Abstract
Over the past decade, the spotted wing Drosophila, Drosophila suzukii, has invaded Europe and America and has become a major agricultural pest in these areas, thereby prompting intense research activities to better understand its biology. Two draft genome assemblies already exist for this species but contain pervasive assembly errors and are highly fragmented, which limits their values. Our purpose here was to improve the assembly of the D. suzukii genome and to annotate it in a way that facilitates comparisons with D. melanogaster. For this, we generated PacBio long-read sequencing data and assembled a novel, high-quality D. suzukii genome assembly. It is one of the largest Drosophila genomes, notably because of the expansion of its repeatome. We found that despite 16 rounds of full-sib crossings the D. suzukii strain that we sequenced has maintained high levels of polymorphism in some regions of its genome. As a consequence, the quality of the assembly of these regions was reduced. We explored possible origins of this high residual diversity, including the presence of structural variants and a possible heterogeneous admixture pattern of North American and Asian ancestry. Overall, our assembly and annotation constitute a high-quality genomic resource that can be used for both high-throughput sequencing approaches, as well as manipulative genetic technologies to study D. suzukii.
Subject terms: Invasive species, Structural variation, DNA transposable elements, Genomics, Comparative genomics, Ecological genetics, Genetic hybridization
Introduction
Drosophila suzukii (Matsumura, 1931), the spotted wing Drosophila (Diptera: Drosophilidae), is an invasive fruit fly species originating from eastern Asia that has spread since 2008 in major parts of America and Europe. This species is still expanding its distribution1,2 and is classified as a major pest on a variety of berries and stone fruit crops3. Its behavior and phenotypic traits are now the subject of intense scrutiny both in the lab and in the field (reviewed in4).
Understanding the biology and the population dynamics of D. suzukii benefits from the production and mining of genomic and transcriptomic data, as well as manipulative genetic technologies including functional transgenesis and genome editing5–7. Yet, the efficacy of these approaches relies critically on high-quality genomic resources. Currently, two D. suzukii genome assemblies, obtained from two different strains, have been generated based on short-read sequencing technologies8,9. The utility of these valuable genomic resources is limited by the extensive and inescapable assembly errors, as well as the high fragmentation rates, that characterize short-read sequencing genome assemblies.
While short-read sequencing has dramatically contributed to the vast repertoire of genomes available nowadays, it has the unsolvable issue that ~ 100 bp-long reads cannot resolve genomic structures with low-complexity or polymorphic regions, and produce a large number of relatively short contigs (e.g.9). The advent of long-read sequencing technology (e.g. nanopore, PacBio) that produces reads that are several dozens of kilobases (kb) long on average has proven an efficient tool to circumvent those limitations and allows to assemble much longer contigs at least for small to medium-sized genomes10–15.
Genome assemblies using long-read sequencing have been generated for at least 18 Drosophila species16–19. Somewhat surprisingly given its economic importance, D. suzukii is missing from this list. In this article, we report the genome assembly of an inbred D. suzukii strain using the long-read Pacific Biosciences (PacBio) technology. The assembly compares favorably to previous ones in terms of general assembly statistics, detailed sequence quality and gene annotation, although some parts, mostly located on regions homologous to the 3R D. melanogaster chromosome arm, remained fragmented and displayed high residual genetic diversity in the strain. The improved assembly allowed us to further explore some specific aspects of the D. suzukii genome including repeats content, structural variants, sequence diversity and population genetic origins.
Results
Available D. suzukii genomic resources contain pervasive local assembly errors
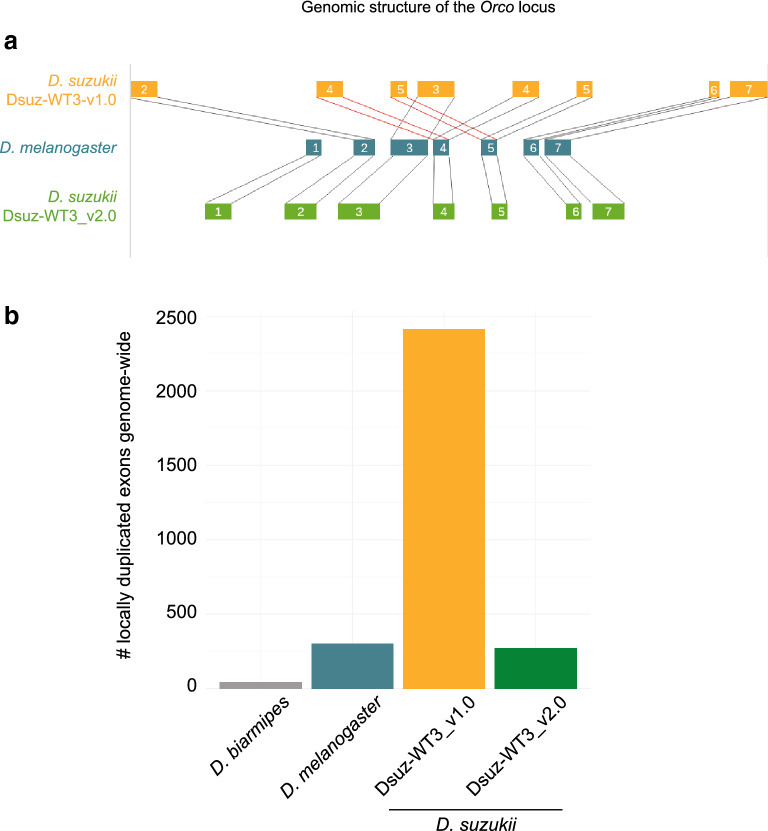
The initial genome assembly of an Italian D. suzukii strain, inbred for 5 generations, was highly fragmented (e.g. N50 of 4.5 kb for the contigs, L50 of 87008). In parallel, a second strain, WT3, established from a single female collected in Watsonville, CA, U.S.A, and inbred for 10 generations by sib-mating, had been sequenced9. The latter assembly, hereafter called Dsuz-WT3_v1.0, was more contiguous than the assembly by Ometto et al., (2013) as judged by summary statistics (e.g. N50 of ~ 27 kb for the contigs and ~ 385 kb for the scaffolds, and L50 of 73 for the scaffolds; Chiu et al. 2013). Nevertheless, we recurrently observed inconsistencies between the Dsuz-WT3_v1.09 assembly and Sanger sequencing data we obtained for specific loci amplified using PCR from the D. suzukii strain used by Chiu et al. (2013). For instance, BLAST alignments of the Orco locus between the Dsuz-WT3_v1.0 assembly and the reference D. melanogaster assembly dm6 indicated that exons 4 and 5 were repeated twice in the middle of the gene (Fig. 1a), a feature not confirmed by Sanger sequencing data. We suspected that the published genome sequence contained a local assembly error corresponding to a ~ 1 kb long region incorrectly repeated twice. To test if such a pattern of locally duplicated exons was widespread in the Dsuz-WT3_v1.0 assembly, we aligned with BLAST all the annotated exons against each other, only retaining hits that were within a 5 kb window of each other and with a near-perfect score (score > 4.8 on a scale ranging from 1 to 5). For comparison, the same procedure was applied to assemblies of D. melanogaster and D. suzukii, and of the sister species D. biarmipes (Fig. 1b). We only found a few locally duplicated exons in the D. melanogaster assembly and almost none in the D. biarmipes assembly. Conversely, the Dsuz-WT3_v1.0 assembly contained thousands of neighboring, nearly identical exons (corresponding to ~ 1,000 transcripts and ~ 600 genes), i.e. at least 10 times more than in D. melanogaster and 50 times more than in D. biarmipes. Assuming that the genome of D. suzukii contains similar levels of near identical adjacent exons as D. melanogaster or D. biarmipes, we suspected that the D. suzukii Dsuz-WT3_v1.0 assembly may contain many local assembly errors. Such errors could be caused, at least partly, by both a high level of residual polymorphism in the sequenced D. suzukii strain, and by the limitations of the short-read sequencing technology.
Figure 1.
(a) Genomic structure of the Orco gene in the Dsuz-WT3_v1.0 genome assembly9 and in the Dsuz-WT3_v2.0 assembly (this article). Genomic structure in D. melanogaster is shown for comparison. The locus encompassing exon 1 is missing in the Dsuz-WT3_v1.0 assembly. (b) Number of nearly identical neighboring exons present in D. suzukii assemblies Dsuz-WT3_v1.0 and Dsuz-WT3_v2.0, and in the D. biarmipes Dbia_1.0 and D. melanogaster dm6 assemblies.
Long-read sequencing and de novo genome assembly
To reduce the genetic diversity of the WT3 strain used for the Dsuz-WT3_v1.0 assembly9, we further isogenized flies from this strain by processing full-sib crosses for six generations, resulting in a total of at least 16 generations of inbreeding. We named this new D. suzukii strain Dsuz-WT3_v2.0 and sequenced genomic DNA extracted from 40 Dsuz-WT3_v2.0 females to a coverage of 160 × using the single molecule real-time sequencing on the Pacific Biosciences technology platform.
We followed a customized approach for the assembly step (see “Materials and methods” for details), paying special attention to both bacterial contamination and the putative presence of different haplotypes of the same locus assembled as separate sequences. The resulting assembly consisted of 546 contigs for an overall size of ~ 270 Mb, which is closer to the estimated genome size of ~ 313 Mb20 than previous assemblies (cf. 232 Mb in Chiu et al., 2013 and 160 Mb in Ometto et al., 2013). Assembly statistics, both in terms of continuity and completeness (N50 of 2.6 Mb, L50 of 15, BUSCO score of 95%), indicated substantial improvements over the Dsuz-WT3_v1.0 assembly (see Supplementary Table S1 for assembly statistics and Supplementary Fig. S1 for BUSCO results). Importantly, the correct non-duplicated structure of the Orco gene was recovered and, more generally, the number of locally duplicated exons in the Dsuz-WT3_v2.0 assembly was similar to that observed in the D. melanogaster assembly (Fig. 1).
For approximately 34 Mb of the Dsuz-WT3_v2.0 assembly two haplotypes of homologous sequence were inferred (see “Materials and methods”) whereas the rest of the assembly was identified as haploid. Distinguishing alternative sequences (or haplotypes) from recent duplicates is notoriously difficult. The coverage at regions that have been assembled as one versus regions with two haplotypes was similar (Supplementary Fig. S2a) providing evidence for the former.
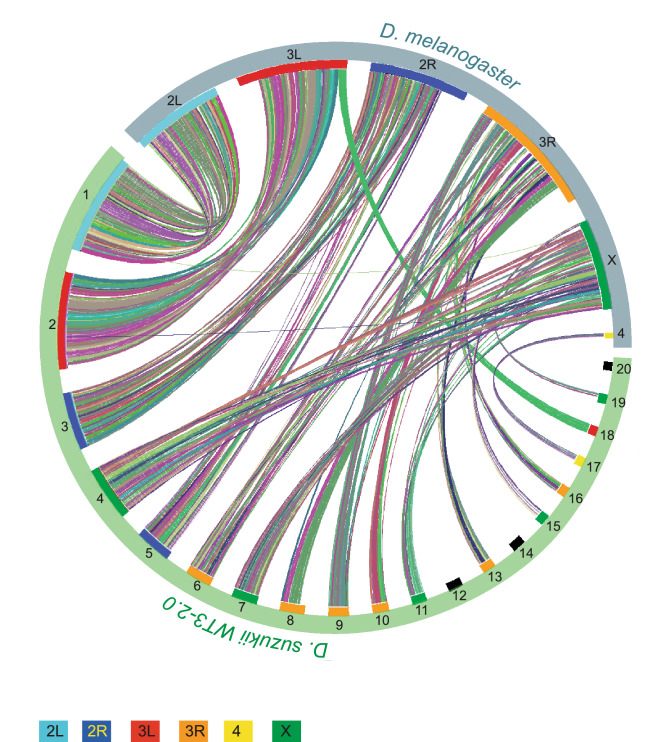
Forty-nine contigs of the Dsuz-WT3_v2.0 assembly could be unambiguously aligned onto the D. melanogaster dm6 genome assembly. Those 49 contigs covered ~ 153 Mb (~ 57% of the Dsuz-WT3-2.0 assembly, ~ 82% of the annotated genes) and corresponded to most of the largest contigs enriched for protein coding genes. For all but one of those 49 contigs, over 99% of the aligned section matched a unique D. melanogaster chromosome. Even the near-chromosome length contigs almost fully aligned to a unique D. melanogaster chromosomal arm (e.g., the 26 Mb-long contig1 aligned to 2L and the 25 Mb-long contig2 aligned to 3L; Fig. 2). This result suggests that few inter-chromosomal rearrangements occurred since the last common ancestor of D. melanogaster and D. suzukii. Still, sequence similarity with D. melanogaster provides a reliable estimate of the chromosomal origin for most D. suzukii contigs (Fig. 2).
Figure 2.
Co-linearity between the 20 longest contigs of the Dsuz-WT3_v2.0 assembly and D. melanogaster chromosome arms. The graph was built using VGST67 (details in the methods section). Colors represent synteny blocks automatically assigned by VGSC.
Next, we sequenced one male and one female with short-reads at approximately 20X coverage to assign contigs to either autosomes or the X chromosome based on the ratio of male to female read coverage. This confirmed previous contig assignment and led to the assignment of six additional contigs (totaling 337 kb) to the X-chromosome and 258 additional contigs (totaling 101 Mb) to autosomes. The list of contig-chromosome associations is described in Supplementary Table S3. The remaining 234 contigs (amounting to ca. 13 Mb and hence representing less than 5% of the assembly) could not be assigned to any autosomes or the X chromosome. Those 234 contigs were small (average size of 57 kb) and tended to contain more repetitive elements (~ 0.3 elements per kb vs 0.17 repetitive elements for the other contigs), which probably explains why they could not be assigned a clear orthologous sequence in D. melanogaster or an autosome/X chromosome.
The consistency between both assignment methods (sequence synteny with D. melanogaster and male over female genomic read coverage) suggested that no large-scale translocations between the X and the autosomes occurred since the split between D. suzukii and D. melanogaster genomes. Accordingly, nomenclature of D. suzukii contigs was based on synteny and contigs were named after the arm to which they align on the D. melanogaster genome. Because the Dsuz-WT3_v2.0 assembly was obtained from female DNA only, the Y chromosome could not be sequenced and assembled.
Genome annotation
We compared the content of various categories of repeated elements in our new D. suzukii assembly with that of other Drosophila species, including D. melanogaster, D. biarmipes and D. takahashi. We found that our D. suzukii assembly has a particularly large repeatome (~ 93 Mb corresponding to more than 35% of our 270 Mb-long genome assembly, Supplementary Fig. S3a and Supplementary Fig. S3b), which was twice the size of the repeatome estimated from the previous Dsuz-WT3_v1.0 D. suzukii assembly. The repeatome in a D. melanogaster assembly made from the same PacBio technology21 was about half less, amounting to 45 Mb in size and corresponding to 25% of the genome. Likewise, the repeatome was around 21% of the genome in both D. biarmipes and D. takahashi. Overall, the ~ 50 Mb inflation of repetitive elements in the ~ 270 Mb D. suzukii genome compared to the ~ 150 Mb-long D. melanogaster genome represents about half of the genome expansion in D. suzukii.
We assessed whether this increase in repetitive sequences was coupled with a change in element repertoire. There are different types of repetitive elements such as satellite DNA, long terminal repeat (LTR) retrotransposons, LINE (long interspersed nuclear element)-like retrotransposons, or terminal inverted repeat (TIR) DNA-based transposons22. The analysis was done for eight Drosophila species scattered along the Drosophila phylogenetic tree: D. suzukii, D. biarmipes, D. takahashi, D. melanogaster, D. yakuba, D. ananassae, D. persimilis and D. grimshawi. The element repertoire followed closely the species phylogeny (Supplementary Fig. S3d, Supplementary Fig. S3f, Supplementary Fig. S3h, Supplementary Fig. S3j, Supplementary Fig. S3l, Supplementary Fig. S3n, Supplementary Fig. S3p, Supplementary Fig. S3r). For instance, the different types of elements were found in the D. suzukii genome in proportions similar to those found in the genome of its most closely related species D. biarmipes and D. takahashii (Supplementary Fig. S3d, f, h) but were very different from those of the most distantly related species D. grimshawi (Supplementary Fig. S3s). And all the values laid in between for the other species. The detailed repertoire from each class was more variable but followed the same phylogenetic signal (Supplementary Fig. S3e, Supplementary Fig. S3g, Supplementary Fig. S3i, Supplementary Fig. S3k, Supplementary Fig. S3m, Supplementary Fig. S3o, Supplementary Fig. S3q, Supplementary Fig. S3s).
Finally, we annotated the genome for coding sequences using three sources of information: (i) de novo predictions, (ii) sequence similarity with D. melanogaster gene annotations, and (iii) D. suzukii RNAseq data that we produced from several embryonic and adult tissues. For compact genomes, gene prediction methods tend to annotate neighboring genes as erroneous chimers23, an issue we also encountered. To partially fix this problem, we systematically searched and corrected those erroneous fusions and also manually curated about 50 genes of particular interest. The resulting annotation was composed of 18,241 genes, 10,557 of them showing a clear orthology with D. melanogaster genes using our genome alignment (Supplementary Table S2). Out of the 10,557 genes found in both Dsuz-WT3_v2.0 and D. melanogaster, 8,576 were also found in the annotation of the Dsuz-WT3_v1.0 assembly (Chiu et al. 2013). The 1,981 missing genes were mostly located in genomic regions that were poorly assembled or absent in the Dsuz-WT3_v1.0 assembly.
Understanding pervasive fragmentation of some parts of the assembly
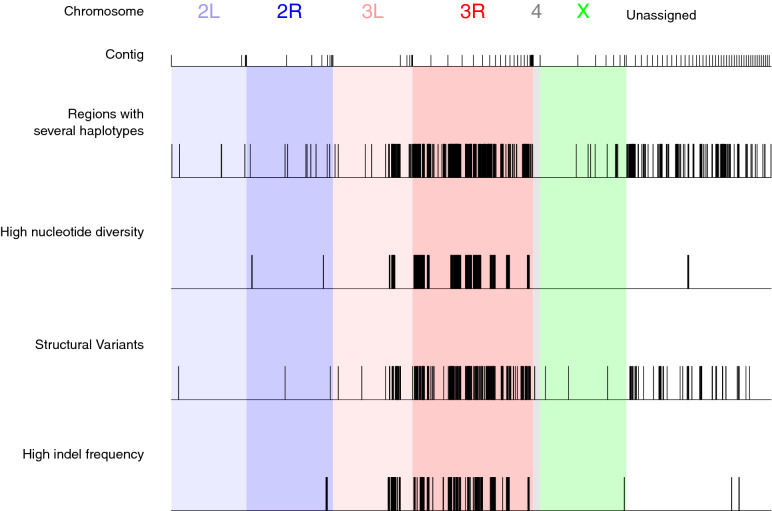
Although improved compared to previous genome assemblies, the quality of the Dsuz-WT3_v2.0 assembly varied along the genome. In particular, the contigs matching chromosome arm 3R were poorly assembled and were the most fragmented part of our assembly (Fig. 2). The longest contig associated with 3R was relatively short (~ 6.5 Mb), contributing to ~ 17% of the total length of contigs associated with 3R, compared to ~ 15 Mb to 26 Mb (i.e., 44 to 93% of total length) for other chromosomes. In addition, the Dsuz-WT3_v2.0 assembly contained ~ 12 Mb of regions with high rates of local sequence errors in the form of small indels, mostly located in the same genomic areas as the regions assembled as distinct haplotypes, that is on chromosome 3 (76% on 3R and 18% on 3L; Fig. 3).
Figure 3.
Location at the contig level of various genomic features on the Dsuz-WT3_v2.0 assembly. Regions assembled as distinct haplotypes, regions of higher nucleotide diversity, regions with structural variants, regions with higher one-nucleotide assembly errors (in the form of indels) are highlighted. The criteria for defining the boundaries of “high indel rate” and “high nucleotide diversity” regions were as follows: a region is initialized if the rate is above 0.005 on at least 5 consecutive windows of 10 kb; the region is closed if the rate drops below 0.001 on at least 5 consecutive 10 kb windows. Using this rule, 64 regions with high indel rates (median length 140 kb) and 27 regions with high nucleotide diversity (median length 420 kb) were identified. Contigs are ordered according to their chromosomal assignment. Only the longest 100 contigs are shown, representing 79% of the total length of the assembly.
About 34 Mb of the Dsuz-WT3_v2.0 genome was assembled as two distinct haplotypes (Fig. 3 and see above), suggesting that residual genetic diversity was maintained in some regions of the D. suzukii strain we sequenced, despite a total of 16 rounds of full-sib crossing. We tested this hypothesis by characterizing the patterns of nucleotide diversity estimated from the sequencing of pools (Pool-seq) of 26 individuals from the Dsuz-WT3_v2.0 strain. We confirmed that those regions had a higher nucleotide diversity compared to other genomic regions (Supplementary Fig. S2b). This was also true when focusing on chromosome 3R (Supplementary Fig. S2c).
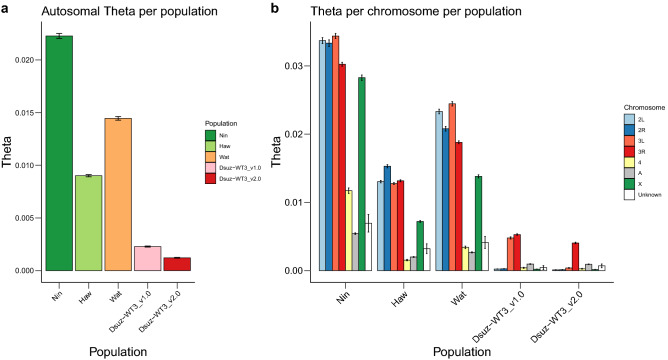
Fraimout et al. (2017)24 showed that the population of Watsonville (USA), from which the inbred strain WT3 was derived, originated from an admixture between the native population from Ningbo (China) and the invasive population from Hawaii (USA). This hybrid origin may have resulted in heterogeneity in the distribution of genetic diversity in the genome, maintained in the Dsuz-WT3_v2.0 assembly despite strong inbreeding. To test this hypothesis, we first used Pool-seq data to compare the patterns of nucleotide diversity in (i) the Dsuz-WT3_v1.0 strain (data from9), (ii) the Dsuz-WT3_v2.0 strain (see above), (iii) a population sample of the Watsonville area (US-Wat) from which the Dsuz-WT3_v1.09 and Dsuz-WT3_v2.0 (this study) strains originate, and (iv) the aforementioned two source populations of the admixed population from Watsonville (US-Haw and US-Nin). As expected, the genome-wide autosomal nucleotide diversity was maximal in the native Chinese population Ningbo (θ = 22.4 × 10–3), lower in the introduced invasive population from Hawaii (θ = 9.05 × 10–3), and intermediate in the admixed population of Watsonville (θ = 14.5 × 10–3) (Fig. 4a). In addition, the nucleotide diversity was strongly reduced in the Dsuz-WT3_v1.0 strain (θ = 2.21 × 10–3), as a result of ten generations of full-sib crossing, and even further reduced in the Dsuz-WT3_v2.0 strain (θ = 1.22 × 10–3), which underwent six additional generations of full-sib crossing (Fig. 4a). Interestingly, at a chromosomal genomic scale, the distribution of nucleotide diversity was heterogeneous, with contigs mapping to chromosome arms 3L and 3R showing substantial residual diversity in the Dsuz-WT3_v1.0 strain with estimated θ = 4.88 × 10–3 and θ = 5.50 × 10–3, respectively, while θ < 0.50 × 10–3 in contigs assigned to other chromosome arms (Fig. 4b). In the Dsuz-WT3_v2.0 strain, the nucleotide diversity dropped to θ = 0.39 × 10–3 in 3L, but 3R conserved almost the same levels of diversity as in the Dsuz-WT3_v1.0 strain (θ = 4.07 × 10–3, Fig. 4b). These results therefore support our hypothesis that residual genetic diversity is maintained in D. suzukii strain Dsuz-WT3_v2.0, notably on chromosome arm 3R. Accordingly, 89% of the regions with several haplotypes that could be assigned to a D. melanogaster chromosome mapped to 3R. This polymorphism is probably one of the reasons why the Dsuz-WT3_v2.0 assembly is more fragmented in 3R and why the Dsuz-WT3_v1.09 contains many poorly assembled regions. In agreement, the nearly identical neighboring exons of the Dsuz-WT3_v1.0 assembly9 (Fig. 1b) mapped preferentially to the Dsuz-WT3_v2.0 regions with high nucleotide diversity or high indel rate, which are described in Fig. 3 (~ sixfold enrichment, Fisher’s p value < 2.2 0.10–16 in both cases, Supplementary Fig. S5a) and more generally to chromosomal arms 3R and 3L (Supplementary Fig. S5b).
Figure 4.
Comparison of nucleotide diversity among D. suzukii strains and populations, and among chromosomes. Nucleotide diversity was estimated from pools of individuals for a Chinese population (Nin), a Hawaiian population (Haw), the Watsonville population (Wat), the Dsuz-WT3_v1.0 strain9 and the Dsuz-WT3_v2.0 strain (this study). Values of nucleotide diversity parameter Theta (θ) were estimated over 10 kb windows (a) for all autosomes or (b) per chromosome. “2L”, “2R”, “3L”, “3R”, “4”, “X”: assigned D. melanogaster chromosome for each Dsuz-WT3_v2.0 contigs. “A”: autosomal contigs with no clear corresponding D. melanogaster chromosome. “Unknown”: contigs for which chromosomal features (i.e., assignment to autosomal or X chromosomes and to D. melanogaster chromosome arm) remain unknown. Error bars correspond to S.E.M.
Next, we tested whether the elevated sequence diversity on chromosomal arm 3R in the Dsuz-WT3_v1.09 and Dsuz-WT3_v2.0 strains could be explained by a heterogeneous pattern of local ancestry origin by characterizing the relative contributions of the Chinese and Hawaiian ancestries to the genome assembly, both at a global and at a chromosomal genomic scale. More specifically, we developed a Hidden Markov Model to determine the genetic origin of the assembled genome at each position using the Pool-seq data produced for the two populations Ningbo and Hawaii mentioned above (see Supplementary Text S2). Overall, the mean fraction α of the assembled genome with a Ningbo origin was found equal to 0.784 (SD = 1.86 × 10–3) for autosomal regions and 0.763 (SD = 8.52 × 10–3) for X-linked regions (Supplementary Table S3). We found no significant differences in α values among the different chromosomes (Supplementary Fig. S4). Remarkably, these α values were close to those found in a previous study based on microsatellite markers for the wild source population from Watsonville (i.e. α = 0.759, 90% credibility interval [0.659; 0.854]) 24. The relative proportions of Chinese and Hawaiian ancestry characterizing the source population from Watsonville have thus been globally preserved in the Dsuz-WT3_v2.0 strain. The regions with several haplotypes, which are mostly concentrated on chromosome arm 3R, only showed a very mild difference in their inferred Hawaiian/Ningbo origin compared to the other regions of the genome (Supplementary Fig. S2d). This means that the elevated nucleotide diversity on 3R (Supplementary Fig. S2b and S2c) cannot be explained by a peculiar pattern of admixture, where for instance a Ningbo ancestry would have been preferentially retained in these genomic regions.
Finally, we tested whether an excess of structural variants on 3R could contribute to the higher levels of residual polymorphism in the contigs mapping to this particular chromosome arm. Structural variants (SV) are an important source of large-scale polymorphism (e.g. 25) but are difficult to detect using short sequencing reads and have been essentially studied by comparing populations that carry different fixed variants (e.g.26,27). To study SVs in our Dsuz-WT3_v2.0 strain, we took advantage of PacBio long reads, which give unprecedented access to such information. We detected a total of 369 SVs, mostly Copy Number Variations (338 CNVs including 219 deletions, 59 insertions and 60 duplications), 23 translocations and 8 inversions. We found that the SVs co-localized well with the highly polymorphic regions (Fig. 3). We noted that the inversions were of relatively large size, with two inversions longer than 100 kb, and an average size of ~ 40 kb (compared to 21 kb in D. melanogaster21). We confidently assigned six inversions to a D. melanogaster chromosome and found that three of them were located on 3R (including one of the longest). In addition, we could confidently assign a D. melanogaster chromosome to both extremities of 10 translocations, out of which five were between contigs of different chromosomes, and five between contigs of 3R. This is probably because 3R is the most fragmented chromosome in our assembly, so both extremities of the translocation event are located on two different contigs that belong to the same chromosome but have not been assembled together. Those translocations were located at the end of contigs and are thus probably not real inter-chromosomal translocations but rather either consecutive contigs on 3R or very large inversions within 3R. Altogether, these results are consistent with the hypothesis that the maintenance of structural variants contributes to the residual sequence polymorphism in some areas of the Dsuz-WT3_v2.0 genome assembly, in particular on 3R.
Discussion
In this article, we used the PacBio long-read technology to re-sequence, assemble and annotate the genome of D. suzukii, an invasive fly species that has caused agricultural damage worldwide. This genome was presumably more difficult to assemble than most other Drosophila genomes because of its larger size and higher content of repetitive elements20. It is hence not surprising that the previous D. suzukii genome assemblies based on short-read sequencing technologies8,9 contained pervasive assembly errors. The long-read assembly presented here constitutes a clear improvement, which is in line with other assemblies obtained using the long-read technology that bypasses the limitations of short-read sequencing (e.g.17,19,21). Besides improving general assembly statistics, we made this assembly as workable as possible, notably for molecular biologists. To this aim, we paid special attention to assembly errors caused by contamination or a poor handling of polymorphism by assembly tools.
We also produced a gene annotation that could be compared with D. melanogaster (orthology table given as Supplementary Table S2). Although our D. suzukii assembly corresponds to the second largest Drosophila genome after D. virilis (333 Mb28), it has a similar gene content compared to D. melanogaster, a feature observed so far for all sequenced Drosophila species (e.g.,29). We found that the D. suzukii genome contains a high amount of repetitive sequences, as previously shown using a genome-assembly free approach20. Our results suggest that this expansion of the repeatome is responsible for at least half of the increase in genome size in D. suzukii (roughly + 100 Mb in our assembly), as compared to the closely-related species D. melanogaster.
We found that, although nucleotide diversity was globally strongly reduced in the inbred strain Dsuz-WT3_v2.0 (and to a lesser extent in the strain Dsuz-WT3_v1.0) compared to the wild source population from Watsonville, the residual diversity was heterogeneously distributed among chromosomes, with the highest levels observed on the chromosome 3R homolog for Dsuz-WT3_v2.0 (in 3R and 3L for Dsuz-WT3_v1.09). This higher residual polymorphism of chromosomal arm 3R is probably responsible, at least partly, for the reduced assembly quality in this genomic region. Because PacBio reads have a very high error rate (~ 15%, mostly insertions30), the assembly algorithms that we used tend to interpret heterozygous SNPs as sequencing errors (i.e., insertions) to be removed. Thus, this results in an “overpolished” assembly that contains small errors in the form of single nucleotide deletions (personal communication from PacBio). In agreement with this, we did detect a higher rate of indels on 3R. As a consequence, special caution should be observed for regions of high polymorphism because they tend to display higher assembly error (in the form of one-nucleotide indels).
The substantial level of residual nucleotide diversity in the Dsuz-WT3_v2.0 strain on the chromosome 3R homolog remains puzzling. This could be explained, at least partly, by selective processes such as balancing selection and associative overdominance that could maintain multiple gene variants at frequencies larger than expected from genetic drift alone31. In addition, a high level of sequence diversity in specific regions could be maintained by local, complex genomic content and structures. For instance, an uneven chromosomal distribution of repetitive elements could have explained part of the elevated nucleotide diversity on 3R. However, we found no enrichment of repetitive elements on contigs assigned to this chromosome (Supplementary Fig. S3c). Large polymorphic inversions have also regularly been shown to maintain sequence polymorphism because they prevent recombination between paired loci (e.g.25,32). We detected some inversions on 3R, but they are too small to fully account for the high residual sequence polymorphism on this chromosome. Large inversions are difficult to identify on 3R because they would likely appear as translocations since both extremities of the inversions would end up on different contigs. In agreement with this, many of the translocations that we detected involved two contigs located on 3R. However, the current state of our assembly does not allow us to provide a clear answer. Assembling chromosome 3R from the genome of the parental populations (i.e., Hawaii and Ningbo), in which such long inversions might be absent or fixed, may help solving this issue because those would be homozygous for the inversions and thus easier to assemble. Long inversions could also be searched using methods that detect physical linkage between regions of the genome (e.g. Hi–C19,33,34). Our results globally suggest that despite the tremendous progress in sequencing technology, the complexity and diversity of genomic structures and sequences, even within an isogenized strain, might make full chromosome-length assemblies difficult to reach for some regions in some species, and the problem worsens for wild individuals.
Conclusion
Our Dsuz-WT3_v2.0 assembly provides a higher quality genomic resource compared to the previous one. It confirms the benefits of long-read sequencing for de novo assembly. As a short-term perspective, we anticipate that our near-chromosome level assembly should be amenable to a chromosome-level assembly. In particular, scaffolding methods using Hi–C data will represent one of the most promising routes to this purpose. We believe that our improved D. suzukii assembly will provide a solid genomic basis to investigate basic biological questions about D. suzukii, using high-throughput sequencing technologies as well as manipulative genetic technologies35.
Materials and methods
Whole-genome long-read sequencing of the Dsuz-WT3_v2.0 D. suzukii strain
The Dsuz-WT3_v2.0 D. suzukii individuals used to produce our genome assembly derived, after six additional generations of full-sib crossing, from the WT3 isofemale strain (here named Dsuz-WT3_v1.0) that was previously established from a female sampled in Watsonville (USA) and sequenced by9. The Dsuz-WT3_v2.0 strain hence went through a total of at least 16 rounds of full-sib crossing.
Genomic DNA extraction
High-molecular weight DNA was extracted from 40 adult D. suzukii females (Dsuz-WT3_v2.0) using the Blood & Cell culture DNA midi kit (Qiagen). The quality and concentration of the DNA was assessed using a 0.5% agarose gel (run for > 8 h at 25 V) and a Nanodrop spectrophotometer (ThermoFisherScientific). PacBio libraries were generated using the SMRTbell™ Template Prep Kit 1.0 according to manufacturer’s instructions. In brief, 10 µg of genomic DNA per library (estimated by Qubit assay) was sheared into 20 kb fragments using the Megaruptor system, followed by an exo VII treatment, DNA damage repair and end-repair before ligation of hair-pin adaptors to generate a SMRTbell™ library for circular consensus sequencing. The library was then subjected to exo treatment and PB AMPure bead wash procedures for clean-up before it was size selected with the BluePippin system (SAGE) with a cut-off value of 9,000 bp. In total 48 units of SMRTcell™ with library was sequenced on the PacBio Sequel instrument using the Sequel 2.0 polymerase and 600 min movie time. The raw data were then imported into the SMRT Analysis software suite (v2.3.0) where subreads shorter than 500 bp and a polymerase read quality below 75 were filtered out.
Genome assembly based on PacBio long reads
We generated two separate assemblies using two approaches: Falcon (https://github.com/PacificBiosciences/FALCON) using the parameters detailed in the Supplementary Text S1 and Canu 1.336 with the default options (except -minReadLength = 7,000 -stopOnReadQuality = 0 -minOverlapLength = 1,000).
The resulting Falcon assembly, hereafter called dsu_f, was 281 Mb long while the Canu assembly, hereafter called dsu_c, was 267 Mb long. We noticed that each assembly lacked different parts of the genome. For instance the gene Abd-B was absent from dsu_c and the gene Or7a was absent from dsu_f; see Supplementary Fig. S1 for more exhaustive BUSCO gene content statistics37. We therefore decided to follow a hybrid strategy (similarly to38) and merged these two assemblies. To that end we proceeded in three successive merging steps (following recommendations provided by Mahul Chakraborty’s) using: (i) the nucmer (with options -l 100) and delta-filter (with options -i 95 -r -q) programs from the MUMmer v3.23 package39 to perform alignment of assemblies on a whole genome scale, and (ii) the Quickmerge program38 (with options -hco 5.0 -c 1.5 -l 660,000 -ml 10,000) to merge assemblies based on their resulting alignment. In the first step, we aligned dsu_c (taken as reference) and dsu_f (taken as query) and obtained the dsu_fc merged assembly. In the second step, we aligned dsu_fc (taken as reference) and dsu_c (taken as query) and obtained the dsu_fc2 merged assembly. In the third and last step, we aligned dsu_fc2 (taken as reference) and dsu_f (taken as query) and obtained the dsu_fc2f merged assembly. We further added a polishing step to account for the high error rate in PacBio reads (above 10%40). This polishing step can be performed after the merging using PacBio reads if they are abundant enough38,41. We mapped back a subset of our PacBio raw data (to obtain 80X coverage) to the dsu_fc2f assembly using pbalign and corrected the assembly using quiver with default parameters (both programs obtained from the SMRT Portal 2.3; https://www.pacbiodevnet.com). The dsu_fc2f_p resulting assembly was ~ 286 Mb long and contained 669 contigs.
We finally sought to remove both exogeneous sequences (e.g., bacterial contaminant) and duplicated sequences resulting from the poor handling of diploidy by assemblers (although Falcon produces a partially diploid genome). We first used BUSCO v2 with the bacterial database (bacteria_odb9 containing 148 genes in 3,663 species) to identify contigs containing bacterial genes. Twenty-two contigs were removed from the assembly, most of them aligning onto the Acetobacter pasteurianus genome. Also, we manually retrieved five additional contigs mapping to Lactobacillus genome leading to a total of 27 bacterial contigs discarded (corresponding to ca. 3 Mb). We then used BUSCO v2 with the Diptera database (diptera_odb9 containing 3,295 genes in 25 species) to identify putative duplicated contigs and flagged the shortest one as redundant. To avoid removing valid contigs, we recovered the contigs that contained at least 10 predicted genes. To this end, we mapped possible ORFs (longer than 200 bp) using the NCBI tool ORFinder v0.4.0 (https://www.ncbi.nlm.nih.gov/orffinder/) that was run with default options. The identified ORFs were then aligned onto the assembly without the redundant contigs using BLAST 42, considering as significant hits with e-value < 10–4 and > 80% of identity. Using this procedure, we removed 69 contigs that had fewer than ten unique ORFs considering that they were likely alternative sequences and we split four contigs in two because the unique ORFs were all located at a contig end with the rest of the contig appearing duplicated. Also, the annotation of structural variants (see below) lead us to remove 27 additional contigs, flagged as translocations but that further scrutiny made us consider as alternative haplotypes. In total 96 redundant contigs plus 4 partial contigs (covering ca. 16 Mb) were removed from the main assembly and were added to the file already containing 457 alternative haplotyes assembled by Falcon, covering approximately 26 Mb and assembled together with dsu_f. In total, this resulted in an assembly of alternative haplotypes of ca. 42 Mb in total.
Only 16 Mb out of the 42 Mb of alternative sequences were assigned to a contig from the main assembly using BUSCO. In addition, this assignation did not provide precise positioning on the main assembly. We therefore decided to precisely map all alternative sequences to our main D. suzukii assembly using the methodology described in the section “Whole genome alignment with other assemblies” below. Aligned regions that varied in size more than two folds between the alternative sequence and the main assembly were filtered out. Following this procedure, we were able to assign 91% of the contigs to the main assembly, covering 97.5% of the alternative sequences (41 Mb/42 Mb).
The final assembly, hereafter called Dsuz-WT3_v2.0 consisted of 546 contigs for an overall size of 268 Mb. The contiguity of the assembly was measured using Quast 4.143 (run with default options) and its completeness was evaluated against the Diptera gene set with BUSCO v2 run with option -c 40.
Assessment of local assembly errors
We used the following procedure to identify local genome assembly errors in the form of short (ca. 1 kb long) sequences duplicated in tandem. We used the genome assemblies of D. melanogaster dm6 (Genbank reference GCA_000001215.4), D. biarmipes Dbia_1.0 (GCA_000233415.1), the previous D. suzukii Dsuz-WT3_v1.0 assembly (GCA_000472105.1) and our assembly Dsuz-WT3_v2.0. We selected exons of an annotation that were within 5 kb of each other but did not overlap. We then blasted them against each other and selected hits that aligned on more than 50% of the shortest among the pair with an e-value below 10–10. For each couple of retained exons, a Needleman/Wunsch global alignment was made using the nw.align 0.3.1 python package (https://pypi.python.org/pypi/nwalign/) and a score was calculated with a NUC.4.4 matrix downloaded from the ncbi website. The score was normalized by the length of the sequence alignment and ranged from -2 (lowest similarity) to 5 (identical sequences).
Identification of autosomal and X-linked contigs using a female- to-male read mapping coverage ratio
To assign contigs of the new Dsuz-WT3_v2.0 assembly to either sex chromosomes or autosomes, we compared the sequencing coverage from whole genome short-read sequence data obtained for one female and one male individual13,44. Two DNA paired-end libraries with insert size of ca. 350 bp were prepared using the Illumina TruSeq Nano DNA Library Preparation Kit following manufacturer protocols on DNA extracted using the Genomic-tip 500/G kit (QIAGEN) for one female (mtp_f19) and one male (mtp_m19) sampled in Montpellier (France). Each individual library was further paired-end sequenced on the HiSeq 2,500 (Illumina, Inc.) with insert size of 125 bp. Base calling was performed with the RTA software (Illumina Inc.). The raw paired-end reads, available at the SRA repository under the SRR10260311 (for mtp_f19) and SRR10260312 (for mtp_m19) accessions, were then filtered using fastp 0.19.445 run with default options to remove contaminant adapter sequences and eliminate poor quality bases (i.e., with a Phred-quality score < 15). Read pairs with either one read with a proportion of low-quality bases over 40% or containing more than five N bases for either of the pairs were removed. After filtering, a total of 78,629,384 (9.379131 Gb with Q > 20) and 52,311,302 (6.342157 Gb with Q > 20) reads remained available for mtp_f and mtp_m respectively with an estimated duplication rate of 0.918% and 0.492%, respectively. Filtered reads were then mapped onto the Dsuz-WT3_v2.0 assembly using default options of the MEM program from the BWA 0.7.17 software46–48. Read alignments with a mapping quality Phred-score < 20 or PCR duplicates were removed using the view (option -q 20) and markdup programs from the SAMtools 1.9 software46, respectively. The resulting total number of mapped reads for mtp_f19 and mtp_m19 was 42,304,522 and 33,301,631 reads with a proportion of properly paired reads of 96.6% and 98.0% respectively.
Sequence coverage at each contig position for each individual sequence was then computed jointly using the default options of the depth program from SAMtools 1.9. To limit redundancy, only one count every 100 successive positions was retained for further analysis and highly covered positions (> 99.9th percentile of individual coverage) were discarded. The overall estimated median coverage was 18 and 21 for mtp_f19 and mtp_m19, respectively.
To identify autosomal and X-linked contigs, we used the ratio ρ of the relative (median) read coverage of contigs between mtp_f19 and mtp_m19 (weighted by their corresponding overall genome coverage). The ratio ρ is expected to equal 1 for autosomal contigs and 2 for X-linked contigs13,44. Note that the inclusion of X-linked positions in the overall estimated male genome coverage to compute the weights in the estimation of ρ result in a downward bias (the higher the actual length of the X-chromosome, the higher the bias). As a matter of expedience, 226 contigs (out of 546) with a coverage lower than 5X (resp. 2X) in mtp_m19 (resp. mtp_f19) or with less than 100 analyzed positions (i.e., < 10 kb) were discarded from further analyses. Conversely, four additional contigs (namely #234, #373, #668 and #638 of length 132 kb, 51 kb, 13 kb and 21 kb respectively) were discarded because they showed outlying coverages (i.e., > Q3 + 1.5(Q3-Q1), where Q1 and Q3 represents respectively the 25% and 75% quantiles of the observed contig coverage distribution) in either mtp_f19 or mtp_m19. The cumulated length of the 316 remaining contigs was 256.1 Mb. Only 11.9 Mb of the Dsuz-WT3_v2.0 assembly were hence discarded. We then fitted a Gaussian mixture model to the estimated ρ distribution of these 316 contigs, with two classes of unknown means and the same unknown variance. The latter parameters were estimated using the Expectation–Maximization algorithm implemented in the mixtools R package49. As expected, the estimated mean of the two classes µ1 = 0.93 and µ2 = 1.90 were slightly lower than that expected for autosomal and X-linked sequences. Our statistical treatment allowed the classification of 296 contigs (223.7 Mb) as autosomal and 13 contigs (31.7 Mb in total) as X-linked with a high confidence (p-value < 0.01), only ca. 12.6 Mb being left unassigned (see Supplementary Table S3 for a complete list of contig-chromosome associations).
Genome annotation for repetitive elements, structural variants and coding sequences
The repertoires of repetitive elements was assessed for the following species and genome assemblies: D. melanogaster dm6 (Genbank reference GCA_000001215.4), D. suzukii Dsuz-WT3_v2.0, D. biarmipes Dbia_1.0 (GCA_000233415.1), D. takahashi Dtak_2.0 (GCA_000224235.2), D. yakuba dyak_caf1 (GCA_000005975.1), D. ananassae dana_caf1 (GCA_000005115.1), D. persimilis dper_caf1 (GCA_000005195.1) and D. grimshawi Dgri_caf1 (GCA_000005155.1). We used the following procedure for each species separately: initial sets of repetitive elements were obtained using RepeatMasker open-4.0.6 (Smit et al., 2013–2015) with default parameters and the large Drosophila repertoire of all classes of repetitive elements from the Repbase database50,51. The number of bases covered by each type of repetitive element was then computed on the repertoire. The set of complete elements was obtained using the output of the programs RepeatMasker and OneCodeToFindThemAll52, from which the number of bases covered by each type of repetitive elements was extracted.
To detect structural variants, we aligned our filtered PacBio reads against our Dsuz-WT3_v2.0 assembly using NGMLR v0.2.653 with the parameter “-i 0.8”. We then used Sniffles v1.0.753 to detect structural variants with the parameter “-s 20 –l 500”. We detected 53 translocations, 59 insertions, 219 deletions, 8 inversions and 60 duplications. During this process, 27 contigs associated with 30 translocations were labelled as alternative sequences upon manual inspection and both the contigs and the associated translocations were hence removed from the main assembly (see section “Genome assembly based on PacBio long reads”).
To annotate protein coding genes, we used sequence-based gene prediction as well as cDNA evidence. RNA was extracted from antennae, ovipositors, proboscis + maxillary palps and tarsi of WT3_1.0 adults females and from pupal ovipositors (collected at 6 h, 24 h and 48 h after puparium formation) using Trizol (Invitrogen) according to manufacturer’s instructions. In total, 8 libraries were prepared using the Truseq stranded kit (Illumina) according to the manufacturer’s instructions, and were sequenced on a Hiseq2500.
We used Maker v2.31.854 to annotate the genome. SNAP55 and AUGUSTUS v3.2.256 with the parameter “augustus_species = fly » were used for ab initio predictions. cDNA evidence was provided from Trinity v2.3.257 and hisat v2.0.458 plus stringtie v 1.2.4 runs59 on the RNAseq data on pupae and the different tissues of female adults. We used the D. melanogaster proteome as protein homology evidence. The repeatmasker parameter was set to “Drosophila” and a general set of 24,916 transposable element proteins was provided. SNAP was trained with two initial runs: the first run used the homology and cDNA evidence (est2genome and protein2genome were set to 1) and the second run used the SNAP HMM file produced after the first maker run. The final maker run combined all the evidence, trained SNAP parameters as well as AUGUSTUS. In order to correct the flawed tendency of assemblers to fuse two neighboring genes together60, we added the following step. A D. suzukii gene was called a “false chimeric” if it could be cut into two parts that each aligned by BLAST to two neighboring genes in D. melanogaster. Based on this criterion, 1,052 genes were identified as chimers and split in two at the position that mimicked the gene limits in D. melanogaster. This method is conservative as it implies that gene structure is conserved between species. This assumption is reasonable owing to the evolution of genes and genomes on the Drosophila phylogeny and was validated by a manual review of modified genes61.
Whole genome alignment with other assemblies
The genome sequences of our D. suzukii assembly Dsuz-WT3_v2.0, the previous D. suzukii Dsuz-WT3_v1.0 assembly (GCA_000472105.1), the D. biarmipes Dbia_1.0 assembly (GCA_000233415.1) and the D. melanogaster dm6 assembly (Genbank reference GCA_000001215.4) were aligned as previously described62. Briefly, we followed the general guideline described in63: we used a large-scale orthology mapping created by Mercator64 with the option to identify syntenic regions of the genomes. Each region was then aligned with Pecan65 with default parameters. This genome assembly was used to assign a chromosome to the different contigs of Dsuz-WT3_v1.0 and Dsuz-WT3_v2.0 and to map the nearly identical neighboring exons in Dsuz-WT3_v1.09 to Dsuz-WT3_v2.0.
To visualize synteny blocks between the 20 longest contigs of the D. suzukii assembly and D. melanogaster chromosomes, we proceeded as follow. We first ran BLASTP between the protein anchors of D. melanogaster and D. suzukii produced during genome alignment with the parameters “-e 1e-10 -b 1 -v 1 -m 8”. We then ran MCScanX66 on the BLASTP output using default parameters. Synteny plots (Fig. 2) were obtained using VGSC67 on the MCScanX output. The results were fully consistent between this method and the genome alignment described above.
Estimating nucleotide diversity in the original WT3 strain, the Dsuz-WT3_v2.0 strain and their populations of origins
We relied on Pool-seq short-read whole-genome shotgun sequencing data (WGS) to estimate nucleotide diversity in the original WT3 strain9 (here referred to as Dsuz-WT3_v1.0), the newly generated Dsuz-WT3_v2.0 strain and three wild populations sampled in Watsonville—USA (US-Wat), Hawaii—USA (US-Haw); and Ningbo—China (CN-Nin). These choices were motivated by the fact that the female initially used to establish the WT3 strain originates from the Watsonville population (Chiu et al., 2013) and that the later population has recently been shown to be of admixed origin between Hawaii and Eastern China (Ningbo) populations24. For the original WT3 strain (Dsuz-WT3_v1.0), WGS data of a pool of tens of females (Chiu, personal communication) used to build the previous genome assembly by9 was downloaded from the SRA under the accession SRR942805. These Pool-seq data were obtained after sequencing of a DNA paired-end (PE) library with insert size of 250 bp on a HiSeq2000 (Illumina, Inc.) sequencers at approximately 40X coverage (see Supplementary Table S1 in9). For the Dsuz-WT3_v2.0 strain, a pool of 26 individual genomes (13 males and 13 females) was sequenced on a HiSeqX sequencer (Macrogen Inc., Seoul, South Korea) targeting a coverage > 30X. The raw paired-end sequences (2 × 150) were made available from the SRA repository under the SRR10260310 accession. Finally, for the US-Wat, the US-Haw and the CN-Nin populations, we relied on the Pool-seq data recently produced by68 from samples of 50 individuals (including 4, 25 and 36 females, respectively) and available from the SRA under the SRR10260026, SRR10260031 and SRR10260027 accessions respectively. The three data sets consisted of 2 × 125 bp PE sequences obtained from a HiSeq 2500 sequencer. Processing and mapping of reads was carried out as described above for the mtp_f19 and mtp_m19 individual WGS. The resulting overall mean coverages were 29.8X, 34.5X, 52.7X, 51.7X and 71.8X for Dsuz-WT3_v1.0, Dsuz-WT3_v2.0, US-Wat, CN-Nin and US-Haw, respectively.
Nucleotide diversity (θ = 4Neµ) was then estimated for non-overlapping 10 kb windows across the genome using the extension of the Watterson estimator69 for Pool-Seq data developed by Ferretti et al. (2013) and implemented in the npstats software. Only positions covered by at least four reads and less than 250 reads with a min quality > 20 were considered in the computations (-mincov 4 -maxcov 250 -minqual 20 options) and windows with less than 9,000 remaining positions were discarded. As a matter of expedience, the haploid pool sample size was set to 50 individuals for the Dsuz-WT3_v1.0 strain. We found, however, that alternative values of 10, 20 or 100 individuals resulted in highly consistent estimates.
Estimating the local ancestry composition of the assembly using an HMM painting model
Because of the admixed origin of the Watsonville population from which the Dsuz-WT3_v1.0 and Dsuz-WT3_v2.0 strains originate, we expected the assembly to be a mosaic of chromosomal segments of ancestral individuals originating from Hawaii (US-Haw) and Eastern China (CN-Nin), its two source populations (Fraimout et al. 2017). To characterize this mosaic, we first called polymorphic sites in the US-Haw and CN-Nin populations. To that end, the US-Haw and CN-Nin Pool-seq BAM files (see above) were processed using the mpileup program from SAMtools 1.9 with default options and -d 5,000 and -q 20. Variant calling was then performed on the resulting mpileup file using VarScan mpileup2cns v2.3.470 with options –min-coverage 50; –min-avg-qual 20 and –min-var-freq 0.001 –variants –output-vcf. The resulting VCF file was processed with the vcf2pooldata function from the R package poolfstats v1.171 retaining only bi-allelic SNPs covered by > 20 and < 250 reads in each of the two sample. For each SNP, we then estimated the frequency of the reference allele (i.e., the one of the assembly) in each population using a Laplace estimator (see Supplementary Text S2). We only retained SNPs displaying an absolute difference in the reference allele frequencies above 0.2 between the two US-Haw and CN-Nin samples (i.e. the most ancestry informative SNPs). This resulted in a total of 2,643,102 autosomal and 540,277 X-linked SNPs.
We further developed a one-order Hidden Markov Model (HMM) to model the assembly as a mosaic of chromosomal segments from either Chinese (“C”) or Hawaiian (“H”) ancestry. This HMM allowed estimation of the local ancestry origin of each reference allele of the assembly based on its estimated frequencies in the CN-Nin and US-Haw samples used as proxies for the “C” and “H” ancestral populations respectively. The model and the parameter estimation method are detailed in Supplementary Text S2.
Supplementary information
Acknowledgments
We thank David Begun for providing us with flies of the WT3 strain, Lasse Bräcker for further inbreeding of the D. suzukii WT3 stock, Mahul Chakraborty for useful suggestions on the assembly procedure of PacBio long reads, and Tom Druet for helpful discussions on HMM modeling. MP, MK, JEG and BP acknowledge financial support from the CNRS, the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 615789; NG and JW acknowledges funding from the Ludwig-Maximilians University of Munich; AE & MG acknowledge financial support by the National Research Fund ANR (France) through the project ANR-16-CE02-0015-01 (SWING), the Languedoc-Roussillon region (France) through the European Union program FEFER FSE IEJ 2014-2020 (project CPADROL), and the INRA scientific department SPE (AAP-SPE 2016 and 2018). JW acknowledges support of the National Genomics Infrastructure (NGI)/Uppsala Genome Center and UPPMAX for providingassistance in massive parallel sequencing and computational infrastructure. Work performed at NGI/Uppsala Genome Center has been funded by RFI/VR and Science for Life Laboratory, Sweden.
Author contributions
A.E., M.G., M.P., N.G. and B.P. conceived the project, M.P., N.G., A.E., M.G. and B.P. designed the experiments. R.B. assembled the genome, M.P. did the genome alignments, M.P. and R.B. annotated and analyzed the genome assembly. M.C. contributed to the analysis of local assembly errors. RJ prepared the samples for PacBio sequencing, J.W. preprocessed the PacBio reads. HP prepared the samples for Pool-seq, M.G. analyzed the Pool-seq data. J.G. and M.K. prepared the samples used for RNA-seq. M.P., A.E., M.G. and B.P. wrote the manuscript, N.G. and J.W. commented on the manuscript. A.E., B.P., M.G., N.G. secured funding.
Data availability
The GenBank accession number of the main assembly is GCA_013340165.1 (Whole Genome Shotgun project WWNF00000000). The GenBank accession number for the alternate haplotype sequences is GCA_013340185.1 (Whole Genome Shotgun project WWNG00000000). The PacBio reads and the RNA-seq reads were submitted to SRA under the Bioproject accession number PRJNA594550. The detailed SRA accession numbers are as follows:
PacBio data (SRA accession numbers):
– SRR10716756 to SRR10716759, SRR10716769 and SRR10716772 to SRR10716814.
RNA-seq data (SRA accession numbers):
– SRR10716760 for male genital discs, 6 h after puparium formation.
– SRR10716761 for female genital discs, 6 h after puparium formation.
– SRR10716762 for male genital discs, 48 h after puparium formation.
– SRR10716763 for female genital discs, 48 h after puparium formation.
– SRR10716764 for male genital discs, 24 h after puparium formation.
– SRR10716765 for female genital discs, 24 h after puparium formation.
– SRR10716766 for adult female tarsae.
– SRR10716767 for adult female proboscis + maxillary palps.
– SRR10716768 for adult female ovipositor.
– SRR10716770 for adult female antennae.
– SRR10716771 for adult male antennae.
Individual Whole Genome Shot-Gun data (SRA accessions numbers):
New to this study:
– SRR10260311 for the female individual mtp_f19.
– SRR10260312 for the male individual mtp_m19.
Pool Whole Genome Shot-Gun data (SRA accessions numbers):
New to this study:
– SRR10260310 for the WT3-2.0 pool.
From Olazcuaga et al. (in prep.):
– SRR10260026 for the US-Wat pool.
– SRR10260031 for the US-Haw pool.
– SRR10260027 for the CN-Nin pool.
From Chiu et al. (2013):
– SRR942805 for the WT3-1.0 pool.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mathieu Gautier, Email: mathieu.gautier@inrae.fr.
Nicolas Gompel, Email: gompel@bio.lmu.de.
Benjamin Prud’homme, Email: benjamin.prudhomme@univ-amu.fr.
Supplementary information
is available for this paper at 10.1038/s41598-020-67373-z.
References
- 1.Cini A, Ioriratti C, Anfora G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012;65:149–160. [Google Scholar]
- 2.Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VLS. The first records of the invasive pest Drosophila suzukii in the South American continent. J. Pest Sci. 2014;2004(87):379–383. [Google Scholar]
- 3.Asplen MK, et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015;2004(88):469–494. [Google Scholar]
- 4.Olazcuaga L, et al. Oviposition preference and larval performance of Drosophila suzukii (Diptera: Drosophilidae), spotted-wing Drosophila: Effects of fruit identity and composition. Environ. Entomol. 2019;48:867–881. doi: 10.1093/ee/nvz062. [DOI] [PubMed] [Google Scholar]
- 5.Karageorgi M, et al. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017;27:847–853. doi: 10.1016/j.cub.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalajdzic P, Schetelig MF. CRISPR/Cas-mediated gene editing using purified protein in Drosophila suzukii. Entomol. Exp. Appl. 2017;164:350–362. [Google Scholar]
- 7.Li J, Handler AM. Temperature-dependent sex-reversal by a transformer-2 gene-edited mutation in the spotted wing drosophila, Drosophila suzukii. Sci. Rep. 2017;7:12363. doi: 10.1038/s41598-017-12405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ometto L, et al. Linking genomics and ecology to investigate the complex evolution of an invasive drosophila pest. Genome Biol. Evol. 2013;5:745–757. doi: 10.1093/gbe/evt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu JC, et al. Genome of Drosophila suzukii, the spotted wing drosophila. G3 Genes Genomes Genet. 2013;3:2257–2271. doi: 10.1534/g3.113.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao Y, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017;546:524–527. doi: 10.1038/nature22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon D, et al. Long-read sequence assembly of the gorilla genome. Science. 2016;352:aae0344. doi: 10.1126/science.aae0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews BJ, et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563:501–507. doi: 10.1038/s41586-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier M, et al. The genomic basis of color pattern polymorphism in the Harlequin ladybird. Curr. Biol. 2018;28:3296–3302.e7. doi: 10.1016/j.cub.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissensteiner MH, et al. Combination of short-read, long-read, and optical mapping assemblies reveals large-scale tandem repeat arrays with population genetic implications. Genome Res. 2017;27:697–708. doi: 10.1101/gr.215095.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korlach, J. et al. De novo PacBio long-read and phased avian genome assemblies correct and add to reference genes generated with intermediate and short reads. Gigascience6 (2017). [DOI] [PMC free article] [PubMed]
- 16.Allen, S. L., Delaney, E. K., Kopp, A. & Chenoweth, S. F. Single-Molecule Sequencing of the Drosophila serrata Genome. [DOI] [PMC free article] [PubMed]
- 17.Miller DE, Staber C, Zeitlinger J, Hawley RS. Highly contiguous genome assemblies of 15 Drosophila species generated using nanopore sequencing. G3 (Bethesda) 2018;8:3131–3141. doi: 10.1534/g3.118.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahajan S, Wei KH-C, Nalley MJ, Gibilisco L, Bachtrog DD. novo assembly of a young Drosophila Y chromosome using single-molecule sequencing and chromatin conformation capture. PLOS Biol. 2018;16:e2006348. doi: 10.1371/journal.pbio.2006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracewell, R., Chatla, K., Nalley, M. J. & Bachtrog, D. Dynamic turnover of centromeres drives karyotype evolution in Drosophila. Elife8 (2019). [DOI] [PMC free article] [PubMed]
- 20.Sessegolo C, Burlet N, Haudry A. Strong phylogenetic inertia on genome size and transposable element content among 26 species of flies. Biol. Lett. 2016;12:20160407. doi: 10.1098/rsbl.2016.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakraborty M, et al. Hidden genetic variation shapes the structure of functional elements in Drosophila. Nat. Genet. 2018;50:20–25. doi: 10.1038/s41588-017-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminker JS, et al. The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol. 2002;3:research00841. doi: 10.1186/gb-2002-3-12-research0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JA, Wang Z. Next-generation transcriptome assembly. Nat. Rev. Genet. 2011;12:671–682. doi: 10.1038/nrg3068. [DOI] [PubMed] [Google Scholar]
- 24.Fraimout A, et al. Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol. Biol. Evol. 2017;34:msx050. doi: 10.1093/molbev/msx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joron M, et al. Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature. 2011;477:203–206. doi: 10.1038/nature10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamichhaney S, et al. Structural genomic changes underlie alternative reproductive strategies in the ruff (Philomachus pugnax) Nat. Genet. 2016;48:84–88. doi: 10.1038/ng.3430. [DOI] [PubMed] [Google Scholar]
- 27.Tusso S, et al. Ancestral admixture is the main determinant of global biodiversity in fission yeast. Mol. Biol. Evol. 2019;36:1975–1989. doi: 10.1093/molbev/msz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory TR, Johnston JS. Genome size diversity in the family Drosophilidae. Heredity (Edinb). 2008;101:228–238. doi: 10.1038/hdy.2008.49. [DOI] [PubMed] [Google Scholar]
- 29.Evolution of genes and genomes on the Drosophila phylogeny. Nature450, 203–218 (2007). [DOI] [PubMed]
- 30.Carneiro MO, et al. Pacific biosciences sequencing technology for genotyping and variation discovery in human data. BMC Genomics. 2012;13:375. doi: 10.1186/1471-2164-13-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Charlesworth B. Resolving the conflict between associative overdominance and background selection. Genetics. 2016;203:1315–1334. doi: 10.1534/genetics.116.188912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas JW, et al. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics. 2008;179:1455–1468. doi: 10.1534/genetics.108.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harewood L, et al. Hi-C as a tool for precise detection and characterisation of chromosomal rearrangements and copy number variation in human tumours. Genome Biol. 2017;18:125. doi: 10.1186/s13059-017-1253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudchenko O, et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science. 2017;356:92–95. doi: 10.1126/science.aal3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCartney MA, Mallez S, Gohl DM. Genome projects in invasion biology. Conserv. Genet. 2019;20:1201–1222. [Google Scholar]
- 36.Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty M, Baldwin-Brown JG, Long AD, Emerson JJ. Contiguous and accurate de novo assembly of metazoan genomes with modest long read coverage. Nucleic Acids Res. 2016;44:e147. doi: 10.1093/nar/gkw654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korlach, J. Perspective—Understanding accuracy in SMRT sequencing. Pac. Biosci. 1–9 (2013).
- 41.Chin C-S, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 42.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 43.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bidon T, Schreck N, Hailer F, Nilsson MA, Janke A. Genome-wide search identifies 1.9 Mb from the polar bear Y chromosome for evolutionary analyses. Genome Biol. Evol. 2015;7:2010–2022. doi: 10.1093/gbe/evv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. (2013).
- 47.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benaglia T, Chauveau D, Hunter DR, Young D. mixtools: An R package for analyzing finite mixture models. J. Stat. Softw. 2009;32:1–29. [Google Scholar]
- 50.Baena-López LA, Baonza A, García-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 51.Bao W, Kojima KK, Kohany O. Repbase update, a database of repetitive elements in eukaryotic genomes. Mob. DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailly-Bechet M, Haudry A, Lerat E. “One code to find them all”: a perl tool to conveniently parse RepeatMasker output files. Mob. DNA. 2014;5:13. [Google Scholar]
- 53.Sedlazeck FJ, et al. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods. 2018;15:461–468. doi: 10.1038/s41592-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korf I. Gene finding in novel genomes. BMC Bioinformatics. 2004;5:59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller O, Kollmar M, Stanke M, Waack S. A novel hybrid gene prediction method employing protein multiple sequence alignments. Bioinformatics. 2011;27(6):757–763. doi: 10.1093/bioinformatics/btr010. [DOI] [PubMed] [Google Scholar]
- 57.Haas B, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Consortium, D. 12 G. Evolution of genes and genomes on the Drosophila phylogeny. Nature450, 203–218 (2007). [DOI] [PubMed]
- 62.Paris, M. et al. Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet.9 (2013). [DOI] [PMC free article] [PubMed]
- 63.Dewey CN. Aligning multiple whole genomes with Mercator and MAVID. Methods Mol. Biol. 2007;395:221–236. doi: 10.1007/978-1-59745-514-5_14. [DOI] [PubMed] [Google Scholar]
- 64.Dewey, C. N. Whole-genome alignments and polytopes for comparative genomics. Thesis 1–110 (2006).
- 65.Paten B, Herrero J, Beal K, Fitzgerald S, Birney E. Enredo and Pecan: Genome-wide mammalian consistency-based multiple alignment with paralogs. Genome Res. 2008;18:1814–1828. doi: 10.1101/gr.076554.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49–e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, et al. VGSC: A web-based vector graph toolkit of genome synteny and collinearity. Biomed Res. Int. 2016;2016:1–7. doi: 10.1155/2016/7823429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olazcuaga, L. et al. A whole-genome scan for association with invasion success in the fruit fly Drosophila suzukii using contrasts of allele frequencies corrected for population structure. Mol. Biol. Evol. (in press). [DOI] [PMC free article] [PubMed]
- 69.Watterson GA. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 1975;7:256–276. doi: 10.1016/0040-5809(75)90020-9. [DOI] [PubMed] [Google Scholar]
- 70.Koboldt DC, et al. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hivert V, Leblois R, Petit EJ, Gautier M, Vitalis R. Measuring genetic differentiation from pool-seq data. Genetics. 2018;210:315–330. doi: 10.1534/genetics.118.300900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GenBank accession number of the main assembly is GCA_013340165.1 (Whole Genome Shotgun project WWNF00000000). The GenBank accession number for the alternate haplotype sequences is GCA_013340185.1 (Whole Genome Shotgun project WWNG00000000). The PacBio reads and the RNA-seq reads were submitted to SRA under the Bioproject accession number PRJNA594550. The detailed SRA accession numbers are as follows:
PacBio data (SRA accession numbers):
– SRR10716756 to SRR10716759, SRR10716769 and SRR10716772 to SRR10716814.
RNA-seq data (SRA accession numbers):
– SRR10716760 for male genital discs, 6 h after puparium formation.
– SRR10716761 for female genital discs, 6 h after puparium formation.
– SRR10716762 for male genital discs, 48 h after puparium formation.
– SRR10716763 for female genital discs, 48 h after puparium formation.
– SRR10716764 for male genital discs, 24 h after puparium formation.
– SRR10716765 for female genital discs, 24 h after puparium formation.
– SRR10716766 for adult female tarsae.
– SRR10716767 for adult female proboscis + maxillary palps.
– SRR10716768 for adult female ovipositor.
– SRR10716770 for adult female antennae.
– SRR10716771 for adult male antennae.
Individual Whole Genome Shot-Gun data (SRA accessions numbers):
New to this study:
– SRR10260311 for the female individual mtp_f19.
– SRR10260312 for the male individual mtp_m19.
Pool Whole Genome Shot-Gun data (SRA accessions numbers):
New to this study:
– SRR10260310 for the WT3-2.0 pool.
From Olazcuaga et al. (in prep.):
– SRR10260026 for the US-Wat pool.
– SRR10260031 for the US-Haw pool.
– SRR10260027 for the CN-Nin pool.
From Chiu et al. (2013):
– SRR942805 for the WT3-1.0 pool.