Abstract
Objective: To study the expression of pyroptosis signaling pathway related proteins in breast cancer tissues and paracancer tissues, analyze their relationship with breast cancer clinicopathologic features, and explore their relationship to prognosis. Methods: Immunohistochemistry ElivisionTM plus was used to detect the expression of caspase-1, IL-1β and Gasdermin-D (GSDMD) in 108 cases of breast cancer and 23 cases of benign lesions adjacent to breast cancer. Results: Using 108 cases of breast cancer and 23 cases of para-cancerous benign tissues, the pyroptosis signaling pathway effector proteins caspase-1, IL-1β, and GSDMD were positively correlated with each other. The higher the expression level, the lower the histophologic grade of breast cancer, the smaller the tumor size, the lower the clinical stage, the lower the possibility of lymph node metastasis, the lower the risk of death, and the better the prognosis. Conclusions: Pyroptosis signaling pathway effectors caspase-1, IL-1β and GSDMD expression may play an important role in the invasion, metastasis, and prognosis of breast cancer.
Keywords: Pyroptosis, caspase-1, IL-1β, GSDMD, breast cancer
Introduction
Breast cancer is a common cancer, with the highest incidence among women, and its morbidity and mortality are expected to increase significantly in the next 5-10 years [1,2]. Therefore, it is still important to explore new treatments for breast cancer. Pyroptosis is a newly described type of cell death that has been discovered and confirmed, which is different from apoptosis and necrosis [3]. It is a new hotspot in cell death research. There are few studies on the expression characteristics and significance of pyroptosis in solid tumors. In this study, the key proteins of caspase-1, IL-1β, and GSDMD in the pyroptosis signaling pathway in breast cancer tissues with a large sample size were targeted and quantitatively analyzed to explore the significance of pyroptosis in the occurrence and development of breast cancer.
Materials and methods
General information
From January 2014 to December 2014, 108 breast cancer archived specimens (paraffin-embedded) and 23 adjacent tissue specimens were collected from the Department of Pathology, the first affiliated hospital of Bengbu Medical College. All the breast cancer patients were from females, who had not received chemotherapy or radiotherapy before surgery, and their ages ranged from 32 to 76 years, with a median age of 50 years. The pathologic classification and clinical staging of all breast cancer patients refer to the 2003 World Health Organization diagnostic criteria for Pathology and Genetics of Breast and Female Genital Tumors. All cases were followed until the patient died or until January 2020, with a minimum of 60 months and a maximum of 72 months. The clinicopathologic data of breast cancer patients are shown in Table 1.
Table 1.
Correlation of caspase1, IL-1β, and GSDMD expressions with clinicopathologic characteristics of patients with breast cancer
| Caspase1 | IL-1β | GSDMD | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Low expression | High expression | P | Low expression | High expression | P | Low expression | High expression | P | |
| Age (year) | |||||||||
| < 50 | 19 | 33 | 0.503 | 19 | 33 | 0.631 | 20 | 32 | 0.228 |
| ≥ 50 | 24 | 32 | 18 | 38 | 28 | 28 | |||
| Pathologic grade | |||||||||
| I | 1 | 13 | 0.001 | 1 | 13 | 0.014 | 4 | 10 | 0.039 |
| II | 21 | 39 | 19 | 41 | 23 | 37 | |||
| III | 21 | 13 | 17 | 17 | 21 | 13 | |||
| Mass size | |||||||||
| < 2 cm | 5 | 28 | 0.001 | 4 | 29 | 0.001 | 8 | 25 | 0.005 |
| ≥ 2 cm | 38 | 37 | 33 | 42 | 40 | 35 | |||
| Lymphatic metastasis | |||||||||
| No | 9 | 40 | 0.000 | 9 | 40 | 0.002 | 15 | 34 | 0.008 |
| Yes | 34 | 25 | 28 | 31 | 33 | 26 | |||
| TNM stage | |||||||||
| I | 5 | 22 | 0.002 | 6 | 21 | 0.023 | 8 | 19 | 0.022 |
| II | 35 | 43 | 28 | 50 | 37 | 41 | |||
| III | 3 | 0 | 3 | 0 | 3 | 0 | |||
Reagent
Rabbit anti-human caspase-1 polyclonal antibody, rabbit anti-human IL-1β polyclonal antibody and rabbit anti-human GSDMD polyclonal antibody were purchased from Proteintech, USA; ElivisionTM plus kit and DAB color development kit were purchased from Fuzhou Maixin Biotechnology.
Experimental method
All breast cancer tissue specimens and control tissue specimens were fixed with 4% neutral formalin solution, embedded in paraffin, and serially sectioned at a thickness of 4 μm, and then dewaxed in a xylene solution and a gradient ethanol solution to water washing. Immunohistochemical staining methods were performed according to Elivision TM plus kit instructions. A known positive film was used as a control, and a PBS solution was used instead of a primary antibody as a negative control.
Result
Based on the combination of the staining intensity and the percentage of positive cells, 0, 1, 2, and 3 points were scored according to the non-yellow, light (light yellow particles), medium (brown yellow particles), and heavy (dark brown) staining. Colored cells accounted for 0% of counted positive cells; 5% to 25% were counted as 1 point; 26% to 50% were counted as 2 points; 51% to 75% were counted as 3 points; > 75% 4 points. Five 400-fold fields of view were randomly taken from each section, and the staining intensity score and the percentage of positive cells were scored for each field. The product of the staining intensity and the percentage of positive cells was < 6 points for low expression and ≥ 6 points for high expression. The results of immunohistochemical staining were determined by two pathologists through independent double-blind method.
Statistical analysis
SPSS 25.0 statistical software package was used for statistical analysis. The survival analysis of caspase-1, IL-1β and GSDMD protein-expressing and low-expressing groups was analyzed by Kaplan-Meier method, and comparison between groups was performed by log-rank test. Multivariate analysis was analyzed by Cox multivariate regression model. In breast cancer tumor tissues, the correlations between the expressions of caspase-1, IL-1β, and GSDMD proteins and adjacent tissues and various clinicopathologic factors were analyzed by χ2 and Spearman rank correlation tests. P < 0.05 was considered significant.
Results
Expression of caspase1 protein in breast cancer and its relationship with clinicopathologic factors
The high expression rate of caspase1 protein in the breast cancer group was 60.19% (65/108, Figure 1A); the low expression rate was 39.81% (43/108, Figure 1B), and the high expression rate of caspase1 protein in the adjacent cancer control group was 82.61% (19/23). The low expression rate was 17.39% (4/23), and the difference between the two groups was significant (P < 0.05). The higher the intensity of caspase1 expression in breast cancer tissues, the lower the tumor tissue pathologic grade, the smaller the tumor size, the lower the clinical stage and the lower likelihood of lymph node metastasis (P < 0.05); the expression intensity of caspase1 protein was independent of the age of breast cancer patients (P > 0.05, Table 1).
Figure 1.
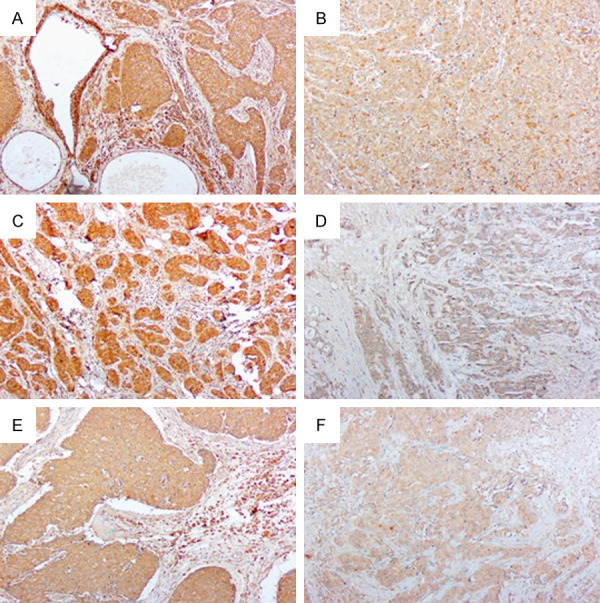
Expression of caspase1, IL-1β, and GSDMD protein in breast cancers (Elivision, original magnification: ×100). A: Caspase1 is highly expressed in breast cancer tissues; B: Low expression of caspase1 in breast cancer; C: IL-1β is highly expressed in breast cancer tissues; D: Low expression of IL-1β in breast cancer; E: GSDMD is highly expressed in breast cancer tissues; F: Low expression of GSDMD in breast cancer.
Expression of IL-1β protein in breast cancer and its relationship with clinicopathologic factors
The high expression rate of IL-1β protein in the control group was 86.96% (20/23), the low expression rate was 13.04% (3/23), and the high expression rate in the breast cancer group was 65.74% (71/108, Figure 1C). The low expression rate was 34.26% (37/108, Figure 1D), and the difference between the two groups was significant (P < 0.05). The expression of caspase1 protein was not related to the age of breast cancer patients (P > 0.05). The higher the IL-1β protein expression intensity, the lower the pathologic grade of breast cancer tumor tissues, the smaller the tumor size, the lower the clinical stage, and the lower likelihood of lymph node metastasis (P < 0.05, Table 1).
Expression of GSDMD protein in breast cancer and its relationship with clinicopathologic factors
The high expression rate of GSDMD protein in the breast cancer group and control group was 55.56% (60/108, Figure 1E) and 78.26% (18/23), and the low expression rate was 44.44% (48/108, Figure 1F) and 21.74% respectively. (5/23); the difference between the two groups was significant (P < 0.05). The lower the pathologic grade of breast cancer tissue, the smaller the tumor size, and the lower the clinical stage, the higher the intensity of GSDMD protein expression, and the difference was significant (P < 0.05). The expression intensity of GSDMD protein was related to lymph node metastasis (P < 0.05); but the expression intensity of GSDMD protein was not related to the age of breast cancer patients (P > 0.05).
Correlation between caspase1, IL-1β, and GSDMD protein expression intensity in breast cancer tissues
Spearman correlation analysis showed a positive correlation between the expression of caspase1 protein and IL-1β protein (r = 0.598, P = 0.000 < 0.05); a positive correlation between the expression of caspase1 protein and GSDMD protein (r = 0.560, P = 0.000 < 0.05); there was a positive correlation between the expression of IL-1β protein and GSDMD protein (r = 0.431, P = 0.000 < 0.05, Table 2).
Table 2.
Relationship of caspase1, IL-1β, and GSDMD expressions in breast cancer
| caspase1 | IL-1β | GSDMD | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Low expression | High expression | Low expression | High expression | Low expression | High expression | |||
| IL-1β | GSDMD | caspase1 | ||||||
| Low expression | 33 | 9 | Low expression | 31 | 25 | Low expression | 38 | 10 |
| High expression | 15 | 74 | High expression | 11 | 64 | High expression | 18 | 65 |
| r | 0.598* | r | 0.431* | r | 0.560* | |||
| P | 0.000 | P | 0.000 | P | 0.000 | |||
positive correlation.
Cox multifactorial analysis
Pathologic classification of breast cancer tissues (divided into groups I, II, and III), age (divided into groups ≥ 50 and < 50 years), tumor size (divided into groups < 2.0 cm and ≥ 2.0 cm), lymph node metastasis (divided into metastasis group and non-metastasis group), clinical stage (divided into group I, group II and group III), caspase1 expression group (divided into high expression group and low expression group), IL-1β expression group (Divided into high expression group and low expression group), GSDMD expression group (high expression group and low expression group) and other parameters were introduced into the Cox multi-factor model for analysis. The results showed whether the expression of caspase1, IL-1β and GSDMD protein was low or not. Expression, clinical stage, pathologic grade, mass size, and lymph node metastasis were independent prognostic factors that affect the survival of breast cancer patients (Table 3).
Table 3.
Multivariate survival analysis of 108 patients with breast cancer
| B | SE | Wald | P | RR | 95% CI | |
|---|---|---|---|---|---|---|
| caspase1 | -1.996 | 0.913 | 4.779 | 0.029 | 0.136 | 0.089-0.679 |
| IL-1β | -0.844 | 0.315 | 7.179 | 0.007 | 0.430 | 0.221-0.806 |
| GSDMD | -0.439 | 0.197 | 4.966 | 0.026 | 0.645 | 0.373-0.915 |
Subsistence analysis
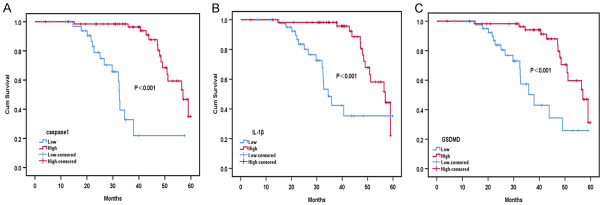
The overall 5-year survival rate for this group of cases was 72.2% (78/108). Kaplan-Meier survival analysis showed that the overall survival time of patients with high expression of caspase1 protein was significantly higher than that of patients in the low expression group; the difference was statistically significant (P < 0.05, Figure 2A). The overall survival time of patients with high expression of IL-1β protein compared to patients with low expression, showed a significant difference (P < 0.05, Figure 2B). The overall survival time of patients with high expression of GSDMD protein was higher than in patients with low expression, and the difference was significant (P < 0.05, Figure 2C).
Figure 2.
Survival curves of breast cancer patients with caspase1 (A), IL-1β (B) and GSDMD (C) protein high expression group and low expression group.
Discussion
Breast cancer has now become the leading cause of cancer death among women worldwide. In China, the annual incidence of breast cancer continues to increase by about 2% [4]. In recent years, there have been many kinds of treatment methods that can effectively treat breast cancer patients, but we are still trying to find new and more effective treatment methods to further improve the disease-free survival rate and improve the quality of life of patients. Therefore, the study of biomarkers that play an important role in the occurrence and development of breast cancer is the main focus of breast cancer research.
In recent years, the research into cell pyroptosis has become frequent. In 2015, Feng’s team discovered GSDMD [5], whose research results were published in Nature, which revealed the molecular mechanism of cell pyroptosis for the first time, which was a breakthrough in the study of cell pyroptosis. This research work first proved that Gasdermin protein was the “killer” protein of cell pyroptosis. It opened up a whole new field of research on cell death and innate immunity.
Differentiation between pyroptosis and apoptosis
Both pyroptosis and apoptosis are programmed cell death modes, which are important ways for the body to maintain immune homeostasis. Programmed cell death can remove unwanted cells. Apoptosis and pyroptosis are different in their pathogenesis, biologic effects, and cell morphology. Apoptosis is mediated by caspase 3/8/9 and occurs as non-inflammatory necrosis. When apoptosis occurs, cytoplasm and nucleus are shriveled, DNA is broken, and cell membrane remains intact. Pyroptosis is an inflammatory cell necrosis mediated by caspase1/4/5/11, in which the cell membrane of pyroptosis forms pores, cells swell, burst, release contents, and cause an inflammatory response [6]. Recent studies have found that cell pyroptosis is involved in the pathogenesis of kidney disease, atherosclerosis, nervous system diseases, infectious diseases, and infectious diseases [7-12].
Cell pyroptosis can be divided into a classical pyroptosis pathway and non-classical pyroptosis pathway, and the classical pyroptosis pathway is the most studied at present [13,14]. The classical pyroptosis pathway is activated after pattern recognition receptors (PRRs) recognize pathogens or inflammatory factors when endogenous or exogenous danger signals such as bacteria and viruses stimulate the body, and apoptosis-associated speck-like protein CARD domain (ASC) and other adaptor proteins interact with each other to recruit pro-caspase1 precursor to form Inflammasomes. Inflammatory bodies activate caspase1, and activated caspase1 cleaves IL-1β and IL-18 precursors into mature IL-1β and IL-18. At the same time, activated caspase1 cleaves GSDMD, causing the N-terminal domain and C terminal domains to be separated. The N-terminal domain of GSDMD gradually moves from the cytoplasm to the cell membrane and aggregates to form holes in the cell membrane. As a result, the integrity of the cell membrane is destroyed and the cells die. IL-1β and IL-18 are secreted from the cell membrane pore to the outside of the cell, and recruit more inflammatory cells outside the cell, thereby expanding the inflammatory response, and eventually causing the cells to undergo osmotic disintegration [15-20]. The non-classical pyroptosis pathway is a complex composed of human caspase4/5 or mouse caspase11 directly binding to bacterial Lipopolysac -- charide (LPS) as intracellular receptors to activate GSDMD and induce pyroptosis [21-23]. Caspase4/5/11 in the non-classical pyroptosis pathway can also indirectly regulate the secretion of mature IL-1 binding and IL-18 through the NLRP3-ASC-caspase-1 pathway [24], suggesting that the classical pyroptosis pathway can interact with the non-classical pyroptosis pathway jointly to promote the secretion of IL-1 binding and IL-18. However, this mechanism is still unclear, but it may be related to the potassium ion outflow after the formation of cell membrane pores, which then leads to the activation of NLRP3 [25].
Caspase1, IL-1, and GSDMD are effector proteins in the classical pyroptosis pathway. Wu et al., in a study using immunohistochemical ElivisionTM plus method to detect 108 cases of breast cancer tissue cell protein caspase1, IL-1 beta and GSDMD protein, found that as the pathologic stage of breast tumor tissue is higher, the greater the mass, the higher the TNM staging, caspase1, the lower the IL-1β and GSDMD protein expression intensity, and the expression was not altered in the lymph node metastases for caspase1, IL-1β and GSDMD protein expression intensity. Kaplan-Meier survival analysis showed that total survival time of patients in the group with high expression of caspase1, IL-1β, and GSDMD protein was significantly higher than that in the group with low expression. Spearman correlation analysis in this study found that the expressions of caspase1, IL-1β, and GSDMD protein in breast cancer tissues were positively correlated with each other, which was in line with the development law of the caustic death pathway. These results suggest that pyroptosis has a significant effect on the progression, invasion, and metastasis of breast cancer, and is beneficial to the prognosis of breast cancer patients. In recent years, the role of pyroptosis in tumorigenesis and development has attracted increasing attention. As a form of programmed death, pyroptosis means that the growth of tumor cells is inhibited, so inducing pyroptosis of tumor cells in breast cancer patients is a way of anti-tumor immunity. However, when excessive pyroptosis occurs, it can also cause a strong pathologic inflammatory response. Studies have shown that when caspase1 is highly expressed, there is more aggregation of inflammatory cells in the tumor interstitial microenvironment (Figure 1A).
Conclusion
In summary, the expression and amount of cytotoxic effector proteins caspase1 and IL-1β may affect the development and prognosis of breast cancer patients, which may provide a new molecular target for the targeted treatment of breast cancer. In this study, the pyroptosis pathway effector proteins caspase1, IL-1 and GSDMD were preliminarily correlated. Further experimental studies are needed to further explore the pathogenesis of pyroptosis.
Acknowledgements
This study was supported by the Bengbu Medical College Graduate Research Innovation Program (Byycx1975), National Innovation and Entrepreneurship Program for College Students (201810367025) and Natural Science Foundation of Bengbu Medical College (BYKY1822ZD).
Disclosure of conflict of interest
None.
References
- 1.Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69:313–317. doi: 10.1007/s13304-017-0424-1. [DOI] [PubMed] [Google Scholar]
- 2.Ribnikar D, Ratosa I, Perhavec A, Amir E. General overview and treatment recommendations for young women with breast cancer. Rev Invest Clin. 2017;69:77–93. doi: 10.24875/ric.17002175. [DOI] [PubMed] [Google Scholar]
- 3.Broz P. Immunology: caspase target drives pyroptosis. Nature. 2015;526:642–643. doi: 10.1038/nature15632. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 6.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Z, Murakami T, Suzuki K, Tamura H, Reich J, Kuwahara-Arai K, Iba T, Nagaoka I. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int Immunol. 2016;28:245–253. doi: 10.1093/intimm/dxv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galloway NL, Doitsh G, Monroe KM, Yang Z, Munoz-Arias I, Levy DN, Greene WC. Cell-to-cell transmission of HIV-1 is required to trigger pyroptotic death of lymphoid-tissue-derived CD4 T cells. Cell Rep. 2015;12:1555–1563. doi: 10.1016/j.celrep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Huang T, Ying L, Han C, Li D, Xu Y, Zhang M, Mou S, Dong Z. MiR-155 is involved in renal ischemia-reperfusion injury via direct targeting of FoxO3a and regulating renal tubular cell pyroptosis. Cell Physiol Biochem. 2016;40:1692–1705. doi: 10.1159/000453218. [DOI] [PubMed] [Google Scholar]
- 10.Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi: 10.1016/j.cca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Mamik MK, Power C. Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain. 2017;140:2273–2285. doi: 10.1093/brain/awx133. [DOI] [PubMed] [Google Scholar]
- 12.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Shou X, Mao X, Dong J, Mohabeer N, Kushwaha KK, Wang L, Su Y, Fang H, Li D. Oxidized low density lipoprotein induced caspase-1 mediated pyroptotic cell death in macrophages: implication in lesion instability? PLoS One. 2013;8:e62148. doi: 10.1371/journal.pone.0062148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circ Res. 2016;118:1525–1539. doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang XF. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: a novel therapeutic potential for ischemia. J Biol Chem. 2015;290:17485–17494. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 19.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kepp O, Galluzzi L, Zitvogel L, Kroemer G. Pyroptosis - a cell death modality of its kind? Eur J Immunol. 2010;40:627–630. doi: 10.1002/eji.200940160. [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Erratum: pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;540:150. doi: 10.1038/nature20106. [DOI] [PubMed] [Google Scholar]
- 22.Place DE, Kanneganti TD. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 2017;13:e1006502. doi: 10.1371/journal.ppat.1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]