Abstract
Background
In late 2019, a novel human coronavirus – severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) – emerged in Wuhan, China. This virus has caused a global pandemic involving more than 200 countries. SARS-CoV-2 is highly adapted to humans and readily transmits from person-to-person.
Aim
To investigate the infectivity of SARS-CoV-2 under various environmental and pH conditions. The efficacies of various laboratory virus inactivation methods and home disinfectants against SARS-CoV-2 were investigated.
Methods
The residual virus in dried form or in solution was titrated on to Vero E6 cells on days 0, 1, 3, 5 and 7 after incubation at different temperatures. Viral viability was determined after treatment with various disinfectants and pH solutions at room temperature (20–25oC).
Findings
SARS-CoV-2 was able to retain viability for 3–5 days in dried form or 7 days in solution at room temperature. SARS-CoV-2 could be detected under a wide range of pH conditions from pH 4 to pH 11 for several days, and for 1–2 days in stool at room temperature but lost 5 logs of infectivity. A variety of commonly used disinfectants and laboratory inactivation procedures were found to reduce viral viability effectively.
Conclusion
This study demonstrated the stability of SARS-CoV-2 on environmental surfaces, and raises the possibility of faecal–oral transmission. Commonly used fixatives, nucleic acid extraction methods and heat inactivation were found to reduce viral infectivity significantly, which could ensure hospital and laboratory safety during the SARS-CoV-2 pandemic.
Keywords: SARS-CoV-2, Stability, Infectivity, High infection rate, Route of transmission
Introduction
The first human coronavirus of confirmed zoonotic origin – severe acute respiratory syndrome coronavirus (SARS-CoV) – emerged in 2003. It spread in over 30 countries and caused SARS [1]. Sixteen years later, another zoonotic coronavirus – SARS-CoV-2 – emerged in Wuhan, China, causing coronavirus disease 2019 (COVID-19) [2,3]. SARS-CoV-2 belongs to the beta-coronavirus lineage B and shares ∼80% identity with SARS-CoV. SARS-CoV-2 is currently causing a global pandemic which, as of mid-April 2020, has affected more than 2 million people and caused more than 150,000 deaths [4]. The actual number of infected cases is believed to be higher due to limited testing of persons requiring hospitalization in several countries during the early stages of the pandemic. It is estimated that 18% of infections are asymptomatic [5]. According to current estimates, the case fatality rate of COVID-19 is lower than that of SARS. However, due to its propensity to cause milder infections, SARS-CoV-2 spreads more efficiently in communities in the absence of rigorous social distancing measures. Previous findings showed that the viability of SARS-CoV degraded and was lost rapidly at higher temperatures and higher relative humidity [6]. This may have impaired its transmission in tropical areas such as Malaysia, Indonesia and Thailand. Judging by the rapidity of its spread, SARS-CoV-2 appears to be affected less by the hot weather and high humidity prevailing in Asian countries, including Malaysia, Thailand and Singapore [4]. However, it is notable that the incidence rates of COVID-19 in these countries are lower compared with countries in Europe and the USA [4].
Understanding the viability of SARS-CoV-2 under various environmental conditions, and the effectiveness of disinfectants against it, is crucial. This is particularly relevant to hospital settings, where highly effective viral inactivation methods are required in wards nursing patients with COVID-19 and laboratories processing samples from patients with COVID-19. In this study, stability of SARS-CoV-2 under various environmental and pH conditions were tested. In addition, the effect of various disinfectant solutions and laboratory inactivation methods on the viability of SARS-CoV-2 was investigated. These factors could play a major role in transmission of disease, and might suggest methods to stop the spread of the virus.
Methods
Virus strains and cell line
Vero E6 cell line was cultured in minimal essential medium (MEM, Gibco, Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS, Gibco), penicillin and streptomycin (Gibco). Virus strains used in the study were SARS-CoV-2 HKU-001a and SARS-CoV HKU39849 [6,7,11]. Virus propagated in Vero E6 cells was maintained in MEM with 1% FBS, and was stored at -80°C until use.
Median tissue culture infectious dose (TCID50) assay
Confluent Vero E6 cells on 96-well plates were incubated with 100 μL of serial 10-fold dilutions of virus in MEM containing 1% FBS for 1 h at 37°C. Next, the virus was removed from the 96-well plates and 100 μL of fresh MEM with 1% FBS was added to the cells. After the change of medium, cells infected with SARS-CoV-2 underwent a 5-day incubation period and cells infected with SARS-CoV underwent a 3-day incubation period; the cytopathic effect was recorded. Median tissue culture infectious dose (TCID50) was determined by the Reed and Muench method [8].
Effect of drying and heat
Ten microlitres of virus (SARS-CoV-2, 106.5 TCID50/mL; SARS-CoV, 107 TCID50/mL) was placed on a glass slide within a shell vial, kept at room temperature (20–25oC and relative humidity of 63%) and allowed to dry in accordance with the authors' previous study with slight modifications [6]. One hundred microlitres of MEM were used to resuspend the virus for 0, 1, 3, 5 and 7 days after incubation at different temperatures: refrigerator (4oC), room temperature (25oC) and two incubators with different temperatures (33oC and 37°C). All the time points were set up in triplicate and incubation was undertaken in the dark. The residual virus infectivity was titrated [8]. Controls were viruses in solution, and stored in closed screw cap tubes with similar treatment.
Effect of pH on viability
Viral transport medium with various pHs from 2 to 13 using 5M and 1M HCl or 5N and 1N NaOH were prepared as described elsewhere [9]. One hundred microlitres of SARS-CoV-2 with 106.5 TCID50/mL was added to each bottle of 0.9 ml viral transport medium (VTM) and incubated at room temperature (20–25°C). All tests were performed in triplicate. Viability of the virus was tested on days 1, 3 and 6. On each testing day, the pH of the VTM bottles was neutralized to pH 7 and the viral titre was measured using the TCID50 assay [8]. An untreated virus stock solution was included as the viral load for the positive control.
Stability in stool
One hundred microlitres of virus with 106.5 TCID50/mL was added to 0.9 mL of watery stool derived from a human patient [10]. Antibiotics (vancomycin 100 μg/mL, amikacin 90 μg/mL and nystatin 40 units/mL) were added to suppress any potential bacterial or fungal growth. The experiment was performed in duplicate. Viability of the virus was titrated as described above [8]. An untreated virus stock solution was included as the viral load for the positive control.
Stability in disinfectants
Thirty microlitres of SARS-CoV-2 (106.5 TCID50/mL) and 270 μL of various disinfectants were mixed and incubated at room temperature (Table I ). After incubation for 1 min and 5 min at room temperature (20–25oC), 900 μL of MEM with 1% FBS was added to 100 μL of virus–disinfectant mixture to dilute the disinfectant immediately before determination of residual virus infectivity by the TCID50 assay [8]. All disinfectants without virus were titrated in parallel to determine the cytotoxic effect. An untreated virus stock solution was included as the viral load for the positive control.
Table I.
Disinfectants used in the study
| Disinfectant | Active ingredient | Supplier | Country or region |
|---|---|---|---|
| Ethanol (75%) | Ethanol 75% | VWR Chemicals BDH | USA |
| Bleach (10%) | Sodium hypochlorite 10%, | Kao | Japan |
| Virkon (2%) | Potassium peroxymonosulfate 21.41%, sodium chloride 1.5% | Lanxess | UK |
| Formalin (10%) | Formaldehyde 4% | Thermo fisher | USA |
| Lysis buffer (EasyMAG) | Guanidine thiocyanate 50%, Triton X-100 <2%, EDTA <1% | Biomerieux | France |
| AVL (viral lysis buffer) | Guanidine thiocyanate 50–70% | Qiagen | USA |
| Liquid hand soap | Biodegradable amphoteric surfactants and DMDM hydantoin | Funchem | HKSAR |
| Hand wash | Sodium laureth sulfate, cocamidopropyl betaine | Manning | China |
| Hand rub (WHO formulation 1) | Ethanol 80% v/v, glycerol 1.45% v/v, hydrogen peroxide 0.125% v/v | HKU in-house | HKSAR |
| Advanced hand sanitizer | Ethyl alcohol 70% | Purell | USA |
| Disinfection solution | Sodium hypochlorite 0.002% and hypochlorous acid 0.013% | Dermo Dacyn | USA |
| Hand wash | Chloroxylenol (PCMX) | Walch | Germany |
HKSAR, Hong Kong Special Administrative Region; HKU, University of Hong Kong; DMDM hydantoin, 1,3-bis(hydroxymethyl)-5,5-dimethylimidazolidine-2,4-dione; WHO, World Health Organization.
Heat inactivation of SARS-CoV-2
Thirty microlitres of SARS-CoV-2 (105.5 TCID50/mL) and 270 μL of FBS were mixed and incubated at 56°C for 30 min. The residual infectivity of the virus was determined by TCID50 assay as described above. The test was performed in triplicate.
Viability after fixation treatment
Vero E6 cells were infected with SARS-CoV-2 at one multiplicity of infection in a six-well plate for 2 days. The infected cells were scraped, spotted on slides and dried. The fixed smears were fixed with chilled acetone (VWR Chemicals BDH, Radnor, PA, USA) for 10 min at -20oC or 4% paraformaldehyde for 30 min at room temperature. The dried acetone and paraformaldehyde fixed smears were washed twice in PBS to remove residual fixatives. The inactivation effects of these fixatives were monitored by scraping cells from fixed smears into culture tubes with Vero E6 cells. The cytopathic effect was examined up to 7 days, and then antinucleoprotein (NP) antigen expression was tested [11].
Results
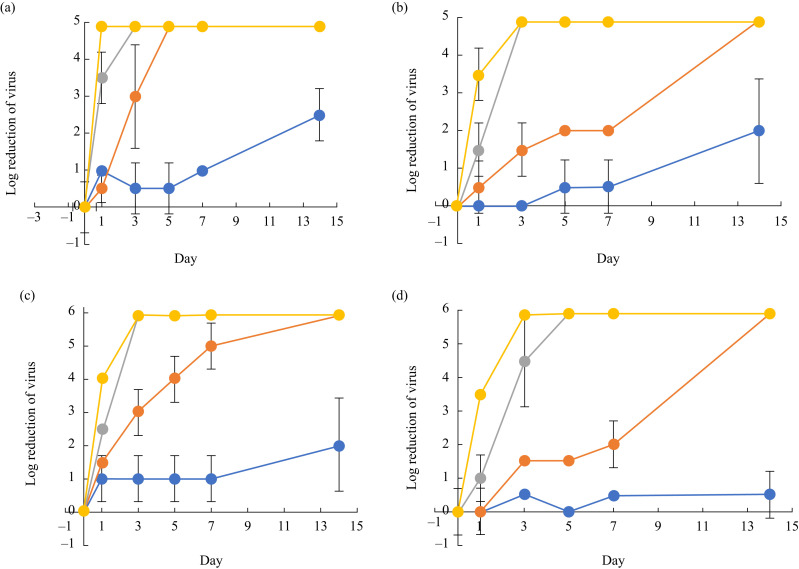
Dried SARS-CoV-2 retained viability for 3–5 days at room temperature (20–25oC) with prolonged survival for >14 days at 4oC (Figure 1 ). The virus lost its infectivity within 1 day at warmer temperatures (∼37°C). SARS-CoV-2 in solution retained viability for 7 days at room temperature (20–25°C) and remained viable for up to 14 days at 4oC. The virus suspended in solution retained viability for 1–2 days at hot temperatures (33–37oC). SARS-CoV had similar viability as SARS-CoV-2 at the same environmental conditions, except that dried SARS-CoV had better survival rates for 7–14 days at room temperature (20–25°C).
Figure 1.
Stability of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and SARS-CoV. (a) Stability of SARS-CoV-2 in dried form. (b) Stability of SARS-CoV-2 in solution. (c) Stability of SARS-CoV in dried form. (d) Stability of SARS-CoV in solution. Blue line, 4oC; orange line, 20–25 oC; grey line, 30 oC; yellow line, 37oC.
When SARS-CoV-2 was added to VTM with pH ranging from pH 2 to pH 13, the virus remained viable for up to 6 days but lost between 2.9 and 5.33 logs of infectivity from pH 5 to pH 9, and remained viable for 1–2 days at pH 4 and pH 11 (Table II ). The virus lost infectivity within 1 day at pH extremes (pH 2–3 and pH 11–12), and lost 5.25 logs of infectivity in stool over a 3-day period.
Table II.
Effects of different pH conditions on infectivity of severe acute respiratory syndrome coronavirus-2a
| pH | Day 1 (log10 reduction ±SD) | Day 3 (log10 reduction ±SD) | Day 6 (log10 reduction ±SD) |
|---|---|---|---|
| 2 | Negative (6.50±0.00) | Negative (6.50±0.00) | ND |
| 3 | Negative (6.50±0.00) | Negative (6.50±0.00) | ND |
| 4 | Positive (2.67±0.29) | Negative (6.50±0.00) | Negative (6.50±0.00) |
| 5 | Positive (1.08±0.52) | Positive (2.33±0.29) | Positive (3.50±0.50) |
| 6 | Positive (1.00±0.50) | Positive (1.67±0.58) | Positive (4.10±0.85) |
| 7 | Positive (0.67±0.29) | Positive (1.50±0.50) | Positive (2.90±0.96) |
| 8 | Positive (1.23±0.25) | Positive (2.73±0.64) | Positive (3.92±0.63) |
| 9 | Positive (1.50±0.87) | Positive (3.23±0.68) | Positive (5.33±0.58) |
| 10 | Positive (2.40±0.36) | Positive (5.13±0.40) | Negative (6.50±0.00) |
| 11 | Positive (3.00±0.70) | Negative (6.50±0.00) | Negative (6.50±0.00) |
| 12 | Negative (6.50±0.00) | Negative (6.50±0.00) | ND |
| 13 | Negative (6.50±0.00) | Negative (6.50±0.00) | ND |
Positive, culture positive; negative, culture negative; ND, not done; SD, standard deviation.
Include untreated virus stock solution as the viral load for the positive control median tissue culture infectious dose/mL=6.50±0.61. All tests were neutralized before testing and performed in triplicate.
Laboratory or domestic disinfectants, including two disinfectants commonly used as a lysis buffer for nucleic acid extraction, were tested for their effects on SARS-CoV-2 propagated in Vero E6 cells (Table III ). Due to the cytotoxicity of certain disinfectants, the detection limit of inactivation ranged from 0.83 to 3.25 log10 reduction for 1 min and from 0.92 to 3.75 log10 reduction for 5 min. This showed that SARS-CoV-2, like SARS-CoV, can be inactivated by common laboratory or domestic disinfectants [10,12,13].
Table III.
Effects of disinfectants on viability of severe acute respiratory syndrome coronavirus-2
| Disinfectantsa | Log10 reduction |
|
|---|---|---|
| 1 min | 5 min | |
| Ethanol (75%) | ≥1.83 ±0.29 | ≥2.00 ±0.00 |
| Bleach (10%) | ≥3.25 ±0.00 | ≥3.25 ±0.00 |
| Virkon (2%) | ≥3.00 ±0.00 | ≥3.00 ±0.00 |
| Formalin (10%) | ≥1.25 ±0.00 | ≥1.25 ±0.00 |
| Lysis buffer (EasyMAG) | ≥2.00 ±0.43 | ≥2.25 ±0.00 |
| AVL (viral lysis buffer, Qiagen) | ≥3.00 ±0.43 | ≥3.25 ±0.00 |
| Liquid hand soap (Funchem) | ≥2.00±1.56 | ≥2.25±0.00 |
| Hand wash (Mannings) | ≥0.83±0.29 | ≥0.92±0.38 |
| Hand rub (WHO formulation 1) | ≥2.17±0.14 | ≥2.25±0.00 |
| Advanced hand sanitizer (Purell) | ≥2.50±0.0 | ≥2.50±0.0 |
| Disinfecting solution (Dermo docyn) | 2.30±0.50 | 3.75±0.43 |
| Hand wash (Walch) | ≥0.83±0.29 | ≥0.92±0.14 |
WHO, World Health Organization.
Include untreated stock solution as the viral load for the positive control (median tissue culture infectious dose/mL=6.50±0.61). The experiment was performed in triplicate.
When the virus was added to 90% FBS or MEM and was heated at 56°C for 30 min, viability in both FBS and MEM was reduced by at least 3 logs (3.58±0.29). This mimics the conditions of heat inactivation, which should inactivate SARS-CoV-2 effectively in human serum for use in immunoassays.
After treatment with chilled acetone or 4% paraformaldehyde, the viability of fixed culture cells was tested. No cytopathic effect was observed and no virus was detected by NP antigen expression. As for SARS-CoV, chilled acetone is required to complete inactivation of cell smears infected with SARS-CoV-2 [10]. In this study, both chilled acetone and 4% paraformaldehyde inactivated SARS-CoV-2 completely, rendering fixed slides safe for further processing in a biosafety level 2 laboratory.
Discussion
The main transmission routes of SARS-CoV-2 are believed to be via: (1) inhalation of aerosols generated by infected persons; (2) direct contact with infected persons; and (3) contact with environmental fomites [13,14]. This study investigated infectiousness of the virus under a variety of environmental conditions. In this study, the dynamic rate of decay of SARS-CoV-2 was similar to that of SARS-CoV (Figure 1). Dried SARS-CoV-2 on glass can retain viability for over 3–4 days at room temperature (22–25°C) and for 14 days at cold temperatures (4°C), but loses viability rapidly within 1 day at warm temperatures (37°C). However, SARS-CoV-2 in solution remained viable for longer than dried SARS-CoV-2 under these temperature conditions. These data demonstrated that SARS-CoV-2 could survive on environmental surfaces, and that contaminated surfaces may act as a reservoir for transmission of the virus if they are not cleaned and disinfected adequately.
This could explain large outbreaks of SARS-CoV-2, such as that on the Diamond Princess cruise ship. This outbreak caused 712 out of 3711 passengers to become infected, with 12 deaths. This ship was placed under quarantine orders from 5th February 2020 [5]. All passengers were confined to the ship with close contact during the quarantine period, and shared various facilities such as the food buffet, water supply, sanitation and air-conditioning systems for many days. SARS-CoV-2 has been shown to have a longer half-life on stainless steel and plastic surfaces [14]. SARS-CoV-2 outbreaks also occurred on military warships in the USA and France. This study clearly illustrates how SARS-CoV-2 can cause long-lasting environmental contamination in such settings.
This study demonstrated that SARS-CoV-2, like SARS-CoV, can survive in stool for 1–2 days but with a 5-log loss of viability [10]. This suggests that viability is lost rapidly in faecal material. SARS-CoV-2 is frequently shed in the stool of infected patients [15]. As the virus remains viable under a wide range of pH and environmental conditions, it is considered likely that it would be able to retain its infectivity on environmental surfaces and, potentially, even in infected food handlers shedding SARS-CoV-2 in faeces. Transmission via the faecal–oral route is theoretically possible, especially in individuals with reduced gastric acidity due to medications such as proton pump inhibitors. In fact, the SARS-CoV-2 host receptor was found in the cytoplasm of gastrointestinal epithelia cells of infected patients [15], and 17.6% of patients with COVID-19 had gastrointestinal symptoms and viral RNA was detected in stool samples from 48.1% of patients [16].
In this study, the results showed that a variety of commonly used disinfectants and laboratory inactivation procedures can reduce viral viability. This is particularly significant for healthcare settings including laboratories that require highly reliable inactivation methods to safeguard staff working with COVID-19 patients and samples. Polymerase chain reaction assays, immunofluorescence staining and serology are all core components of the BSL-2 virology laboratory. This study confirmed that commonly used fixatives, nucleic acid extraction methods and heat inactivation can significantly abrogate viral infectivity. This study, therefore, has a direct impact for hospital and laboratory safety during the COVID-19 pandemic.
A limitation of this study is that residual cytotoxicity from disinfectants may have been present, as dilution of active compounds was performed, rather than neutralization, before virus titration.
In conclusion, this study contributes to a better understanding of the stability of SARS-CoV-2 in different environmental situations. The stability of SARS-CoV-2 is similar to that of SARS-CoV. This study showed that SARS-CoV-2 can survive on contaminated environmental surfaces for days, and can survive for prolonged periods when in fluid suspensions. This has implications for the transmission of infection in health care, but also in terms of transmission related to food handlers and workers in meat and poultry processing facilities [17]. Finally, commonly used viral inactivation methods in clinical virology laboratories, and disinfectant solutions used in healthcare settings, were shown to be sufficient to reduce the viability of SARS-CoV-2 drastically.
Conflict of interest statement
None declared.
Funding sources
This study was supported, in part, by donations from May Tam Mak Mei Yin, Richard Yu and Carol Yu, the Shaw Foundation Hong Kong; Michael Seak-Kan Tong, Respiratory Viral Research Foundation Limited; Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited; Chan Yin Chuen Memorial Charitable Foundation; Marina Man-Wai Lee, Hong Kong Hainan Commercial Association South China Microbiology Research Fund; Jessie & George Ho Charitable Foundation; Perfect Shape Medical Limited; Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for the Department of Health of the Hong Kong Special Administrative Region Government; Theme-Based Research Scheme (T11/707/15) of the Research Grants Council; Hong Kong Special Administrative Region; Sanming Project of Medicine in Shenzhen, China (No. SZSM201911014); and High Level-Hospital Program, Health Commission of Guangdong Province, China. The funding sources had no role in the study design, data collection, analysis, interpretation or writing of the report.
References
- 1.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worldometer Coronavirus updates. Available at: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas [last accessed April 2020].
- 5.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K.H., Peiris J.S.M., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed L.J., Muench H. A simple method of estimating fifty percent end points. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 9.Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO; Geneva: 2020. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network.https://www.who.int/csr/sars/survival_2003_05_04/en/ Available at: [last accessed July 2020] [Google Scholar]
- 11.Chu H., Chan J.F.W., Yuen T.T.T., Shuai H., Yuan S., Wang Y. An observational study on the comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV: implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19. Lancet Microb. 2020;1:e14–23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eleraky N.Z., Potgieter L.N., Kennedy M.A. Virucidal efficacy of four new disinfectants. J Am Anim Hosp Assoc. 2002;38:231–234. doi: 10.5326/0380231. [DOI] [PubMed] [Google Scholar]
- 13.Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Hui P.Y., Yen H.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microb. 2020;1:e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindson J. COVID-19: faecal–oral transmission? Nat Rev Gastroenterol Hepatol. 2020;1 doi: 10.1038/s41575-020-0295-7. doi:10.1038/s41575-020-0295-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyal J.W., Grant M.P., Broadwater K., Bjork A., Waltenburg M.A., Gibbins J.D. COVID-19 among workers in meat and poultry processing facilities — 19 states. Morb Mortal Wkly Rep. 2020;69:557–561. doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]