Abstract
Atypical or malignant transformation (AT/MT) has been described in WHO grade I meningiomas. Our aim was to identify predictive factors of AT/MT at recurrence. A total of N = 15 WHO grade increases were observed in N = 13 patients (0.96% of the study population, risk of transformation of 0.12% per patient-year follow-up). Patients with and without progression at recurrence were similar regarding age, gender distribution, skull-base location, bone infiltration, and Simpson grades. Recurrence-free survival was lower in patients with transformation (5 ± 4.06 years versus 7.3 ± 5.4 years; p = 0.03). Among patient age, gender, skull base location, extent of resection or post-operative RT, no predictor of AT/MT was identified, despite a follow-up of 10,524 patient-years. The annual risk of transformation of WHO grade I meningiomas was 0.12% per patient-year follow-up. Despite the important number of patients included and their extended follow-up, we did not identify any risk factor for transformation. A total of 1,352 patients with surgically managed WHO grade I meningioma from a mixed retro-and prospective database with mean follow-up of 9.2 years ± 5.7 years (0.3–20.9 years) were reviewed. Recurring tumors at the site of initial surgery were considered as recurrence.
Subject terms: Surgical oncology, Cellular neuroscience, Risk factors, Epidemiology
Introduction
Meningiomas are the most frequent intracranial, extra-cerebral tumors and represent up to 40% of primary intracranial tumors in surgical series1,2. Whilst their etiology is still not fully understood, it is known that genetic inherited dispositions, hormonal effects, ionizing radiation exposure, as well as immunological factors are implicated in their genesis3,4.
According to the latest recommendations5, the management of meningiomas is primarily surgical and aims for a complete/maximal resection of the tumor, including its dural tail. A meningioma management according to the highest standards of care eventually offers high rates of disease-control and globally favorable outcomes1,6–9.
The European Association of Neuro-Oncology (EANO) guidelines5 advises a close and diligent post-operative clinico-radiological follow-up, as even long-term recurrences may occur10. The 14 monosomy and 1p36 chromosomal deletion11, the location of the meningioma1,11. a sub-total resection (STR)1,8,11 and higher World Health Organization (WHO) grade1,12,13 are amongst the patient-, tumor- and surgery-related factors of recurrence identified and described in the literature.
The grading of the meningiomas is based on the WHO classification, ranging from grade I to III, with respective overall and progression-free survivals (OS & PFS)5,14. Although up to 3% are malignant (WHO grade III)5,15,16 and 5–7% atypical (WHO grade II), the vast majority of meningiomas are benign (WHO grade I) and show an indolent progression6. Therefore, meningiomas can be perceived as a chronic disease in most of the cases10,17.
However, similarly to what can occur in low-grade gliomas (LGG), malignant transformation (MT) has previously been described in formally identified WHO grade I meningiomas18–21. The overall rate of progression from benign to non-benign histology ranges from 0.16% to 2.0%, according to the most recent data available in the literature22–25. In the case of LGGs, risk factors for malignant transformation (age, multiple tumor location, management by chemotherapy alone and STR) have been described25. Similarly, age and non skull-base location has been suggested as predictors for atypical or malignant transformation (AT/MT) at recurrence in meningiomas9,15,26–28. This statement is of paramount importance, since patients with recurring meningioma with AT/MT may need more aggressive treatment. Therefore, it is primordial that neurosurgeons and neuro-oncologists are able to identify properly those patients at risk for AT/MT.
In this study, a large cohort of patients with recurring WHO grade I meningiomas is presented, focusing on the progression toward higher WHO grades at recurrence. Our aim is to identify the predictive factors of AT/MT at recurrence, using a large dataset with an exhaustive and very long follow-up, representing a total of 10, 524 patient-years.
Results
WHO grade increase in recurrent meningiomas and population characteristics
Baseline demographics, clinical and radiological characteristics of the study cohort are summarized in Table 1. A total of N = 15 WHO grade increases at recurrence were observed in N = 13 patients, representing 0.96% of the study population and corresponding to a risk of transformation of 0.12% per patient-year follow-up. Patients with and without progression at recurrence were similar regarding age, gender distribution, skull-base location and bone infiltration (Table 1). The Simpson grades were also similar between the two groups.
Table 1.
Baseline clinico-radiological data, overall survival and progression-free survival of patients with and without WHO grade increase at first recurrence.
| WHO grade increase | No WHO grade increase | p | |||
|---|---|---|---|---|---|
| n = 13 | % | n = 1,342 | % | ||
| Age | 55.5 ± 11.6 | – | 58 ± 13.2 | – | 0.44 |
| Sex | 8 F/5 M | – | 957 F/382 M | – | 0.53 |
| Skull base tumor | 5 | 38.5 | 653 | 48.7 | 0.58 |
| Bone infiltration | 1 | 7.7 | 248 | 18.5 | 0.48 |
| Simpson grade after initial surgery | 0.28 | ||||
| I | 4 | 30.8 | 532 | 39.8 | |
| II | 6 | 46.2 | 458 | 34.2 | |
| III | 0 | 0 | 70 | 5.3 | |
| IV | 3 | 23 | 272 | 20.4 | |
| V | 0 | 0 | 5 | 0.3 | |
| Postop RT after 1st surgery | 0 | 0 | 28 | 2.1 | 0.96 |
| OS | 11.1 ± 6.2 | – | 9.2 ± 5.6 | – | 0.29 |
| RFS | 5 ± 4.1 | – | 7.3 ± 5.4 | – | 0.03 |
OS overall survival, Neuro. Worsening neurological worsening, Postop RT post-operative radiation therapy, PFS progression-free survival.
The distributions of WHO grades at second and third surgery are shown in Table 2. A total of N = 11 transformations were observed between the first and the second surgery; 9 increases to WHO grade II and 2 increases to WHO grade III. At the third surgery, N = 4 new transformations were observed: N = 2 from WHO grade I to WHO grade II and N = 2 from WHO I already transformed in grade II to WHO grade III. Two patients were operated 3 times with an increase of WHO grade at each surgery.
Table 2.
Distribution of the WHO grades at the second and the third surgery in originally WHO grade I meningiomas.
| WHO grade | 2nd surgery | 3rd surgery |
|---|---|---|
| I | 76 (87.4%) | 14 (70%) |
| II | 8 (9.2%) | 3 (15%) |
| III | 3 (3.4%) | 3 (15%) |
| Total | 87 | 20 |
In the WHO grade progression group, no patient had post-operative radiation therapy (RT). In the group with WHO grade stability, a total of N = 28 patients had an adjuvant RT after the first surgery. No difference was observed between the groups, as shown in Table 1.
Retreatment-free survival
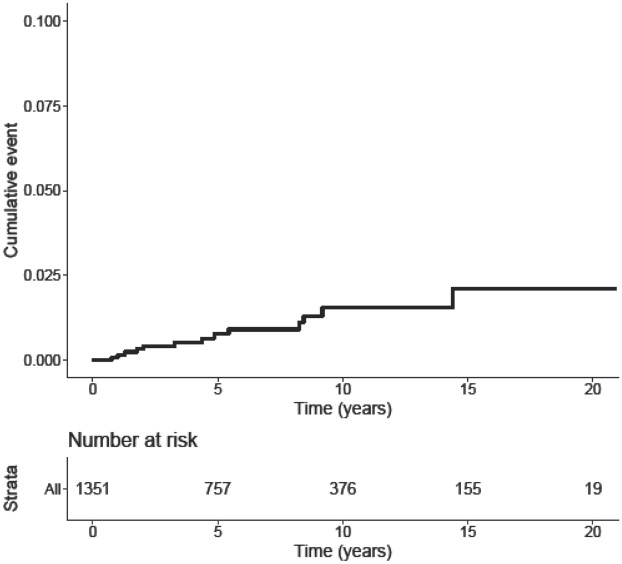
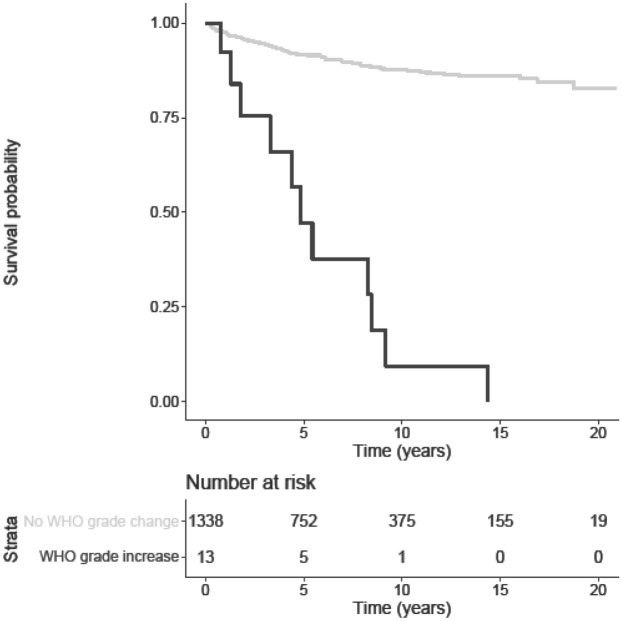
Data on RFS and OS are shown in Table 1. RFS was significantly lower in patients with transformation when compared to patients without transformation (5.0 ± 4.1 years versus 7.3 ± 5.4 years; p = 0.03). The mean RFS in tumors showing a progression of one WHO grade was 4.8 ± 4.1 years, whereas the mean RFS in tumors showing a progression of two WHO grades was 8.1 ± 8.9 years. Two patients presented outlying time-to-progression (1.8 years and 14.4 years). Interestingly, the OS did not significantly differ between the two cohorts. The Kaplan–Meier curve of WHO grade progression probability over time is shown in Fig. 1. The Kaplan–Meier curves of PFS in progressing and non-progressing recurrences are shown in Fig. 2.
Figure 1.
Kaplan–Meier curve of progression in WHO grade over time, with the respective number at risk.
Figure 2.
Kaplan–Meier curves of PFS in patients with (dark grey) and without (light grey) WHO grade change.
Predictive factors of WHO grade transformation
No predictive factor for WHO grade progression at recurrence was found amongst the age, gender, skull base location, the extent of resection as well as the post-operative RT, as shown in Table 3.
Table 3.
Predictive factors of WHO grade increase after first surgery.
| WHO grade increase | ||
|---|---|---|
| Odd ratio | p | |
| Age | 0.98 [0.94; 1.02] | 0.43 |
| Sex (male) | 1.54 [0.46; 4.69] | 0.46 |
| Skull base location | 0.71 [0.21; 2.18] | 0.56 |
| Simpson grade of first surgery | 1.05 [0.63; 1.66] | 0.84 |
| RT after first surgery | 0.10 [0.01; 5.06] | 0.99 |
RT radiation therapy.
Discussion
In this study, we in investigated the risk of atypical or malignant transformation (AT/MT) in recurring intracranial WHO grade I meningiomas, using a database of 1,352 patients treated by surgery with a cumulated total of 10,524 patient-years follow-up. The annual risk of AT/MT was 0.12% per patient-year follow-up, representing approximately 1% of surgically managed patients, and 19.5% of the patients treated for a recurrence.
Previous studies have identified advanced age and non-skull base location as predictors for AT/MT in recurring meningiomas15,29. Despite the large number of patients included and their long and complete follow-up, no risk factor for transformation among gender, skull-base location, Simpson grade after first surgery, radiotherapy after initial surgery, and advanced age could be identified. We therefore conclude that should risk factors of AT/MT exist, their effect is probably minor.
According to the latest EANO recommendations, the post-operative follow-up of meningiomas should be performed by a senior neurosurgeon; the interval between follow-up visits can vary considerably, depending on the Simpson grade, the initial size of the lesion, its location, the age of the patient, as well as its general neurological condition5. In the case of WHO grade I meningiomas with documented GTR, the recurrence rate at 10 year ranges from 20 to 39%7,30,31. Therefore, an annual follow-up is recommended, up to 5 years after the treatment, then every 2 years. Our results corroborate these data. However, one of our patients had a meningioma recurrence with AT/MT increase more than ten years after initial diagnosis. According to the literature, this is no exception; in their long-term follow-up of surgically managed parasagittal meningiomas, Petersson-Segerlind et al. stated that the 25 years recurrence rate was up to 47%. More specifically, the 10- and 25-years recurrence rates for Simpson grade I–II resections of parasagittal meningiomas were 13% and 48%, respectively. The authors found that the 10- and 25-years mortality rates were as high as 33% and 63%, respectively, of which 50% and 48% of the mortality were directly attributable to the tumor at 10 and 25 years, respectively10. Data on incidentally, observed meningiomas can support these findings. Jadid et al. followed a cohort of consecutive patients referred with incidentally discovered, asymptomatic meningiomas for at least 10 years and found that 35.4% of the tumors showed growth (regardless of tumor size), resulting in a 75% 15-year growth-rate by life-table statistics17.
To our knowledge, this is the first population-based study assessing AT/MT in originally and formally identified WHO grade I meningiomas using a large cohort with a very long and complete follow-up. Our findings are of paramount importance for neurosurgeons, as they should be able to estimate and inform the patient of the rate of atypical or malignant transformation of meningiomas, that may need an earlier second surgery, but without impact on OS.
Strengths and limitations
Our dataset offers a comprehensive and very extended source of data on patients with intra-cranial meningiomas with complete follow-up, since only one patient was lost during the study period.
The small number of histological transformations (N = 15 WHO increases in N = 13 patients) limits the finding of statistically associated prognostic factors. However, our cohort is one of the largest, to our knowledge. As WHO classification changed several times over the duration of the study, re-classification of the tumors carries risk of mis-grading, also representing a limitation.
The main limitation is the missing of data on molecular characteristics (MIB-1), because of the extended period of inclusion and could not be retrieved for the analysis. The importance of genetics and biomarkers such as MIB-1 and Ki-67 is very well established in the management of meningiomas, at first surgery as well as whenever recurrence occurs15,32,33. For instance, McGovern et al.15 found that WHO grade I non-skull base meningiomas have a higher MIB-1 index than grade I skull base meningiomas (1.77 ± 1.86% versus 3.44 ± 3.53%), correlating with lower RFS. Matsuno et al.32 assessed the proliferative potentials of meningiomas using the anti-Ki-67 monoclonal antibody and the MIB-1. The authors observed a statistically significant difference in the MIB-1 index related to gender and age as male patients showed a mean index of 5.5%, versus 2.7% in female patients, and higher MIB-1 indexes were observed in younger patients. Meningiomas with a MIB-1 index ≥ 3% were found to have a significantly high tendency for recurrence within the first 10-year follow-up periods. These results were corroborated by Perry et al.33 Unfortunately, were not able to retrieve information about MIB-1 status of the tumor, as these data were not available for the whole cohort, due to the extended time of recruitment. Certainly, the future will open new perspectives in molecular diagnosis in meningioma management, including the development of biomarkers for AT/MT.
Lastly, analysis of the methylation profile has recently been developed and reported by Sahm et al.34 It is a new yet powerful method for analyzing epigenetic changes in tumor DNA. The analysis of methylation is increasingly used in CNS tumors overall, with excellent diagnostic and prognosis values in terms of biological behavior, open new perspectives towards the identification of predictors of grade changes in initially WHO grade I recurring meningiomas, for which patient-, tumor- and surgery-related characteristics are not sufficient to identify predictors for AT/MT. Unfortunately, we were not able to retrieve information about methylation status of the tumor.
Conclusion
The annual risk of transformation of WHO grade I meningiomas is 0.12% per patient-year follow-up. Despite the important number of patients included in the study and their extended follow-up, we were not able to identify any risk factor for transformation, among gender, skull-base location, advanced age, Simpson grade after first surgery and first postoperative RT.
Methods
Patient cohort
We performed a review of a cohort of intracranial meningiomas consecutively treated by surgery at Oslo University Hospital (OUH). OUH is a tertiary referral center including two neurosurgical units (Rikshospitalet and Ullevaal), covering a total of 3 million inhabitants (representing circa 56% of the Norwegian population). A total of 1,469 consecutive patients were identified from a mixed retro-and prospective database (from 1990 to 2002 and from 2003 to 2010, respectively). For this study, only patients with formally identified, surgically managed WHO grade I intracranial meningioma were considered, representing a total of 1,352 patients.
In most cases, surgery aimed at complete tumor resection, considering both patient and tumor characteristics. Based on surgical reports and the post-operative imaging studies, the extent of resection (EOR) was assessed using the Simpson grade scale30. Gross total resection (GTR) was defined as a Simpson grade I–III resection, according to the latest guidelines of the European Association of EANO5.
The histopathological diagnosis of meningioma was confirmed by a senior pathologist on a case-by-case basis. Since the WHO criteria changed during the study period, the tumors included between 1990 to 2001 were re-classified as WHO grade I, WHO grade II and WHO grade III by a neuropathologist (previously benign, atypical, and anaplastic, respectively).
The mean follow-up was 9.2 years ± 5.7 years (range 0.3–20.9 years), with a cumulated total of 10,524 patient-years follow-up. One patient was lost of follow-up due to moving abroad. Regarding the post-operative clinico-radiologic follow-up, patients were generally followed yearly or after 1 and 3 years and then every 5 years. However, the follow-up did not follow a rigorous policy since the initial size of the lesion, its location, the Simpson grading of the resection, the age of the patient and the neurological status were considered on a case-by-case basis.
Recurring tumors at the site of initial surgery with radio-clinical correlations were considered as recurrence. Retreatment-free survival (RFS or time-to-retreatment) was defined as the time between the first surgery and the second surgical procedure. Radiological recurrences without clinical impact not requiring any adjuvant treatment, as well as tumors occurring remotely to the primary site, were not considered as recurrence and thus not included in the analysis. In recurring tumors, histo-pathological data was recorded up to the third surgery. In cases where patients were operated more than three times, data on following surgeries were not included in the analysis. As a result, from a dataset comprising N = 1,352 meningiomas, a total of N = 87 and N = 20 patients with first and second recurrence were available, respectively.
Ethics
The study was regulated by the Personal Data Act/Personal Health Data Filing System Act and approved by the Data Protection Official, registered Norwegian institutional review board at OUH (2017/5204). Under this regulation, patient’s written informed consent was not required to collect and analyze data. The study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
The statistical analysis was performed using R v3.5.1 (https://www.r-project.org). The statistical significance threshold was set at p = 0.05. A binomial logistic regression following a general linear model approach was used to identify predictive factors for AT/MT at recurrence. A Kaplan–Meier approach was used to assess the risk of transformation over time.
Acknowledgements
The authors would like to thank Eirik Helseth MD, PhD, David Scheie MD, PhD, Bernt Filip Hasseleid MD, Andreas Mathisen MD, Andreas Hessen Schei MD, and Kristina M. Ødegaard MD for their valuable contributions in collecting data for this manuscript and the neurosurgeons of Rikshospitalet and Ullevaal for their dedicated patient care.
Author contributions
Data Collection: T.R.M. Statistics: J.M.L. Manuscript drafting: M.V.C., T.R.M., J.M.L. Critical revisions: all authors.
Data availability
If requested by the editors, the authors will fully cooperate in obtaining and providing the data on which the manuscript is based without any restriction.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Corniola MV, et al. Posterior fossa meningiomas: perioperative predictors of extent of resection, overall survival and progression-free survival. Acta Neurochir. 2019 doi: 10.1007/s00701-019-03862-z. [DOI] [PubMed] [Google Scholar]
- 2.Meling TR, Da Broi M, Scheie D, Helseth E, Smoll NR. Meningioma surgery-are we making progress? World Neurosurg. 2019;125(5):e205–e213. doi: 10.1016/j.wneu.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J. Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D. Radiation-induced meningioma: a descriptive study of 253 cases. J. Neurosurg. 2002;97:1078–1082. doi: 10.3171/jns.2002.97.5.1078. [DOI] [PubMed] [Google Scholar]
- 5.Goldbrunner R, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 6.Claus EB, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 7.Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson grade I resection as the goal: clinical article. J. Neurosurg. 2012;117:999–1006. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]
- 8.Konglund A, et al. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurol. Scand. 2013;127:161–169. doi: 10.1111/j.1600-0404.2012.01692.x. [DOI] [PubMed] [Google Scholar]
- 9.Meling TR, Da Broi M, Scheie D, Helseth E. Meningiomas: skull base versus non-skull base. Neurosurg. Rev. 2019;42:163–173. doi: 10.1007/s10143-018-0976-7. [DOI] [PubMed] [Google Scholar]
- 10.Pettersson-Segerlind J, Orrego A, Lonn S, Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011;76:564–571. doi: 10.1016/j.wneu.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Maillo A, et al. Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol. 2007;9:438–446. doi: 10.1215/15228517-2007-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemee JM, et al. Extent of resection in meningioma: predictive factors and clinical implications. Sci. Rep. 2019;9:5944. doi: 10.1038/s41598-019-42451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayerbe J, et al. Risk factors predicting recurrence in patients operated on for intracranial meningioma. A multivariate analysis. Acta Neurochir. 1999;141:921–932. doi: 10.1007/s007010050398. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 15.McGovern SL, et al. A comparison of World Health Organization tumor grades at recurrence in patients with non-skull base and skull base meningiomas. J. Neurosurg. 2010;112:925–933. doi: 10.3171/2009.9.JNS09617. [DOI] [PubMed] [Google Scholar]
- 16.Lorez, M., Nanieva, R., Arndt, V., Rohrmann, S. & Group, T. N. W. Benign and malignant primary brain tumours in the Swiss Population (2010–2014). Schweizer Kreisbulletin2, 2018 (2018).
- 17.Jadid KD, et al. Long-term follow-up of incidentally discovered meningiomas. Acta Neurochir. 2015;157:225–230. doi: 10.1007/s00701-014-2306-3. [DOI] [PubMed] [Google Scholar]
- 18.Tsuda K, et al. Benign spinal meningioma without dural attachment presenting delayed CSF dissemination and malignant transformation. Brain Tumor Pathol. 2013;30:185–191. doi: 10.1007/s10014-012-0116-y. [DOI] [PubMed] [Google Scholar]
- 19.Cimino PJ. Malignant progression to anaplastic meningioma: neuropathology, molecular pathology, and experimental models. Exp. Mol. Pathol. 2015;99:354–359. doi: 10.1016/j.yexmp.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Baeesa SS, et al. Malignant transformation and spine metastasis of an intracranial grade I meningioma: in situ immunofluorescence analysis of cancer stem cells case report and literature review. World Neurosurg. 2018;120:274–289. doi: 10.1016/j.wneu.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Barkhoudarian G, Whitelegge JP, Kelly DF, Simonian M. Proteomics analysis of brain meningiomas in pursuit of novel biomarkers of the aggressive behavior. J. Proteomics Bioinform. 2016;9:53–57. doi: 10.4172/jpb.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HD, Choi CY, Lee DJ, Lee CH. Intraventricular atypical meningiomas. J. Korean Neurosurg. Soc. 2011;49:292–295. doi: 10.3340/jkns.2011.49.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantle RE, Lach B, Delgado MR, Baeesa S, Belanger G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J. Neurosurg. 1999;91:375–383. doi: 10.3171/jns.1999.91.3.0375. [DOI] [PubMed] [Google Scholar]
- 24.Singh J, Kharosekar H, Velho V. Recurrent intraventricular meningioma with malignant transformation. Asian J. Neurosurg. 2017;12:551–555. doi: 10.4103/1793-5482.149996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy ES, et al. Risk factors for malignant transformation of low-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:965–971. doi: 10.1016/j.ijrobp.2017.12.258. [DOI] [PubMed] [Google Scholar]
- 26.Iwami K, et al. Anaplastic meningioma with rapid growth after omental flap transposition: a case report and experimental study. Brain Tumor Pathol. 2015;32:137–144. doi: 10.1007/s10014-014-0190-4. [DOI] [PubMed] [Google Scholar]
- 27.LeMay DR, Bucci MN, Farhat SM. Malignant transformation of recurrent meningioma with pulmonary metastases. Surg. Neurol. 1989;31:365–368. doi: 10.1016/0090-3019(89)90068-2. [DOI] [PubMed] [Google Scholar]
- 28.Kruse F., Jr Meningeal tumors with extracranial metastasis: a clinicopathologic report of 2 cases. Neurology. 1960;10:197–201. doi: 10.1212/wnl.10.2.197. [DOI] [PubMed] [Google Scholar]
- 29.Cornelius JF, et al. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir. 2013;155:407–413. doi: 10.1007/s00701-012-1611-y. [DOI] [PubMed] [Google Scholar]
- 30.Nishizaki T, et al. Prognostic implications of meningiomas in the elderly (over 70 years old) in the era of magnetic resonance imaging. Acta Neurochir. 1994;126:59–62. doi: 10.1007/BF01476411. [DOI] [PubMed] [Google Scholar]
- 31.Raizer JJ, et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J. Neurooncol. 2014;117:93–101. doi: 10.1007/s11060-014-1358-9. [DOI] [PubMed] [Google Scholar]
- 32.Matsuno A, et al. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol. 1996;91:504–510. doi: 10.1007/s004010050458. [DOI] [PubMed] [Google Scholar]
- 33.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82:2262–2269. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2262::AID-CNCR23>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Sahm F, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18:682–694. doi: 10.1016/S1470-2045(17)30155-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If requested by the editors, the authors will fully cooperate in obtaining and providing the data on which the manuscript is based without any restriction.