Abstract
The pro-inflammatory cytokine interleukin-1 (IL-1) plays a key role in many physiological processes and during the inflammatory and immune response to most common diseases. IL-1 exists as two agonists, IL-1α and IL-1β that bind to the only signaling IL-1 type 1 receptor (IL-1R1), while a second decoy IL-1 type 2 receptor (IL-1R2) binds both forms of IL-1 without inducing cell signaling. The field of immunology and inflammation research has, over the past 35 years, unraveled many mechanisms of IL-1 actions, through in vitro manipulation of the IL-1 system or by using genetically engineered mouse models that lack either member of the IL-1 family in ubiquitous constitutive manner. However, the limitation of global mouse knockout technology has significantly hampered our understanding of the precise mechanisms of IL-1 actions in animal models of disease. Here we report and review the recent generation of new conditional mouse mutants in which exons of Il1a, Il1b, Il1r1, and Il1r2 genes flanked by loxP sites (fl/fl) can be deleted in cell-/tissue-specific constitutive or inducible manner by Cre recombinase expression. Hence, IL-1αfl/fl, IL-1βfl/fl, IL-1R1fl/fl, and IL-1R2fl/fl mice constitute a new toolbox that will provide a step change in our understanding of the cell-specific role of IL-1 and its receptor in health and disease and the potential development of targeted IL-1 therapies.
Keywords: Inflammation, Immunity, IL-1, IL-1 receptors, Cre/loxP, Conditional deletion
Introduction
Interleukin-1 is a master pro-inflammatory cytokine implicated in a wide range of physiological processes including development [1], regulation of neuroimmune and neuroendocrine functions [2], and central processes such as sleep and memory [3] and plays a key role in the initiation and orchestration of the inflammatory response to most, if not all, pathological inflammatory conditions, including infections and non-communicable diseases such as atherosclerosis or stroke (see [4] for review). The IL-1 family comprises two IL-1 agonists (IL-1α and IL-1β) and the naturally IL-1 receptor antagonist (IL-1Ra) that bind primarily to the IL-1 type 1 receptor (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) leading to cell signaling (reviewed in [5]), while a second IL-1 type 2 receptor (IL-1R2) binds both IL-1 isoforms without inducing cell signaling, acting therefore as a decoy receptor [6].
Since their discovery, IL-1α and IL-1β are believed to have similar and often overlapping biological functions, since they bind to the same receptor inducing similar cell signaling mechanisms. However, there are marked differences in the regulation of expression and mechanisms of actions of these two cytokines. Although both isoforms require enzymatic cleavage for generation of their mature forms, IL-1β is the main secreted isoform, whereas IL-1α remains cytoplasmic but can also be released during cell death or by mechanisms that are different from that of IL-1β (reviewed in [7]). Furthermore, both IL-1α and IL-1β have been thought to exert similar biological activities primarily through binding to IL-1R1. However, several previous studies have demonstrated differential actions of both cytokines in various paradigms of inflammation; for instance, IL-1α and IL-1β exert differential potency at inducing fever when administered exogenously [8], while IL-1α, but not IL-1β, triggers sepsis lethality in mouse [9] and is required for T cell activation during allergen-induced hypersensitivity [10]. Further, IL-1α, but not IL-1β, induces brain cells to generate the LG3 neuroprotective protein fragment of the extracellular matrix component perlecan, a prominent component of the blood-brain barrier [11]. Of interest, polymorphisms in the human Il1a, but not Il1b gene, is associated with higher incidence of vascular malformation and/or higher risk of ischemic stroke [12, 13]. In contrast, IL-1β, but not IL-1α, activates IL-6 expression in neurons [14], selectively mediates the response to vascular injury [15], while IL-1α- and IL-1β-specific actions have also been identified in acute colon inflammation in mice [16]. Taken together, these observations suggest that IL-1α and IL-1β may be differentially expressed during inflammation and may exert non-overlapping ligand-specific differential actions dependent on the disease paradigm.
Mouse genetic models to understand the role of IL-1α and IL-1β in disease
For decades, the field of inflammation research has unraveled key mechanisms of IL-1 actions using traditional global gene targeting knockout technology in animal models. Indeed, IL-1α-deficient (−/−), IL-1β−/−, and IL-1α/β−/− (as well as IL-1Ra−/−) mice generated by Horai and collaborators in 1998 [17] have proven useful to identify some selective mechanisms of actions of both isoforms in some pathological conditions. In those genetic models, disruption of the Il1a and Il1b genes was achieved by deletion of the NH2-terminal coding region for mature IL-1α (exon 5–intron 5) and IL-1β (exon 3–5), leading to ubiquitous constitutive inhibition of expression of either genes. These genetic models have been used widely in many disease models and have subsequently led to the identification of some IL-1α- and IL-1β-specific mechanisms as described above. Further, IL-1R1−/− mice, originally generated by Immunex by targeted deletion of exon 1 and 2 of the Il1r1 gene [18], showed that most, but not all, IL-1 actions are mediated by IL-1R1 (see [19] for review). Indeed, studies using IL-1R1−/− mice in animal models of gut infection with helminth Trichuris muris [20] and experimental stroke [21] found that IL-1β can function in an IL-1R1-independent manner, while IL-1β exacerbates neuronal apoptosis caused by status epilepticus through a mechanism independent of IL-1R1 [22]. Further, some neuroprotective actions of IL-1 are believed to be triggered independently of IL-1R1 via activation of the neuroprotective PI3K/Akt signaling pathway [23], while we have reported IL-1R1-independent IL-1 actions in glial cells [24]. Those IL-1R1-independent actions, primarily observed in the original IL-1R1−/− mice, are known to be mediated through a spliced variant of the Il1r1 gene leading to a truncated IL-1R1 isoform still expressed upon exon 1–2 deletion, due to the activation of an additional internal promoter positioned upstream of exon 1–2 [25]. This truncated isoform of the receptor has been fully characterized and lacks part of the extracellular IL-1 binding region but is still capable of inducing an intracellular signal in response to IL-1 that is known to mediate the neuroprotective actions of IL-1 in the brain via activation of the PI3K/Akt pathways [25]. Ubiquitous Il1rap gene deletion, that encodes IL-1RAcP, has also been achieved in mice, by targeting exon D1 and part of exon D2 that encode the first Ig-like and part of the second Ig-like extracellular domains, resulting in complete inhibition of IL-1 signaling in response to IL-1α and IL-1β [26]. In accordance with the phenotypic responses observed in IL-1R1−/− mice, IL-1RAcP−/− mice show reduced neuroimmune and febrile responses to IL-1 [27, 28]. Finally, IL-1R2−/− mice in which exon 2–4 are deleted using conventional gene targeting method have also been generated [29]. These mice show increased susceptibility to collagen-induced arthritis, while IL-1β-induced cytokine response was enhanced in macrophages. In agreement with its inhibitory function, IL-1Ra−/− mice develop spontaneous autoimmune arthritis [30] and psoriasis-like cutaneous inflammation [31] and show increased brain injury to experimental stroke [32] and atherosclerotic lesion in experimental atherosclerosis [33]. Taken together, these observations demonstrate the complexity of the IL-1 system and point to important, yet undiscovered, mechanisms of actions of IL-1 ligands and their receptors, which cannot be explored by using classical pharmacological or genetic approaches.
Generation of a new toolbox to allow cell-specific conditional deletion of IL-1 ligands and their receptors
Germline gene deletion in mice has yielded important discoveries regarding the role of IL-1 ligands and their receptors in various inflammatory paradigms. However, this approach has important limitations such as viability and fertility of progeny, subtle phenotypic changes, and/or compensatory mechanisms that may alter steady-state immune responses. In relation to the IL-1 system, IL-1α−/−, IL-1β−/−, IL-1R1−/−, IL-1RAcP−/−, and IL-1R2−/− mice have all been reported to be viable with no obvious altered fertility. However, some reports suggested that IL-1 regulates ovulation, oocyte maturation, and early embryonic development [34, 35], which could lead to long-term significant phenotypic changes. Indeed, normal bone growth and remodeling are altered in IL-1R1−/− and IL-1α/β−/− mice [36, 37], whereas decrease body fat mass is reduced in IL-1Ra−/− mice [38], strongly suggesting that significant phenotypic changes occur after ubiquitous deletion of IL-1 family members. Finally, significant compensatory changes are known to occur after ubiquitous gene deletion, and microarray analysis demonstrated that expression of several genes is altered in IL-1R1−/− mice [24]. To overcome these limitations, the Cre/loxP system that allows selective/conditional deletion of targeted genes was recently used to generate new mouse mutant lines to allow cell-specific conditional deletion of IL-1 ligands and its receptors in a Cre recombinase-dependent manner (loxP-flanked, abbreviated as fl/fl). To this end, we have recently reported the generation and characterization of new IL-1αfl/fl and IL-1βfl/fl mouse lines [39, 40] generated from Il1atm1a(EUCOMM)Wtsi (clone EPD0822-4-H02) or Il1btm1a(EUCOMM)Hmgu (clone HEPD0840-8-E03) embryonic stem cells purchased from the European Mouse Mutant Cell Repository (EuMMCR). The full description of the gene targeting strategies for both IL-1αfl/fl and IL-1βfl/fl mice as well as experimental procedure from initial culturing and microinjection of ES cells leading to the generation of mice allowing for the conditional deletion of IL-1α and IL-β are published [39, 40]. In these new alleles, exon 4 of the Il1a gene (for IL-1αfl/fl mice) or exon 4–5 of the Il1b gene (for IL-1βfl/fl mice) flanked with loxP sites can be deleted by Cre recombinase, leading to exon 4 or 4–5 deletion and generation of cell-specific IL-1α and IL-1β-deficient allele, respectively (Fig. 1A and B).
Fig. 1.
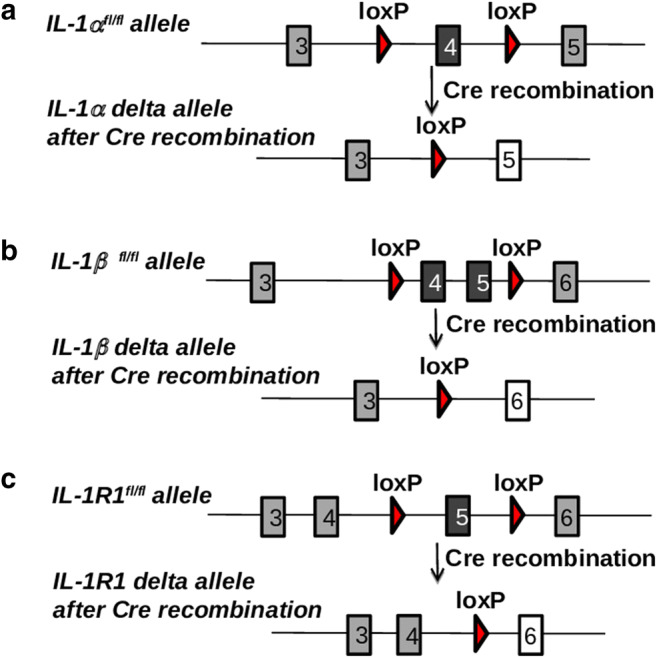
Generation of IL-1αfl/fl, IL-1βfl/fl, and IL-1R1fl/fl mice. A Exon 4 of the Il1a gene (for IL-1α fl/fl), B exon 4–5 of the Il1b gene (for IL-1β fl/fl mice), or C exon 5 of the Il1r1 gene (for IL-1R1fl/fl) flanked with loxP sites is excised upon Cre recombination, resulting in cell-specific IL-1α-, IL-1β-, or IL-1R1-deficient allele, respectively
Recently, two new mouse mutants, allowing for the conditional deletion of Il1r1 (IL-1R1fl/fl), have been described. Robson and collaborators [41] have generated a new IL-1R1fl/fl, in which exon 3–4 of the Il1r1 gene (encoding part of the extracellular binding region) can be deleted by Cre recombination and demonstrated ubiquitous inhibition of IL-1R1 signaling by the crossing of the conditional allele to the CMV-Cre mice, which mediated recombination in early embryogenesis. Concomitantly, we have generated a new IL-1R1fl/fl mouse (developed by Taconic, Cologne, Germany), in which exon 5 that also encodes part of the extracellular binding region of the receptor is flanked by LoxP sites [42] (Fig. 1C). In those two new IL-1R1 mutants, deletion of exon 3–4 or exon 5 inactivates the two previously described functional IL-1R1 gene transcripts (including the full-length IL-1R1 and truncated IL-1R3) upon Cre- mediated recombination [25]. In our study, we have also reported the generation of a new ubiquitous IL-1R1−/− mouse as well as myeloid cell-specific IL-1R1-deficient mice by crossing IL-1R1fl/fl with mice expressing Cre recombinase under the promoter of keratin 14 (K14-Cre) and the Vav promoter, respectively [42]. Of importance, an advanced genetic tool based on restoration of Il1r1 gene expression has been developed by Liu and collaborators [43]. In this advanced model, excision of a disruptive intronic sequence in the Il1r1 gene under Cre recombination in a global IL-1R1−/− background allows functional restoration of IL-1 signaling under cell-specific promoters and has been important in the discovery of mechanisms of IL-1 signaling in the brain in the broad context IL-1-driven central inflammation [44]. Finally, generation of IL-1R2fl/fl targeting exon 3 of the Il1r2 gene was also reported [45], and we now report in our hand the generation of a similar IL-1R2fl/fl mouse that targets exon 3 of Il1r2 gene and further generation of IL-1R2−/− by crossing IL-1R2fl/fl with mice expressing Cre recombinase under the promoter of keratin 14 (K14-Cre) (Fig. 2A).
Fig. 2.
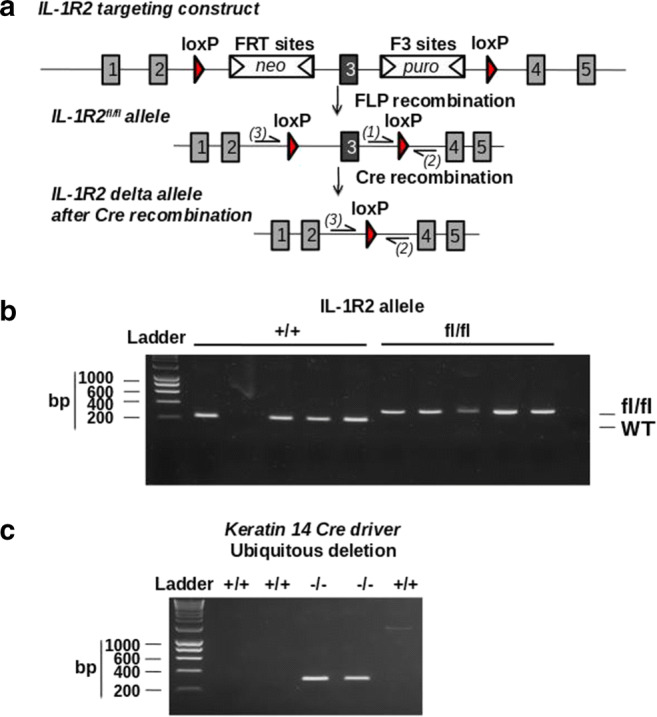
Generation of IL-1R2fl/fl and IL-1R2−/− mice. A Genetic approach to generate IL-1R2fl/fl mice was designed to induce deletion of exon 3 encoding part of the extracellular binding domain, generating a frameshift from exon 4 to all downstream exons leading to genetic inhibition of IL-1R2. B Genotyping identification of IL-1R2fl/fl mice was carried out by PCR using the following primers: Forward, TGTCTCCATCAGACTGACTTTAGG, depicted (1), and reverse, ACCATGTCTGCCTGTTCACC, depicted (2) on genomic DNA. Amplification product size obtained was as follows: wild type (228 bp) and IL-1R2fl/fl (347 bp). C Genotypic identification of exon 3 deletion in IL-1R2−/− mice (obtained by crossing IL-1R2fl/fl mice with mice expressing Cre recombinase under a keratin 14 promoter) was carried out by PCR on isolated genomic DNA using the following primers: Forward, GTAGTGGGCAATCAGATGGAC, depicted (3), and reverse, ACCATGTCTGCCTGTTCACC, depicted (2). Amplification product size obtained was 300 bp in the IL-1R2−/− mice after Cre recombination
Generation of IL-1R2fl/fl and IL-1R2−/− mice
IL-1R2 conditional (IL-1R2fl/fl) mice were generated at Taconic (Cologne, Germany) by gene targeting using BAC clones as the targeting vector from the C57BL/6J RPCI-23 BAC library encoding two loxP sites flanked exon 3 of the Il1r2 gene and subsequent homologous recombination in C57BL/6 N embryonic stem (ES) cells. From targeted C57BL/6 N ES cells, as verified by southern blotting, chimeric mice were generated and bred to C57BL/6 females. Germline transmission was identified by genotyping PCR sample analysis using a Caliper LabChip GX device (details are available upon request). Genotyping identification of IL-1R2fl/fl mice was carried out on genomic DNA by PCR (see Fig. 2B) (details of primers used in Fig. 2 legend and protocol of DNA amplification available upon request). Amplification product size obtained were as follows: wild type (228 bp) and IL-1R2fl/fl (347 bp).
A new ubiquitous IL-1R2−/− mouse was generated by crossing the IL-1R2fl/fl mice with mice expressing Cre recombinase under the control of the human keratin 14 promoter in oocytes as described [46], leading to genetic deletion of exon 3 in all tissues. The deletion of exon 3 causes a frame shift from exon 4 to all downstream exons. Genotypic identification of exon 3 deletion in IL-1R2−/− mice was carried out by PCR on isolated genomic DNA (see Fig. 2C) (details of primers used in figure legend and protocol of DNA amplification available upon request).
Advanced discoveries in mechanisms of IL-1 actions in disease using the toolbox
The new toolbox comprising IL-1αfl/fl, IL-1βfl/fl, IL-1R1fl/fl, and IL-1R2fl/fl mice allows for the first time the generation of new mouse lines in which IL-1 and its receptors can be deleted in a cell/tissue specific manner. To date, IL-1αfl/fl, IL-1βfl/fl, IL-1R1fl/fl, and IL-1R2fl/fl mice have been crossed with specific Cre drivers leading to cell- or tissue-specific deletion of either gene in a constitutive (Cre) or inducible (Cre-ER) manner, revealing new mechanisms of IL-1 actions. Table 1 provides a list of the cell-/tissue-specific deficient mice in IL-1α, IL-1β, IL-1R1, and IL-1R2 generated to date that have been tested under different inflammatory paradigms. While the generation of cell-specific IL-1α and IL-1β-deficient lines is limited, due to the recent generation of IL-1αfl/fl and IL-1βfl/fl mice, the first studies using those models have demonstrated the critical role of microglial IL-1β in the establishment of pain in the context of complex regional pain syndrome [39], while cardiomyocyte-derived IL-1α has been found not to contribute to tissue remodeling during myocardial infarction [40]. In contrast, IL-1R1fl/fl mice have generated various cell-/tissue-specific IL-1R1-deficient lines, most studies showing a critical role for IL-1 signaling in immune cell activation and vascular activation in various models of peripheral infection and chronic inflammation (see Table 1). For instance, IL-1 signaling in cells of the hematopoietic lineage is required for the IL-17 and IL-22 response to gut infection by the nematode Trichuris Muris [42], whereas IL-1 signaling in T cells [50] and in GM-CSF producing cells [52] plays a critical role in experimental autoimmune encephalomyelitis. Furthermore, IL-1 signaling in T cells plays a key role in the systemic immune response to injection of CD3 antibodies [49]. Inhibition of IL-1 signaling by ColVI-Cre driver in intestinal mesenchymal cells showed that IL-1R1 in these cells has no important role in the development of intestinal carcinogenesis [60]. Furthermore, no direct role of IL-1 signaling in CD45+ hematopoietic was found in IL-1-mediated resistance to Mycobacterium tuberculosis [62]. In contrast, IL-1R1 on hepatocytes reduces liver injury in a model of acute liver failure [61], while deletion of IL-1R1 in pancreatic cells alters glucose homeostasis and triggers β-cell de-differentiation [57]. Finally, a study using Ly6G-Cre mice found that IL-1R1 in neutrophils plays a key role in reducing the tumorigenic effects of IL-1 [51]. In peripheral vascular beds, cadherin-Cre mediated IL-1R1 deletion in endothelial cells contributes to the anti-tumor function of adoptively transferred T cells regulated by IL-1β [63], whereas IL-1R1 signaling in smooth muscle cells contributes to the atheroprotective effect of IL-1 in advanced atherosclerotic lesions [56].
Table 1.
List of cell-/organ-specific deficient mice for IL-1 isoforms or their receptors and main effects observed
| Gene | Cre driver | Cell/tissue targeted | Main effects | References |
|---|---|---|---|---|
| Il1a | Myh6-Cre | Cardiomyocytes | No effect on cardiac tissue remodeling after MI | Bageghni et al. [40] |
| Il1b | CMV-Cre | Ubiquitous deletion | Reduces bone metastasis during breast cancer | Tulotta et al. [47] |
| CX3CR1-CreER | Microglial cell | Reduces the development of pain | [39] | |
| Il1r1 | K14-Cre | Ubiquitous deletion | Mediates peripheral immune response to T. Muris infection | [42] |
| Inhibits melanoma inflammatory niche | Young et al. [48] | |||
| CMV-Cre | Ubiquitous deletion | Decreases inflammatory responses to systemic challenges | Mufazalov et al. [49]; Mufazalov et al. [50] | |
| NI | Robson et al. [41] | |||
| PGK-Cre | Ubiquitous deletion | Regulates cardiac tissue remodeling after MI | Bageghni et al. [40] | |
| Col1a2-CreER | Fibroblasts | Regulates cardiac tissue remodeling after MI | Bageghni et al. [40] | |
| CD4-Cre | T cells | Regulates immune response to CD3 antibody injection | Mufazalov et al. [49] | |
| Regulates neuroinflammation in EAE | Mufazalov et al. [50] | |||
| Vav-Cre | Myeloid cells | Mediates peripheral immune response to T. Muris infection | Adbulaal et al. [42] | |
| Ly6G-Cre | Neutrophils | Reduces the tumorigenic effect of IL-1 | Dmitrieva-Posocco et al. [51] | |
| Csf2-Cre | GM-CSF positive cells | Regulates inflammation after EAE | Komuczki et al. [52] | |
| CX3CR1-CreER | Microglial cells | Reduces renewal of microglial population | Bruttger et al. [53] | |
| Regulates microglial activation after CNS inflammation | Zhu et al. [54]* | |||
| No effect on febrile response to IL-1 | Knoll et al. [55]* | |||
| Myh11-CreER | Smooth muscle cells | Reduces atheroprotective effect of IL-1 after atherosclerosis | Gomez et al. [56] | |
| Pdx1-Cre | Pancreatic cells | Alters glucose homeostasis and triggers β-cell de-differentiation | Burke et al. [57] | |
| Slco1c1-CreER | Brain endothelial cells | Reduces CNS inflammation and brain damage after stroke | Wong et al. [58] | |
| Reduces fever response to IL-1 | Matsuwaki et al. [59] | |||
| Nestin-Cre | Neuronal cells | No effect on febrile response to IL-1 | Matsuwaki et al. [59] | |
| Reduces brain damage after stroke | Wong et al. [58] | |||
| Trpv1-Cre | Nociceptor sensory neurons | No effect on febrile response to IL-1 | Matsuwaki et al. [59] | |
| htPA-Cre | Neural crest cells | No effect on febrile response to IL-1 | Matsuwaki et al. [59] | |
| ChAT-Cre | Catecholaminergic neurons | Decreases brain damage after stroke | Wong et al. [58] | |
| PF4-Cre | Platelets | No effect on brain damage after stroke | Wong et al. [58] | |
| ColVI-CreER | Intestinal mesenchymal cells | No effect on development of intestinal cancer | Koliaraki et al. [60] | |
| Hep-Cre | Hepatocytes | Reduces liver injury after acute liver failure | Gehrke et al. [61] | |
| CD45-Cre | Leukocytes | No role in IL-1-mediated resistance to Mycobacterium tuberculosis | Bohrer et al. [62] | |
| Cdh5-Cre | Vascular endothelial cells | Mediates the anti-tumor properties of T cells. Regulates IL-1-induced brain inflammation | Lee et al. [63] | |
| Tie2-Cre | Endothelial cells | Decreases IL-1-induced brain inflammation | Liu et al. [44]# | |
| Il1r2 | CMV-Cre | Ubiquitous deletion | Reduces inflammation after arthritis | Martin et al. [45] |
| K14-Cre | Ubiquitous deletion | NI | Unpublished** |
For IL-1R1fl/fl mice, all cell-/tissue-specific IL-1R1−/− mice have been generated by IL-1R1fl/fl mouse from Abdulaal et al. [42], except those marked (*), generated by IL-1R1fl/fl mouse from Robson et al. [41]. #In the study of Liu and collaborators (2019), the following mouse lines have also been generated: IL-1R1fl/fl x LysM-Cre, IL-1R1fl/fl x CX3CR1-Cre, IL-1R1fl/fl x Camk2a-Cre, IL-1R1fl/fl x Vglut2-Cre, and IL-1R1fl/fl x GFAP-Cre. **IL-1R2fl/fl mice crossed with K14-Cre mice are reported in the present publication. Cre, constitutive deletion by Cre drivers; Cre-ER, inducible deletion by Cre-ER drivers; EAE, experimental autoimmune encephalomyelitis; MI, myocardial infarction; NI, not indicated
In the brain, IL-1 signaling in microglia is required for the renewal properties of microglial progenitor cells [53]. Brain endothelial IL-1R1 is essential in the initiation of the fever response elicited by IL-1, whereas deletion of IL-1R1 on central or peripheral neurons (including catecholaminergic neurons and nociceptor sensory neurons) had no noticeable effect on the febrile response [59]. An interesting work by Knoll and collaborators [55] confirmed that endothelial IL-1R1 signaling is critical in the establishment of the febrile response to IL-1, whereas IL-1R1 signaling in microglia of the brain parenchyma has no role. Further, endothelial IL-1R1 is essential for endothelial activation in the context of IL-1-driven brain inflammation [44] and a further study using microglial-specific IL-1R1-deficient mice showed that IL-1 actions on brain endothelia triggers the production of endothelial-derived factors that are able to activate microglial cells [54]. In the context of stroke, brain endothelial IL-1R1, but also neuronal IL-1R1, is critical in mechanisms of cerebrovascular inflammation and brain damage after experimental cerebral ischemia, whereas no involvement of IL-1 signaling in peripheral cells, including platelets on stroke outcome was observed [58].
Finally, little work has been conducted regarding IL-1R2fl/fl mice, and to date, only ubiquitous constitutive deletion of IL-1R2 (IL-1R2−/−) has been achieved, including that of our work. The only work reporting the use of IL-1R2−/− in disease is that of Martin and collaborators [45], who demonstrated that IL-1R2 deletion plays an important inhibitory role on IL-1-regulated inflammation in a model of arthritis, in accordance with its known inhibitory function, as recently reviewed [64].
Concluding remarks and future directions
IL-1 is a key cytokine regulating many physiological processes as well as the inflammatory responses to infection or injury, and global constitutive IL-1-deficient mouse models, which is a fairly recent approach, have to date helped unraveling key mechanisms of IL-1 actions in disease but have significant limitations. The recent generation of new mouse mutants allowing conditional deletion of IL-1 and its receptors has led to the discovery of unexpected new mechanisms of inflammation regulated by IL-1. To date, a limited number of cell-/tissue-specific IL-1-deficient mice have been generated, and this is mainly due to the fact that IL-1αfl/fl, IL-1βfl/fl, IL-1R1fl/fl, and IL-1R2fl/fl mice have only been recently produced. However, several projects targeting the IL-1 system in other cells/tissues are currently ongoing. Importantly, to the best of our knowledge, IL-1Rafl/fl and IL-1RAcPfl/fl mice have not been generated yet, and future generation of new lines in which all IL-1 ligands and their receptors can be targeted in other cell/tissue and other disease models will lead to new mechanisms to be discovered, providing a step change in our understanding of IL-1 actions disease and the potential development of new targeted IL-1 therapies.
Acknowledgements
The authors would like to thank the Transgenic Facility of the University of Manchester and Dr. Elena Redondo-Castro for helping with the culturing of ES cells for the generation of IL-1αfl/fl and IL-1βfl/fl mice.
Funding information
E.P. and S.F. received funding from the British Heart Foundation (BHF), grant PG/13/55/30365 for the generation of IL-1α fl/fl and IL-1β fl/fl mice. A.W is a member of the Research Center for Immunotherapy (FZI) Mainz and was supported by the Deutsche Forschungsgemeinschaft (DFG) grants CRC/TRR128 and CRC1292. W.A. received funding from King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia. W.M. received funding from the European Union (FP7/2012–2017) under grant agreement n° 305,564, SysmedIBD. I.A.M. received intramural funding (Stufe1) provided by the University of Mainz.
Compliance with ethical standards
Conflict interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Werner Müller and Ari Waisman are joint senior authors
References
- 1.Blomberg L, Hashizume K, Viebahn C. Blastocyst elongation, trophoblastic differentiation, and embryonic pattern formation. Reproduction. 2008;135:181–195. doi: 10.1530/REP-07-0355. [DOI] [PubMed] [Google Scholar]
- 2.Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013;65:1655–1662. doi: 10.1016/s1734-1140(13)71527-5. [DOI] [PubMed] [Google Scholar]
- 3.Marshall L, Born J. Brain-immune interactions in sleep. Int Rev Neurobiol. 2002;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello CA. Introduction to the interleukin-1 family of cytokines and receptors: drivers of innate inflammation and acquired immunity. Immunol Rev. 2018;281:5–7. doi: 10.1111/imr.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahan CJ, Slack JL, Mosley B, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brough D, Denes A. Interleukin-1α and brain inflammation. IUBMB Life. 2015;67:323–330. doi: 10.1002/iub.1377. [DOI] [PubMed] [Google Scholar]
- 8.Anforth HR, Bluthe RM, Bristow A, et al. Biological activity and brain actions of recombinant rat interleukin-1alpha and interleukin-1beta. Eur Cytokine Netw. 1998;9:279–288. [PubMed] [Google Scholar]
- 9.Benjamin JT, Moore DJ, Bennett C, van der Meer R, Royce A, Loveland R, Wynn JL. Cutting edge: IL-1α and not IL-1β drives IL-1R1-dependent neonatal murine sepsis lethality. J Immunol. 2018;201:2873–2878. doi: 10.4049/jimmunol.1801089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakae S, Naruse-Nakajima C, Sudo K, et al. IL-1 alpha, but not IL-1 beta, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int Immunol. 2001;13:1471–1478. doi: 10.1093/intimm/13.12.1471. [DOI] [PubMed] [Google Scholar]
- 11.Saini MG, Pinteaux E, Lee B, Bix GJ. Oxygen-glucose deprivation and interleukin-1α trigger the release of perlecan LG3 by cells of neurovascular unit. J Neurochem. 2011;119:760–771. doi: 10.1111/j.1471-4159.2011.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Um JY, Moon KS, Lee KM, Yun JM, Cho KH, Moon BS, Kim HM. Association of interleukin-1 alpha gene polymorphism with cerebral infarction. Mol Brain Res. 2003;115:50–54. doi: 10.1016/s0169-328x(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 13.Um JY, Moon KS, Lee KM, Kim HM. Interleukin-1 gene cluster polymorphisms in cerebral infarction. Cytokine. 2003;23:41–46. doi: 10.1016/s1043-4666(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Differential effects of interleukin-1 alpha and beta on interleukin-6 and chemokine synthesis in neurones. Mol Cell Neurosci. 2008;38:259–265. doi: 10.1016/j.mcn.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, Francis S. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–1403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA, Apte RN, Voronov E. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 17.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaccum MB, Stocking KL, Charrier K, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 19.Boutin H, Kimber I, Rothwell NJ, Pinteaux E. The expanding interleukin-1 family and its receptors: do alternative IL-1 receptor/signaling pathways exist in the brain? Mol Neurobiol. 2003;27:239–248. doi: 10.1385/MN:27:3:239. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys NE, Grencis RK. IL-1-dependent, IL-1R1-independent resistance to gastrointestinal nematodes. Eur J Immunol. 2009;39:1036–1045. doi: 10.1002/eji.200838938. [DOI] [PubMed] [Google Scholar]
- 21.Touzani O, Boutin H, Lefeuvre R, et al. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor. J Neurosci. 2002;22:38–43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rincón-López C, Tlapa-Pale A, Medel-Matus J-S, Martínez-Quiroz J, Rodríguez-Landa JF, López-Meraz ML. Interleukin-1β increases neuronal death in the hippocampal dentate gyrus associated with status epilepticus in the developing rat. Neurologia. 2017;32:587–594. doi: 10.1016/j.nrl.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Diem R, Hobom M, Grötsch P, Kramer B, Bähr M. Interleukin-1β protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Mol Cell Neurosci. 2003;22:487–500. doi: 10.1016/s1044-7431(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 24.Andre R, Moggs JG, Kimber I, Rothwell NJ, Pinteaux E. Gene regulation by IL-1beta independent of IL-1R1 in the mouse brain. Glia. 2006;53:477–483. doi: 10.1002/glia.20302. [DOI] [PubMed] [Google Scholar]
- 25.Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F, Herness S, Quan N. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc Natl Acad Sci U S A. 2012;109:12189–12194. doi: 10.1073/pnas.1205207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cullinan EB, Kwee L, Nunes P, et al. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol. 1998;161:5614–5620. [PubMed] [Google Scholar]
- 27.Liège S, Layé S, Li KS, Moze E, Neveu PJ. Interleukin 1 receptor accessory protein (IL-1RAcP) is necessary for centrally mediated neuroendocrine and immune responses to IL-1β. J Neuroimmunol. 2000;110:134–139. doi: 10.1016/s0165-5728(00)00331-3. [DOI] [PubMed] [Google Scholar]
- 28.Zetterström M, Lundkvist J, Malinowsky D, Eriksson G, Bartfai T. Interleukin-1-mediated febrile responses in mice and interleukin-1 beta activation of NFκB in mouse primary astrocytes, involves the interleukin-1 receptor accessory protein. Eur Cytokine Netw. 1998;9:131–138. [PubMed] [Google Scholar]
- 29.Shimizu K, Nakajima A, Sudo K, Liu Y, Mizoroki A, Ikarashi T, Horai R, Kakuta S, Watanabe T, Iwakura Y. IL-1 receptor type 2 suppresses collagen-induced arthritis by inhibiting IL-1 signal on macrophages. J Immunol. 2015;194:3156–3168. doi: 10.4049/jimmunol.1402155. [DOI] [PubMed] [Google Scholar]
- 30.Akitsu A, Ishigame H, Kakuta S, Chung SH, Ikeda S, Shimizu K, Kubo S, Liu Y, Umemura M, Matsuzaki G, Yoshikai Y, Saijo S, Iwakura Y. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2 + Vγ6 + γδT cells. Nat Commun. 2015;6:7464. doi: 10.1038/ncomms8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd J, Little MC, Nicklin MJH. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J Invest Dermatol. 2004;122:665–669. doi: 10.1111/j.0022-202X.2004.22305.x. [DOI] [PubMed] [Google Scholar]
- 32.Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- 33.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 34.Gérard N, Caillaud M, Martoriati A, et al. The interleuking-1 system and female reproduction. J Endocrinol. 2004;180:203–212. doi: 10.1677/joe.0.1800203. [DOI] [PubMed] [Google Scholar]
- 35.Caillaud M, Duchamp G, Gérard N. In vivo effect of interleukin-1 beta and interleukin-1 RA on oocyte cytoplasmic maturation, ovulation, and early embryonic development in the mare. Reprod Biol Endocrinol. 2005;3:26. doi: 10.1186/1477-7827-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simsa-Maziel S, Zaretsky J, Reich A, Koren Y, Shahar R, Monsonego-Ornan E. IL-1RI participates in normal growth plate development and bone modeling. Am J Physiol Endocrinol Metab. 2013;305:E15–E21. doi: 10.1152/ajpendo.00335.2012. [DOI] [PubMed] [Google Scholar]
- 37.Lee YM, Fujikado N, Manaka H, Yasuda H IY IL-1 plays an important role in the bone metabolism under physiological conditions. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/?term=20679512. Accessed 15 May 2020 [DOI] [PubMed]
- 38.Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJH, Meier CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 39.Helyes Z, Tékus V, Szentes N, Pohóczky K, Botz B, Kiss T, Kemény Á, Környei Z, Tóth K, Lénárt N, Ábrahám H, Pinteaux E, Francis S, Sensi S, Dénes Á, Goebel A. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc Natl Acad Sci U S A. 2019;116:13067–13076. doi: 10.1073/pnas.1820168116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bageghni SA, Hemmings KE, Yuldasheva NY et al (2019) Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI insight 5 [DOI] [PMC free article] [PubMed]
- 41.Robson MJ, Bin ZC, Quinlan MA, et al. Generation and characterization of mice expressing a conditional allele of the interleukin-1 receptor type 1. PLoS One. 2016;11:e0150068. doi: 10.1371/journal.pone.0150068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdulaal WH, Walker CR, Costello R, Redondo-Castro E, Mufazalov IA, Papaemmanouil A, Rothwell NJ, Allan SM, Waisman A, Pinteaux E, Müller W. Characterization of a conditional interleukin-1 receptor 1 mouse mutant using the Cre/LoxP system. Eur J Immunol. 2016;46:912–918. doi: 10.1002/eji.201546075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Yamashita T, Chen Q, et al. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci. 2015;35:2860–2870. h. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Nemeth DP, McKim DB, et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50:317–333.e6. doi: 10.1016/j.immuni.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin P, Palmer G, Rodriguez E, Seemayer CA, Palomo J, Talabot-Ayer D, Gabay C. Deficiency in IL-1 receptor type 2 aggravates K/BxN serum transfer-induced arthritis in mice but has no impact on systemic inflammatory responses. J Immunol. 2017;198:2916–2926. doi: 10.4049/jimmunol.1600855. [DOI] [PubMed] [Google Scholar]
- 46.Hafner M, Wenk J, Nenci A, Pasparakis M, Scharffetter-Kochanek K, Smyth N, Peters T, Kess D, Holtkötter O, Shephard P, Kudlow JE, Smola H, Haase I, Schippers A, Krieg T, Müller W. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis. 2004;38:176–181. doi: 10.1002/gene.20016. [DOI] [PubMed] [Google Scholar]
- 47.Tulotta C, Lefley DV, Freeman K, et al (2019) Endogenous production of IL-1beta by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin Cancer Res 25:2769–2782 [DOI] [PubMed]
- 48.Young HL, Rowling EJ, Bugatt M et al (2017) An adaptive signaling network in melanoma inflammatory niches confers tolerance to MAPK signaling inhibition. J Exp Med 214:1691–1710 [DOI] [PMC free article] [PubMed]
- 49.Mufazalov IA, Regen T, Schelmbauer C, Kuschmann J, Muratova AM, Nikolaev A, Müller W, Pinteaux E, Waisman A. Generation of a novel T cell specific interleukin-1 receptor type 1 conditional knock out mouse reveals intrinsic defects in survival, expansion and cytokine production of CD4 T cells. PLoS One. 2016;11:e0161505. doi: 10.1371/journal.pone.0161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mufazalov IA, Schelmbauer C, Regen T, Kuschmann J, Wanke F, Gabriel LA, Hauptmann J, Müller W, Pinteaux E, Kurschus FC, Waisman A. IL-1 signaling is critical for expansion but not generation of autoreactive GM-CSF+ Th17 cells. EMBO J. 2017;36:102–115. doi: 10.15252/embj.201694615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dmitrieva-Posocco O, Dzutsev A, Posocco DF, et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity. 2019;50:166–180.e7. doi: 10.1016/j.immuni.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komuczki J, Tuzlak S, Friebel E, et al. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1β. Immunity. 2019;50:1289–1304.e6. doi: 10.1016/j.immuni.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Müller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Zhu L, Liu X, Nemeth DP, DiSabato DJ, Witcher KG, Mckim DB, Oliver B, le X, Gorantla G, Berdysz O, Li J, Ramani AD, Chen Z, Wu D, Godbout JP, Quan N. Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav Immun. 2019;81:292–304. doi: 10.1016/j.bbi.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knoll GJ, Krasnow SM, Marks DL (2017) Interleukin-1β signaling in fenestrated capillaries is sufficient to trigger sickness responses in mice. J Neuroinflammation 14: 219 [DOI] [PMC free article] [PubMed]
- 56.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St. Hilaire C, Müller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H, Owens GK. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. doi: 10.1038/s41591-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burke SJ, Batdorf HM, Burk DH, Martin TM, Mendoza T, Stadler K, Alami W, Karlstad MD, Robson MJ, Blakely RD, Mynatt RL, Collier JJ. Pancreatic deletion of the interleukin-1 receptor disrupts whole body glucose homeostasis and promotes islet β-cell de-differentiation. Mol Metab. 2018;14:95–107. doi: 10.1016/j.molmet.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong R, Lénárt N, Hill L, Toms L, Coutts G, Martinecz B, Császár E, Nyiri G, Papaemmanouil A, Waisman A, Müller W, Schwaninger M, Rothwell N, Francis S, Pinteaux E, Denés A, Allan SM. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons. Brain Behav Immun. 2019;76:126–138. doi: 10.1016/j.bbi.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuwaki T, Shionoya K, Ihnatko R, Eskilsson A, Kakuta S, Dufour S, Schwaninger M, Waisman A, Müller W, Pinteaux E, Engblom D, Blomqvist A. Involvement of interleukin-1 type 1 receptors in lipopolysaccharide-induced sickness responses. Brain Behav Immun. 2017;66:165–176. doi: 10.1016/j.bbi.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Koliaraki V, Chalkidi N, Henriques A, et al. Innate sensing through mesenchymal TLR4/MyD88 signals promotes spontaneous intestinal tumorigenesis. Cell Rep. 2019;26:536–545.e4. doi: 10.1016/j.celrep.2018.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gehrke N, Hövelmeyer N, Waisman A, Straub BK, Weinmann-Menke J, Wörns MA, Galle PR, Schattenberg JM. Hepatocyte-specific deletion of IL1-RI attenuates liver injury by blocking IL-1 driven autoinflammation. J Hepatol. 2018;68:986–995. doi: 10.1016/j.jhep.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Bohrer AC, Tocheny C, Assmann M, Ganusov VV, Mayer–Barber KD. Cutting edge: IL-1R1 mediates host resistance to mycobacterium tuberculosis by trans-protection of infected cells. J Immunol. 2018;201:1645–1650. doi: 10.4049/jimmunol.1800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee PH, Yamamoto TN, Gurusamy D, Sukumar M, Yu Z, Hu-Li J, Kawabe T, Gangaplara A, Kishton RJ, Henning AN, Vodnala SK, Germain RN, Paul WE, Restifo NP. Host conditioning with IL-1β improves the antitumor function of adoptively transferred T cells. J Exp Med. 2019;216:2619–2634. doi: 10.1084/jem.20181218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlüter T, Schelmbauer C, Karram K, Mufazalov IA. Regulation of IL-1 signaling by the decoy receptor IL-1R2. J Mol Med (Berl) 2018;96:983–992. doi: 10.1007/s00109-018-1684-z. [DOI] [PubMed] [Google Scholar]


