Abstract
Hepatocellular carcinoma (HCC) is the leading cause of tumor death in China with high mortality since its strong metastatic potential. Currently, treatment against advanced HCC is poorly efficient and thus screening new drugs to prevent the HCC invasion is of great significance to improve the survival rate of patients with HCC. From the results of this study, we concluded that propofol, a widely used anesthetics could prevent the proliferation by MTT assay. The scratch wound and invasion assays showed that migratory property and invasiveness in HCC cells SMMC-7721 was inhibited by propofol. This process was probably mediated by NET1 since NET1 overexpression offset the repressive effect of propofol on the invasiveness and migratory ability of SMMC-7721 cells. Furthermore, propofol treatment also reduced p-ERK1/2 and VEGF level by western blot analysis. Similar observation was found when NET1 was silenced. Thus, the results of this study provided valuable clinical therapy potential of propofol against liver cancer. We also disclosed molecular mechanism underlying the regulation of invasion and migration in HCC cells by NET1.
Subject terms: Biogeochemistry, Genetic engineering
Introduction
Liver cancer is a fatal cancer and presented the second mortality rate in the world1,2. Hepatocellular carcinoma (HCC) belonging to primary liver cancer3 acts as the third leading mortality of tumor‑related deaths in China4. HCC in China accounts for more than 50% out of incidence around the world5. Since HCC are with great invasiveness of HCC6, great progresses have been made in more than 70% of patients after the diagnosis of 1 year7. Therefore, therapy against advanced HCC is less efficient and survival of patients with advanced HCC is low8. Accordingly, it is of great significance to find novel antitumor medicine to prevent the HCC invasion.
Propofol (2, 6-diisopropylphenol) is a commonly used short-term sedative anesthetic9. Recently, its potential clinical application other than anesthesia attracts more attentions. Studies have shown that propofol could inhibit the tumor progression10–13. In HCC, propofol can inhibit proliferation, migration and invasion of liver cancer cells14. Meanwhile, propofol inhibited tumor progression of hepatocellular carcinoma xenografts in BALB/C mice15. Propofol also induces apoptosis of hepatocellular carcinoma cells16. All these studies indicated that propofol maybe a candidate drug for liver cancer. However, its underlying mechanismof the anticancer effect remains elusive.
As concluded above, we investigated the role of propofol in HCC by regulating the NET1 expression. NET1 silencing decreases the formation of new blood vessels and development of cervical squamous cell carcinoma17. NET1 also regulated chemoresistance in bladder cancer cells18. Thus, NET1 may correlate with the tumor progression and growth. Here, we indicated the inhibitory effect of propofol on HCC cell invasion and migration mediated by NET1. Meanwhile, NET1 also affects the expression of p-ERK1/2 and VEGF. Thus, this work validated the potential value of propofol in the treatment of liver cancer.
Methods and materials
Collection of HCC tissues and maintenance of HCC cell lines
All experimental procedures were approved by the Shanghai Xuhui Hospital Ethics Committee, while human tissue experiments were conducted with the patients’ written consent. All tumor tissues were kindly provided by Shanghai Xuhui hospital. The Ethics Committee of this hospital approved all bioassays and all patients signed the written consent. All experiments were performed in accordance with Shanghai Xuhui Hospital’ guidelines and regulations. And all participants were informed consent for study participation. HEK293 cell line and hepatic cancer cell lines Hep-G2, Huh7, SMMC-7721 and HL-7702 were purchased from ATCC. All HCC cell lines were maintained and passaged in Dulbecco's Modified Eagle's Medium (Gibco) added with 10% fetal bovine serum (FBS; HyClone), and incubated in a warm and moisture refrigerator supplied with 5% CO2.
NET1 silencing by RNAi
The p Silencer™ siRNA expression vector (ThermoFisher) was applied to clone NET1 siRNA or its specific scramble control. The recombinant plasmids were transfected into SMMC-7721 cells using Lipofectamine 2000 (Life Technologies, USA) based on the user guidelines. 24 h later, qRT-PCR and western blot were utilized to measure the silencing effect of siRNA respectively.
Western blot
Twenty ug of total cell lysates were quantified and separated on polyacrylamide gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane. Then, the PVDF membrane was preincubated with 5% nonfat dry milk prepared by 1 × TBST for 1 h at room temperature, and then incubated with the specific primary antibodies against NET1, p-ERK1/2, ERK1/2, VEGF and GAPDH (purchased from Cell Signaling Technologies, USA) respectively. Then membrane was then incubated with peroxidase-conjugated anti-rabbit or anti-goat IgG (purchased from ThermoFisher). These protein bands were visualized by adding ECL solution droply (Amersham Biosciences).
RNA extraction and qRT-PCR
TRIzol reagent (ThermoFisher) was used to extract total RNA from HCC cell lines or tissues according to the manufacturer’s user guidelines. Reverse transcription (RT) and one-step RT-PCR kit (Takara) were used to synthesize the first strand of cDNA. qRT-PCR was performed by SYBR Green and ABI apparatus (Thermofisher) according to the principles of manufactures. The relative level of gene expression was calculated by the 2−∆∆Ct method. Primers forward and reverse are chemically synthesized as follows: NET1, 5′-CTG TTC ACC TCG GGA CAT TT-3′ and 5′-TGG AGC TGT CAG ACG TTT TG-3′, GAPDH (5′-GGTATCGTGGAAGGACTCATGAC-3′ and 5′-ATGCCAGTGAGCTTCCCGT TCAGC-3′).
MTT assay
MTT was used to detect the effects of propofol on SMMC-7721 cell lines. Cells treated with the indicated propofol at doses of 0, 10, 25, 50, 100 μM for 48 h, and then incubated with MTT at room temperature for 4 h to produce formazan. Then, SDS-HCl was used to dissolve the formazan, and the absorbance at 570 nm was measured with a Universal Microplate Reader (Thermo). The cell viability was calculated by the following formula: Value of OD = OD of propofol-treated group/OD of blank control group.
IC50 values were the dose required to propofol inhibitory activity of 50% of the cell population and calculated from logarithmic sigmoidal dose–response curves generated using GraphPad Prism 5.0 software (GraphPad Inc).
Scratch wound assay
SMMC-7721 cells treated with or without propofol were seeded on 60 mm tissue-culture plastic dishes. When cells reached 80% cell confluence, a scratch wound was created using a sterile pipette tip. At 12, 24 and 48 h post-wound scratching, the cells among different groups were stained with 0.1% Crystal Violet and photographed in the same field of view. Wound closure was calculated according to the ratio of areas uncovered by cells before and after wound scratching.
Invasion assay
24-well plate of BD transwells was utilized to perform invasion assay. In brief, 5 × 104 cells in 100 μl serum-free medium were seeded on the upper chamber of matrigel-coated transwell, which were embedded into medium of the lower chamber. Six hundred μL 10% FBS serum medium was added to the lower chamber as a chemoattractant. After 48 h incubation, non-migrating cells were removed by wiping the upper chamber with a cotton swab. Migrated cells on the bottom side of the well were stained with Giemsa, and were counted in five random fields under a microscope (Olympus, Japan) at 40 × magnification.
Statistical analysis
Each assay was repeated at least in triplicates. Data were scientifically analyzed by Microsoft Excel and Graphpad Prism software, and presented as mean ± SEM. The intensity of western blot bands was measured by Image J software. Unpaired t test or one-way ANOVA was used to determine significant differences between two groups or multiple groups respectively.
Results
Differential expression of NET1 in hepatocellular carcinoma
To determine if NET1 was differentially expressed in hepatocellular carcinoma, we measured NET1 expression level in hepatocellular carcinoma tissue as well as hepatic cancer cell lines. The results of western blot analysis further confirmed that the relative expression of NET1 was higher in hepatic tumor when compared with that of normal tissue (Fig. 1A,B). Meanwhile, we also found that NET1 level in hepatic cancer cell line including Hep-G2, Huh7, SMMC-7721 and HL-7702 was significantly higher than that in normal HEK293T cells (Fig. 1C,D). Thus, we concluded that NET1 was significantly higher in hepatic tumors and cancer cell lines, implying its potential role in hepatic tumor growth.
Figure 1.
Aberrant expression of NET1 in hepatocellular carcinoma. (A, B) NET1 was significantly increased in hepatic tumor compared to the normal tissue. (C, D) NET1 expression was obviously elevated in hepatic cancer cell lines compared to that in HEK293 cell line.
Propofol suppressed hepatocellular carcinoma growth, migration and invasion
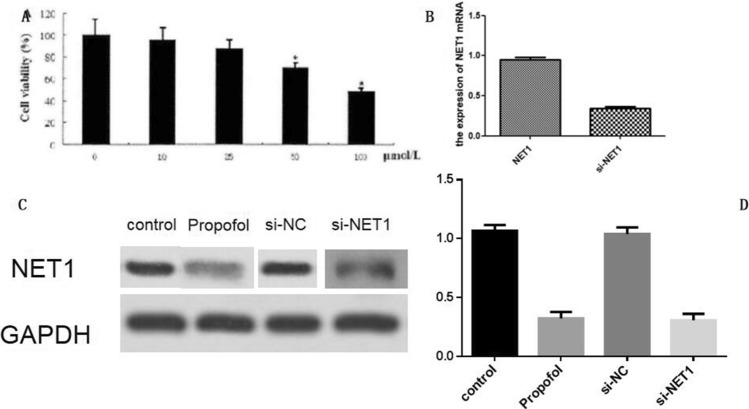
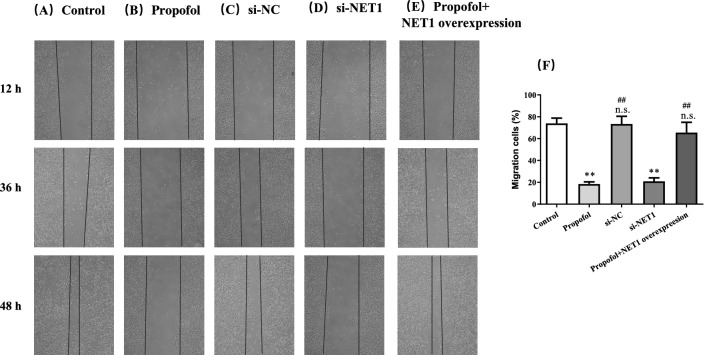
Then, we asked whether propofol affected hepatocellular carcinoma growth. Since the NET1 expression in SMM-7721 cells was the highest among HCC cell lines, SMM-7721 cells were employed for the subsequent experiments. The CCK-8 assay showed that SMMC-7721 cell viability was significantly impaired in the presence of 50 or 100 μM propofol (Fig. 2A).The IC50 of propofol was calculated as 91.12 ± 3.27 μM. Considering the cell viability was reduced to almost 50% when cells were incubated with 100 μM propofol, the concentration of propofol in subsequent experiments was set as 100 μM. Furthermore, propofol treatment led to a significant decrease in NET1 level (Fig. 2B–D). This showed that the repressive property of propofol on hepatic tumor growth was highly associated with NET1 expression. In the presence of propofol, migration of SMMC-7721 cells was negatively affected (Fig. 3A,B). By contrast, this inhibitory effect was offset with NET1 overexpression in SMMC-7721 cells (Fig. 3E). However, when NET1 was silenced, the migratory capacity in SMMC-7721 cells was negatively regulated (Fig. 3D) compared to that in scramble control group (Fig. 3C). Meanwhile, the effect of NET1 silencing on SMMC-7721 migration was comparable with that in propofol-treated cells, showing that downregulation of NET1 led to impairment in hepatic cancer cell migration.
Figure 2.
Propofol inhibited SMMC-7721cell viability through regulating NET1 expression. (A) Cells treated with the indicated propofol at doses of 0, 10, 25, 50, 100 μM for 48 h exhibited decreased cell viability. (B) NET1 mRNA level was decreased by NET1-siRNA. (C, D) NET1 protein level was decreased by propofol treatment and NET1-siRNA.
Figure 3.
The treatment of 100 μM Propofol (Panel B) or NET1 silencing (Panel D) significantly inhibited SMMC-7721 cell migration compared to control group (Panel A) and NC group (Panel C).While the inhibitory effect of propofol was offset by NET1 overexpression (Panel E). Statistical analysis on SMMC-7721 cell migration with indicted treatment (Panel F). **P < 0.01 compared with control group, n.s. represented no significance compared with control group, # and ## denote P < 0.05, 0.01 compared with si-NET1 group.
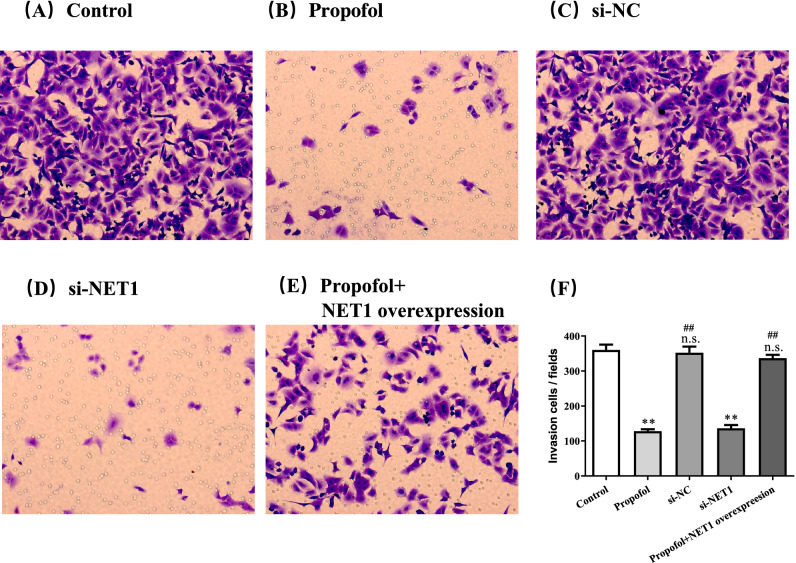
Transwell assay indicated that propofol treatment inhibited invasion of SMMC-7721 cells (Fig. 4A,B,F). With overexpression of NET1, alteration in invasion by propofol was counteracted (Fig. 4C,F). Meanwhile, NET1 silencing in SMMC-7721 cells inhibited invasion compared to that in cells transfected with scramble control (Fig. 4D–F). Therefore, we dropped a conclusion that propofol or NET1 silencing could inhibit the growth, migration and invasion, while overexpression of NET1 offset the inhibitory effect of propofol on migration and invasion in SMMC-7721 cells.
Figure 4.
The treatment of 100 μM Propofol for 48 h (Panel B) or NET1 silencing (Panel D) significantly inhibited SMMC-7721 cell invasion compared to control group (Panel A) and NC group (Panel C).While (E) the inhibitory effect of propofol was offset by NET1 overexpression (Panel E). Statistical analysis on SMMC-7721 cell invasion with indicted treatment (Panel F). **P < 0.01 compared with control group, n.s. represented no significance compared with control group, # and ## denote P < 0.05, 0.01 compared with si-NET1 group.
Propofol affected NET1 downstream pathway
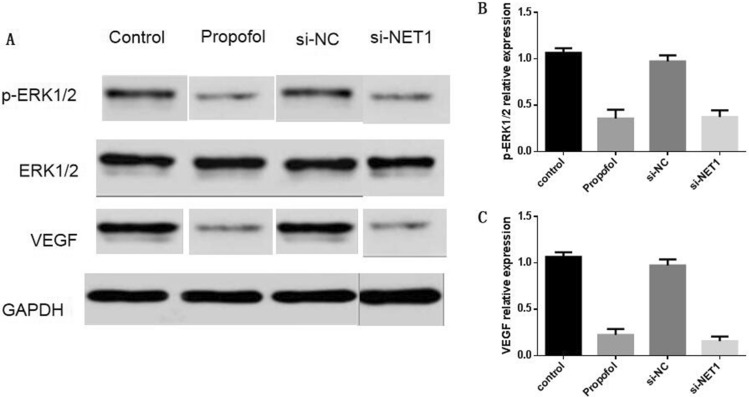
Since propofol treatment regulated NET1 expression, we determined whether it alter the expression level of key elements in NET1 downstream pathway. Western blot analysis showed that propofol decreased phosphorylation level of ERK1/2 in SMMC-7721 cells (Fig. 5). The decrease in phosphorylated ERK1/2 level was also observed when NET1 was silenced (Fig. 5). Meanwhile, propofol or NET1 silencing also decreased VEGF level (Fig. 5). Therefore, propofol treatment affected NET1 downstream target genes.
Figure 5.
(A) The treatment of 100 μM Propofol for 48 h or NET1 silencing downregulated p-ERK1/2 or VEGF protein level. (B) Statistical analysis on p-ERK1/2protein level under various conditions. (C) Statistical analysis on VEGF protein level under various conditions.
Discussion
In this study, we found NET1 regulated propofol-induced inhibitory effect on liver cancer cell invasion and migration. This work provided mechanistic evidence to support this inhibitory effect was mediated by ERK/VEGF signaling. Thus, a new mechanism underlying the influence of propofol on HCC cell migration and invasion was disclosed and this implied potential new therapeutic target against HCC.
A bunch of studies reported therapeutic role of propofol in cancer development. In lung cancer cells, propofol induced apoptosis depending on ERK1/2 signaling19. This is consistent with our findings that propofol could inhibit cell viability and decrease the p-ERK1/2 level. It was also reported that silencing of NET1 and VEGF inhibited HCC growth20. This was also similarly shown in the current study that NET1 silencing inhibited the invasion and migration in HCC cells. More importantly, we integrated propofol, NET1 and ERK/VEGF and disclosed the correlation among them to regulate the HCC cell viability, migration and invasion.
NET1 is a new member of the tetraspanins group and correlated with malignant tumor development21. To elucidate the molecular mechanism underlying NET-1, we examined and found that siNET-1 could regulate the VEGF and p-ERK1/2 level. HCC express a large number of VEGF to initiate angiogenesis to that it receives enough oxygen and nutrients supply22. VEGF is a major contributor to the tumor progression and therefore, controlling the VEGF expression may function as a promising mean to treat the HCC. Since propofol treatment or siNET1 decreased the VEGF level, further indicating propofol may function as a potential anti-HCC medicine and NET1 is likely to become a therapeutic target to HCC. Thus, more works will be focused on the in vivo study to show whether propofol or NET-1 will regulate the HCC growth and invasion in xenografted tumors.
Ethics, consent and permissions
All experimental procedures were approved by the Shanghai Xuhui Hospital Ethics Committee, while human tissue experiments were conducted with the patients’ written consent.
Consent to publish
All authors agreed to publish the study.
Author contributions
Each author has made important scientific contributions to this research and assisted in drafting or revising of the manuscript. Conceived and designed the experiments: G.F., C.G. Performed the experiments: G.F., C.G. Analyzed the data: G.F., C.G., M.C., L.C. Contributed reagents/materials/analysis tools: M.C., L.C.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Guoxiong Fei and Meili Cao.
References
- 1.Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. 2017;12:153–186. doi: 10.1146/annurev-pathol-052016-100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto A, Furuta M, Totoki Y, et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016;48:500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- 3.Tamai T, Hayato S, Hojo S, et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J. Clin. Pharmacol. 2017;57:1138–1147. doi: 10.1002/jcph.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharmacol. Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 5.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72(Suppl 1):30–44. doi: 10.1159/000111705. [DOI] [PubMed] [Google Scholar]
- 7.Castroagudin JF, Delgado M, Villanueva A, et al. Safety of percutaneous ethanol injection as neoadjuvant therapy for hepatocellular carcinoma in waiting list liver transplant candidates. Transplant Proc. 2005;37:3871–3873. doi: 10.1016/j.transproceed.2005.09.168. [DOI] [PubMed] [Google Scholar]
- 8.Gluer AM, Cocco N, Laurence JM, et al. Systematic review of actual 10-year survival following resection for hepatocellular carcinoma. HPB (Oxford). 2012;14:285–290. doi: 10.1111/j.1477-2574.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleck T, Schubert S, Ewert P, et al. Propofol effect on cerebral oxygenation in children with congenital heart disease. Pediatr. Cardiol. 2015;36:543–549. doi: 10.1007/s00246-014-1047-7. [DOI] [PubMed] [Google Scholar]
- 10.Mammoto T, Mukai M, Mammoto A, et al. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–170. doi: 10.1016/S0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 11.Miao Y, Zhang Y, Wan H, et al. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother. 2010;64:583–588. doi: 10.1016/j.biopha.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet. Mol. Res. 2015;14:7529–7537. doi: 10.4238/2015.July.3.28. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZT, Gong HY, Zheng F, et al. Propofol suppresses proliferation and invasion of gastric cancer cells via downregulation of microRNA-221 expression. Genet. Mol. Res. 2015;14:8117–8124. doi: 10.4238/2015.July.17.20. [DOI] [PubMed] [Google Scholar]
- 14.Liu SQ, Zhang JL, Li ZW, et al. Propofol inhibits proliferation, migration, invasion and promotes apoptosis through down-regulating miR-374a in hepatocarcinoma cell lines. Cell Physiol. Biochem. 2018;49:2099–2110. doi: 10.1159/000493814. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang N, Cao Q, et al. The effects of propofol on the growth behavior of hepatoma xenografts in Balb/c mice. Biomed. Pharmacother. 2017;90:47–52. doi: 10.1016/j.biopha.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Wu GQ, Zhang Y, et al. Propofol induces apoptosis of hepatocellular carcinoma cells by upregulation of microRNA-199a expression. Cell. Biol. Int. 2013;37:227–232. doi: 10.1002/cbin.10034. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Xia P, Zhang W, et al. Short interfering RNA targeting Net1 reduces the angiogenesis and tumor growth of in vivo cervical squamous cell carcinoma through VEGF down-regulation. Hum. Pathol. 2017;65:113–122. doi: 10.1016/j.humpath.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Xiao J, Niu S, Zhu J, et al. miR223p enhances multichemoresistance by targeting NET1 in bladder cancer cells. Oncol. Rep. 2018;39:2731–2740. doi: 10.3892/or.2018.6355. [DOI] [PubMed] [Google Scholar]
- 19.Xing SG, Zhang KJ, Qu JH, et al. Propofol induces apoptosis of non-small cell lung cancer cells via ERK1/2-dependent upregulation of PUMA. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4341–4349. doi: 10.26355/eurrev_201807_15431. [DOI] [PubMed] [Google Scholar]
- 20.Wu YY, Chen L, Wang GL, et al. Inhibition of hepatocellular carcinoma growth and angiogenesis by dual silencing of NET-1 and VEGF. J Mol Histol. 2013;44:433–445. doi: 10.1007/s10735-012-9480-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Ji Q. Net1, a therapeutic potential target for cancer. Hum. Pathol. 2018;71:168. doi: 10.1016/j.humpath.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Ng IO, Poon RT, Lee JM, et al. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am. J. Clin. Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]