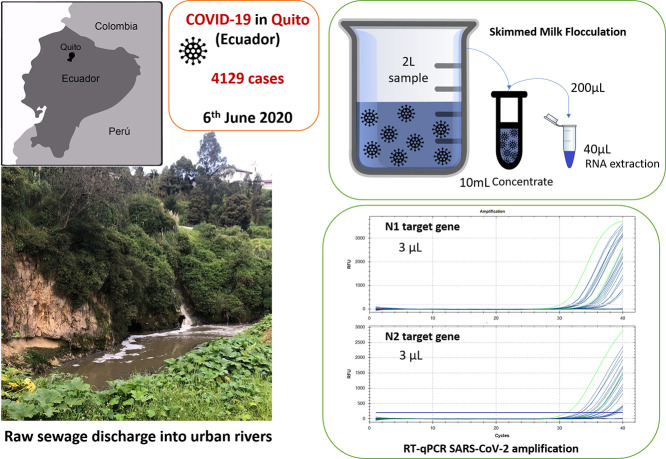
Abstract
Since the beginning of COVID-19 pandemic studies on viral shedding have reported that this virus is excreted in feces in most patients. High viral loads are found at the sewage pipeline or at the entrance of wastewater treatment plants from cities where the number of COVID-19 cases are significant. In Quito (Ecuador) as in many other cities worldwide, wastewater is directly discharged into natural waters. The aim of this study was to evaluate SARS-CoV-2 presence in urban streams from a low sanitation context. Three river locations along the urban rivers of Quito were sampled on the 5th of June during a peak of COVID-19 cases. River samples were evaluated for water quality parameters and afterwards, concentrated for viral analysis using skimmed milk flocculation method. The viral concentrates were quantified for SARS-CoV-2 (N1 and N2 target regions) and Human Adenovirus as a human viral indicator. The results showed that SARS-CoV-2 was detected for both target regions in all samples analyzed in a range of 2,91E+05 to 3,19E+06 GC/L for N1 and from 2,07E+05 to 2,22E+06 GC/L for N2. The high values detected in natural waters from a low sanitation region have several implications in health and ecology that should be further assessed.
Keywords: COVID-19, Andean rivers, Wastewater, Viral dissemination, Ecuador, HAdV
Graphical abstract
1. Introduction
Since the first detected case of unknown pneumonia in Wuhan (China), in half a year, SARS-CoV-2 has caused millions of confirmed cases and more than 500.000 deaths worldwide (WHO, 2020). Currently, Latin America is considered the epicenter of the pandemic (UN, 2020). Brazil reported Latin America's first case in late February 2020, and since then, COVID-19 cases and deaths have increased widely throughout nearly every country in the region. In Ecuador, official reports declare more than 61.000 cases and 4700 deaths in the first week of July (SNGRE, 2020).
SARS-CoV-2 transmission in humans is mainly airborne (Zhang et al., 2020) but recent studies suggest that extended shedding of SARS-CoV-2 in feces is a potential health risk (Lodder and de Roda Husman, 2020). As most COVID-19 cases fecally excreted the virus, SARS-CoV-2 has been detected in wastewater treatment plants from several cities around the world and some countries have started to monitor the viral load in sewage systems in an attempt to design early warnings for future outbreaks (Bivins et al., 2020). However, the fecal shedding in wastewater could be especially relevant in low sanitation countries where insufficient or nonexistent treatments of wastewater are applied.
Studies analyzing SARS-CoV-2 in sewage have tested the efficiency of wastewater treatment in the removal of SARS-CoV-2 since most effluents' samples did not show its presence (Randazzo et al., 2020a). Regarding natural streams, in Japan, Haramoto et al. (2020) did not detect SARS-CoV-2 in river waters polluted with sewage. A study in Milan, Italy detected the presence of SARS-CoV-2 genetic material in sewage and in river water (Rimoldi et al., 2020) but found that the virus was not vital, although a quantification of the virus was not performed.
In most countries of Latin America an average of 30% of wastewaters are treated before discharging in water bodies (Rodriguez et al., 2020). In particular, Ecuador has less than 20% of treatment coverage for its wastewaters (Rodriguez et al., 2020) and its capital city, Quito with almost 3 million inhabitants only treats 3% of its sewage (EPMAPS, 2020). Therefore, the urban rivers of Quito, receiving the fecal waters from the whole city, are highly contaminated by human microorganisms and other pollutants, disseminating them along the river basin and crossing from up 2800 masl to the Pacific coast (Guerrero-Latorre et al., 2018; Voloshenko-Rossin et al., 2015). Previous studies have described the viral diversity of human pathogens present in the urban rivers of Quito and highlighting the health risk of populations that can use downstream waters for irrigation or recreational purposes (Guerrero-Latorre et al., 2018). In the context of the COVID-19 pandemic, very little information has emerged from low sanitation countries about the presence of SARS-Cov-2 in sewage and natural waters. Therefore, this is the first study in a low sanitation country that attempted to analyze the presence of SARS-CoV-2 in river waters impacted by urban wastewater.
2. Material and methods
2.1. Sampling
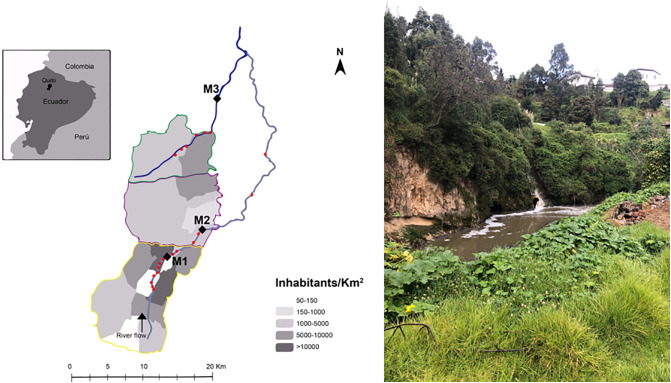
The 5th of June 2020 three locations from Quito's river were sampled. Sampling locations are representative from south-center (M1), north-center (M2) and north (M3) accumulated wastewater discharges into the natural streams passing through the city (Fig. 1 ; EPMAPS, 2011). At each location, 2 L samples were collected by hand for viral analysis. Sterile plastic bottles treated with 1 N HCl solution were used, to eliminate any genetic material that could alter the sample. At the moment of the sample collection, bottles were previously washed with river water before filling each bottle. Additionally, 2 L were collected for other biological and chemical measurements: DBO5, DQO, pH and conductivity. Samples were preserved at 4 °C and taken to the laboratory in less than 3 h.
Fig. 1.
Left. Sampling locations in urban rivers from Quito (Guerrero-Latorre et al., 2018). M1 (0°,14′00.27″S; 78°30′42.43″O), M2 (0°11′52.47″S;78°28′06.46″O), M3 (0°00′42.98″S; 78°26′25.86″O). Right. Picture of direct discharge of wastewater into Machángara River (sampling point M1).
2.2. Water concentration and RNA extraction
Collected water was concentrated for viral analysis using an adapted Skimmed Milk Flocculation method using 2 L of water (Fernandez-Cassi et al., 2018). Briefly, sample was preconditioned at pH 3.5 (with 1 N HCl) and conductivity above 1.5 mS/cm2 (adding sea salts, Sigma-Aldrich). Then, 10 mL of pre-flocculated skimmed milk (Difco, Detroit, USA) 1% (w/w) solution was added per liter of water sample. After 8 h of stirring, flocks were centrifuged at 8000 ×g for 40 min using conical tubes in an Eppendorf 5804R centrifuge (Hamburg, Germany). Pellets collected from each water sample (2 L) were suspended as a pool in 10 mL of phosphate buffer. Nucleic acids, RNA and DNA, were extracted using AccuPrep® Universal RNA Extraction Kit (Bioneer, Daejeon, Korea) from 200 μL of the viral concentrates into 40 μL of eluted solution according to manufacturer's instruction. Two independent nucleic acid isolations from each viral concentrate were conducted.
2.3. qRT-PCR analysis
Nucleic acid extractions from viral concentrates were used to analyze SARS-CoV-2 (CDC, 2020) and Human Adenovirus as a microbial indicator of human fecal contamination (Hernroth et al., 2002). Every quantitative PCR assay included a negative control and was performed in a CFX96 Real-Time detection system (Bio-Rad, California, USA). SARS-CoV-2 RNA was detected by using TaqMan™ Fast Virus 1-Step Master Mix (ThermoFisher Scientific, Massachusetts, USA) and the RT-qPCR diagnostic panel assays for N1 and N2 regions of the N gene validated by the US Centers for Disease Control and Prevention (CDC, 2020). The sets of primers and probes (2019-nCoV CDC EUA Kit) as well as the positive control (2019-nCoV_N_Positive Control) were purchased from IDT (Integrated DNA Technologies, Leuven, Belgium). Reaction mix (15 μL) consisted of 3,75 μL 4× master mix, 0,75 μL BSA (1 mg/mL), 1,12 μL primer (6,7 μM) and probe (1,7 μM) mix, 3 μL RNA sample and 6,82 μL RNAse free H2O water. The thermal cycling conditions were as follows: reverse transcription at 50 °C for 5 min, preheating at 95 °C for 1 min and 42 cycles of amplification at 95 °C for 15 s and 55 °C for 1 min. HAdV was quantified by Environmental TaqMan MasterMix (ThermoFisher Scientific, Massachusetts, USA) as previously described (Calgua et al., 2013).
All target genes were analyzed by quadruplicate: two reactions with direct volume of extraction and two reactions with 1/10 dilution from extraction. This approach is used to evaluate the presence of inhibitors in complex matrices (McKee et al., 2015).
Viral loads were quantified as GC by plotting the Ct value to an external standard curve built with tenfold serial dilution of plasmid control/gBlock (IDT) and referred to L of water collected.
2.4. Cases per sampling point
In order to relate SARS-CoV-2 viral load in river water samples with reported COVID-19 cases in Quito, we gathered information from the sectors of Quito that discharge sewage to the sampling points according to the Potable Water and Sanitation Enterprise of Quito (EPMAPS, 2011). Since the city of Quito reports its cases by these sectors we gather current and historical information from 14 days prior to the sampling points (https://twitter.com/MunicipioQuito/ and https://coe-pichincha.senescyt.gob.ec/situacion-pichincha/). This information was validated with the official government reports (SNGRE, 2020).
3. Results
Water quality parameters of samples analyzed show an important anthropogenic impact in the rivers (Table 1 ). Sampling points M2 and M3 do not meet the national requirement for aquatic life preservation for DQO and DBO5 (Ministerio del Ambiente de Ecuador, 2015). Human Adenovirus, a viral indicator used to evaluate the microbial impact in river water present concentrations between 1,13E+04 and 2,60E+05 GC/L corresponding to strong impact (Rusiñol et al., 2014).
Table 1.
Water quality parameters at sampling locations.
| Sampling points | pH | Conductivity | DBO5 mg O2/L |
DQO mg O2/L |
HAdV GC/L |
|---|---|---|---|---|---|
| M1 | 7,73 | 653,07 | 13 | 35.706 | 1,13E+04 |
| M2 | 8,16 | 703,2 | 28a | 63.353a | 2,18E+04 |
| M3 | 7,83 | 696,48 | 16 | 43.941a | 2,60E+05 |
Parameters that do not comply with the Ecuadorian normative for aquatic life preservation (Ministerio del Ambiente de Ecuador, 2015).
SARS-CoV-2 N nucleocapsid (N) protein gene was present in the three locations and show concentrations ranging from 2,84E+05 to 3,19E+06 GC/L for N1 target region and from 2,07E+05 to 2,23E+06 for N2 target region (Table 2 ).
Table 2.
Viral loads of SARS-CoV-2 per location and cumulative and active COVID-19 cases in the influence area.
| Sampling points | SARS-CoV-2 N1 GC/L |
SARS-CoV-2 N2 GC/L |
Cumulative cases (total COVID-19 cases until 5th June) | Active cases (COVID-19 cases from the prior 14 days) |
|---|---|---|---|---|
| M1 | 3,19E+06 | 2,23E+06 | 2077 | 579 |
| M2 | 2,84E+05 | 2,07E+05 | 580 | 81 |
| M3 | 2,91E+06 | 8,55E+05 | 358 | 90 |
These values are clearly related to COVID-19 cases reported in the contributing areas for each point, showing higher values in M1 (south-center city) where higher active cases were registered (Table 2). The collection of samples was during a peak of the outbreak, since the active cases (considered the notified cases in the last 14 days prior to the sampling day) represent 25% of the total cases of COVID19 reported in the city since the beginning of the outbreak.
4. Discussion
This is the first study that quantifies SARS-CoV2 in river water, and at important levels as expected for rivers receiving untreated sewage. Previous studies attempting to detect the virus in river water, have not found (Haramoto et al., 2020) or detected it without quantification (Rimoldi et al., 2020). This could be due to the fact that both studies are from areas that treat its wastewaters, although overflows from combined sewage can occur, loads are considerably lower than untreated waters. The urban rivers of Quito are impacted by the direct discharge of sewage water from a population of almost 3 million inhabitants. The water quality parameters described in Table 1 indicate the high levels of organic and inorganic contamination from those urban rivers. The water parameters measured in 2 of the 3 locations studied were above the national regulations for aquatic life preservation (Ministerio del Ambiente de Ecuador, 2015). However, all environmental values are higher than those registered for reference sites in this same basin (Ríos-Touma et al., 2014).
Viral human contamination analyzed by HAdV indicator shows the great impact of human excreta in the urban rivers of Quito. Values are very similar from the samples collected in the same locations in 2017 (Guerrero-Latorre et al., 2018) pointing out that sanitation conditions have not improved. In that study, a metagenomic analysis was carried out in the same locations and sequences from 26 human viral pathogens were obtained, revealing the microbial consequences of sewage discharge without previous treatment into natural streams. Both studies have used the skimmed milk flocculation as a concentration method for highly polluted waters, showing good performance and results. This inexpensive method can be used for SARS-CoV-2 analysis of water samples in laboratories that do not have specialized equipment for viral concentration.
Nowadays, in the peak of COVID-19 pandemic in Ecuador, levels of SARS-CoV-2 found in urban rivers of Quito are similar as found in sewage of Valencia (Spain) with more than 5000 active cases (Randazzo et al., 2020b) and Paris in the epidemic peak of cases with more than 10,000 hospitalized cases (Wurtzer et al., 2020). However, at the sampling date, in the urban area of Quito only 750 COVID-19 active cases were notified, suggesting an important underdiagnosed as seen in many other regions worldwide. Also, these high values can be related to asymptomatic cases since other studies have reported a considerable amount of them. For example, at the Diamond Princess cruise ship 17.9% of the cases were asymptomatic (Mizumoto et al., 2020) and in a Long-Term Care Skilled Nursing Facility in King County, WA, 57% of the cases were asymptomatic or pre-symptomatic (Kimball et al., 2020).
The implications from our study can be extended to other cities where the sewage is directly discharged into natural streams. Firstly, the presence of SARS-CoV-2 together with other waterborne pathogens discharged in open river water might pose a risk of infection for the population in contact with the water downstream. The risk has been recently approached in a QMRA study of WWTP workers exposed to a different concentration of SARS-CoV-2 in sewage (Zaneti et al., 2020). However, it is important to remark that only genomic material has been detected in waters and the viability of the virus is not known for polluted waters. Secondly, the viral dissemination into the environment has an unknown impact into livestock and wildlife health, as the spillover events of zoonotic links are frequent in the Coronaviridae family (Franklin and Bevins, 2020).
Finally, the presence of the virus can be also used as a surveillance tool for an early warning system using main sewage discharges along the city helping to control the pandemic where diagnostic tools are limited (Bivins et al., 2020). Also, the method implemented in the present publication can be used in other cities where sewage is not possible to sample and wastewaters are discharged to streams or rivers.
5. Conclusions
We have detected for the first time important viral loads of SARS-CoV-2 from urban streams of Quito. The loads found suggest that cases are probably a lot higher than the official data. Also, implications in dissemination of SARS-CoV-2 in low sanitation countries by polluted freshwaters should be further considered. The low degree of wastewater treatment coverage in the region, might be a factor of increased risk for COVID-19 pandemic.
CRediT authorship contribution statement
Laura Guerrero-Latorre: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft, Writing - review & editing. Isabel Ballesteros: Methodology, Validation, Formal analysis, Investigation, Writing - review & editing. Irina Villacrés-Granda: Methodology, Validation, Investigation. M. Genoveva Granda: Methodology, Validation, Investigation. Byron Freire-Paspuel: Validation, Investigation. Blanca Ríos-Touma: Conceptualization, Methodology, Validation, Investigation, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was performed under the MAE-DNB-CM-2018-093 authorization conceded by the Environment Ministry of Ecuador. We are grateful to Gabriel Iturralde for the support and resources from the Research Laboratories at UDLA. Special thanks to Xavier Amigo and Nature Experience for its invaluable service during the sampling campaign.
Funding
This work was supported by research funds from Universidad de Las Américas, Ecuador and Fideicomiso Sumar Juntos del Banco del Pichincha.
Editor: Damia Barcelo
References
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., et al. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Calgua B., Rodriguez-Manzano J., Hundesa A., Sunen E., Calvo M., Bofill-Mas S., Girones R. New methods for the concentration of viruses from urban sewage using quantitative PCR. J. Virol. Methods. 2013;187(2):215–221. doi: 10.1016/j.jviromet.2012.10.012. [DOI] [PubMed] [Google Scholar]
- CDC US Centers for Disease Control and Prevention; 2020. 2019-Novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html
- Empresa Pública Metropolitana de Agua Potable y Saneamiento Estudios de Actualización del Plan Maestro Integrado de Agua Potable y Alcantarillado para el DMQ. 2011. https://www.aguaquito.gob.ec/sites/default/files/documentos/plan_maestro_agua_potable.pdf (Accessed June 1st, 2020)
- Empresa Pública Metropolitana de Agua Potable y Saneamiento Programa para la descontaminación de los ríos de Quito. 2020. https://www.aguaquito.gob.ec/programa-para-la-descontaminacion-de-los-rios-de-quito/ (Accessed June 13th, 2020)
- Fernandez-Cassi X., Timoneda N., Martínez-Puchol S., Rusiñol M., Rodriguez-Manzano J., Figuerola N., et al. Metagenomics for the study of viruses in urban sewage as a tool for public health surveillance. Sci Total Environ. Mar. 2018;15(618):870–880. doi: 10.1016/j.scitotenv.2017.08.249. [DOI] [PubMed] [Google Scholar]
- Franklin A.B., Bevins S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020;733:139358. doi: 10.1016/j.scitotenv.2020.139358. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Romero B., Bonifaz E., Timoneda N., Rusiñol M., Girones R., Rios-Touma B. Quito’s virome: metagenomic analysis of viral diversity in urban streams of Ecuador’s capital city. Sci. Total Environ. 2018;645:1334–1343. doi: 10.1016/j.scitotenv.2018.07.213. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. 2020. First Environmental Surveillance for the Presence of SARS-CoV-2 RNA in Wastewater and River Water in Japan. medRxiv 2020.06.04.20122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernroth B.E., Conden-Hansson A.C., Rehnstam-Holm A.S., Girones R., Allard A.K. Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: the first Scandinavian report. Appl. Environ. Microbiol. 2002;68(9):4523–4533. doi: 10.1128/AEM.68.9.4523-4533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K., Bardossy A.C., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, march 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A., Spear S., Pierson T. The effect of dilution and the use of a post-extraction nucleic acid purification column on the accuracy, precision, and inhibition of environmental DNA samples. Biol. Conserv. 2015;183:70–76. doi: 10.1016/j.biocon.2014.11.031. [DOI] [Google Scholar]
- Ministerio del Ambiente de Ecuador . 2015. 097-A Refórmese el Texto Unificado de Legislación Secundaria. Registro Oficial. Año III - No 387.Quito, miércoles 4 de noviembre de 2015. (P 6 a 78) [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. 15 August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Cuevas-Ferrando E., Sanjuan R., Domingo-Calap P., Sanchez G. 2020. Metropolitan Wastewater Analysis for COVID-19 Epidemiological Surveillance. medRxiv 2020.04.23.20076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Moja L., Gismondo M.R., Salerno F. 2020. Presence and Vitality of SARS-CoV-2 Virus in Wastewaters and Rivers. medRxiv 2020.05.01.20086009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Touma B., Acosta R., Prat N. The Andean biotic index (ABI): revised tolerance to pollution values for macroinvertebrate families and index performance evaluation. Rev. Biol. Trop. 2014;62:249–273. doi: 10.15517/rbt.v62i0.15791. [DOI] [PubMed] [Google Scholar]
- Rodriguez Hector, Delgado Alexander, Nolasco Anna, Saltiel Daniel, Gustavo D.J.S. World Bank; 2020. From Waste to Resource. Water Papers. [DOI] [Google Scholar]
- Rusiñol M., Fernandez-Cassi X., Hundesa A., Vieira C., Kern A., Eriksson I., et al. Application of human and animal viral microbial source tracking tools in fresh and marine waters from five different geographical areas. Water Res. 2014;59:119–129. doi: 10.1016/j.watres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- SNGRE Informes de Situación e Infografías – COVID 19 – desde el 29 de Febrero del 2020. 2020. https://www.gestionderiesgos.gob.ec/informes-de-situacion-covid-19-desde-el-13-de-marzo-del-2020/ (Accessed 5th July 2020)
- UN Central and South America now ‘intense zones’ for COVID-19 transmission. 1 June 2020. 2020. https://news.un.org/en/story/2020/06/1065252 (Accessed 13th June 2020)
- Voloshenko-Rossin A., Gasser G., Cohen K., Gun J., Cumbal-Flores L., Parra-Morales W., Sarabia F., Ojeda F., Lev O. Emerging pollutants in the Esmeraldas watershed in Ecuador: discharge and attenuation of emerging organic pollutants along the San Pedro–Guayllabamba–Esmeraldas rivers. Environ Sci Process Impacts. 2015;17:41–53. doi: 10.1039/C4EM00394B. [DOI] [PubMed] [Google Scholar]
- WHO WHO coronavirus disease (COVID-19) dashboard. 2020. https://covid19.who.int/
- Wurtzer S., Marechal V., Mouchel J.M., Maday Y., Teyssou R., Richard E., et al. 2020. Evaluation of Lockdown Impact on SARS-CoV-2 Dynamics Through Viral Genome Quantification in Paris Wastewaters. medRxiv 2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneti R.N., Girardi V., Spilki F.R., Mena K., Campos-Westphalen A.P., da Costa Colares W.R., et al. 2020. QMRA of SARS-CoV-2 for Workers in Wastewater Treatment Plants. medRxiv 2020.05.28.20116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. 2020:202009637. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]