals who had colonoscopy but no CRC diagnosis, because this offered the advantage of ensuring that the control comparison group was free of CRC and other neoplasia. However, individuals completing colonoscopy under age 50 may differ from the general population of individuals under age 50 in CRC risk, as well as distribution of purported risk factors for CRC. As such, risk factor associations for CRC in our study, which focused on a study base of individuals exposed to colonoscopy under age 50, may not be generalizable to the general population of individuals under age 50. Observed risk factors in this study might be less or more closely associated with CRC risk were a comparison to be made to a group of non-colonoscopy controls.
Indication for colonoscopy was not available for analysis. In a post-hoc analysis, we conducted manual chart review to identify indication at colonoscopy for 100 cases and 100 CRC-free colonoscopy controls (Appendix Figure 1). Among cases and controls combined, the two most common indications for colonoscopy were rectal bleeding (46%) and abdominal pain (19.5%). Weight loss (9%) was also a prevalent indication. When separated by case/control status, the proportion with rectal bleeding (54% for cases, 38% for controls, p=0.03) and abdominal pain (24% for cases, 15% for controls, p=0.15) were more similar than the proportion with weight loss (17% for cases, 1% for controls, p<0.01). Additional statistical differences in proportions were found for indications of screening for a family history of CRC (p<0.01), iron deficiency anemia (p<0.01), abnormal imaging finding suggestive of malignancy (p<0.01), and fatigue (p=0.03).
These observations support that a high proportion of the study base exposed to colonoscopy had potential clinical signs of CRC, and that indications such as rectal bleeding, weight loss, iron deficiency anemia, imaging findings suggestive of malignancy and fatigue were more prevalent among CRC cases than CRC-free colonoscopy controls. These exploratory data are also consistent with: (1) the inverse relationship we described for BMI and EOCRC risk in our adjusted analyses, and (2) our post-hoc analysis demonstrating weight loss as an early clinical finding of EOCRC.
Absence of indication for colonoscopy could also impact interpretation of our observation of no association between race/ethnicity and CRC risk. For example, if African Americans were disproportionately referred for the indication of early initiation of screening at age 45, this could have impacted our observation of no association between race/ethnicity and CRC risk, either towards (due to subclinical CRC detection) or away from a closer association (due to detection and removal of large adenomas). Family history was also not completely characterized. While family history could serve as a potential risk factor for EOCRC, data on family history of CRC within usual care VHA data are not coded and available for large-scale analysis. As such, we were unable to examine the association of family history of CRC with risk for CRC. Similarly, the usual VHA data accessed did not contain coded data on any germline testing for hereditary CRC syndromes, precluding analysis of prevalence of germline mutations in CRC cases or controls. Previous research has demonstrated that up to 16% of individuals with EOCRC may have a pathogenic germline mutation associated with increased risk for cancer.53, 54
The study was cross-sectional and risk factors were measured at time of baseline colonoscopy, precluding the ability to examine impact of longitudinal status of candidate risk factors on EOCRC risk. Variation over time for predictor variables, such as prior history of diabetes that had resolved by time of colonoscopy, could not be accounted for in our analyses. Additionally, our results are subject to residual confounding due to unmeasured factors. For example, while we postulate that weight loss may be an early clinical sign of CRC, we cannot rule out presence of an unmeasured confounder associated with both weight loss and CRC diagnosis as an explanation for our observed finding.
Strengths of this study include the large sample, and our strategy of utilizing normal colonoscopy controls to optimize exclusion of prevalent neoplasia among controls. Further, measurement approaches for all predictors used in our analyses had been previously validated by our group or other investigators.30–33
CONCLUSIONS
In a large study of US Veterans with EOCRC compared to normal colonoscopy controls, we found age and male sex were associated with increased cancer risk, consistent with observations reported for individuals both over and under age 50. Our results extend prior observations of CRC risk associated with aspirin exposure by demonstrating that reduced risk associated with exposure applies specifically to individuals younger than 50. In contrast to epidemiologic findings for individuals under and over age 50, we found no relationship between race/ethnicity and EOCRC among US Veterans. We provide one of the first analyses of the association of diabetes and smoking and EOCRC risk, and found no association, in contrast to the well-established relationship between these factors and increased risk for CRC older than age 50. On initial analyses, increased BMI and weight were associated with reduced, rather than increased odds for EOCRC. However, our post-hoc analyses suggest that cases were more likely to have had significant reductions in weight in the 5-year period preceding colonoscopy, consistent with weight loss being a potential early clinical sign of EOCRC. Taken together, these findings provide new insights into risk factors for EOCRC, but also highlight that much work remains to be done to identify the key drivers and clinical signs of EOCRC. Elucidating risk factors and key clinical signs may serve to clarify which individuals under age 50 may benefit from screening and diagnostic evaluation that may lead to early detection and prevention of EOCRC.
Supplementary Material
What you need to know.
BACKGROUND AND CONTEXT
Colorectal cancer (CRC) incidence and mortality are increasing among persons in the United States younger than 50 years old, but risk factors associated with early-onset CRC (EOCRC) have not been well determined.
NEW FINDINGS
In a case–control study of United States veterans, we found increasing age and male sex to be significantly associated with increased risk of EOCRC, and aspirin use to be significantly associated with decreased risk; these factors also affect risk for CRC onset after age 50. Weight loss may be an early clinical sign of EOCRC.
LIMITATIONS
This was a retrospective analysis of veterans.
IMPACT
Weight loss may be an early sign of EOCRC, but more studies are needed to identify features that can be used to identify individuals at highest risk.
Acknowledgments
Grant Support: VA HSR&D 5 I01 HX 001574–04 (Gupta, PI); NIH/NCI 5 R37 CA 222866–02 (Gupta, PI); NIH/NCI 1 F32 CA 239360–01 (Demb, PI); NIH/NIDDK DK120515 (Eckman/Schnabl PI); NIH/NCI 1UG3CA233314–01A1 (Martinez, PI)
Footnotes
Disclosures: All authors listed above have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N NA, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD: National Cancer Institute, 2017. [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thursfield VFH. Cancer in Victoria: Statistics and trends 2010. Victoria, Melbourne: Cancer Council Victoria, 2012. [Google Scholar]
- 4.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 5.Myers EA, Feingold DL, Forde KA, et al. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J Gastroenterol 2013;19:5651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Ma J, Miller KD, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI: Journal of the National Cancer Institute 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adelstein BA, Macaskill P, Chan SF, et al. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol 2011;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol 1999;94:3039–45. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 10.Fancher TT, Palesty JA, Rashidi L, et al. Is gender related to the stage of colorectal cancer at initial presentation in young patients? J Surg Res 2011;165:15–8. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg BA, Marshall JL. Colon Cancer in Young Adults: Trends and Their Implications. Curr Oncol Rep 2019;21:3. [DOI] [PubMed] [Google Scholar]
- 12.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 13.Moore HG. Colorectal cancer: what should patients and families be told to lower the risk of colorectal cancer? Surg Oncol Clin N Am 2010;19:693–710. [DOI] [PubMed] [Google Scholar]
- 14.Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SE, Jo HB, Kwack WG, et al. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. . World Journal of Gastroenterology 2016;22:2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clin Gastroenterol Hepatol 2019;17:115–122. [DOI] [PubMed] [Google Scholar]
- 17.You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med 2012;172:287–9. [DOI] [PubMed] [Google Scholar]
- 18.Griffin PM, Liff JM, Greenberg RS, et al. Adenocarcinomas of the colon and rectum in persons under 40 years old. A population-based study. Gastroenterology 1991;100:1033–40. [DOI] [PubMed] [Google Scholar]
- 19.Betes M, Munoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol 2003;98:2648–54. [DOI] [PubMed] [Google Scholar]
- 20.Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011;128:1668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy CC, Sanoff HK, Stitzenberg KB, et al. Patterns of Sociodemographic and Clinicopathologic Characteristics of Stages II and III Colorectal Cancer Patients by Age: Examining Potential Mechanisms of Young-Onset Disease. J Cancer Epidemiol 2017;2017:4024580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med 2015;4:1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol 2016;34:2148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013;24:335–41. [DOI] [PubMed] [Google Scholar]
- 25.La Vecchia C, Negri E, Decarli A, et al. Diabetes mellitus and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 1997;6:1007–10. [PubMed] [Google Scholar]
- 26.Tsong WH, Koh WP, Yuan JM, et al. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. British Journal of Cancer 2007;96:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Affairs USDoV. Veteran Population. Natl. Cent. Veterans Anal. Stat. 2018. Volume 2018, 2018. [Google Scholar]
- 28.Gupta S, Liu L, Patterson OV, et al. A Framework for Leveraging “Big Data” to Advance Epidemiology and Improve Quality: Design of the VA Colonoscopy Collaborative. EGEMS (Wash DC) 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earles A, Liu L, Bustamante R, et al. Structured Approach for Evaluating Strategies for Cancer Ascertainment Using Large-Scale Electronic Health Record Data. JCO Clinical Cancer Informatics 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27 Suppl 2:B10–21. [DOI] [PubMed] [Google Scholar]
- 31.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bustamante R EA, Murphy JD, et al. Ascertainment of Aspirin Exposure Using Structured and Unstructured Large-Scale Electronic Health Record Data. Med Care 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev 2010;47:739–50. [DOI] [PubMed] [Google Scholar]
- 34.Brenner H, Hoffmeister M, Stegmaier C, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut 2007;56:1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 36.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner H, Hoffmeister M, Arndt V, et al. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer 2007;96:828–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmeister M, Schmitz S, Karmrodt E, et al. Male sex and smoking have a larger impact on the prevalence of colorectal neoplasia than family history of colorectal cancer. Clin Gastroenterol Hepatol 2010;8:870–6. [DOI] [PubMed] [Google Scholar]
- 39.Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999–2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1967–76. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CC, Wallace K, Sandler RS, et al. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019;156:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–87. [DOI] [PubMed] [Google Scholar]
- 42.Peeters PJ, Bazelier MT, Leufkens HG, et al. The risk of colorectal cancer in patients with type 2 diabetes: associations with treatment stage and obesity. Diabetes Care 2015;38:495–502. [DOI] [PubMed] [Google Scholar]
- 43.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. Jama 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 44.Zisman AL, Nickolov A, Brand RE, et al. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med 2006;166:629–34. [DOI] [PubMed] [Google Scholar]
- 45.Poynter JN, Haile RW, Siegmund KD, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev 2009;18:2745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bibbins-Domingo K Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–45. [DOI] [PubMed] [Google Scholar]
- 47.Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Jama 2005;294:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook NR, Lee IM, Zhang SM, et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med 2013;159:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50. [DOI] [PubMed] [Google Scholar]
- 50.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Center for Chronic Disease Revention and Health Promotion, Division of Nutrition, Physical Activity and Obesity. Data, Trend and Maps [online]. Centers for Disease Control and Prevention. [Google Scholar]
- 52.Yano EM HP, Wright S, et al. Integration of Women Veterans into VA Quality Improvement Research Efforts: What Researchers Need to Know. Journal of General Internal Medicine. 2010:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018;154(4):897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.