Summary
Background
In the wake of the SARS-CoV-2 pandemic and unprecedented global demand, clinicians are struggling to source adequate access to personal protective equipment. Respirators can be in short supply, though are necessary to protect workers from SARS-CoV-2 exposure. Rapid decontamination and reuse of respirators may provide relief for the strained procurement situation.
Method
In this study, we investigated the suitability of 70°C dry heat and microwave-generated steam (MGS) for reprocessing of FFP2/N95-type respirators, and Type-II surgical face masks. Staphylococcus aureus was used as a surrogate as it is less susceptible than enveloped viruses to chemical and physical processes.
Results
We observed >4 log10 reductions in the viability of dry S. aureus treated by dry heat for 90 min at 70°C and >6 log10 reductions by MGS for 90 s. After 3 reprocessing cycles, neither process was found to negatively impact the bacterial or NaCl filtration efficiency of the respirators that were tested. However, MGS was incompatible with Type-II surgical masks tested, as we confirmed that bacterial filtration capacity was completely lost following reprocessing. MGS was observed to be incompatible with some respirator types due to arcing observed around some types of metal nose clips and by loss of adhesion of clips to the mask.
Conclusion
Considering the advantages and disadvantages of each approach, we propose a reprocessing personal protective equipment/face mask workflow for use in medical areas.
Keywords: Reprocessing, PPE, Face masks, Dry heat, Microwave, COVID-19
Introduction
Respiratory personal protective equipment (PPE) such as respirators, and surgical masks (SMs) are essential for infection control and the protection of healthcare workers. Following the spread of the SARS-CoV-2 pandemic, worldwide demand has raised procurement issues for healthcare providers. Frontline workers and media outlets widely report that supply has become restricted in many facilities, potentially jeopardising the health of workers and patients. One approach for addressing supply shortages is the re-use of PPE and face masks, which are normally regarded as single-use items. Re-use could dramatically increase the number of masks available during emergency situations, until production capabilities are able to meet demand.
There are key challenges posed by the reuse of masks which must be considered to make informed decisions on whether to utilize such an approach. During use, micro-organisms are deposited on to masks from the environment and the wearer. Although SARS-CoV-2 is the current concern, as an enveloped virus, it does not necessarily present a challenge for disinfection or sterilization processes [1,2]. A number of reprocessing solutions have recently been considered to eliminate SARS-CoV-2 from PPE and/or face masks including a steam-based approach [3], dry and steam sterilization [4], ionized H2O2 [5] and ultraviolet radiation [6]. Additional information on N95-type respirator decontamination is summarized in https://multimedia.3m.com/mws/media/1824869O/decontamination-methods-for-3m-filtering-facepiece-respirators-technical-bulletin.pdf (last accessed June 2020). However, it is essential that if PPE are to be reprocessed, they are decontaminated effectively between users because other microorganisms less susceptible to desiccation (such as Staphylococcus aureus) would otherwise persist on masks. Indeed, approximately a third of UK health workers are nasopharyngeal carriers of S. aureus [7]. The method of decontamination must also be compatible with the mask so as not to impair its effectiveness [8]. Under the EN 14683:2019 standard [9], these masks are validated by how efficiently they prevent the penetration of S. aureus through the gauze material. Type-II and II-R surgical masks, which are designated for medical use, should provide at least 98% protection against the penetration of bacterial aerosols.
Respirators such as the FFP2/N95-type respirators are designed to protect the wearer from particles and aerosols in the environment. The BS EN 149:2001+A1:2009 standard [10] validates these masks by their ability to filter aerosols of sodium chloride (NaCl). The sub-micrometre dimensions of these aerosols allow NaCl filtration efficiency to be used as a surrogate for their effectiveness against viruses, which may become damaged by the aerosolization process. FFP2 respirators are to provide >94% filtration efficiency against NaCl [10].
This study aimed to establish effective protocols for the decontamination of respirators using dry heat or microwave-generated steam (MGS).
Materials and methods
Type-II surgical masks and FFP2/N95-type respirators
Type-II surgical masks (Cosy Cloud; Lot: 72190801N) were kindly provided by Hardshell UK Limited and the School of Medicine (Cardiff University). FFP2/N95-type respirators (Kimberly-Clark; Fluidshield) were kindly provided by Public Health Wales. Prior to use, masks and respirators were inspected for defects to ensure there were no rips or tears in the substrate.
Test organism
S. aureus NCTC 10788 was used at the test organism. Cultures were maintained and recovered on pre-poured tryptone soya agar (TSA) plates (E&O Laboratories).
Suspensions of S. aureus were prepared by inoculating 20 mL tryptone soya broth (TSB) with a single colony from the refrigerated stock. Inoculated media was then placed in an orbital shaker incubator and incubated at 37°C for 18–24 h. Following incubation, cultures were centrifuged at 3500 g for 10 min. Spent media was discarded and cells were washed in sterile tryptone sodium chloride (TSC; 8.5 g/L sodium chloride, 1 g/L tryptone). After washing, samples were recentrifuged and pellets resuspended in fresh TSC supplemented with 0.3% w/v bovine serum albumin (BSA), to simulate soiling of PPE with salivary proteins. Bacterial cell density was adjusted to 1–5 × 109 cfu/mL by equilibrating to A630 2.0–2.2 using an Amersham UV/Vis Ultrospec 3100 pro spectrophotometer.
Preparation of contaminated samples
To reduce the number of masks and respirators used for this study, 100-μL aliquots of S. aureus suspension (1–5 × 109 cfu/mL) were inoculated on to 30-mm-diameter cellulose filter membranes (Whatman), which were placed in a laminar flow cabinet for 30 min to produce a dried inoculum. Control consisted of an inoculated membrane not subjected to decontamination processes.
Decontamination with MGS
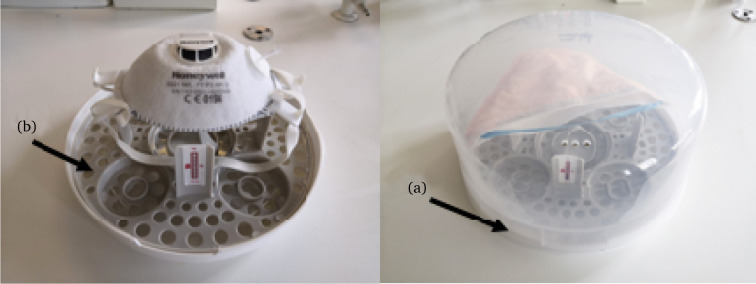
An industrial-grade 2.45-GHz microwave oven (NE-1853; Panasonic), with adjustable power settings up to a maximum output of 1800 W was used as a microwave irradiation source. The oven has two 900-W magnetrons (top and bottom) and microwaves are coupled into the oven space via rotating antennas, yielding a more uniform distribution of microwave electric field and consistent microwave exposure from sample to sample for exposure times in excess of 60 s. A microwave steam ‘sterilizer’ (Tommy Tipee; Figure 1 ) was used to provide moist heat. Prior to use, the base of the sterilizer was filled with either 100 or 200 mL of 20°C deionized water. The power could be varied via changing the duty cycle and 1800-W (100% duty cycle) and 900-W (50% duty cycle) exposures were assessed in this study. The final decontamination procedure used to prepare masks and respirators for downstream testing were prepared using 1800 W power for 90 s alongside 200 mL of water in the reservoir, For decontamination, inoculated membranes were carefully placed on the inner and outer sides of a folded N95 respirator (i.e. on the top and within the area within the fold); this was to identify whether generated steam would sufficiently disinfect the internal surfaces of the respirator. Samples were irradiated for 60, 90 and 120 s using either 900- or 1800-W power settings. To verify that PPE reached a minimum target temperature of 70°C, irreversible heat indicator stickers (71–110°C; RS Components) were placed within the steam sterilizers and inspected following irradiation.
Figure 1.
N95 respirator loaded into the microwave steam ‘sterilizer’. Water was added to the reservoir (a) in the base of the unit whilst the mask was placed atop the grating (b). Note the temperature indicator sticker placed within the sterilizer to monitor temperature between 71 and 110°C.
Dry heat decontamination
A laboratory MINI/6 incubator (Genlab Ltd) was used to provide dry heat and was set to 70°C. Temperature was monitored with a glass thermometer whilst relative humidity was monitored with a digital hygrometer (Traceable; Fisher Brand). Relative humidity remained below the lower limit of detection of this device (25%) throughout the experiments. Samples were placed in the dry heat for 5–90 min. Inoculated membranes were placed on a folded N95 respirator as described above, and the respirator placed on a shelf in the centre of the incubator.
Recovery of viable organisms from membranes
Following decontamination processes, inoculated membranes were aseptically removed from the masks and placed individually in 50-mL centrifuge tubes containing 5 g sterile glass beads and 10 mL resuspension medium (TSC supplemented with 0.1% polysorbate-80). Tubes were vortexed for 1 min to dislodge cells into the resuspension medium. Suspensions were serially diluted in a 10-fold series using sterile TSC. Aliquots of 20 μL volume of each dilution were plated in triplicate on a TSA plate. To increase the lower limit of detection, 500-μL aliquots of the resuspension media (i.e. the 100 dilution) were also used to prepare spread plates on TSA using sterile L-shaped spreaders. Following overnight incubation at 37°C, colonies were counted and log10 reductions were calculated relative to the untreated controls. The lower limit of detection using this approach was calculated as 2 cfu/mL. A >4 log10 reduction in cfu/mL was used as a target value for determining whether the approaches we explored for reprocessing were effective.
Bacterial filtration efficiency via aerosol nebulization
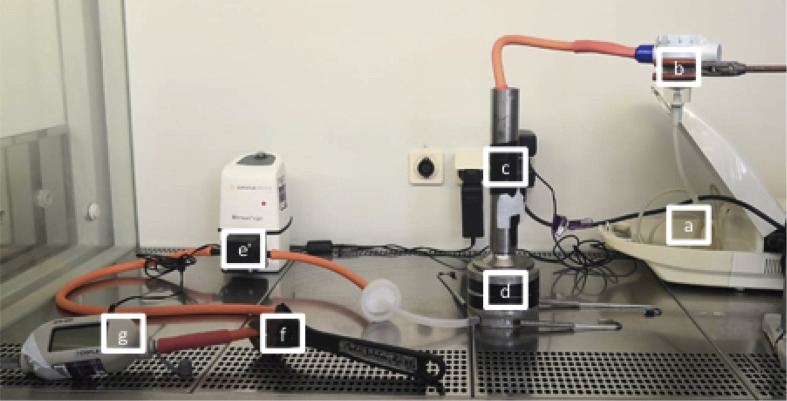
Bacterial filtration efficiency (BFE) of masks was ascertained using an approach based on the requirements of EN 14683:2019 [9]. A Microsart e.jet pump (Sartorious), Andersen Cascade Impactor (ACI; Copley scientific), DFM2000 flow meter (Copley Scientific) and a nebulizer (Philips Respronics, Best, The Netherlands) were assembled (Figure 2 ) inside a biosafety level-2 cabinet.
Figure 2.
Test set-up for delivery of microbial aerosol on to sample mask material surfaces. Air flow is in the direction from (a) to (g). (a) Nebulizer; (b) nebulizer chamber; (c) face mask insertion point; (d) Andersen cascade impactor; (e) vacuum pump; (f) adjustable clamp; (g) flow meter.
Three sterile AISI 430 stainless-steel coupons were arranged evenly around the edge of the collecting plate to check for homogeneity of nebulized inoculum. The collecting plate was placed lip down on top of stage 0 which was placed on top of stage F. All levels of the ACI were closed before testing and PVC tape was applied to seal all joints susceptible to leaks.
Surgical face masks and respirators were reprocessed either once or three times by dry heat- and microwave-based methods. Each sample of pristine or reprocessed mask material (of area 5 × 7.5 cm) was placed in the metal tubing in the ACI (Figure 2c) at a 45° angle and secured with PVC tape to prevent leaking. The surface area of mask material exposed to the nebulized air was 4 cm2. A flow meter (Copley Scientific DFM2000) was placed after the vacuum pump to measure the air flow (L/min) through the system. The adjustable clamp was used to obtain an appropriate flow rate. The nebulizer ran for 15 min before each cycle to ensure consistent flow rate of 6.5 ± 0.5 L/min. This equated to a face velocity of 0.24 m/s across the 4-cm2 test area, which is approximately 2.5 times the face velocity (0.09 m/s) described in EN 14683:2019 [9]. Testing was limited to this face velocity due to the use of an isovolumetric pump, which could not be adjusted.
Ten millilitres of 1–5 × 109 cfu/mL bacterial suspension were decanted into the nebulizer chamber (Figure 2b) and the system was set to aerosolize for 15 min whilst maintaining the aforementioned flow conditions. Following nebulization, the individual coupons were transferred to 50-mL centrifuge tubes containing 5 g glass beads and 10 mL TSC. Tubes were vortexed for 1 min to resuspend bacterial cells and viable bacteria enumerated as described above.
Assessment of mask NaCl filtration efficiency
Under BS EN149:2001+A1:2009 [10], assessment of respirator filtration efficiency is determined by measuring penetration of sub-micrometre NaCl aerosols at a flow rate of 95 SLPM over a respirator or face mask. In the case of a typical respirator which has a filtration area of circa 225 cm3, this is equivalent to an average respirator face velocity of 0.07 m/s.
The methodology used for the determination of NaCl filtration efficiency was based on parameters used in the BS EN149:2001+A1:2009 test [10]. Rather than fitting an entire respirator or face mask for testing, a cut 47-mm diameter sample section which afforded a 35-mm diameter was tested. NaCl was nebulized to an average mobility size of 56 nm. Size distribution was determined by differential mobility spectrometry, rather than photo-optical methods of mass measurement.
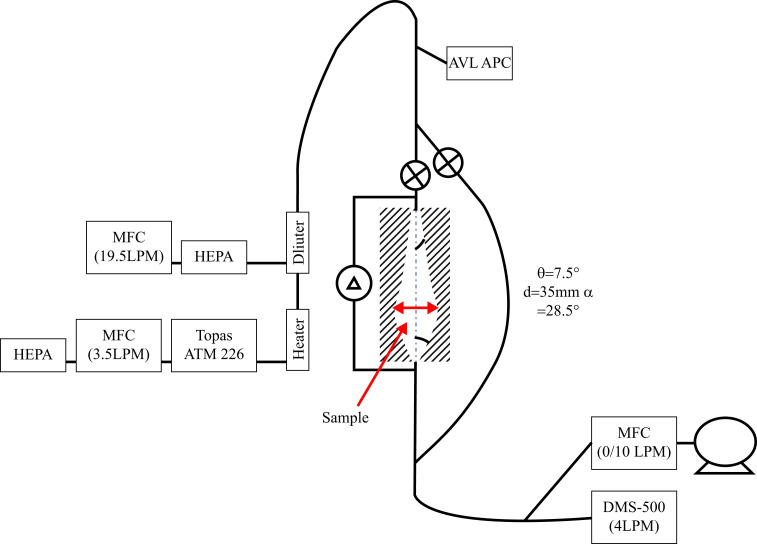
Particle penetration was assessed in a manner similar that which has been previously reported [11]. The cut sections of pristine and reprocessed respirators or masks were sequentially tested within a stainless-steel filter holder within the NaCl penetration measurement system (Figure 3 ).
Figure 3.
Schematic representation of the NaCl penetration test set up. AVL, particle counter; DMS, differential mobility spectrometer; HEPA, high-efficiency particulate air filter; MFC, mass flow controller. Sample insertion point marked in red.
A sterile NaCl solution (0.9% w/v) was atomized using a Topas ATM 226 collision nebulizer head, supplied with compressed HEPA filtered air at a flowrate of 3.5 SLPM controlled using a Bronkhorst EL-Flow mass flow controller (±0.6% of reading + 0.1% FS = 0.0033 SLPM). The nebulized aerosol was subsequently heated to 60°C using a PID controlled, non-vitiating electric heater (Watlow CAST-X) to ensure evaporation of the distilled water. To supress re-nucleation of the water and provide sufficient flow for analysis, the aerosol was diluted using a Palas VKL-10 stainless-steel educator diluter with HEPA filtered dilution air supplied at a constant flow rate of 19.5 SLPM (0.41 g/s) using a Bronkhorst cori-flow (±0.5% of reading).
The diluted sample was fed into a Y splitter using conductive silicon tubing (1.2 m). Full-bore plug valves (Swagelok 3/8″) were used to select the flow path facilitating sequential measurement before and after the filter. The filter flow path was sampled through a stainless-steel filter holder with an inlet angle (θ) of 7.5°and sampling surface diameter of 35 mm (0.000962 m2). Pressure drop across the filter holder was measured using a Druck 700 mbar deltaP transducer. The bypass section around the filter was constructed from conductive silicon (0.6 m). The aerosol flow paths were subsequently recombined using a secondary Y splitter with the sample measured using a Cambustion DMS-500 particle sizer using an internal dilution rate of 2, facilitating a constant inlet sample flow rate of 3.86 SLPM (±0.077 SLPM). This flow rate corresponded to a face velocity of circa 0.07 m/s. To ensure consistency of the test aerosol, an AVL APC with a condensation particle counter (TSI 3790 D50 = 10nm) continuously sampled at the inlet Y splitter.
For each sample of material, NaCl penetration measurements were performed three times on three separate filter sections. These technical repeats were used to calculate a mean value for NaCl penetration for each sample prior to statistical analysis.
Effect of essential oils on filtration efficiency
The end-user experience is a likely determinant for acceptability and uptake of reprocessed PPE by healthcare personnel. Communication with a frontline healthcare worker suggested that potential malodour associated with past users may negatively impact staff morale and frustrate respirator usage. To cover potential malodour issues, a set of PPE was reprocessed by microwaving, with the addition of 250 μL (five drops) of lemon-scented essential oil to the reservoir of the bottle sterilizer. The NaCl filtration capacity of the PPE was then determined as described above.
Compatibility of different respirator models with MGS-reprocessing
Four respirator models were subjected to a single cycle of microwave-reprocessing and visually inspected for defects indicative of reprocessing failure. This included assessment of burns, loss of adhesion and effects on the elastic fasteners. We stress that though no arcing was observed in our experiments for the unbranded respirator, this may not apply if a different microwave oven and/or different exposure protocol were used.
Statistical analysis
Statistical analysis was performed on bacterial and NaCl filtration efficiency data. Reprocessed masks were compared with pristine (new) controls using a one-way analysis of variance (ANOVA) and Dunnett's post hoc test. Three biological replicates were performed with all experiments.
Results
Efficacy of decontamination procedures against simulated bacterial contamination
Two approaches were successful at achieving the target of >4 log10 reductions in S. aureus dry inoculum viability (Table I ). Dry heat at 70°C was a relatively slow method and took 90 min until a satisfactory reduction was observed. Conversely, microwaving at 1800 W was rapid and >6 log10 reductions were attained within 60–90 s, depending on the volume of water added to the base of the sterilizer.
Table I.
Bactericidal effect of decontamination procedure against dry inocula of Staphylococcus aureus (108 cfu with 0.3% w/v bovine serum albumin) on coupons placed on the respirator (see text)
| Reprocessing method | Parameters | Time | Log10 reduction (S. aureus) | SD (±) | N∗ |
|---|---|---|---|---|---|
| Dry heat | 70°C/<25% relative humidity | 30 min | 1.77 | 0.54 | 3 |
| 60 min | 3.50 | 0.34 | 3 | ||
| 90 min | 4.49 | 0.57 | 5 | ||
| Microwave (200 mL in ‘sterilizer’) | 900W | 90 s | 0.30 | 0.28 | 3 |
| 1800W | 90 s | 6.85 | 0.51 | 3 | |
| Microwave (100 mL in ‘sterilizer’) | 900 W | 120 s | 3.96 | 1.10 | 3 |
| 1800 W | 60 s | 6.14 | 0.35 | 5 |
Procedures that achieved target reduction of ≥4.0 log10 are highlighted in bold. SD, standard deviation.
N, number of replicates.
Dry heat inactivation of S. aureus inocula at 70°C followed a first-order kinetic, in which there was a logarithmic increase in bacterial death over time (data not shown). At 90 min, a 4.49 log10 reduction was observed, indicating that an acceptable degree of sanitization occurs around this time point. Shorter exposure times were insufficient for achieving the desired reduction in S. aureus viability.
BFE of reprocessed masks
BFE of S. aureus through Type-II surgical masks was unaffected by dry heat decontamination (Table II ). Pristine masks achieved an average BFE of 99.0%, whilst single and triple dry heat reprocessed masks had a filtration efficiency of 95.0 and 99.2%, respectively. There were no statistically significant differences (P>0.05; ANOVA, Dunnett) in performance compared with the pristine control. In contrast, surgical masks reprocessed via MGS exhibited a complete loss of BFE. Microwave-reprocessed face masks were observed to lose their rigidity immediately following reprocessing and were noted to become significantly more wet during the nebulization procedure, possibly indicating reduced water-repellent properties.
Table II.
Bacterial filtration efficiency of decontaminated surgical masks
| Decontamination procedure | Average log10 cfu recovery | SD | Bacterial filtration efficiency (%) |
|---|---|---|---|
| No mask in place | 4.16 | ±0.10 | 0.00 |
| Pristine | 2.18 | ±1.10 | 99.0 |
| Dry heat × 1 | 2.86 | ±0.61 | 95.0 |
| Dry heat × 3 | 2.05 | ±0.45 | 99.2 |
| MGS × 1 | 4.59 | ±0.97 | <0.00 |
| MGS × 3 | 4.19 | ±0.63 | <0.00 |
MGS, microwave-generated steam; SD, standard deviation. × 1: reprocessed once; × 3: reprocessed three times.
N95 respirators offered superior bacterial filtration performance compared with surgical masks at the tested face velocity of 0.24 m/s (Table III ). Decontamination of respirators with either dry heat or MGS was not found to have a negative impact on BFE, even after three reprocessing cycles. In all cases, the number of bacteria recovered from processed surfaces was below the lower limit of detection.
Table III.
Bacterial filtration efficiency of decontaminated FFP2/N95-type respirators tested (N = 3)
| Decontamination procedure | Log10 cfu recovery from sterile discs | SD | Bacterial filtration efficiency (%) |
|---|---|---|---|
| No respirator in place | 4.16 | ±0.10 | 0.00 |
| Pristine | ≤0.30 | ±0.00 | ≥99.9 |
| Dry heat × 1 | ≤0.30 | ±0.00 | ≥99.9 |
| Dry heat × 3 | ≤0.30 | ±0.00 | ≥99.9 |
| MGS × 1 | ≤0.30 | ±0.00 | ≥99.9 |
| MGS × 3 | ≤0.30 | ±0.00 | ≥99.9 |
MGS, microwave-generated steam; SD, standard deviation. × 1: reprocessed once; × 3: reprocessed three times.
Impact of decontamination procedures on NaCl aerosol filtration efficiency
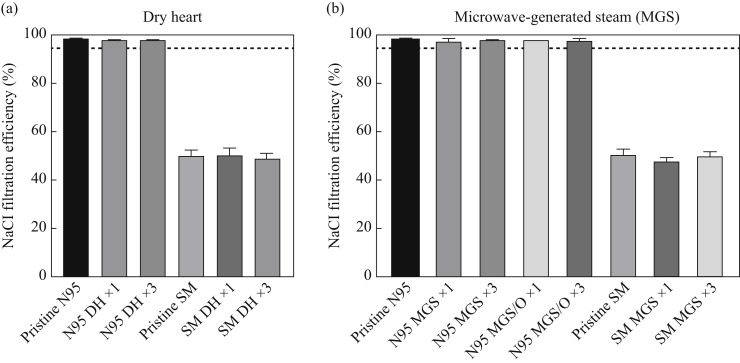
Thirty-five-millimetre cut sections of pristine surgical masks provided 50% NaCl filtration efficiency whilst pristine N95 respirators provided 98% NaCl filtration efficiency (Figure 4 ). There were no detectable changes (P>0.05; ANOVA, Dunnett) in performance between pristine and reprocessed masks of any type, even after three reprocessing cycles. Notably, N95 masks could be reprocessed with MGS in combination with essential oil.
Figure 4.
NaCl filtration efficiency of pristine and reprocessed Type-II surgical masks (SM) and FFP2/95-type respirators (N95) at 0.07 m/s face velocity with (a) dry heat (70°C for 90 min) and (b) microwave-generated steam (MGS; 1800 W, 90 s, 100 mL water in sterilizer). DH, dry heat; MGS/O, microwave-generate steam with essential oil. Numbers denote number of reprocessing cycles.
Visual inspection of microwave compatibility of respirators
Different types of respirator were noted for their varying compatibility with MGS reprocessing (Figure 5 ). Kimberly–Clark N95 respirators have a flat metal clip across the bridge of the nose and were compatible with microwave reprocessing, as was the respirator which contained no metal components. The Honeywell FFP3 respirator was incompatible as the nose clip arced during the microwave procedure. This led to heating of the material which created holes along the bridge of the nose. A generic unbranded respirator with a flat metal nose clip did not cause arcing. However, there was a loss of adhesion between the clip and respirator following a single MGS-reprocessing cycle, which may be due to degradation of the adhesive. Elastic fasteners did not appear to be negatively impacted by MGS-reprocessing.
Figure 5.
Compatibility of different respirator models with MGS reprocessing at 1800 W for 90 s. Kimberley–Clark N95 respirators (A) and a metal-free FFP2 respirator (C) showed no signs of damage following reprocessing. A Honeywell FFP3 respirator (B) and generic unbranded PPE (D) were both damaged by the microwave procedure.
Discussion
Thermal methods of decontamination such as microwave irradiation and dry heat may potentially facilitate decontamination of PPE without impinging on bacterial and NaCl filtration efficiency. Dry heat at 70°C has been shown to reduce the viral infectivity of SARS-CoV-2-contaminated respirators to below the limit of detection within 60 min [12]. MGS offers a potentially more rapid approach, although the use of an inappropriate setting or microwave device would cause irreversible damage to PPE [8]. Lore and colleagues [13] showed that the use of microwave reprocessing for 2 min was effective in reducing influenza virus load by >4 log median tissue culture infective dose from contaminated N95-type respirators, without affecting their NaCl filtration performance. Heimburg et al. [14] also reported a 4 log10 reduction of viable H1N1 on N95-type respirator following MGS reprocessing. This reprocessing method was also found to be efficient in reducing MS2 bacteriophage (a surrogate used for non-enveloped viruses such as the poliovirus) by 4 log10 within 45 s on respirator filter coupons [15]. Using different respirator models and steam bags, Fisher et al. [16] observed at least a 3 log10 reduction in MS2 following microwaving (2450 MHz microwave oven; 1100 W, 90 s).
Here, we tested the microbicidal efficacy of dry heat (70°C for 90 min) and MGS (1800W, 90 sec, 100 or 200 mL water in ‘sterilizer’) against S. aureus as a test microorganism. S. aureus should be less susceptible to chemical and physical microbicidal processes than enveloped viruses including SARS-CoV-2, although it might be more susceptible to these processes than other microorganisms including mycobacteria [1]. If other microorganisms, such as mycobacteria, were a concern, these should be tested.
We found that MGS (1800 W, 90 s, 100 or 200 mL water in ‘sterilizer’) and dry heat (70°C for 90 min) were both effective in decontaminating surgical masks and respirators. Whilst dry heat was not found to negatively impact function of PPE or face masks, MGS was incompatible with surgical masks and some models of respirator. The Kimberly–Clark FFP2/N95-type respirators used in this study were able to be reprocessed with either method and could be effectively disinfected within 90 s. In addition, Type-II surgical face masks and FFP2/N95-type respirators were able to be reprocessed up to three times without negatively impacting their function. The addition of lemon-scented essential oil to the MGS process was not found to negatively impact on NaCl filtration efficiency, indicating that such oils may be added to a microwave steam sterilizer to impart a fresh fragrance on to the PPE if desired. It might not however cover strong malodours. Dry heat reprocessing is less manually intensive and may be more easily scaled-up to high-volume reprocessing workflows. A summary of the advantages and disadvantages of each method are included in Table IV .
Table IV.
Overview of advantages and disadvantages of dry heat and microwave-generated steam (MGS) decontamination methods
| Advantages | Disadvantages | |
|---|---|---|
| Dry heat | Can be used on both surgical masks and N95-type respirators | Slower disinfection rate compared to MGS |
| Likely to be compatible with all PPE types | Lower efficacy in field trials on used PPE | |
| Large dry-heat sources available with capacity to reprocess many PPE at once | May not eliminate malodour | |
| Microwave-generated steam | Very rapid disinfection rate | More labour intensive due to manual loading of sterilizer and microwave |
| Greater efficacy in field trials may be due to broader spectrum of activity | Incompatible with surgical masks | |
| Essential oils can be added to impart fragrance and avoid PPE smells associated with past users | Incompatible with some respirator models∗ |
PPE, personal protective equipment.
Wire nose-bridges may cause arcing damage to mask and some adhesives degraded by steam.
It should be noted that the bacterial and saline filtration tests used in this study were based on, but were not identical to, the BS EN14683:2019 [9] and BS En149:2001+A1:2009 [10] standard tests. Standard tests might give different results; in particular the particle counting method used in the saline filtration test weights the relative contribution of different size particles differently from the spectrophotometric method used in EN149:2001+A1:2009, which measures the mass of NaCl rather than the number of particles and therefore gives greater emphasis to larger particles. In addition, while it might be surprising that the NaCl filtration of reprocessed surgical masks was not affected by MGS, bacterial filtration was. It is important to understand that the filter does not just act as a sieve which would allow smaller particles through while stopping larger ones, but captures nanoparticles by electrostatic interaction, and this mechanism could still operate even if bacteria were able to penetrate the material.
It is also important to note that other PPE brands might respond differently to dry heat and MGS processes, and that different microwave ovens would need to be validated; in particular, domestic microwave ovens typically have much lower power (600 W) and use rotating turntables rather than rotating antenna, thus a longer exposure time would be needed and they might not achieve the same results. Here, we observed that the use of an industrial-grade 2.45-GHz microwave oven at an 1800-W setting for 90 s with 100 mL water in the sterilizer was incompatible with some FFP2/N95-type respirators. The deleterious effect of MGS on different types of N95-type respirators has been observed by others [17]. Viscusi et al. [8] reported that the use of a microwave oven with a revolving glass carousel, 1100 W (manufacturer rated; 750 W ft3 experimentally measured) for 2 min total exposure melted the tested N95 type respirator.
In this study, we only tested some aspects of the performance of surgical masks and respirators, and other tests might be needed to ensure that there was no degradation of other aspects such as the fit to the face. It is noted that in these tests only a section of PPE was tested hence the impact of the reprocessing methods on PPE fit, exhalation valves, mask seams, etc., would need to be appraised to confirm that the suggested reprocessing methods do not impact on the overall integrity of a given PPE. However, given the BS EN 149:2001+A1:2009 standard specifies that filtering respirators should be exposed to a thermal cycle (a) for 24 h to a dry atmosphere of 70 ± 3°C; and (b) for 24 h to a temperature of -30 ± 3°C, before being allowed to return to room temperature for at least 4 h, ensuring that no thermal shock occurs, prior to testing, it is supported that the 70°C dry heat method discussed in this paper should not be detrimental.
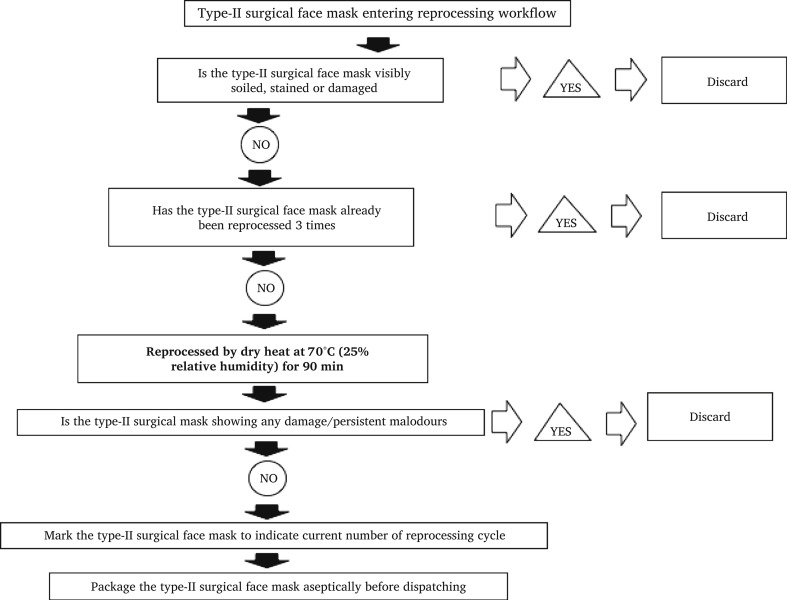
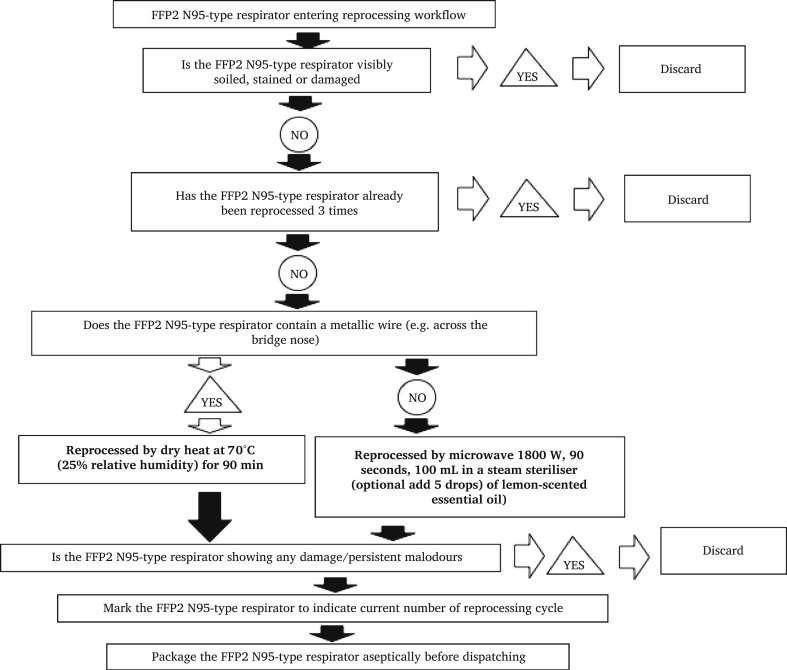
This study was designed to test the efficacy of two methods for the reprocessing of surgical masks and PPE and we are proposing feasible workflows for the reprocessing of Type-II surgical face masks (Figure 6 ) and N95-type respirators (Figure 7 ). Were these methods to be considered, information on handling and logistics as well as oven or MGS capacity would need to be addressed.
Figure 6.
Proposed workflow for Type-II surgical face masks reprocessing.
Figure 7.
Proposed workflow for FFP2/N95-type respirator reprocessing.
In conclusion, we found that MGS (industrial-grade 2.45 GHz microwave oven; 1800 W, 90 s, 100 or 200 mL water in a ‘sterilizer’) was potentially effective in decontaminating some types of FFP2/N95-type respirators, whilst dry heat (70°C for 90 min) was potentially effective for the reprocessing of either N95-type respirators or Type-II surgical face masks, providing possible safe reprocessing methods should the procurement of unused PPE fail.
Acknowledgements
The authors wish to thank Public Health Wales for kindly donating FFP2/N95-type respirator and Hardshell UK Limited and the School of Medicine, Cardiff University, for donating Type-II surgical face masks. We would also like to acknowledge the European Union Aviation Safety Agency (EASA), for the loan of components of the European nvPM mobile reference system under contract EASA.2015.C01.AM01.
Conflict of interest statement
None declared.
Funding
Staff conducting the NaCl filtration efficiency testing were part-funded by the H2020-EU.3.4. AVIATOR project under grant agreement number 814801.
References
- 1.Maillard J.-Y., McDonnell G. Selection and use of disinfectants. In Practice. 2012;34:292–299. [Google Scholar]
- 2.Maillard J.-Y., Sattar S., Pinto F. Virucidal activity of biocides. In: Fraise A.P., Maillard J.-Y., Sattar S., editors. Principles and practice of disinfection, preservation and sterilization. 5th edition. Blackwell Science; London: 2013. [Google Scholar]
- 3.Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Donskey C.J. It's not the heat, it's the humidity: effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;48:854–855. doi: 10.1016/j.ajic.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Straten B., de Man P., van den Dobbelsteen J., Koeleman H., van der Eijk A., Horeman T. Sterilization of disposable face masks by means of standardized dry and steam sterilization processes; an alternative in the fight against mask shortages due to COVID-19. J Hosp Infect. 2020;105:356–357. doi: 10.1016/j.jhin.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng V.C.C., Wong S.-C., Kwan G.S.W., Hui W.-T., Yuen K.-Y. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hops Infect. 2020;105:358–359. doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card K.J., Crozier D., Dhawan A., Dinh M., Dolson E., Farrokhian N. UV Sterilization of Personal Protective Equipment with Idle Laboratory Biosafety Cabinets During the Covid-19 Pandemic. MedRxiv. 2020 doi: 10.1101/2020.03.25.20043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price J.R., Cole K., Bexley A., Kostiou V., Eyre D.W., Golubchik T. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis. 2017;17:207–214. doi: 10.1016/S1473-3099(16)30413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viscusi D.I., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Annals Occupat Hygiene. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.BS EN14683 . The British Standards Institution; London: 2019. Medical face masks. Requirements and test methods. [Google Scholar]
- 10.BS EN 149:2001+A1:2009 . The British Standards Institution; London: 2020. Respiratory protective devices. Filtering half masks to protect against particles. Requirements, testing, marking. [Google Scholar]
- 11.Durand E., Crayford A.P., Johnson M. Experimental validation of thermophoretic and bend nanoparticle loss for a regulatory prescribed aircraft nvPM sampling system. Aerosol Sci Technol. 2020 doi: 10.1080/02786826.2020.1756212. [DOI] [Google Scholar]
- 12.Fischer R., Morris D.H., van Doremalen N., Sarchette S., Matson J., Bushmaker T. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. MedRxiv. 2020 doi: 10.1101/2020.04.11.20062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., Hinrichs S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56(1):92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 14.Heimbuch B.K., Wallace W.H., Kinney K.R., Lumley A.E., Wu C.Y., Woo M.-H. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:1–9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Fisher E., Rengasamy S., Viscusi D., Vo E., Shaffer R. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Appl Environ Microbiol. 2009;75:1500–1507. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher E.M., Williams J.L., Shaffer R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS One. 2011;15(4) doi: 10.1371/journal.pone.0018585. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R., Shaffer R.E. Evaluation of multiple (3-Cycle) decontamination processing for filtering Facepiece respirators. JEFF. 2010;4:33–41. [Google Scholar]