Abstract
The novel and highly pathogenic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has become a continued focus of global attention due to the serious threat it poses to public health. There are no specific drugs available to combat SARS-CoV-2 infection. Natural products (carolacton, homoharringtonine, emetine, and cepharanthine) and natural product-inspired small molecules (ivermectin, GS-5734, EIDD-2801, and ebselen) are potential anti-SARS-CoV-2 agents that have attracted significant attention due to their broad-spectrum antiviral activities. Here, we review the research on potential landmark anti-SARS-CoV-2 agents, systematically discussing the importance of natural products and natural-product-inspired small molecules in the research and development of safe and effective antiviral agents.
Keywords: COVID-19, SARS-Cov-2, natural products, natural-product-inspired, potential anti-SARSCoV-2 agents
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to be the subject of global attention due to the serious threat it poses to public health (Wang et al., 2020; Zhu et al., 2020). Currently, there are no specific drugs available to combat SARS-CoV-2 (Kupferschmidt and Cohen, 2020). The epidemic is ongoing and, as of 12 June, 2020, the World Health Organization (WHO) reported that there had been 7,410,510 confirmed cases worldwide, including 418,294 deaths (Coronavirus disease (COVID-2019)).
This means that more aggressive trials of drugs to prevent and treat SARS-CoV-2 infection should be intensely pursued across the globe (Kalil, 2020). Therefore, there is an urgent need to identify agents for treating SARS-CoV-2 infection (Li and De Clercq, 2020). As a major source for drugs and drug leads, natural products and natural-product-inspired agents have attracted significant attention, and they have played an integral role in the treatment of many different conditions (Newman and Cragg, 2020). Since the start of the multinational COVID-19 outbreak, significant progress has been made in identifying natural products and natural-product-inspired small molecules that may serve as anti-SARS-CoV-2 drugs. This review systematically discusses the current progress regarding potential anti-SARS-CoV-2 natural products and natural-product-inspired small molecules.
Promising Natural Products for Treating SARS-CoV-2 Infection
Natural products possess tremendous structural diversity and unique chemical diversity, and they continue to serve as excellent starting points for inspiring new drug discovery (Shen, 2015). The history of the modern pharmaceutical industry includes many stories about how natural products profoundly inspired drug discovery (Li and Weng, 2017). With the current technological advances, natural products remain potentially transformative drugs for many health conditions. The growing understanding of efficient antiviral drug development has led to the exploration of natural products as an important tactic for identifying effective COVID-19 treatments.
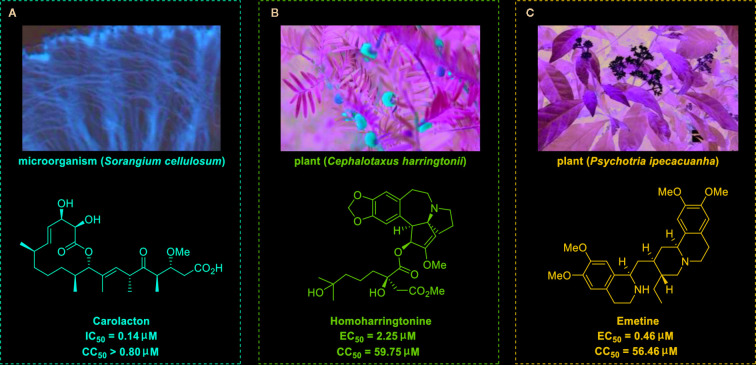
Carolacton, produced by the myxobacterium Sorangium cellulosum, is an antibacterial macrolide keto-carboxylic acid ( Figure 1A ) (Jansen et al., 2010). In vitro, it demonstrates significant bioactivity against the human pathogen Streptococcus mutans by reducing the number of viable cells in biofilms (Fu et al., 2020), and it has been found to be a methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) inhibitor (Anderson et al., 2020). SARS-CoV-2 may have originated in bats (Shi et al., 2020) (as a study has shown that its genome is similar to that of the bat coronavirus RaTG13, with 96.2% identity (Zhou et al., 2020)) and, very recently, Tan’s group demonstrated that MTHFD1 is a critical host factor for the viral RNA replication of a broad spectrum of viruses in both bats and humans (Anderson et al., 2020). Based on in-depth research, Tan’s group demonstrated that MTHFD1 is a potential target for developing anti-SARS-CoV-2 agents and that the MTHFD1 inhibitor carolacton strongly inhibited SARS-CoV-2 replication in Vero cells at a half maximal inhibitory concentration (IC50) of as low as 0.14 μM and a moderate cytotoxicity (50% cytotoxic concentration [CC50] >0.80 μM) (Anderson et al., 2020). Thus, there is hope that this antimicrobial natural product may be useful in the COVID-19 epidemic.
Figure 1.
Promising natural products for treating COVID-19. (A) Carolacton was isolated from the myxobacterium Sorangium cellulosum. (B) Homoharringtonine was isolated from the plant Cephalotaxus harringtonii. (C) Emetine was isolated from the plant Psychotria ipecacuanha.
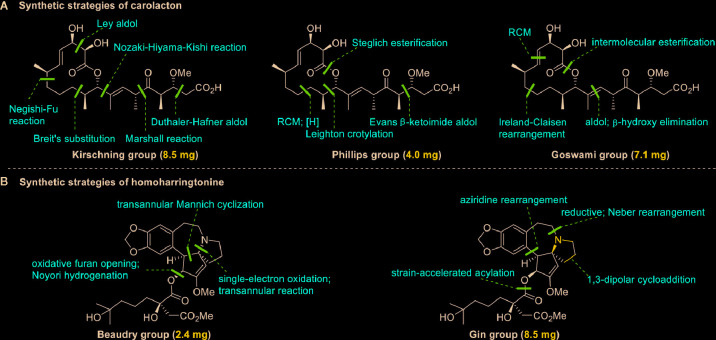
Regarding the total synthesis of carolacton, three examples of milligram-scale processes have been reported. In 2012, Kirschning’s group (Schmidt and Kirschning, 2012) reported on the first total synthesis of carolacton (with an overall yield of 4.3%) on an 8.5-mg scale in 22 linear steps. The key transformations involved asymmetric Nozaki-Hiyama-Kishi cross-coupling and Negishi-Fu coupling as well as the metal-mediated Ley aldol reaction, Duthaler-Hafner aldol reaction, Marshall reaction, and Breit’s substitution. Two years later, Phillips’s group and Wuest’s group (Hallside et al., 2014) collaboratively developed an efficient synthesis of carolacton (with an overall yield of 7.9%) on a 4.0-mg scale in just 14 linear steps. This synthesis process involved ring-closing metathesis (RCM), selective reduction, Leighton crotylation, and Steglich esterification. In 2017, Goswami’s group (Kuilya and Goswami, 2017) reported a third total synthesis (with an impressive overall yield of 18.8%) on a 7.1-mg scale in just 13 linear steps. The key strategies included Urpi acetal aldol reaction, β-hydroxy elimination, intermolecular esterification, and RCM. The three elegant synthesis strategies for producing carolacton are shown in Figure 2A . However, in the current research and clinical contexts, the development of a ton-scale process to synthesize carolacton is urgently needed.
Figure 2.
Key strategies in the synthesis of carolacton and homoharringtonine. (A) Synthetic strategies of carolacton by Kirschning’s group, Phillips’s group and Goswami’s group, respectively. (B) Synthetic strategies of homoharringtonine by Gin’s group and Beaudry’s group, respectively.
Homoharringtonine (omacetaxine) is a natural product that was isolated from the plant Cephalotaxus harringtonii in 1970 (Powell et al., 1970). It was approved by the US Food and Drug Administration (FDA) in 2012 as an effective anti-cancer agent to treat chronic myeloid leukemia ( Figure 1B ) (Mullard, 2013). Additionally, it exhibits broad-spectrum activities against viruses, such as mouse hepatitis virus (MHV) at an IC50 of 0.012 μM (Cao et al., 2015), herpes simplex virus type 1 (HSV-1) at an IC50 of 0.14 μM (Dong et al., 2018), foot and mouth disease virus (FMDV) at an IC50 of 3.05 μM (Gong et al., 2019), and echovirus 1 (EV1) at a half maximal effective concentration (EC50) of 0.12 μM (Andersen et al., 2019). Two elegant milligram-scale total syntheses of homoharringtonine have been reported by Gin’s group (Eckelbarger et al., 2008) and Beaudry’s group (Ju and Beaudry, 2019), respectively ( Figure 2B ). In 2008, Gin’s group reported a novel total synthesis of homoharringtonine on an 8.5-mg scale, using aziridine rearrangement and 1,3-dipolar cycloaddition as key strategies. In 2019, Beaudry’s group developed an excellent total synthesis of homoharringtonine involving 12 linear steps on a 2.4-mg scale. The key strategies were oxidative furan ring opening with spontaneous transannular Mannich reaction as well as the Noyori hydrogenation reaction.
The tetrahydroisoquinoline alkaloid emetine is an older natural product that has potential cardiotoxicity and was isolated from the plant Psychotria ipecacuanha ( Figure 1C ) (Wiegrebe et al., 1984). As a protein synthesis inhibitor, emetine has been widely used in pharmacology (Akinboye et al., 2012). Furthermore, emetine has recently been recognized as a promising broad-spectrum antiviral drug with in vitro activity against multiple viruses, including MHV-A59 at an EC50 of 0.12 μM (Shen et al., 2019), severe acute respiratory syndrome coronavirus (SARS-CoV) at an EC50 of 0.051 μM (Dyall et al., 2014), Middle East respiratory syndrome coronavirus (MERS-CoV) at an EC50 of 0.014 μM (Dyall et al., 2014), and Ebola virus (EBOV) at an IC50 of 0.017 μM (Yang et al., 2018). Very recently, Peterson’s group showed that emetine concentrations could be 300 times higher in the lungs compared to in the blood, and emetine may achieve therapeutic concentrations at viral infection sites, especially in the lungs (Bleasel and Peterson, 2020).
Yen’s group recently revealed that both homoharringtonine and emetine could effectively inhibit the replication of SARS-CoV-2 in Vero E6 cells at an EC50 of 2.55 and 0.46 μM, respectively (Choy et al., 2020). Additionally, the combination of emetine and the C-nucleoside analog GS-5734 exhibited a synergistic inhibitory effect against SARS-CoV-2 replication (Choy et al., 2020). Multiple lines of evidence have shown the potential usefulness of homoharringtonine and emetine as treatments for viral infections, but further research is needed to explore whether they exhibit anti-SARS-CoV-2 activity in vivo.
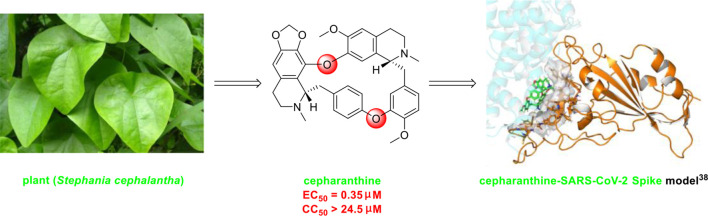
The bisbenzylisoquinoline alkaloid cepharanthine is a natural product isolated from the plant Stephania cephalantha, which is used as a traditional herbal medicine ( Figure 3 ) (Bailly, 2019). Specifically, this approved drug has significant bioactivity against several diseases, with anti-viral, anti-malarial, and anti-cancer effects (Fang et al., 2013). It has IC50 values of 0.026 μM and 9.5 μg/mL against HIV-1 (Okamoto et al., 1998) and SARS-CoV (Zhang et al., 2005), respectively. Furthermore, it dramatically blocked the viral replication in human coronavirus OC43 (HCoV-OC43)-infected MRC-5 human lung cells (with an IC50 of 0.83 μM) and inhibited viral S and N protein expression (Kim et al., 2019). Watashi’s group recently revealed that cepharanthine could effectively inhibit SARS-CoV-2 replication in vitro with an EC50 at 0.35 μM with minimal toxicity (selectivity index >70) (Ohashi et al., 2020). It is worth noting that cepharanthine is an approved drug with a good safety profile, highlighting a new potential role for cepharanthine regarding inhibiting SARS-CoV-2 replication.
Figure 3.
Promising natural product cepharanthine for treating COVID-19 (image reproduced from ref. 38, bioRxiv, doi: 10.1101/2020.04.14.039925).
There are currently no data on drug–drug interactions involving the abovementioned natural products (The liverpool drug interaction group), and close attention should be paid to these interactions during therapeutic use because drug–drug interactions will play significant roles in the safety and effectiveness of anti-COVID-19 agents.
Promising Natural Product-Inspired Agents for Treating SARS-CoV-2 Infection
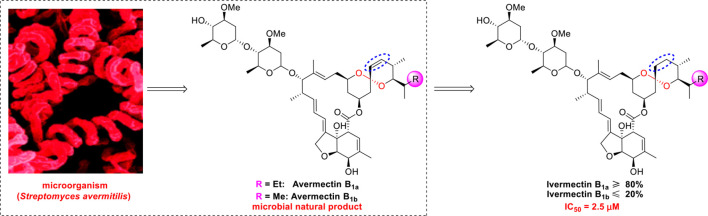
Ivermectin (brand name: Stromectol) is a landmark broad-spectrum anti-parasitic drug that was developed by Ōmura’s group along with Merck Sharp and Dohme Research Laboratories in 1978 (Burg et al., 1979). It has been demonstrated to be highly effective (94.9% efficacy at 24 hours and 73.8% efficacy at 2 weeks) as an oral drug for the treatment of head lice (Pariser et al., 2012). Ivermectin is the semisynthetic 22,23-dihydro derivative of the natural product avermectin B1 (B1a and B1b), which is produced by Streptomyces avermitilis ( Figure 4 ) (Campbell et al., 1983). Ivermectin is one of the most widely used antibiotics for both animals and humans, and the researchers who discovered it received the Nobel Prize in Physiology or Medicine in 2015 (Campbell, 2016).
Figure 4.
Promising natural-product-inspired ivermectin for treating COVID-19.
The use of ivermectin is currently being expanded. For example, Wagstaff’s group highlighted that ivermectin is highly effective at controlling SARS-CoV-2 RNA replication in vitro, with a 5000-fold reduction in the virus within 48 hours (Caly et al., 2020). Recently, Lohmer’s group found that a single oral dose of ivermectin is unlikely to reach the IC50 (2.5 μM) in the lungs (predicted lung concentration: 0.0857 μM), and they suggested that combination therapy or inhaled treatment (to increase the concentration in the lungs) could be considered as potential solutions (Schmith et al., 2020). Nevertheless, its safety in humans has been continuously documented (Buonfrate et al., 2019), and it is hoped that it will become a key component of COVID-19 treatment regimens.
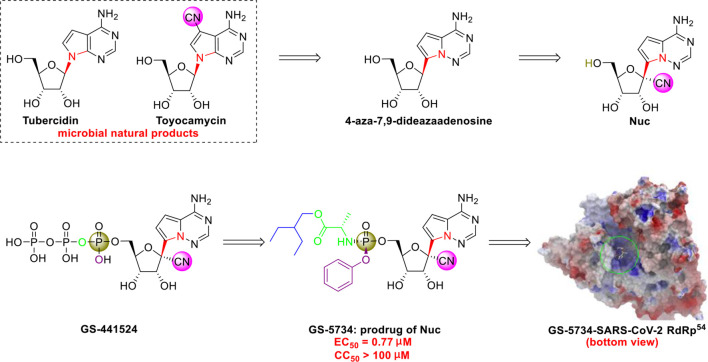
The C-nucleoside analog GS-5734 (remdesivir), a broad-spectrum antiviral agent developed by Gilead Sciences (Warren et al., 2016), exhibited promising clinical efficacy in the treatment of the first US case of SARS-CoV-2 infection (Holshue et al., 2020). Initial research on synthesizing GS-5734 (which is a prodrug) began with structural modification of tubercidin (an antibiotic and adenosine analog that is isolated from Streptomyces tubercidicus) by replacing the C-N linkage with a C-C bond to create 4-aza-7,9-dideazaadenosine ( Figure 5 ) (Patil et al., 1994). 4-aza-7,9-dideazaadenosine has equal cytotoxicity against HL-60 cells to tubercidin (50% infectious does [ID50] = 0.82 nM) and increased hydrolytic stability (Patil et al., 1994).
Figure 5.
Promising natural-product-inspired GS-5734 for treating COVID-19 (image reproduced with permission from ref. 54, Acta Pharm. Sin. B, 2020, 10, 766-788).
However, an important contribution to antiviral drug design is 1’-CN substituted Nuc, which can be viewed as a new structure that was inspired by the natural cyanide toyocamycin (isolated from Streptomyces toyocmnsis) (Nishimura et al., 1956). Nuc strongly inhibits hepatitis C virus in vitro (EC50 = 4.1 μM) (Cho et al., 2012). The active form of Nuc in virus-infected cells has been reported to be GS-441524, but the monophosphate conversion of Nuc to GS-441524 is a rate-limiting step (Warren et al., 2016). To overcome this issue, the monophosphorylated prodrug GS-5734 was developed to achieve better cellular uptake in vivo (Warren et al., 2016).
GS-5734 has been recognized as a promising broad-spectrum antiviral drug against multiple viruses including SARS-CoV (EC50 = 69 nM) (Sheahan et al., 2017) and MERS-CoV (EC50 = 20 nM) (Sheahan et al., 2017). Furthermore, Xiao’s group (Wang et al., 2020) and Yen’s group (Choy et al., 2020) recently revealed that GS-5734 is highly effective against SARS-CoV-2 infection in vitro (EC50 = 0.77 μM, viral load fitted in linear scale; or EC50 = 23.15 μM, viral load fitted in logarithmic scale) and has low toxicity (selectivity index >130). Regarding the mechanism of action, Li’s group highlighted that GS-5734 can bind to the RNA-binding channel of the SARS-CoV-2 RNA-dependent RNA polymerase (Wu et al., 2020) ( Figure 5 ). The 1’-ribose CN substitution observed in GS-5734 plays an important role in inhibiting the viral RNA replication of SARS-CoV-2 (Zhang et al., 2020).
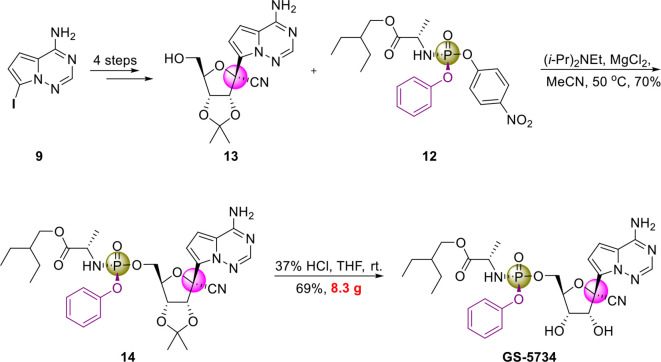
Notably, since 2016, GS-5734 has been reported to be safe and exhibit clinical efficacy against EBOV infection (Jacobs et al., 2016; Dörnemann et al., 2017) and SARS-CoV-2 infection (Beigel et al., 2020; Holshue et al., 2020). On April 10, 2020, Gilead Sciences reported that 68% of patients with severe COVID-19 who were treated with GS-5734 (via the compassionate use program) exhibited clinical improvement, and no new safety issues were detected (Grein et al., 2020). In addition, in May, 2020, the US FDA issued an emergency use authorization (EUA) for GS-5734 for treating SARS-CoV-2 infection (Mullard, 2020). To increase the efficiency of pharmaceutical research on GS-5734, researchers at Gilead Sciences developed a scalable process for synthesizing GS-5734 (Warren et al., 2016) ( Figure 6 ). Currently, there are no drug–drug interaction data on GS-5734 (Summary on compassionate use remdesivir Gilead), but the potential for clinically significant interactions is low (The liverpool drug interaction group). It is hoped that GS-5734 will be confirmed to be a safe and effective drug against SARS-CoV-2.
Figure 6.
Gram-scale synthesis of GS-5734.
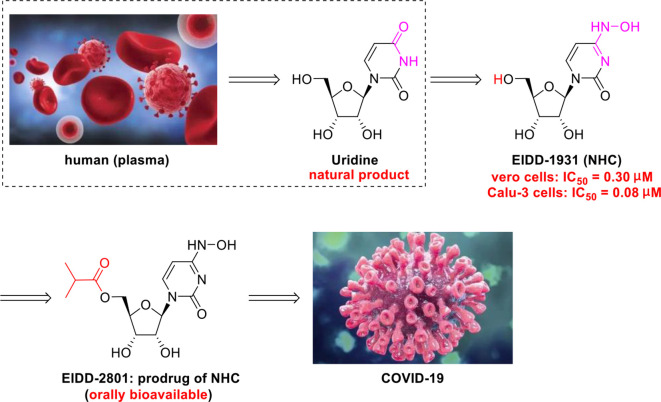
The N-nucleoside analog EIDD-2801, a promising orally bioavailable antiviral agent, was discovered by Plemper’s group at Emory University (Toots et al., 2019). Initial research on the synthesis of EIDD-2801 began with structurally modifying the broad-spectrum antiviral agent N 4-hydroxycytidine (NHC, EIDD-1931) (Agostini et al., 2019), which was in turn derived from the essential natural product uridine found in human plasma ( Figure 7 ) (Yamamoto et al., 2011).
Figure 7.
Promising natural-product-inspired EIDD-2801 for treating COVID-19.
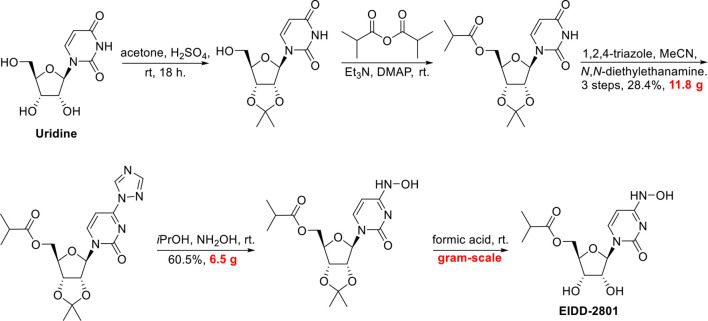
More recently, researchers at Emory University reported a scalable process for synthesizing EIDD-2801 (Painter et al., 2019) ( Figure 8 ). There is currently widespread interest in the use of EIDD-2801 as a promising anti-COVID-19 agent (Rothan and Byrareddy, 2020). Baric’s group (Cho et al., 2012) highlighted that EIDD-1931 is very effective at controlling SARS-CoV-2 replication. EIDD-1931 exhibited potent anti-SARS-CoV-2 activity in Calu-3 cells (IC50 = 0.08 μM) and Vero cells (IC50 =0.30 μM), with no observable cytotoxicity at doses of up to 5 μM (Rothan and Byrareddy, 2020). Furthermore, EIDD-2801 is an orally bioavailable prodrug that is efficiently hydrolyzed in vivo and it exhibits remarkable selectivity (therapeutic window >1713) (Sheahan et al., 2020).
Figure 8.
Gram-scale synthesis of EIDD-2801.
In addition, Baric’s group showed that EIDD-2801 significantly improved the pulmonary function of mice infected with MERS-CoV or SARS-CoV (Sheahan et al., 2020). Thus, EIDD-2801 has demonstrated potential effectiveness, even though there is a lack of good evidence in humans. It is hoped that researchers will work to overcome the obstacles (such as produced on a manufacturing scale, efficacy and safety in vivo) to allow it to be assessed in clinical research.
Other Small Molecules With In Vitro Activity Against SARS-Cov-2
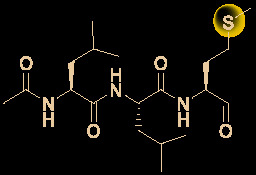
Growing understanding of efficient antiviral drug development has led to the use of small molecules being recognized as an important potential tactic for treating COVID-19. Rao’s group (Jin et al., 2020) showed that the organoselenium compound ebselen ( Table 1 ) and other inhibitors of the SARS-CoV-2 main protease (Mpro) exhibited potent activities, with IC50 values at micromolar or sub-micromolar levels (0.67–21.4 μM). Ebselen is highly effective at inhibiting SARS-CoV-2 infection (EC50 = 4.67 μM) and has low toxicity (median lethal dose [LD50] in rats >4,600 mg/kg). Most importantly, its safety in humans has been continuously evaluated in multiple clinical trials (Kil et al., 2020). Besides the abovementioned small molecules, several other natural products and natural product-inspired potential small molecules have also exhibited notable anti-SARS-CoV-2 activities ( Table 1 ).
Table 1.
Other small molecules with in vitro activity against SARS-Cov-2.
| No. | Name | Structure | EC50 or IC50 (μM) | SI | Ref. |
|---|---|---|---|---|---|
| 1 | Abiraterone acetate |
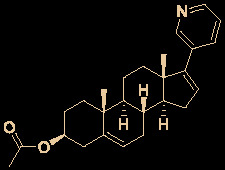
|
1.94 | 47.6 | (Yuan et al., 2020) |
| 2 | ALLM |
|
2.07 | 48.3 | (Ma et al., 2020) |
| 3 | Amodiaquine |
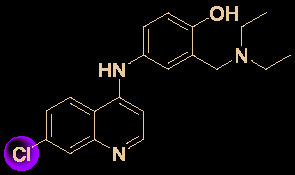
|
4.20 | >7.1 | (Ianevski et al., 2020) |
| 4 | Auranofin |
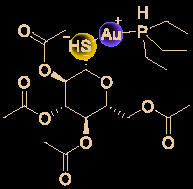
|
1.40 | 4.1 | (Rothan et al., 2020) |
| 5 | Azithromycin |
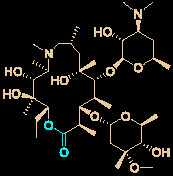
|
2.12 | 19.0 | (Touret et al., 2020) |
| 6 | Baicalein |
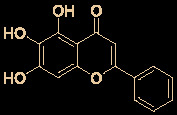
|
12.5-25.0 | 2.0 | (Liu et al., 2020) |
| 7 | Baicalin |
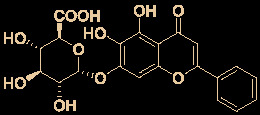
|
10.27 | 19.0 | (Su et al., 2020) |
| 8 | Boceprevir |
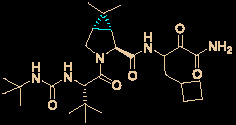
|
1.90 | 52.6 | (Ma et al., 2020) |
| 9 | Carmofur |
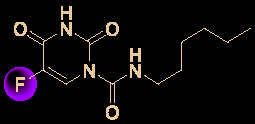
|
24.30 | 5.4 | (Jin et al., 2020) |
| 10 | Cinanserin |
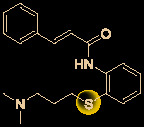
|
20.61 | 9.7 | (Jin et al., 2020) |
| 11 | CVL218 |
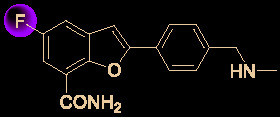
|
5.12 | 17.8 | (Ge et al., 2020) |
| 12 | Digitoxin |
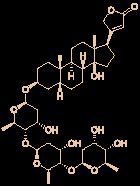
|
0.23 | 214.1 | (Jeon et al., 2020) |
| 13 | Digoxin |
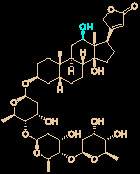
|
0.19 | 256.6 | (Jeon et al., 2020) |
| 14 | Diiodohydroxyquinoline |
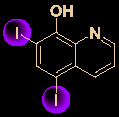
|
1.38 | >72.5 | (Ianevski et al., 2020) |
| 15 | Ebselen |
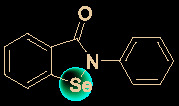
|
4.67 | – | (Jin et al., 2020) |
| 16 | Fluspirilene |
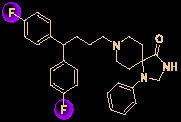
|
3.16 | 9.6 | (Weston et al., 2020) |
| 17 | GC-376 |
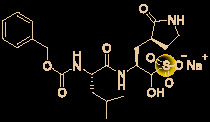
|
3.37 | 29.7 | (Ma et al., 2020) |
| 18 | Hexachlorophene |
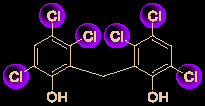
|
0.90 | 21.6 | (Jeon et al., 2020) |
| 19 | MDL28170 |
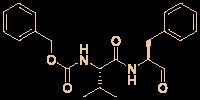
|
0.49 | 204.0 | (Ma et al., 2020) |
| 20 | Nafamostat |
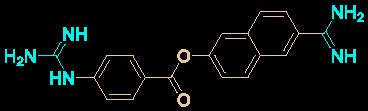
|
0.0022 | 11363 | (Ko et al., 2020) |
| 21 | Nelfinavir |
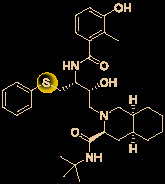
|
0.77 | 83.1 | (Ohashi et al., 2020) |
| 22 | Niclosamide |
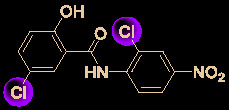
|
0.28 | 176.7 | (Jeon et al., 2020) |
| 23 | Ouabain |
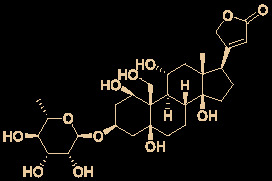
|
0.097 | 515.5 | (Jeon et al., 2020) |
| 24 | Salinomycin sodium |
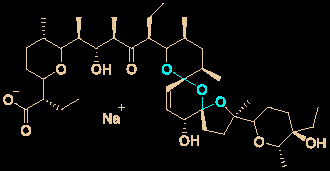
|
0.24 | 211.0 | (Jeon et al., 2020) |
| 25 | S312 |
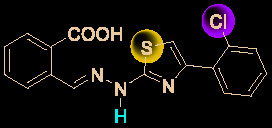
|
1.55 | >64.6 | (Xiong et al., 2020) |
| 26 | S416 |
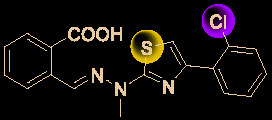
|
0.017 | >5882 | (Xiong et al., 2020) |
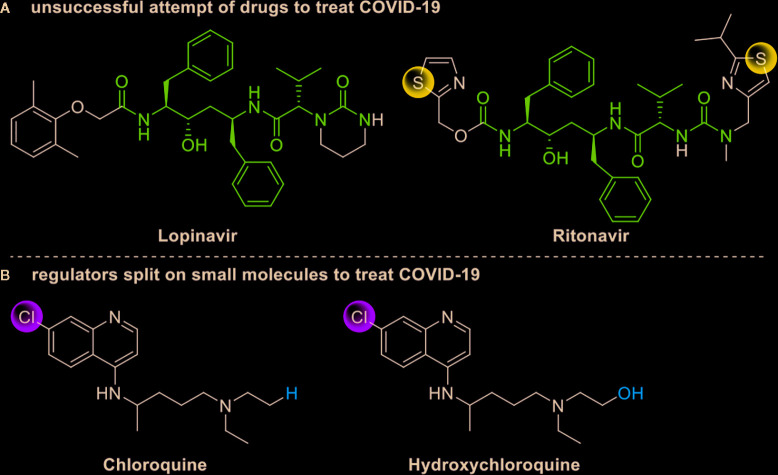
Drug development carries high risks. For example, although the approved protease inhibitors lopinavir and ritonavir ( Figure 9A ) were thought to be potentially effective against SARS-Cov-2 (as they have been reported to be active against SARS6), Wang’s group showed that lopinavir combined with ritonavir does not seem to be highly effective in patients with COVID-19 (Cao et al., 2020).
Figure 9.
Other small molecules for treating COVID-19. (A) Unsuccessful attempt of lopinavir and ritonavir to treat COVID-19. (B) Regulators split on chloroquine and hydroxychloroquine to treat COVID-19.
Chloroquine and hydroxychloroquine ( Figure 9B ) have garnered considerable attention due to their ability to effectively inhibit SARS-CoV-2 (Yamamoto et al., 2011; Liu et al., 2020; Yao et al., 2020). For example, Xiao’s group (Wu et al., 2020) showed that chloroquine potently blocked SARS-CoV-2 infection at a low concentration in vitro (EC50 = 1.1 μM). Although the US FDA has authorized the use of chloroquine and hydroxychloroquine, the WHO has stated that the clinical data do not support their clinical use in COVID-19 patients (Jaffe, 2020; Taccone et al., 2020). In addition, chloroquine can be lethal (narrow therapeutic window) in children, and caution is warranted when it is used for critical illness (Smit et al., 2020).
Conclusion and Outlook
Currently, global attention continues to be focused on COVID-19 due to its serious threat to public health. Scientists have discovered that SARS-CoV-2 has developed mutations (in 149 sites from the 103 sequenced SARS-Cov-2 strain) that have substantially changed its pathogenicity (Tang et al., 2020; Yao et al., 2020). Therefore, rapid discovery of safe, effective, and broad-spectrum anti-COVID-19 drugs is urgent. However, it is well-known that the development of a new drug usually takes more than 10 years (Ashburn and Thor, 2004). Very recently, potential anti-SARS-CoV-2 natural products and natural product-inspired small molecules have attracted significant attention due to their broad-spectrum antiviral activities. Here, we reviewed the research on potential landmark anti-SARS-CoV-2 natural products (carolacton, homoharringtonine, cepharanthine, and emetine) and natural product-inspired small molecules (ivermectin, GS-5734, EIDD-2801, and ebselen). In-depth research on potential anti-COVID-19 natural product-inspired small molecules has led to the development of multiple lines of evidence demonstrating their effects on SARS-CoV-2 infection (Ferner and Aronson, 2020; Pruijssers et al., 2020; Toots et al., 2020; Williamson et al., 2020; Xing et al., 2020).
While the current COVID-19 pandemic has led to more rapid natural product-based drug discovery and development, it is also worth noting that ton-scale total synthesis strategies for the abovementioned potential anti-SARS-CoV-2 natural products (such as carolacton and homoharringtonine) are urgently needed. However, significant challenges (for example, attainment of clinical evidence regarding the anti-SARS-CoV-2 effects of the agents in patients, and traditional drug development approach in SARS-CoV-2 is becoming untenable) will need to be overcome in order for successful clinical research to be completed. We hope that natural products and natural product-inspired small molecules will be shown to be safe and effective for treating SARS-CoV-2 infection.
Author’s Note
Figures were reproduced with permission through Copyright Clearance Center’s RightsLink® service.
Author Contributions
ZW conceived the review. LY collected the literatures. ZW, and LY wrote the manuscript. ZW edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
Project supported by the PhD research start-up funds of Qufu Normal University, China (Grant Nos. bsqd20190164, and bsqd20190060) and the project of introduction and cultivation for young innovation talents in colleges and universities of Shandong Province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agostini M. L., Pruijssers A. J., Chappell J. D., Gribble J., Lu X. T., Andres E. L., et al. (2019). Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 93, e01348–e01319. 10.1128/JVI.01348-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinboye E. S., Rosen M. D., Denmeade S. R., Kwabi-Addo B., Bakare O. (2012). Design, synthesis, and evaluation of pH-dependent hydrolyzable emetine analogues as treatment for prostate cancer. J. Med. Chem. 55, 7450–7459. 10.1021/jm300426q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Krpina K., Ianevski A., Shtaida N., Jo E., Yang J., et al. (2019). Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses 11, 964. 10.3390/v11100964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. E., Cui J., Ye Q., Huang B. Y., Zu W. H., Gong J., et al. (2020). Orthogonal genome-wide screenings in bat cells identify MTHFD1 as a target of broad antiviral therapy. bioRxiv. 10.1101/2020.03.29.014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T. T., Thor K. B. (2004). Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discovery 3, 673–683. 10.1038/nrd1468 [DOI] [PubMed] [Google Scholar]
- Bailly C. (2019). Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 62, 152956. 10.1016/j.phymed.2019.152956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J. H., Tomashek K. M., Dodd L. E., Mehta A. K., Zingman B. S., Kalil A. C., et al. (2020). Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- Bleasel M. D., Peterson G. M. (2020). Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals 13, 51. 10.3390/ph13030051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonfrate D., Salas-Coronas J., Muñoz J., Maruri B. T., Rodari P., Castelli F., et al. (2019). Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 19, 1181–1190. 10.1016/S1473-3099(19)30289-0 [DOI] [PubMed] [Google Scholar]
- Burg R. W., Miller B. M., Baker E. E., Birnbaum J., Currie S. A., Hartman R., et al. (1979). Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 15, 361–367. 10.1128/AAC.15.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J. D., Catton M. G., Jans D. A., Wagstaff K. M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 178, 104787. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. C., Fisher M. H., Stapley E. O., Albers-Schonberg G., Jacob T. A. (1983). Ivermectin: a potent new antiparasitic agent. Science 221, 823–828. 10.1126/science.6308762 [DOI] [PubMed] [Google Scholar]
- Campbell W. C. (2016). Ivermectin: a potent new antiparasitic agent. Angew . Chem. Int. Ed. 55, 10184–10189. 10.1002/anie.201601492 [DOI] [PubMed] [Google Scholar]
- Cao J., Forrest J. C., Zhang X. (2015). A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res. 114, 1–10. 10.1016/j.antiviral.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y. M., Wen D. N., Liu W., Wang J. L., Fan G. H., et al. (2020). A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 382, 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A., Saunders O. L., Butler T., Zhang L. J., Xu J., Vela J. E., et al. (2012). Synthesis and antiviral activity of a series of 1’-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 22, 2705–2707. 10.1016/j.bmcl.2012.02.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K. T., Wong A. Y. L., Kaewpreedee P., Sia S. F., Chen D. D., Hui K. P. Y., et al. (2020). Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro . Antivir. Res. 178, 104786. 10.1016/j.antiviral.2020.104786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus disease (COVID-2019) situation reports 1-144; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200612-covid-19-sitrep-144.pdf?sfvrsn=66ff9f4f_2
- Dörnemann J., Burzio C., Ronsse A., Sprecher A., De Clerck H., Van Herp M., et al. (2017). First newborn baby to receive experimental therapies survives Ebola virus disease. J. Infect. Dis. 215, 171–174. 10.1093/infdis/jiw493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H. J., Wang Z. H., Meng W., Li C. C., Hu Y. X., Zhou L., et al. (2018). The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses 10, 10. 10.3390/v10110601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall J., Coleman C. M., Hart B. J., Venkataraman T., Holbrook M. R., Kindrachuk J., et al. (2014). Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 58, 4885–4893. 10.1128/AAC.03036-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckelbarger J. D., Wilmot J. T., Epperson M. T., Thakur C. S., Shum D., Antczak C., et al. (2008). Synthesis of antiproliferative Cephalotaxus esters and their evaluation against several human hematopoietic and solid tumor cell lines: uncovering differential susceptibilities to multidrug resistance. Chem. Eur. J. 14, 4293–4306. 10.1002/chem.200701998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z. H., Li Y. J., Chen Z., Wang J. J., Zhu L. H. (2013). Inhibition of signal transducer and activator of transcription 3 and cyclooxygenase-2 is involved in radiosensitization of cepharanthine in HeLa cells. Int. J. Gynecol. Cancer 23, 608–614. 10.1097/IGC.0b013e31828a05fd [DOI] [PubMed] [Google Scholar]
- Ferner R. E., Aronson J. K. (2020). Remdesivir in covid-19: A drug with potential-don’t waste time on uncontrolled observations. BMJ 369, m1610. 10.1136/bmj.m1610 [DOI] [PubMed] [Google Scholar]
- Fu C. Z., Sikandar A., Donner J., Zaburannyi N., Herrmann J., Reck M., et al. (2020). The natural product carolacton inhibits folatedependent C1 metabolism by targeting FolD/MTHFD. Nat. Commu. 8, 1529. 10.1038/s41467-017-01671-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y. Y., Tian T. Z., Huang S. L., Wan F. P., Li J. X., Li S. Y., et al. (2020). A data-driven drug repositioning framework discovered a potential therapeutic agent targeting COVID-19. bioRxiv. 10.1101/2020.03.11.986836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M. J., Li S. F., Xie Y. L., Zhao F. R., Shao J. J., Zhang Y. G., et al. (2019). Inhibitory effects of homoharringtonine on foot and mouth disease virus in vitro. J. Med. Virol. 91, 1595–1601. 10.1002/jmv.25494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. (2020). Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 382, 2327–2336. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallside M. S., Brzozowski R. S., Wuest W. M., Phillips A. J. (2014). A concise synthesis of carolacton. Org. Lett. 16, 1148–1151. 10.1021/ol500004k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M. L., DeBolt C., Lindquist S., Lofy K. H., Wiesman J., Bruce H., et al. (2020). First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382, 929–936. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianevski A., Yao R. A., Fenstad M. H., Biza S., Zusinaite E., Lysvand H., et al. (2020). Antiviral options against SARS-CoV-2 infection. bioRxiv. 10.1101/2020.05.12.091165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rodger A., Bell D. J., Bhagani S., Cropley I., Filipe A., et al. (2016). Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388, 498–503. 10.1016/S0140-6736(16)30386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe S. (2020). Regulators split on antimalarials for COVID-19. Lancet. 395, 1179–1179. 10.1016/s0140-6736(20)30817-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Irschik H., Huch V., Schummer D., Steinmetz H., Bock M., et al. (2010). Carolacton-a macrolide ketocarbonic acid that reduces biofilm formation by the caries- and endocarditis-associated bacterium Streptococcus mutans. Eur. J. Org. Chem. 2010, 1284–1289. 10.1002/ejoc.200901126 [DOI] [Google Scholar]
- Jeon S., Ko M., Lee J., Choi I., Byun S. Y., Park S., et al. (2020). Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Ch. 64, e00819–20. 10.1128/AAC.00819-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z. M., Du X. Y., Xu Y. C., Deng Y. Q., Liu M. Q., Zhao Y., et al. (2020). Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 582, 289–293. 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- Jin Z. M., Zhao Y., Sun Y., Zhang B., Wang H. F., Wu Y., et al. (2020). Structural basis for the inhibition of COVID-19 virus main protease by carmofur, an antineoplastic drug. bioRxiv. 10.1101/2020.04.09.033233 [DOI] [PubMed] [Google Scholar]
- Ju X., Beaudry C. M. (2019). Total synthesis of (-)-cephalotaxine and (-)-homoharringtonine via furan oxidation-transannular mannich cyclization. Angew. Chem. Int. Ed. 58, 6752–6755. 10.1002/anie.201902174 [DOI] [PubMed] [Google Scholar]
- Kalil A. C. (2020). Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 323, 1897–1898. 10.1001/jama.2020.4742 [DOI] [PubMed] [Google Scholar]
- Kil J., Lobarinas E., Spankovich C., Griffiths S. K., Antonelli P. J., Lynch E. D., et al. (2020). Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 390, 969–979. 10.1016/S0140-6736(17)31791-9 [DOI] [PubMed] [Google Scholar]
- Kim D. E., Min J. S., Jang M. S., Lee J. Y., Shin Y. S., Song J. H., et al. (2019). Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules 9, 696. 10.3390/biom9110696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M., Jeon S., Ryu W. S., Kim S. (2020). Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate. bioRxiv. 10.1101/2020.05.12.090035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilya T. K., Goswami R. K. (2017). Stereoselective total synthesis of carolacton. Org. Lett. 19, 2366–2369. 10.1021/acs.orglett.7b00903 [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K., Cohen J. (2020). Race to find COVID-19 treatments accelerates. Science 367, 1412–1413. 10.1126/science.367.6485.1412 [DOI] [PubMed] [Google Scholar]
- Li G. D., De Clercq E. (2020). Therapeutic options for the 2019 novel coronavirus, (2019-nCoV). Nat. Rev. Drug Discovery 19, 149–150. 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- Li F. S., Weng J. K. (2017). Demystifying traditional herbal medicine with modern approaches. Nat. Plants 3, 17109. 10.1038/nplants.2017.109 [DOI] [PubMed] [Google Scholar]
- Liu H. B., Ye F., Sun Q., Liang H., Li C. M., Lu R. J., et al. (2020). Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. bioRxiv. 10.1101/2020.04.10.035824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R. Y., Xu M. Y., Wang X., Zhang H. Y., Hu H. R., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery 6, 16. 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. L., Hurst B., Hu Y. M., Szeto T., Tarbet B., Wang J. (2020). Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. bioRxiv. 10.1101/2020.04.20.051581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. (2013). 2012 FDA drug approvals. Nat. Rev. Drug Discovery 12, 87–90. 10.1038/nrd3946 [DOI] [PubMed] [Google Scholar]
- Mullard A. (2020). Hints of hope with remdesivir. Nat. Rev. Drug Discovery 19, 373. [DOI] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. 10.1021/acs.jnatprod.9b01285 [DOI] [PubMed] [Google Scholar]
- Nishimura H., Katagiri K., Sato K., Mayama M., Shimaoka N. (1956). Toyocamycin, a new anti-candida antibiotic. J. Antibiot. 9, 60–62. [PubMed] [Google Scholar]
- Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., et al. (2020). Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv. 10.1101/2020.04.14.039925 [DOI] [Google Scholar]
- Okamoto M., Ono M., Baba M. (1998). Potent inhibition of HIV type 1 replication by an antiinflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res. Hum. Retrovir. 14, 1239–1245. 10.1089/aid.1998.14.1239 [DOI] [PubMed] [Google Scholar]
- Painter G. R., Guthrie D. B., Bluemling G. R., Natchus M. R. (2019). N4-hydroxy cytidine and derivatives and anti-viral uses related thereto. WO 2019/113462 A1.
- Pariser D. M., Meinking T. L., Bell M., Ryan W. G. (2012). Topical 0.5% ivermectin lotion for treatment of head lice. N. Engl. J. Med. 367, 1687–1693. 10.1056/nejmoa1200107 [DOI] [PubMed] [Google Scholar]
- Patil S. A., Otter B. A., Klein S. (1994). 4-Aza-7,9-dideazaadenosine, a new cytotoxic synthetic C-nucleoside analogue of adenosine. Tetrahedron Lett. 35, 5339–5342. 10.1016/S0040-4039(00)73494-0 [DOI] [Google Scholar]
- Powell R. G., Weisleder D., Smith C. R., Rohwedder W. K. (1970). Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 11, 815–818. 10.1016/S0040-4039(01)97839-6 [DOI] [PubMed] [Google Scholar]
- Pruijssers A. J., George A. S., Schäfer A., Leist S. R., Gralinski L. E., Dinnon III K. H., et al. (2020). Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. bioRxiv. 10.1101/2020.04.27.064279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H. A., Byrareddy S. N. (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H. A., Stone S., Natekar J., Kumari P., Arora K., Kumar M. (2020). The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 547, 7–11. 10.1016/j.virol.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T., Kirschning A. (2012). Total synthesis of carolacton, a highly iotent biofilm inhibitor. Angew. Chem. Int. Ed. 51, 1063–1066. 10.1002/anie.201106762 [DOI] [PubMed] [Google Scholar]
- Schmith V. D., Zhou J., Lohmer L. R. L. (2020). The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin. Pharmacol. Ther. 10.1002/CPT.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T. P., Sims A. C., Graham R. L., Menachery V. D., Gralinski L. E., Case J. B., et al. (2017). Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653. 10.1126/scitranslmed.aal3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T. P., Sims A. C., Zhou S. T., Graham R. L., Pruijssers A. J., Agostini M. L., et al. (2020). An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12, eabb5883. 10.1126/scitranslmed.abb5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Niu J. W., Wang C. H., Huang B. Y., Wang W. L., Zhu N., et al. (2019). High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 93, e00023–e00019. 10.1128/JVI.00023-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. (2015). A new golden age of natural products drug discovery. Cell 163, 1297–1300. 10.1016/j.cell.2015.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. Z., Wen Z. Y., Zhong G. X., Yang H. L., Wang C., Huang B. Y., et al. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368, 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit C., Peeters M. Y. M., Anker J. N., Knibbe C. A. J. (2020). Chloroquine for SARS−CoV−2: implications of its unique pharmacokinetic and safety properties. Clin. Pharmacokinet. 59, 659–669. 10.1007/s40262-020-00891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. X., Yao S., Zhao W. F., Li M. J., Liu J., Shang W. J., et al. (2020). Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 10.1101/2020.04.13.038687 [DOI] [Google Scholar]
- Summary on compassionate use remdesivir Gilead https://www.ema.europa.eu/en/documents/other/summary-compassionate-use-remdesivir-gilead_en.pdf (Accessed May 5, 2020).
- Taccone F. S., Gorham J., Vincent J. L. (2020). Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir. Med. 8, 539–541. 10.1016/S2213-2600(20)30172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. L., Wu C. C., Li X., Song Y. H., Yao X. M., Wu X. K., et al. (2020). On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 7, 1012–1023. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The liverpool drug interaction group Evaluating the interaction risk of experimental COVID-19 therapies. https://undark.org/2020/05/07/drug-interaction-covid-19/ (Accessed May 28, 2020).
- Toots M., Yoon J. J., Cox R. M., Hart M., Sticher Z. M., Makhsous N., et al. (2019). Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 11, eaax5866. 10.1126/scitranslmed.aax5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toots M., Yoon J. J., Hart M., Natchus M. G., Painter G. R., Plemper R. K. (2020). Quantitative efficacy paradigms of the influenza clinical drug candidate. Transl. Res. 218, 16–28. 10.1016/j.trsl.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret F., Gilles M., Barral K., Nougairède A., Decroly E., de Lamballerie X., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. bioRxiv. 10.1101/2020.04.03.023846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P. W., Hayden F. G., Gao G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395, 470–473. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. L., Cao R. Y., Zhang L. K., Yang X. L., Liu J., Xu M. Y., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus, (2019-nCoV) in vitro . Cell Res. 30, 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T. K., Jordan R., Lo M. K., Ray A. S., Mackman R. L., Soloveva V., et al. (2016). Therapeutic Efficacy of The Small Molecule GS-5734 Against Ebola virus in rhesus monkeys. Nature 531, 381–385. 10.1038/nature17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston S., Coleman C. M., Haupt R., Logue J., Matthews K., Frieman M. B. (2020). Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. bioRxiv. 10.1101/2020.03.25.008482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegrebe W., Kramer W. J., Shamma M. (1984). The emetine alkaloids. J. Nat. Prod. 47, 397–408. 10.1021/np50033a001 [DOI] [Google Scholar]
- Williamson B. N., Feldmann F., Schwarz B., Meade-White K., Porter D. P., Schulz J. (2020). Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv. 10.1101/2020.04.15.043166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. R., Liu Y., Yang Y. Y., Zhang P., Zhong W., Wang Y. L., et al. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 10, 766–788. 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J., Shankar R., Drelich A., Paithankar S., Chekalin E., Dexheimer T., et al. (2020). Reversal of infected host gene expression identifies repurposed drug candidates for COVID-19. bioRxiv. 10.1101/2020.04.07.030734 [DOI] [Google Scholar]
- Xiong R., Zhang L. K., Li S. L., Sun Y., Ding M. Y., Wang Y., et al. (2020). Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. bioRxiv. 10.1101/2020.03.11.983056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Koyama H., Kurajoh M., Shoji T., Tsutsumi Z., Moriwaki Y. (2011). Biochemistry of uridine in plasma. Clin. Chim. Acta 412, 1712–1724. 10.1016/j.cca.2011.06.006 [DOI] [PubMed] [Google Scholar]
- Yang S., Xu M., Lee E. M., Gorshkov K., Shiryaev S. A., He S. H., et al. (2018). Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discovery 4, 31. 10.1038/s41421-018-0034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. T., Ye F., Zhang M., Cui C., Huang B. Y., Niu P. H., et al. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H. P., Lu X. Y., Chen Q., Xu K. J., Chen Y., Cheng L. F., et al. (2020). Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. 10.1101/2020.04.14.20060160 [DOI] [Google Scholar]
- Yuan S. F., Chan J. F. W., Chik K. K. H., Chan C. C. Y., Tsang J. O. L., Liang R. H., et al. (2020). Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system. Pharmacol. Res. 159, 104960. 10.1016/j.phrs.2020.104960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. H., Wang Y. F., Liu X. J., Lu J. H., Qian C. W., Wan Z. Y., et al. (2005). Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 118, 493–496. [PubMed] [Google Scholar]
- Zhang L., Zhang D., Yuan C., Wang X. W., Li Y. F., Jia X. L., et al. (2020). Role of 1’-ribose cyano substitution for remdesivir to effectively inhibit both nucleotide addition and proofreading in SARS-CoV-2 viral RNA replication. bioRxiv. 10.1101/2020.04.27.063859 [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. (2020). A novel coronavirus from patients with pneumonia in China 2019. N. Engl. J. Med. 382, 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]