Abstract
Oligoasthenospermia is one of the main causes of infertility in reproductive-age men. This study aimed to explore the feasibility of exogenous testosterone supplemental therapy (TST) for adult male rats with oligoasthenospermia model. The rats (n=40) were randomized equally into 4 groups: control group, model group, low-dose and high-dose groups (n=10, respectively). After establishment of an oligoasthenospermia model that was treated with glucosides of tripterygium wilfordii (GTWs), the low-dose and high-dose groups were treated with 2 testosterone undecanoate (TU) injections at doses of 7.5 mg and 15 mg for 8-week period (4-week intervals). Body weights, serum reproductive hormone levels, sperm measurements in the epididymis, and testis histology were monitored. The TU injections increased serum testosterone levels steadily. The epididymis sperm concentration and motility increased slowly in high dose group at 4-weeks whereas sperm measurements increased significantly in the TST groups at 8 weeks. In addition, exogenous TST increased the intra-testicular testosterone concentration somewhat and alleviated the testicular oxidative stress markers of Malondialdehyde (MDA) and level of GSH-PX (Glutathione Peroxidase) after 8 weeks treatment. The improvement of sperm and testicular function acted mainly by curbing mitochondrial apoptosis in the testis by modulation of Bcl-2, Bax, Caspase-3, and Caspase-9 expression. However, the results of immunohistochemistry and western blotting in the low-dose group were still lower than control values. TST at an appropriate dose within a period of 8 weeks was effective to stimulate spermatogenesis and alleviate inflammation, oxidative stress, and apoptosis through suppression of testis damage in this rat model of oligoasthenospermia.
Keywords: Oligoasthenospermia, sperm, testosterone undecanoate (TU), glucosides of tripterygium wilfordii (GTWs)
Introduction
According to reports from the WHO, approximately 15% of couples of childbearing age suffer from infertility, in which male factors account for 40% to 60% [1]. In male infertility, oligoasthenospermia accounted for nearly 50% of cases [2]. The etiology of oligoasthenospermia is complex, and many factors affecting the integrity of reproductive endocrine axis, reproductive organ development and spermatogenesis may lead to oligoasthenospermia, such as varicocele, reproductive tract infection, testicular injury, medicine taken and physicochemical factors, oxidative stress and other factors [3-5].
Spermatogenesis is regulated by endogenous testosterone (T) and FSH. Many studies have revealed that supra-physiological level of TST has an adverse impact on the spermatogenesis [6-9], whereas physiological dosage of TST as an experimental therapy for idiopathic oligoasthenospermia is commonly used in clinics [10-12]. According to the 2019 European Association of Urology (EAU) guidelines on male infertility, testosterone replacement is still strictly contraindicated for men who are considering parenthood and the treatment of male infertility with low levels of LH and FSH [13].
On the contrary, some studies had reported that a small dosage of TST could improve the semen measurements and semen quality owing to their unclear mechanism on anti-oxidant stress [14-18]. For this reason, it is uncertain whether the physiological dosage of TST can be suitably used for oligoasthenospermia with hypotestosteronemia [19]. In this study, the rat model of oligoasthenospermia was used to evaluate the effect of physiologic TST on spermatogenesis and sperm quality, and to explore a possible mechanism for the indication of TST in the clinical application of treatment of oligoasthenospermia.
Materials and methods
Animal models and experimental groups design
Male Sprague-Dawley rats (280-320 g, n=40) aged 8 weeks were purchased from the HFK Bioscience CO., LTD (Beijing, China, Certification SYXK2014-0004). The experiment procedures were carried out in accordance with the Ethical Principles of Animal Research. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study protocol was approved by the Professional Committee on Ethics of Experimental Animal Welfare, Research Institute of National Health Commission. Rats were housed under standard conditions (Room temperature 23°C±2°C on 12 h-light/dark cycles at 55%±5% relative humidity and 2 air change cycles/h) and ad libitum to standard rodent chow and filtered water. All rats were grown and kept in cages in the same condition up to 6 months without exposure to any experiments, stress or birth [20,21].
Thereafter the rats were assigned into 4 groups that using computer generated block randomization including the control group rats (n=10, group A, were administered physiologic saline through the modeling and treatment procedure); the model group (n=10, group B), low-dose TU group (n=10, group C) and high-dose TU group (n=10, group D) were first administered GTWs to induce oligoasthenospermia. The usage of the GTWs (Fudan Fuhua Pharmacy Co., Shanghai, China; Batch NO. 160301) for intragastric administration to induce rat model of oligoasthenospermia was determined based on previous studies conducted in rats at a dose of 40 mg/kg/d for 4 week [22-24].
After establishing the oligoasthenospermia model, the low-dose TU group (7.5 mg/kg) and high-dose TU group (15 mg/kg) were received intramuscular injecteion (im) of TU (Xian-Ju Pharmacy Co., Zhejiang, China; Batch NO. H10900063) twice for 8 weeks (4-week intervals), respectively. As a long-acting testosterone ester, the TU injection can maintain serum T within the physiological range for 4 weeks and could be almost equivalent pharmacokinetics produced by oral TU administration (equivalent to 40 mg/d for the low-dose and 80 mg/d for the high-dose group for a 4-week period). Simultaneously, the control group and model group were intramuscularly injected with 0.2 ml saline in the same procedure instead. After subsequent 8 weeks’ treatment, all rats were weighed and then sacrificed after anesthesia (Figure 1).
Figure 1.
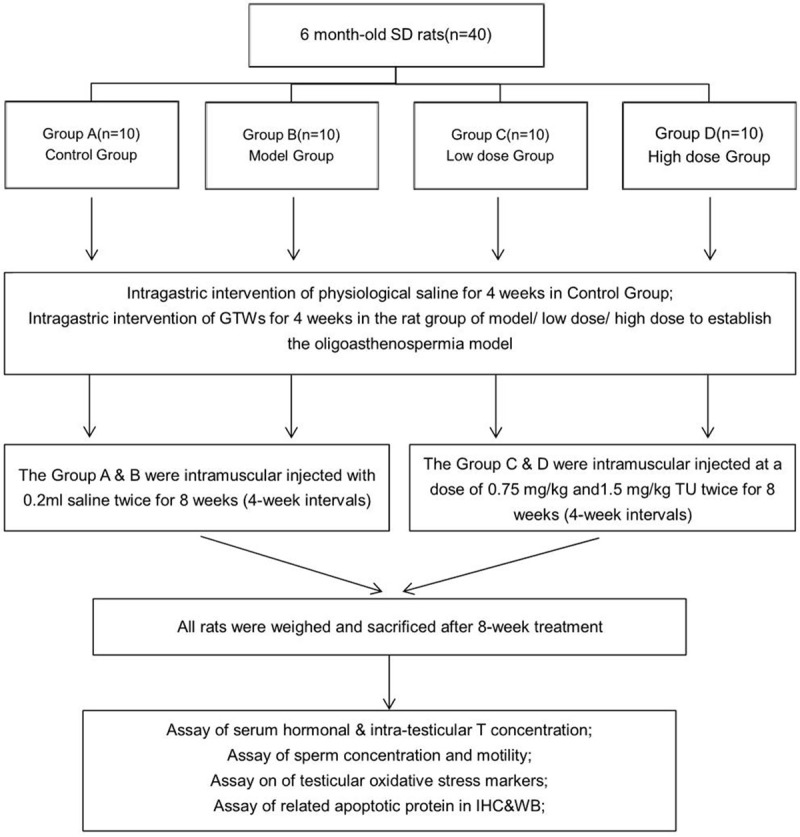
Flow chart showing the study protocol. The rats were assigned into 4 groups that used computer generated block randomization including the control group rats (n=10, group A, were administered physiologic saline through the modeling and treatment procedure). The model group (n=10, group B), low-dose TU group (n=10, group C), and high-dose TU group (n=10, group D) were first administered GTWs to induce oligoasthenospermia.
Blood collection and histologic procedures
Blood samples were collected from the sublingual vein in the animals after being anaesthetized during the experiment. At the end of the TST, large blood samples were withdrawn from the rat heart and abdominal aorta after they were anaesthetized. The serum samples were collected and stored at -80°C until assayed. The hormonal analyses for serum T, LH, FSH, E2 and SHBG were performed using commercially available kits according to the manufacturer’s instructions. The detection ranges were as follows: FSH (2.47-200 ng/mL, LSBio, WA, USA); LH (1.2-280 mIU/mL, Novus, OX, UK); E2 (3.12-200 ng/mL, Aviva, CA, USA); T (0.13-25.6 ng/mL, Aviva, CA, USA); and SHBG (0.312-20 ng/mL, Aviva, CA, USA), respectively. The intra-assay and inter-assay coefficients of variation (CVs) were 10% and 15.5%, respectively.
The testes and epididymides were removed, of which left testis and epididymis were cleaned of blood and weighed. The left testis of each animal was fixed in Bouin’s Solution for 48 h before a routine procedure to embed the tissue in paraffin. The tissue block was cut into sections at 4 μm thickness before stained with hematoxylin and eosin (H&E). A part of right testis was quickly flash-frozen in liquid nitrogen and afterwards transferred to store at -80°C for subsequent analysis.
The testicular tissue homogenate was prepared and used for determination of intra-testicular T and testicular oxidative stress markers. The detection ranges were as follows: T (0.13-25.6 ng/mL, Aviva, CA, USA); Glutathione Peroxidase (GSH-PX, 20-330 U/mL, Jiancheng, NJ, CN); Total Superoxide Dismutase (T-SOD, 5.0-122.1 U/mL, Jiancheng, NJ, CN); Malondialdehyde (MDA, 0-113.0 nmol/mL, Jiancheng, NJ, CN); Nitric Oxide (NO, 0-800 μmol/L, Jiancheng, NJ, CN) and Nitric Oxide Synthase (NOS, 0.2-81.9 U/mL, Jiancheng, NJ, CN) levels, for which commercially available kits were used according to the instructions of the manufacturer. The intra-assay and inter-assay coefficients of variation (CVs) were 5.8% and 10.5%, respectively.
Analysis of sperm concentration and motility
Spermatozoa obtained from the epididymis immediately placed in a Petri dish containing M199 (Gibco, USA) supplemented with 2.0 g/L bovine serum albumin (Sigma, USA). 10 μL of the suspension was drawn into a Makler counting chamber (Sefi Medical Instruments ltd., Israel) and the sperm concentration were analyzed using the Computer-Assisted Sperm Analysis (CASA; Hamilton Thorne-TOX IVOS, USA) system in rat semen analysis module. The sperm motility measurements observed were as follows: curvilinear velocity (VCL), average path velocity (VAP), and straight-line velocity (VSL).
Immunohistochemistry (IHC)
The sections were dewaxed, rehydrated through graded series of ethanols, and rinsed with PBS. Endogenous peroxidase activity was inhabited by incubation and then nonspecific antibody binding was blocked with 5% bovine serum albumin (BSA, Sigma, USA). Primary antibodies were detected, including, Bcl-2 (1:1000, Abcam, CB, UK), Bax (1:1000, Abcam, CB, UK), Caspase 3 (1:1000, ABclonal, BOS, USA), Caspase 9 (1:1000, ABclonal, BOS, USA), and GAPDH (1:5000, REK, Tianjin, China) after that the sections were stained with diaminobenzidine (DAB; ZSGB-BIO, Beijing, China), and the images were examined.
Western blotting (WB)
Frozen testis tissue was homogenized in assay buffer containing protease inhibitor cocktail (Roche, USA). Proteins were separated and transferred to a polyvinylidene difluoride membrane that was blocked overnight, and then probed with rabbit anti-rat antibodies against the following proteins: Bcl-2, Bax, Caspase 3, and Caspase 9. The Image J software was employed to quantify the protein expression according to gray levels of the bands.
Statistical analysis
Data were expressed as the mean ± standard errors of the mean (SEM), and sperm routine analysis were expressed as median (25th and 75th percentile) values. The one-way ANOVA and repeated measurement analysis comparison between each group was performed using a Turkey’s test with variance homogeneity test. The Welch’ ANOVA was used to calculate the differences between groups when the variance homogeneity was not satisfied, and Post hoc analysis was conducted by using Games-Howells test. Statistical analysis and graph creation were performed with GraphPad Prism version 8.02 (GraphPad Software Inc., San Diego, CA, USA), and P<0.05 was considered significant.
Results
Change of body weight and sperm quality
Significantly, there still were significant differences in body weight among the model group, low-dose and high-dose groups compared with that of the control group at the end of the experiment (Table 1). In this study, the sperm concentration, motility, and sperm movement measurements were lower in model group than that in the control group, indicating that the oligoasthenospermia model was successfully established. The sperm concentration and motility of the progressive sperm (PR, %) were increased by TST in both low-dose and high-dose groups relative to that of the model group. The sperm concentration and motility of high-dose group were basically recovered to the level of the control group. However, TST had no effect on sperm movement measurements (VAP, VSL and VCL; P>0.05).
Table 1.
Change of body weight, organ index & sperm measurements in each group after 8-week TST
| Measurements | Control Group (n=10) | Model Group (n=10) | Low-dose Group (n=10) | High-dose Group (n=10) | ANOVA Value |
|---|---|---|---|---|---|
| Tissue Weight | |||||
| Body Weight (g) | 674.43±9.754 | 568.90±21.216† | 589.50±20.403† | 594.80±17.247† | 5.032 (P=0.006) |
| Epididymis (g) | 1.427±0.040 | 1.443±0.138 | 1.633±0.111 | 1.612±0.224 | 7.993 (P=0.145) |
| Testicular Organ Index | 0.0055±0.00013 | 0.0061±0.00046 | 0.0061±0.00029 | 0.0060±0.00046 | 0.852 (P=0.506) |
| Epididymis Organ Index | 0.0023±0.00008 | 0.0024±0.00021 | 0.0027±0.00020 | 0.0027±0.00033 | 8.212 (P=0.082) |
| Sperm measurements | |||||
| Concentration (106/mL) | 240.00 (202.50, 275.00)* | 69.68 (57.03, 83.18) | 182.25 (196.90, 240.15)* | 238.65 (233.68, 259.80)*,# | 23.07 (P<0.0001) |
| Motility (%) | 60.50 (50.00, 71.00)* | 12.88 (10.12, 17.25) | 49.50 (37.00, 54.00)* | 61.50 (53.00, 65.25)* | 21.64 (P<0.0001) |
| PR (%) | 41.00 (35.00, 46.75)*,# | 10.75 (9.18, 14.26)# | 33.00 (25.50, 40.00)* | 34.50 (32.25, 37.00)* | 21.64 (P<0.0001) |
Note: Data are expressed as the mean ± standard errors of the mean (SEM), and the sperm routine analysis are expressed in median (25th and 75th percentile) values.
Compared with control group, P<0.05;
Compared with model group, P<0.05;
Compared with low-dose group, P<0.05.
Histopathologic damage and reversal
A histologic analysis of testicular tissues from the control group revealed a normal process of spermatogenesis (Figure 2A and 2E). In contrast, the model group exhibited testicular damage including loss, disorganization, and sloughing of spermatogenic cells, degeneration of interstitial cells, and vacuolization in the cytoplasm of Sertoli cells, which was consistent with oligoasthenospermia (Figure 2B and 2F). TST partially restored the normal morphology of Leydig cells, Sertoli cells, and spermatogenic cells (Figure 2C and 2G), with the most obvious improvement recorded in high-dose group (Figure 2D and 2H). In contrast with the model group, the Johnsen score of the low-dose and high-does groups showed better pathogenic manifestations (Figure 3A). Moreover, TST increased the size of the nucleus of spermatogenic cells and the diameter of the testicular SfT (Figure 3B and 3C), as well as greatly increased the thickness of spermatogenic epithelium in the high-dose group compared to the model group (Figure 3D).
Figure 2.
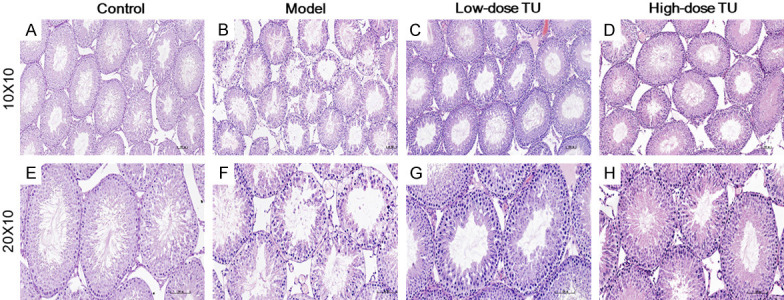
Histologic changes of rat testes with H&E staining in each group. Control group with the normal histology of SfT and interstitial cells (A and E). The model group with loss of spermatogenic cells, degeneration of interstitial cells, and vacuolization in the cytoplasm of Sertoli cells (B and F). Low-dose TU group with increased numbers of spermatogenic cells, hyperplasia of interstitial cells, and decreased vacuolization in the cytoplasm of Sertoli cells (C and G). High-dose TU group with further enhancement of tissue recovery as compared to the low-dose TU group (D and H). TST promoted the morphology of testicular SfT, increased the number of spermatogenic cells and interstitial cells, and decreased the number of vacuoles in Sertoli cells after 8-week TST (Magnification ×100: A-D and ×200: E, F). Scale bar =100 μm.
Figure 3.
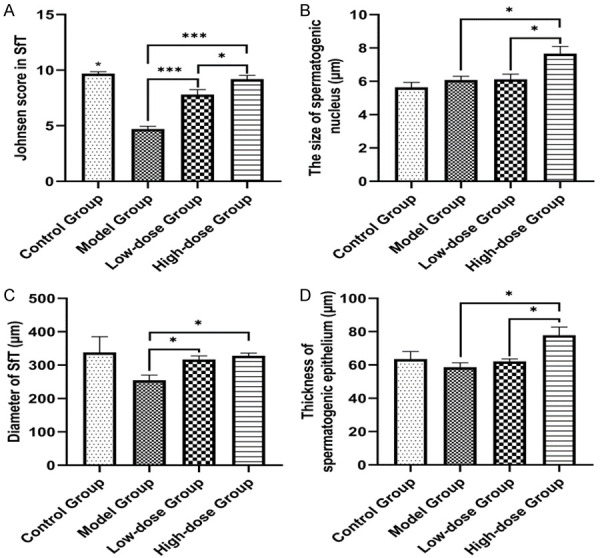
Change in spermatogenic cells and testicular SfT measurement in each group. TST (testosterone supplemental therapy) improved the Johnsen score of SfT in testes (A), increased the diameter of spermatogenic nucleus (B) and the size of SfT (C), and markedly promoted the thickness of spermatogenic epithelium (D) in the high-dose group compared to the model group (×400). The data in the figure are presented as mean ± standard error (SE); *P<0.05, **P<0.01, ***P<0.001.
Change of serum hormone levels in each group after 8-week TST
The levels of serum FSH, LH, and SHBG in the model group increased when compared with that of the control group, while the levels of serum T and E2 decreased remarkably after establishment of the oligoasthenospermia model at the 4th week (Figure 4). At the end of first TU administration (the 8th week), the level of serum T, E2, and SHBG increased in low-dose and high-dose groups (Figure 4A, 4B and 4E). It was worth noting that FSH and LH level were also increased at the 4th week in the TST groups (Figure 4C and 4D). In addition, the serum LH and SHBG in the low-dose and high-dose groups showed a significant difference of downward trend (P<0.05) compared with that of the control group at the end of TST (Figure 4D and 4E).
Figure 4.
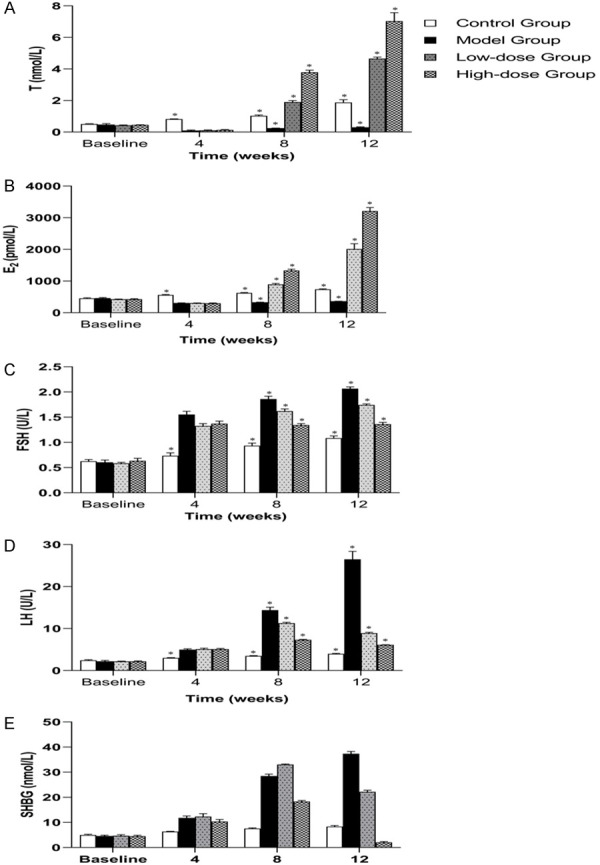
The change of serum hormones at the baseline model established in the 4th week, and TST period from the 8th week to 12th week. TST increased the serum T and E2 levels significantly in peripheral blood throughout the experiment (A and B). Moreover, the growth trend of serum FSH, LH, and SHBG had been effectively inhibited or reduced by TST (C-E). * designates P<0.05.
Intra-testicular T and testicular oxidative stress markers
GTWs induced oxidative stress in rat testes, as evidenced by the significant increase in MDA, with a compensatory increase of GSH-PX and NO concentrations in the model group to eliminate the superoxide compared to those of the control group were observed at the end of experiment (Figure 5B, 5C and 5F). Exogenous TST could promote intra-testicular T in the low-dose and high-dose groups, and improve oxidative stress markers of MDA and GSH-PX levels after 8-week TST compared with that of the model group (Figure 5A-C). The level of NO was observed a downward trend in the TST groups at the end of experiment without significant difference when compared with that of the model group (Figure 5F). The T-SOD and T-NOS remained unchangeable when compared with that of the control group and the model group (Figure 5D and 5E).
Figure 5.
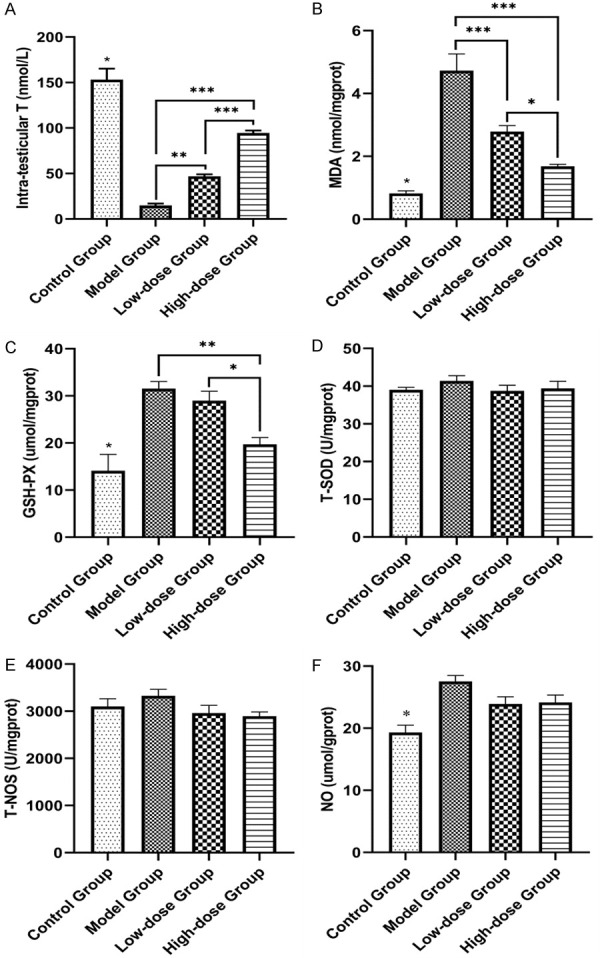
The changes in oxidative stress markers of rat testes in each group. TST could promote the intra-testicular T in the low-dose and high-dose groups (A) and alleviated the testicular oxidative stress markers of MDA and GSH-PX at the end of TST (B and C). The T-SOD and T-NOS remained unchangeable when compared to that of the control group and the model group (D and E). The level of NO remained basically unchanged through 8-week treatment after a compensatory increase at the 4th week (F). *P<0.05, **P<0.01, ***P<0.001.
Effect of TST on mitochondrial apoptosis-related pathway
The damage of the testicular tissues was evidenced by apoptosis proteins (Bax, Caspase-9, and Caspase-3) with immunohistochemistry, markedly increased in the model group compared to that of the other 3 groups at the end of TST (Figure 6). Bax immune-positive cells were mainly located in spermatogenic and Sertoli cells (Figure 6A-D); Bcl-2 immuno-positive cells were mainly located in sperm and spermatogenic cells (Figure 6E-H); Caspase-9 & 3 immuno-positive cells were mainly located in sperm, spermatocytes and Leydig cells (Figure 6I-L, 6M-P). Moreover, the damage was partially reversed in the TST groups compared with that of the model group. However, Bcl-2 expression displayed similar changes between each group (Figure 6E-H). To further quantify these changes, western blotting was used to evaluate the expression levels of these apoptosis proteins (Figure 7). Bax, caspase-9 and -3, and Cleaved- caspase-3 were upregulated in the model group (Figure 7A, 7D-F), whereas Bcl-2 was downregulated in rats with oligoasthenospermia (Figure 7B), which was reversed by TST after 8-week intervention (Figure 7G). Bax/Bcl-2 ratio was increased in the model group and decreased in the TST groups (Figure 7C), prompting the Bax/caspase-9/caspase-3 cascade in oligoasthenospermia.
Figure 6.
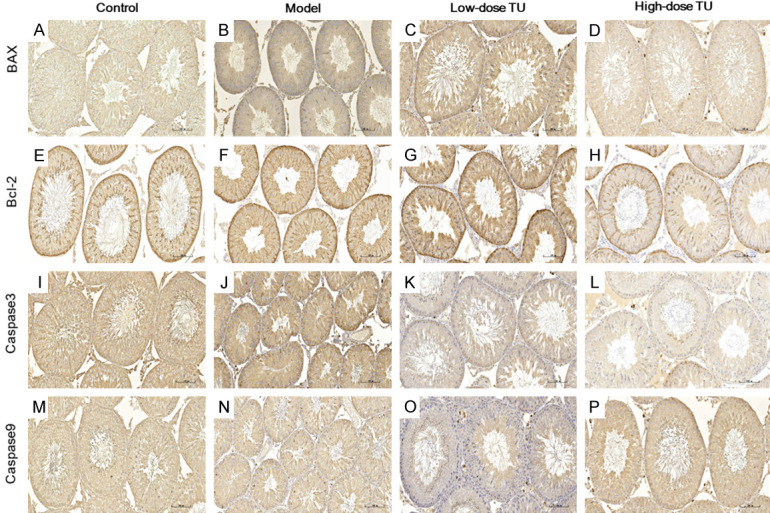
Localization of apoptosis proteins was detected by immunohistochemistry in each group. The apoptosis protein expression of Bax, Cleaved Caspase-3 and Caspase-9 protein in high-dose group was significantly reduced and alleviated compared to the model group (A-D, I-L and M-P), but without significant difference in Bcl-2 protein between each group (E-H) (Magnification ×200). Scale bar =100 μm.
Figure 7.
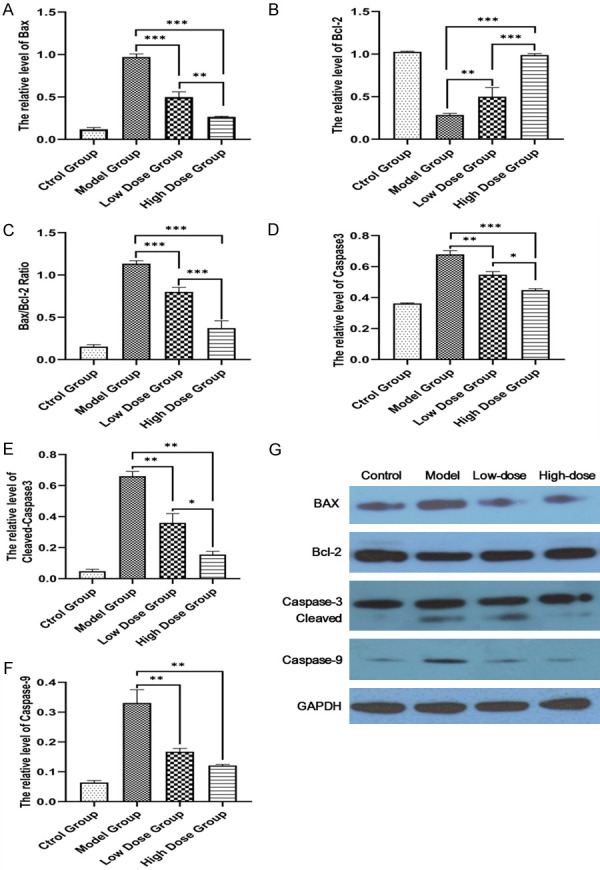
Change of apoptosis proteins detected by western blotting in each group. GADPH was used as the control for band density normalization. Bax, Cleaved-caspase-3 and caspase-3 and -9 were upregulated (A, D-F), whereas Bcl-2 was downregulated in the model group (B), which was reversed by TST after 8-week intervention (G). Bax/Bcl-2 ratio was increased in the model group and decreased in the TST groups (C), *P<0.05, **P<0.01, ***P<0.001.
Discussion
The serum FSH, LH, and T secreted by the gonadal axis directly regulate human sexual function and spermatogenesis, while Sertoli cells and Leydig cells in the testis can impact hormone secretion and spermatogenesis [25,26]. Moreover, this dosage of the GTWs had been confirmed to successfully act on inducing and imitating the oligoasthenospermia state, and the pathologic results showed that there was still a significant difference between the normal after 8 weeks of drug withdrawal [23]. Therefore, the GTWs have long been broadly applied to induce the oligoasthenospermia model of rats in China. In our study, the testicular seminiferous epithelium in the model group became thinner, and the seminiferous cells showed loss, disorganization, interstitial space edema, and spermatogenic cells atrophy, resulting in the decrease of epididymis sperm concentration and sperm activity after administrated GTWs 40 mg/(kg·d) for 4 weeks. The levels of serum FSH and LH were observed to be increased to varying degrees after GTWs modeling, while the levels of serum T and testicular tissue T were significantly reduced. Sertoli cells and Leydig cells were denatured and apoptotic, suggesting that the model of oligoasthenospermia with endocrine damage was successfully established [23,27,28].
According to guidance from Chinese experts and suggestions from multicenter clinical studies, TU 40 mg/d or 80 mg/d for androgen supplementation plays an important role in the treatment of idiopathic male infertility, and there is no need to worry too much about the negative feedback of androgen on spermatogenesis [19,29,30]. The therapeutic effects of TST on this kind of oligoasthenospermia were mainly reflected in an improvement in the number and activity of epididymis sperm, partial recovery or improvement of testicular histopathology, and serum sex hormone levels. After TST intervention, the levels of serum FSH and LH decreased, the level of serum T increased significantly, and the concentration of testicular T partially recovered when compared to that of the control group. In addition, the results of testicular histopathology also showed that the number and structure of Sertoli cells and Leydig cells had been restored, suggesting that TST could improve the pathologic morphology of testis, promote spermatogenesis, and increase the number of spermatozoa [31-34].
According to the existing consensus, the production and clearance of ROS are in a dynamic balance under the normal physiologic conditions [35]. When various harmful endogenous or exogenous stimuli disrupt this balance, especially when it exceeds the scavenging capacity of antioxidant system, the body will produce an oxidative stress response [36]. In this state, excess free radicals produced by the body will lead to lipid peroxidation, and the end product is MDA. In the present experiment, MDA in the model group was increased significantly, indicating that GTWs successfully induced oxidative stress damage of the testes and produced excessive ROS. Due to the cytotoxicity of MDA, it will affect the activity of mitochondrial respiratory chain complex and mitochondrial key enzymes, and aggravate membrane damage, which could be the mechanism of spermatogenesis damage induced by GTWs.
In order to prevent the destruction of oxygen free radicals to the cell, almost all cells have a complete protective system to eliminate various reactive oxygen species produced by cell metabolism. GSH-PX is an important enzyme that catalyzes the decomposition of hydrogen peroxide, and its specific catalytic GSH can protect the structural and functional integrity of the cell membrane [37]. Therefore, with the generation of excessive free base, the concentration of GSH-PX also presented a compensatory increase to improve the scavenging ability. The levels of GSH-PX and MDA in the treatment groups gradually decreased, indicating a significant improvement trend in cell damage after TST. Therefore, TST could not only reduce the damage caused by oxidative stress to a certain extent, but also play a role in inhibiting cell apoptosis [38,39]. This could be one of the mechanisms by which TST repaired such cellular damage.
NO is a bioactive free radical that catalyzes the production of L-arginine in the presence of NADPH by NOS, and is closely related to the testis microcirculation regulation, T secretion, and sperm maturation [40,41]. Compared with the control group, the NO level of the other three groups increased significantly after modeling. However, concentrations of NO in the low-dose and high-dose groups were decreased slightly compared with that of the model group after TST, which indicated that the testicular damage degree was also improved [42]. Therefore, our results confirmed that TST partially restored the testicular oxidative damage caused by GTWs, and showed reparative effects on the testicular tissue and spermatogenesis.
In order to explore the molecular mechanism of oxidative damage leading to oligoasthenospermia, key proteins of mitochondrial apoptosis pathway were selected for verification. Apoptosis of mitochondria is controlled by Bcl-2 family proteins, including Bcl-2 and Bax [43]. The former inhibits apoptosis, while the latter antagonizes this cyto-protective effect [44,45]. By immunohistochemistry and western blotting, we further verified that GTWs induced apoptosis in this kind of oligoasthenospermia model. Especially the level of Bax, an apoptotic protein related to the activation of mitochondrial apoptosis pathway, was significantly up-regulated, which led to the increase of Bax/Bcl-2 ratio in the model group, and was related to the enhancement of mitochondrial membrane permeability [46,47]. Thus, the downstream of caspase-3 and -9 induction of apoptosis is activated [48,49]. Furthermore, Caspase-3 can activate other caspase proteins by cleavage or proteolysis [50]. When compared with the model group, we found the protein Bcl-2 and Bax levels of the treatment groups were increased and decreased significantly, respectively, resulting in a decrease in Bax/Bcl-2 ratio after TST. Such a decrease indicated that TST effectively prevent Bax translocation to mitochondria, thus inhibiting the apoptosis. This could be the molecular target of spermatogenesis partial recovery after TST.
In conclusion, we confirmed that GTWs can cause the drug-induced oligoasthenospermia with low serum T, and TST can partially restore spermatogenesis in male SD rats. Our results provided an experimental basis and indications for the clinical empirical therapy of this kind of oligoasthenospermia by TST. In addition, our study also clarified the possible mechanism of GTWs for the spermatogenesis damage. TST for damage repair serves to reduce apoptosis of the testicular cells and restore the function of Leydig cells by regulating the antioxidant system in vivo. As well, the molecular mechanism of reducing testicular cell apoptosis was achieved by inhibiting the Bax/Caspase mitochondrial apoptosis pathway.
Acknowledgements
This study was supported by Science and Technology Projects of Research Institute for National Health Commission, China (No. 2016JY004) and Guangdong Science and Technology Project of Guangzhou Women and Children’s Medical Center (NO. 2017A030223003). The authors thank Xiaowei Wang, RA, Fang Zhou, PhD, Xiaowei Liang, MD (the department of male clinical research), Guo Bao, PhD (the department of animal research center) and Linyuan Zhang, AP (Department of Occupational Health and Poison Control, National Center for Disease Prevention and Control) for their friendly assistance in the laboratory guidance and animal experiments.
Disclosure of conflict of interest
None.
References
- 1.Niederberger C. WHO manual for the standardized investigation, diagnosis and management of the infertile male. Urology. 2001;57:208. [Google Scholar]
- 2.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C European Association of Urology Working Group on Male Infertility. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Nieschlag E, Behre HM. In: Andrology: male reproductive health and dysfunction. Nieschlag E, editor. Berlin: Springer; 2001. [Google Scholar]
- 4.Du Plessis SS, Agarwal A, Sabanegh ES. In: Male infertility: a complete guide to lifestyle and environmental factors. Du Plessis SS, editor. New York: Springer; 2014. [Google Scholar]
- 5.Siddiq FM, Sigman M. A new look at the medical management of infertility. Urol Clin North Am. 2002;29:949–63. doi: 10.1016/s0094-0143(02)00085-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GY, Gu YQ, Wang XH, Cui YG, Bremner WJ. A clinical trial of injectable testosterone undecanoate as a potential male contraceptive in normal Chinese men. J Clin Endocrinol Metab. 1999;84:3642–7. doi: 10.1210/jcem.84.10.5957. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, Bo L, Xiong C, Wang X, Liu X, Peng L, Yao K. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–5. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 8.Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, Huang ZJ, Zhang GY. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562–8. doi: 10.1210/jc.2002-020447. [DOI] [PubMed] [Google Scholar]
- 9.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, Weidner W EAU Working Group on Male Infertility. EAU guidelines on male infertility. Eur Urol. 2005;48:703–11. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Bhasin S. Approach to the infertile man. J Clin Endocrinol Metab. 2007;92:1995–2004. doi: 10.1210/jc.2007-0634. [DOI] [PubMed] [Google Scholar]
- 11.Cavallini G. Male idiopathic oligoasthenoteratozoospermia. Asian J Androl. 2006;8:143–57. doi: 10.1111/j.1745-7262.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Madhukar D, Rajender S. Hormonal treatment of male infertility: promises and pitfalls. J Androl. 2009;30:95–112. doi: 10.2164/jandrol.108.005694. [DOI] [PubMed] [Google Scholar]
- 13.Jungwirth A, Diemer T, Kopa Z, Krausz C, Minhas S, Tournaye H. EAU guidelines on male infertility. In: Jungwirth A, editor. European Association of Urology guidelines. Arnhem, the Netherlands: Springer; 2019. [Google Scholar]
- 14.Makary S, Abdo M, Fekry E. Oxidative stress burden inhibits spermatogenesis in adult male rats: testosterone protective effect. Can J Physiol Pharmacol. 2018;96:372–81. doi: 10.1139/cjpp-2017-0459. [DOI] [PubMed] [Google Scholar]
- 15.Pusch HH. Oral treatment of oligozoospermia with testosterone-undecanoate: results of a double-blind-placebo-controlled trial. Andrologia. 1989;21:76–82. [PubMed] [Google Scholar]
- 16.Adamopoulos DA, Pappa A, Billa E, Nicopoulou S, Koukkou E, Michopoulos J. Effectiveness of combined tamoxifen citrate and testosterone undecanoate treatment in men with idiopathic oligozoospermia. Fertil Steril. 2003;80:914–20. doi: 10.1016/s0015-0282(03)01123-3. [DOI] [PubMed] [Google Scholar]
- 17.Ridley AJ, Blasco L. Testosterone and gossypol effects on human sperm motility. Fertil Steril. 1981;36:638–42. [PubMed] [Google Scholar]
- 18.Shieh CC, Chang SC, Tzeng CR, Huang JJ, Nir WJ, Hong CY. Measurement of testosterone in seminal plasma, saliva and serum by solid-phase enzyme immunoassay. Andrologia. 1987;19:614–9. doi: 10.1111/j.1439-0272.1987.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 19.Li HJ. More attention should be paid to the treatment of male infertility with drugs--testosterone: to use it or not? Asian J Androl. 2014;16:270–273. doi: 10.4103/1008-682X.122343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–7. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Andreollo NA, Santos EF, Araújo MR, Lopes LR. Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig. 2012;25:49–51. doi: 10.1590/s0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- 22.Ma HF, Li HS, Wang B, Mo XW, Zhao B, Liu Y. Research progress in the model of spermatogenic impairment rats induced by tripterygium glycosides. Chin J Hum Sex. 2014;23:54–7. [Google Scholar]
- 23.Ma HF, Li HS, Zhao ZJ, Wang B, Zhao B, Mo XW, Liu Y, Chen MX, Yang MJ. Establishment of rat model of spermatogenesis disorder induced by tripterygium wilfordii glycoside. Zhonghua Nan Ke Xue. 2015;21:179–84. [Google Scholar]
- 24.Wang T, Huang J, Wu D, Li Q, Liu X, Chen H, Qiao L, Li D, Li J. Effect of wuziyanzong pill on sperm quality and calcium ion content in oligoasthenospermia rats. J Tradit Chin Med. 2012;32:631–5. doi: 10.1016/s0254-6272(13)60083-7. [DOI] [PubMed] [Google Scholar]
- 25.Dohle GR, Smit M, Weber RF. Androgens and male fertility. World J Urol. 2003;21:341–5. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- 26.Lombardo F, Sgrò P, Salacone P, Gilio B, Gandini L, Dondero F, Jannini EA, Lenzi A. Androgens and fertility. J Endocrinol Invest. 2005;28(Suppl):51–5. [PubMed] [Google Scholar]
- 27.Lan ZJ, Gu ZP, Lu RF, Zhuang LZ. Effects of multiglycosides of tripterygium wilfordii (GTW) on rat fertility and leydig and sertoli cells. Contraception. 1992;45:249–61. doi: 10.1016/0010-7824(92)90069-6. [DOI] [PubMed] [Google Scholar]
- 28.Lu QX. Effect of glycosides of tripterygium wilfordii Hook on the reproductive system and major organs of male rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1990;12:203–7. [PubMed] [Google Scholar]
- 29.He XY, Li G, Zhang X, Jiang H, Zhao LM, Dai DX, Xie Y. Multicenter clinical study of a small dose of androgen for the treatment of oligo-asthennospermatism. Chin J Androl. 2009;23 42-5+48. [Google Scholar]
- 30.He XY, Song T, Li G, Yu MB, Wang XX, Hong BF. Clinical study of a small dose of androgen for the treatment of oligo-asthennospermatism. Chin J Androl. 2006:28–32. [Google Scholar]
- 31.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fok KL, Chen H, Ruan YC, Chan HC. Novel regulators of spermatogenesis. Semin Cell Dev Biol. 2014;29:31–42. doi: 10.1016/j.semcdb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 34.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30:119–32. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto H, Doita M, Nishida K, Yamamoto T, Sumi M, Kurosaka M. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine (Phila Pa 1976) 2006;31:4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- 37.Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet. 2014;31:341–54. doi: 10.1007/s10815-013-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makary S, Abdo M, Fekry E. Oxidative stress burden inhibits spermatogenesis in adult male rats: testosterone protective effect. Can J Physiol Pharmacol. 2018;96:372–81. doi: 10.1139/cjpp-2017-0459. [DOI] [PubMed] [Google Scholar]
- 39.Tian M, Liu F, Liu H, Zhang Q, Li L, Hou X, Zhao J, Li S, Chang X, Sun Y. Grape seed procyanidins extract attenuates cisplatin-induced oxidative stress and testosterone synthase inhibition in rat testes. Syst Biol Reprod Med. 2018;64:246–59. doi: 10.1080/19396368.2018.1450460. [DOI] [PubMed] [Google Scholar]
- 40.Staicu FD, Lopez-Úbeda R, Romero-Aguirregomezcorta J, Martínez-Soto JC, Matás Parra C. Regulation of boar sperm functionality by the nitric oxide synthase/nitric oxide system. Version 2. J Assist Reprod Genet. 2019;36:1721–36. doi: 10.1007/s10815-019-01526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zini A, O’Bryan MK, Magid MS, Schlegel PN. Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol Reprod. 1996;55:935–41. doi: 10.1095/biolreprod55.5.935. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, He Q, Yan X, Cai Y, Chen J. Effect of exogenous nitric oxide on sperm motility in vitro. Biol Res. 2014;47:44. doi: 10.1186/0717-6287-47-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb RA. Role of mitochondria in apoptosis. Crit Rev Eukaryot Gene Expr. 2000;10:231–9. doi: 10.1615/critreveukargeneexpr.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- 44.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–9. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–86. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–37. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 48.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–83. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 49.Fang M, Wang XD. The mitochondrial pathways of apoptosis. J Beijing Medical University. 2002;34:1–10. [Google Scholar]
- 50.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–70. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]


