A rapidly evolving sweeping pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leading to coronavirus disease 2019 (COVID-19) has dominated the first half of 2020 and continues to do so. Cases of COVID-19 were first described in late 2019, when a series of previously unidentified pneumonia-related deaths emerged in Hubei province, China [1]. COVID-19, subsequently spread to almost all countries with a current count of > 11.5 million cases and > 535,000 confirmed deaths worldwide (https://www.worldometers.info/coronavirus/). Although virus’ origin, cellular entry and epidemiology [2–4] have rapidly been clarified, in situ observations of the actual viral interactions within human organs and tissues in patients suffering from COVID-19 have for a long time been addressed at the level of case reports or small series of ≤ 4 cases, as reviewed by Calabrese et al. [5] in the current issue of Virchows Archiv. Indeed, by the end of April 2020 when 150,000 patients had already died of COVID-19, only 16 autopsy cases had been reported in the peer-reviewed literature, with nine publications presenting limited autopsies, assessment of postmortem core-needle or incisional collections of tissue. In the absence of reliable data regarding the degree of SARS-CoV-2 infectivity in dead individuals, various authorities discouraged the conduction of autopsies. This, combined with the ill-adjusted attitudes of pathologists, clinicians and societies towards autopsies, locked down scientific activities to elucidate the actual underlying mechanisms of COVID-19 [6]. This seems incomprehensible, given the fundamental and time-proven role of autopsies in re-emerging, emerging or unknown diseases [e.g., 7]. Only after 170,000 reported COVID-19 deaths and 4 months of pandemic, the first autopsy series of > 10 patients (n = 21) was put forward published [8], and only after another 280,000 deaths and one more month, finally a series of > 50 patients (n = 80) was released [9].
The paper by Heinrich et al. in this volume [10] very fittingly illustrates the overcoming of the above mentioned hindrance of autopsies from the German perspective, reporting the systematic postmortem examination, including CT scan, autopsy, histology, and virology assessments, of the first (German) patient to die from COVID-19. As suggested by the fact that the deceased had to be transported to Hamburg within 12 days of death and examined at the Department of Legal Medicine, the overcoming of the blocked position regarding COVID-19 autopsies, at least in Germany, was largely due to the personal commitment and professional conviction of the involved authors. They detected a rather characteristic [5, 8] morphologic pattern with deep-red, slightly nodular, hyperemic, and very heavy lungs with prominent diffuse alveolar damage (DAD), microvascular thrombosis, capillary congestion, and acute hemorrhagic tracheo-bronchitis.
In the light of absence of sound evidences regarding the degree of infectivity of COVID-19 cadavers, various labs adjusted their autopsy practices [e.g., 8] in order to ensure safety precaution against infection. In the current issue of Virchows Archiv, Basso et al. [11] describe and nicely illustrate the procedure, which has been used to perform the first series of postmortem examinations at Padua University Hospital, Italy, to minimize the risk of infection for pathologists and technicians. The paper clearly shows that if autopsies of COVID-19 patients are performed under well thought-out conditions, they are indeed feasible and at minimal risk, even if an oscillator saw with special suction device instead of a handsaw is utilized to open the neurocranium. Moreover, the authors report a very important finding that helps clarification whether patients who died on COVID-19 are still infectious: cultures demonstrated vital viruses in lung samples obtained even 6 days after death.
Bösmüller et al. [12] meticulously describe the pulmonary findings in four cases of fatal COVID-19 with a spectrum of histopathologic changes of the lungs, which probably reflect disease course and duration, invasive therapies and laboratory features: early phases of disease were characterized by neutrophilic, exudative capillaritis with microthrombosis; later stages displayed DAD and progressive intravascular thrombosis in small to medium-sized pulmonary vessels, occasionally with infarction, which were accompanied by laboratory parameters of disseminated intravascular coagulation; at later stages, extensive intra-alveolar proliferation of fibroblasts and marked metaplasia of alveolar epithelium corresponding to organized DAD was observed. The paper contains a nice electron microscopic microphotograph describing properly sized (i.e., 75–120 nm) virus-like particles in endothelial cells and, most importantly, located in membrane-bound compartments that likely represent endosomes, and not, as repeatedly put forward elsewhere, as freely floating particles in the cytoplasm. Importantly, the observations by Heinrich et al. [10] and Bösmüller et al. [12] of profound pathologic changes in the pulmonary microvascular compartment perfectly fit with and are probably the net reflection by conventional light microscopy of the severe microangiopathic changes that have been documented by micro–computed tomographic imaging, corrosion casting, scanning electron microscopy, and molecular analysis of autopsy-collected materials [13] expanding the evidence that COVID-19 most likely represents an angiocentric disease with drastic proneness to thrombosis. Indeed, not only are virus-like particles detectable in endothelial cells, but applying anti-SARS-CoV-2-spike-protein antibodies, one will detect highly preferential presence of virus antigens in the endothelial, but not in the epithelial compartment of the alveolar units (Fig. 1), arguing in favor of the above hypothesis.
Fig. 1.
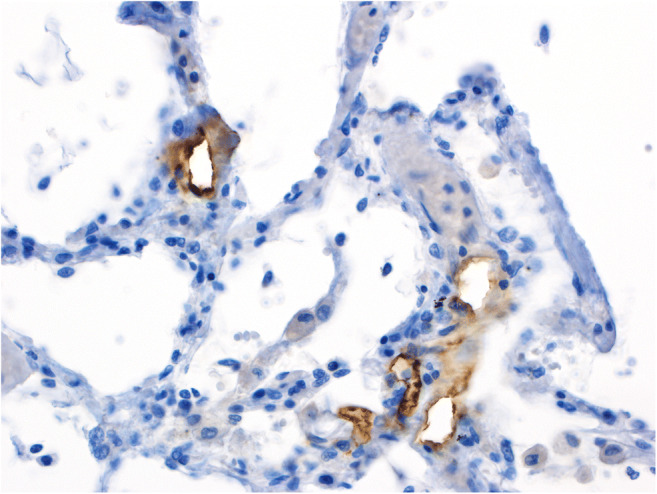
Immunohistochemical staining of a lung specimen for SARS-CoV-2 Spike protein with the clone 007 from Sino Biological on an autopsy case from our published cohort [8] showing presence of spiked viruses mainly in the endothelial compartment. The staining has been performed by Mattia Bugatti in the lab of Fabio Facchetti
Finally, Calabrese et al. [5] precisely summarize lessons learned regarding the pulmonary pathology of COVID-19 from autopsy studies in the last 4 months, and present a very useful appropriate table. Their paper nicely illustrates the shift of paradigms with respect to COVID-19 pathophysiology by gathering knowledge that mirrors the unlocking of autopsy-based research carried out in this period, from unspecified “pneumonia” over DAD in the clinical context of an acute respiratory distress syndrome to a far more complex scenario, including angiopathy [13] and a wide spectrum of associated pathologies. A valuable paragraph addresses advantages and caveats of electron microscopic examinations concerning SARS-CoV-2 in COVID-19 (Fig. 2).
Fig. 2.
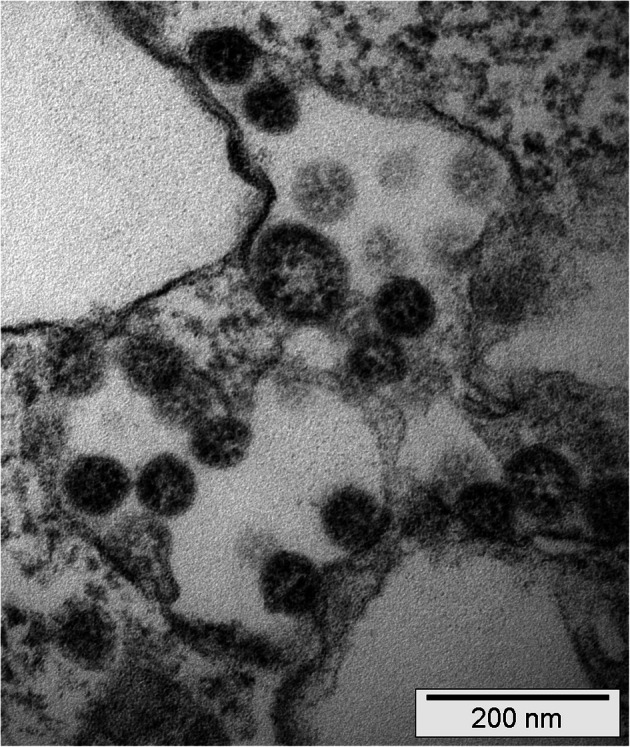
Electron microscopic images of patient-derived SARS-CoV-2 obtained from COVID-19-affected individuals of a published cohort from the University Hospital Basel, Switzerland [8] cultured ex vivo in virus-susceptible Vero cells (kidney epithelial cells from Cercopithecus aethiops) from ATCC (https://www.lgcstandards-atcc.org/Products/All/CRL-1586.aspx?geo_country=ch). The image taken at 110,000× magnification shows virus structures of characteristic size (75–120 nm) (1) within membranous intracellular structures of the trans-Golgi apparatus/endoplasmic reticulum with characteristic, (2) nucleocapsid structures on the inner side (~ 20 nm measuring dark dots), and (3) merely perceptible surrounding projections; the presence of all four features being considered diagnostic of coronaviruses. Courtesy of Martin Herzig, Juergen Hench from the Institute of Medical Genetics and Pathology, and Reiner Gosert and Hans Hirsch from the Clinical Virology at the University Hospital Basel, Basel, Switzerland
Shoulder to shoulder, clinical and forensic pathologists overcame the obstruction of autopsy studies in COVID-19 victims and hereby generated valuable knowledge on the pathophysiology of the interaction between SARS-CoV-2 and the human body, thus contributing to our understanding of this deadly pandemic.
Contributions
AT conceptualized and wrote the paper; DJ partially wrote, critically read, and edited the paper.
Funding information
AT is supported by the Botnar Research Centre for Child Health.
Compliance with ethical standards
Statement of informed consent/ethics committee approval
Not applicable to this type of papers.
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513 [DOI] [PMC free article] [PubMed]
- 2.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280 [DOI] [PMC free article] [PubMed]
- 4.Sun J, He WT, Wang L et al (2020) COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med 26:483–495 [DOI] [PMC free article] [PubMed]
- 5.Calabrese F, Pezzuto F, Fortarezza F et al (2020) Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 10.1007/s00428-020-02861-1 [DOI] [PMC free article] [PubMed]
- 6.Salerno M, Sessa F, Piscopo A, et al. No autopsies on COVID-19 deaths: a missed opportunity and the lockdown of science. J Clin Med. 2020;9:E1472. doi: 10.3390/jcm9051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reye RDK, Morgan G, Baral J. Encephalopathy and fatty degeneration of the viscera: a disease entity in childhood. Lancet. 1963;II:749–752. doi: 10.1016/S0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- 8.Menter T, Haslbauer JD, Nienhold R et al (2020) Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 10.1111/his.14134 [DOI] [PMC free article] [PubMed]
- 9.Edler C, Schröder AS, Aepfelbacher M et al (2020) Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 134:1275–1284 [DOI] [PMC free article] [PubMed]
- 10.Heinrich F, Sperhake J, Heinemann A et al (2020) Germany’s first COVID-19 deceased: a 59-year old man presenting with diffuse alveolar damage due to SARS-CoV-2 infection. Virchows Arch [DOI] [PMC free article] [PubMed]
- 11.Basso C, Calabrese F, Sbaraglia M et al (2020) Feasibility of postmortem examination in the era of COVID-19 pandemic: the experience of a Northeast Italy University Hospital. Virchows Arch. 10.1007/s00428-020-02861-1 [DOI] [PMC free article] [PubMed]
- 12.Bösmüller H, Traxler S, Bitzer M et al (2020) The evolution of pulmonary pathology in fatal COVID-19 disease. An autopsy study with clinical correlation. Virchows Arch 10.1007/s00428-020-02881-x [DOI] [PMC free article] [PubMed]
- 13.Ackermann M, Verleden SE, Kuehnel M et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed]


