Abstract
Cancer stem cells (CSCs) are essential in every step of tumorigenesis and progression. As an important process in cancer development, epithelial-mesenchymal transition (EMT) has been reported to promote stem-like cells. Bladder cancer is one of the most common cancers in the urinary tract, and cigarette smoke (CS) is a preventable risk factor. In the present study, we tested the hypothesis that CS could promote stemness and EMT in bladder cancer. Bladder cancer UM-UC-3 and EJ cell lines were maintained in serum-free medium to grow as tumor spheres, characteristic of CSCs. Results demonstrated that CS enhanced tumor sphere formation capacity, upregulated expression of CSC markers, increased the proportion of the CD44+ cell population, and promoted EMT. Mechanistically, the Sonic Hedgehog (SHH) pathway regulated CS-triggered EMT and stemness. More importantly, among bladder cancer patients, smokers harbored higher levels of CSC markers and proteins for SHH signaling than non-smokers. Collectively, findings in this study highlight the critical role of CS in the stemness and EMT of bladder cancer. Smoking cessation and intervening in the SHH pathway may both be strategies to prevent bladder cancer.
Keywords: Bladder cancer, cigarette smoke, cancer stem cells, epithelial-mesenchymal transition, sonic hedgehog pathway
Introduction
Bladder cancer is one of the most common cancers in the urinary tract. Annually, there are estimated 430,000 newly diagnosed cases and 180,000 deaths related to bladder cancer [1]. Approximately 80,500 bladder cancer patients were diagnosed in 2015 in China [2]. Due to recurrence and resistance to chemotherapy, the outcome for bladder cancer is poor. It has been reported that environmental factors participate in the tumorigenesis of bladder cancer [3,4]. More specifically, many lines of evidence have shown that cigarette smoke (CS) strongly correlates with the prevalence of bladder cancer, which has been recognized as the most important single risk factor [5].
Cancer stem cells (CSCs) are a subpopulation of cells with the characteristics of self-renewal, inducing tumor growth and resistance to treatment [6]. A growing body of research has shown that CSCs are responsible for progression and recurrence of various types of tumors including bladder cancer [7-9]. Currently, strategies targeting CSCs have been widely utilized to treat refractory cancer. Functions of CSCs are regulated by crucial signal pathways such as Sonic Hedgehog (SHH), Wnt/β-catenin, and Notch pathways [10]. The SHH pathway is essential for the maintenance and functions of CSCs [11]. The SHH ligand binds 12-pass transmembrane Patched 1 (Patch1) receptor and then represses the associated 7-pass transmembrane Smoothened (Smo). Patch1, upon activation, executes its inhibitory action on Smo and induces the activation of Gli family, which ultimately upregulates target genes [12]. Epithelial-mesenchymal transition (EMT) contributes to cancer invasion and metastasis by various mechanisms [13,14]. EMT can not only exhibit a mesenchymal-like phenotype, but also induce cells to acquire stem-cell-like characteristics [15,16].
CS has been confirmed to promote EMT in various cancers. It has been reported that long-term smoke exposure enhances EMT and acquires CSCs properties in the SV-40 urothelial cell line [17]. Recently, induction of lung, renal, pancreatic, and oral cancer stem-cell-like cells by CS exposure has also been reported [18-23]. Nevertheless, it remains unknown whether CS promotes bladder CSCs and by what mechanism. Therefore, we conducted the present study to test the roles of CS on bladder CSC properties and the role of the SHH pathway in this process. Here, we report that cigarette smoke extract (CSE) promotes the stemness of bladder CSCs and their EMT. Activation of SHH signaling is essential for CSE-induced stemness of bladder CSCs and EMT. Moreover, bladder cancer patients who smoke harbor increased levels of markers for CSCs and SHH signaling in their bladder cancer compared to non-smoker patients. These results highlight the importance of CS in progression of bladder cancer.
Materials and methods
Cell culture and reagents
Human bladder cancer cell lines EJ and UM-UC-3 were obtained from American Type Culture Collection (ATCC, Wiltshire, USA) and maintained in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco). Cells were maintained at 37°C with 5% CO2. Antibodies CD44, CD133, ALDH1A1, Nanog, Oct4, CyclinD1, PCNA, Smo, Shh, Gli1, Gli2, E-cadherin, N-cadherin, ZO-1, Vimentin, and GAPDH were purchased from Proteintech (Rocky Hill, NJ, USA). Anti-rabbit or anti-mouse secondary antibodies were purchased from ZSGBBIO (Beijing, China).
Acquirement of cigarette smoke extract (CSE)
CSE was prepared freshly for every round of experiments, according to the protocol of a reported method, by combusting one filterless 3R4F research cigarette (University of Kentucky) [24,25].
Tumor sphere formation assay
Bladder cancer cells were seeded into 24-well plates with the density of 5000 cells per well and cultured in serum-free medium RPMI-1640 (Gibco) with 20 ng/mL fibroblast growth factor (FGF), 20 ng/mL epidermal growth factor (EGF), 5 μg/ml insulin and 2% B27 (all from Gibco). Formation of tumor spheres was photographed under a microscope (Nikon, Japan). In order to investigate the effects of CSE on the tumor sphere formation, UM-UC-3 and EJ cells were treated with a gradient of concentrations of CSE (0, 0.01%, 0.05%, and 0.1%). After 7 days of treatment, the number of UM-UC-3 and EJ tumor spheres that had formed larger than 50 μm in diameter was counted.
Western blotting
Cells were washed with phosphate-buffered saline (PBS), followed by solubilization in the RIPA buffer containing protease inhibitors (Roche, Basel, Switzerland). The protein concentration was measured by BCA Protein Assay kits (Pierce, Rockford, WI, USA). Protein was separated by SDS-PAGE (8%-12%) electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, USA). The membranes were blocked with 5% skim milk at room temperature, and then incubated overnight with primary antibodies (1:500-1:1000 dilution) at 4°C overnight. Afterwards, the membranes were incubated with secondary antibodies (1:1000) at room temperature for one hour. GAPDH was used as the loading control.
RNA extraction and quantitative real-time PCR
Total RNA was isolated by Trizol reagent (Invitrogen). A total of 1 μg of RNA was used for reverse transcription according to the manufacturer’s instructions (Abm, Canada). The quantitative real-time PCR (qRT-PCR) was performed using the Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and LC96 real-time PCR detection system (Roche, Biosystems).
The primers of CD44, CD133, ALDH1A1, Nanog, Oct4, GAPDH, PCNA and CyclinD1 were acquired from the Beijing Genomics Institute (Beijing, China), whose sequences are listed in Table 1. Fold changes in the expression of each gene were calculated through 2-(ΔΔCt) method with GAPDH serving as the housekeeping gene.
Table 1.
Sequences of primers
| Gene | Primers | |
|---|---|---|
| CD44 | Forward | 5’-GACACATATTGTTTCAATGCTTCAGC-3’ |
| Backward | 5’-GATGCCAAGATGATCAGCCATTCTGGAAT-3’ | |
| CD133 | Forward | 5’-TACAACGCCAAACCACGACTGT-3’ |
| Backward | 5’-TCTGAACCAATGGAATTCAAGACCCTTT-3’ | |
| Oct4 | Forward | 5’-TGGGATATACACAGGCCGATG-3’ |
| Backward | 5’-TCCTCCACCCACTTCTGAG-3’ | |
| Nanog | Forward | 5’-TTTGTGGGCCTGAAGAAAACT-3’ |
| Backward | 5’-AGGGCTGTCCTGAATAAGCAG-3’ | |
| ALDH1A1 | Forward | 5’-GCACGCCAGACTTACCTGTC-3’ |
| Backward | 5’-CCTCCTCAGTTGCAGGATTAAAG-3’ | |
| CyclinD1 | Forward | 5’-AGGCCCTGGCTGCTACAAG-3’ |
| Backward | 5’-ACATCTGAGTGGGTCTGGAG-3’ | |
| PCNA | Forward | 5’-CTGAAGCCGAAACAGCTAGACT-3’ |
| Backward | 5’-TCGTTGATGAGGTCTTGAGTGC-3’ | |
| GADPH | Forward | 5’-CAAGGTCACCATGACAACTTTG-3’ |
| Backward | 5’-GTCCACCACCCTGTTGCTGTAG-3’ | |
Cell proliferation assay
Cells were plated into 96-well plates with a density of 1,000 cells per well and maintained in the serum-free medium. After treatment with various concentrations of CSE (0, 0.01%, 0.05%, 0.1%, 0.25%, 0.5%, 0.75% and 1.0%) for 7 days, cell proliferation assay was conducted by cell counting kit 8 (CCK-8) assay (Beyotime, China).
Detection of CD44 positive cells by flow cytometry
Briefly, adherent cells and tumor sphere-forming bladder cancer cells were collected and then washed twice with ice-cold PBS. Approximately 1×106 cells were stained with 1 μL of PE-conjugated CD44 (Miltenyi Biotech, Teterow, Germany) antibody or isotype control antibody (Mouse IgG1) (Miltenyi Biotech) in darkness at 4°C for 15 minutes. Subsequently, the number of CD44 positive cells was applied by a FACSCalibur flow cytometer (BD Biosciences, NJ, USA). Flow cytometry data were analyzed by FlowJo (version 10.5, Windows edition, BD).
Immunofluorescence staining
The sphere-forming cells were fixed in 4% paraformaldehyde and incubated with CD44 antibodies at 4°C overnight. After washing with Tris-buffered saline Tween, cells were stained with Cy3-conjugated goat anti-rabbit secondary antibodies for 2 h and DAPI for 15 min. The fluorescent images were captured using a fluorescence microscope (Nikon, Japan).
Transwell assay
Bladder cancer tumorsphere cells were pretreated with CSE (0, 0.01%, 0.05%, and 0.1%) for 7 days, and were then added to the upper transwell chambers (Millipore, USA) with Matrigel (BD, USA). After 24 h, cells that had migrated through the membrane were stained with 0.1% crystal violet. Images of the stained cells were photographed under a microscope (Nikon, Tokyo, Japan) and counted.
Transfection with small interfering RNA (siRNA)
Bladder cancer cells were seeded at a density of 1×105 cells/well into six-well plates in the RPMI-1640 medium containing 10% FBS without antibiotics. Following 12 h of incubation, cells were then transiently transfected with Gli1-siRNA (75 nM) or non-targeting control siRNA (75 nM) with Lipofectamine 2000 reagents (Invitrogen, Carlsbad, California, USA) according to standard protocols. Twelve hours later, cells were trypsinized and cultured in the serum-free medium for 5 more days. Targeting sequences of siRNA were described as follows: Gli1 siRNA, 5’-CCAGGAAUUUGACUCCCAATT-3’. Gli1-siRNA and control-siRNA were obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
SHH signaling inhibition
Vismodegib, a SHH signaling specific inhibitor, was purchased from Sigma (Oakville, ON, Canada). Vismodegib, resolved in DMSO to the designated concentrations, was added to cell culture in order to test the role of the SHH pathway.
Clinical patients and bladder specimens
A total of 38 bladder cancer patients, diagnosed according to the WHO classification without any treatment, were enrolled in the Second Affiliated Hospital of Anhui Medical University. The ethics committee of the Second Affiliated Hospital of Anhui Medical University, China approved and supervised the current project. A written consent form was explained, agreed to, and signed by each participant. Surgically resected samples from bladder cancer patients were prepared as formalin-fixed paraffin-embedded tissue. Serial sections (4 to 6 μm in thickness) were cut. A fraction of freshly resected tumor (8 cm3) was stored in -80°C until utilized for qPCR and western blotting.
Immunohistochemistry (IHC)
The paraffin sections were deparaffinized with xylene and hydrated in a graded ethanol series. After inhibition of the endogenous peroxidase, the sections were then incubated with antibodies against CD133 (1:200), CD44 (1:200), Oct4 (1:100), Nanog (1:100), Gli1 (1:200) and Smo (1:200). Visualization of targeting proteins was performed using a DAB kit. Subsequently, slides were imaged under an optical microscope (Nikon, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Student t-tests were used for comparison between two groups, and one-way ANOVA with Dunnett post-hoc tests were used for analyses among more than two groups by Graph-Pad Prism 7.0 software (GraphPad Software, San Diego, CA, US). A p value < 0.05 was considered significant.
Results
Acquisition of bladder cancer stem cells (CSCs) by serum-free medium culture in vitro
Culture using serum-free medium (SFM) is widely used in isolation of CSCs in vitro. UM-UC-3 and EJ cells were maintained in serum-free medium with FGF and EGF. As shown in Figure 1A, these two bladder cell lines formed tumor spheres after SFM culturing. Both protein and mRNA levels of bladder CSCs markers, including CD44, CD133, ALDH1A1, Oct4, and Nanog, were markedly upregulated in tumor sphere-formed cells when compared with adherent cells maintained in the serum-supplied medium (SSM) (Figure 1B and 1C). In addition, flow cytometry analysis showed that an increased percentage of CD44-positive cells observed in those sphere-forming cells compared to adherent cells (Figure 1D). These results demonstrated the characteristics of bladder CSCs in UM-UC-3 and EJ sphere-forming cells.
Figure 1.
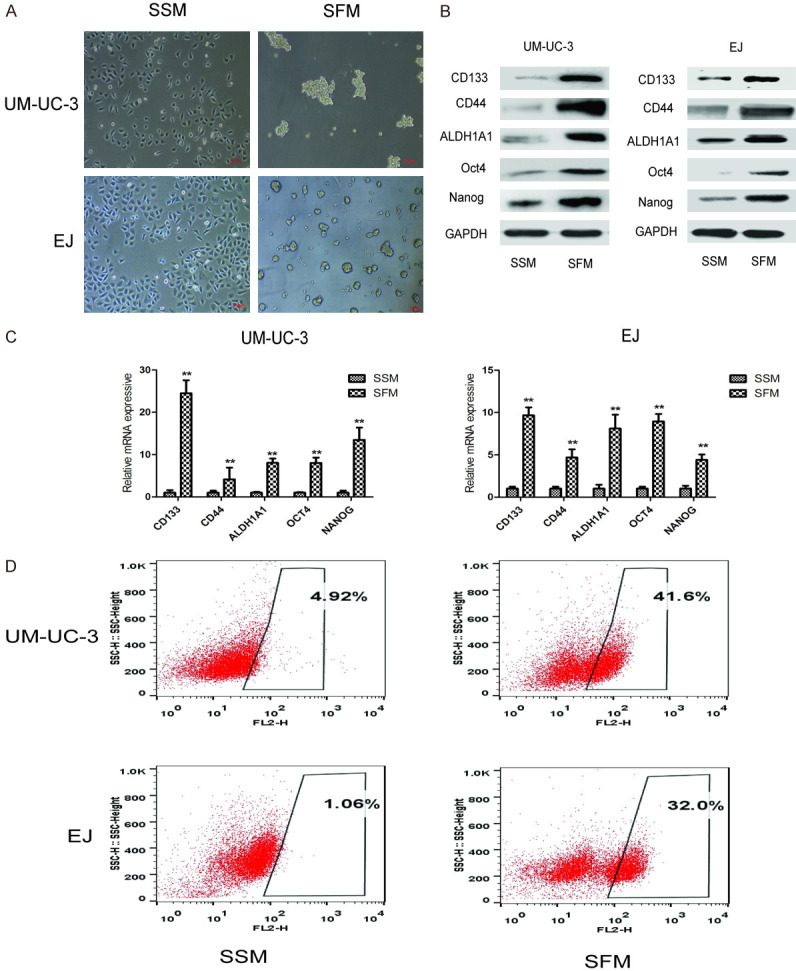
Tumor sphere formation assay of bladder CSCs by serum-free medium culture. UM-UC-3 and EJ cells were cultured in serum-supplied medium and serum-free medium for 7 days, respectively. A. Representative images of tumor spheres after 7 days of culture. Bar = 100 μm. B. The protein levels of bladder CSCs markers (CD44, CD133, ALDH1A1, Oct4, and Nanog) were measured by western blot. C. The mRNA levels of bladder CSCs markers were measured by qRT-PCR. D. The number of CD44+ cells was detected by flow cytometry. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with control group.
CSE promoted the stemness of bladder CSCs
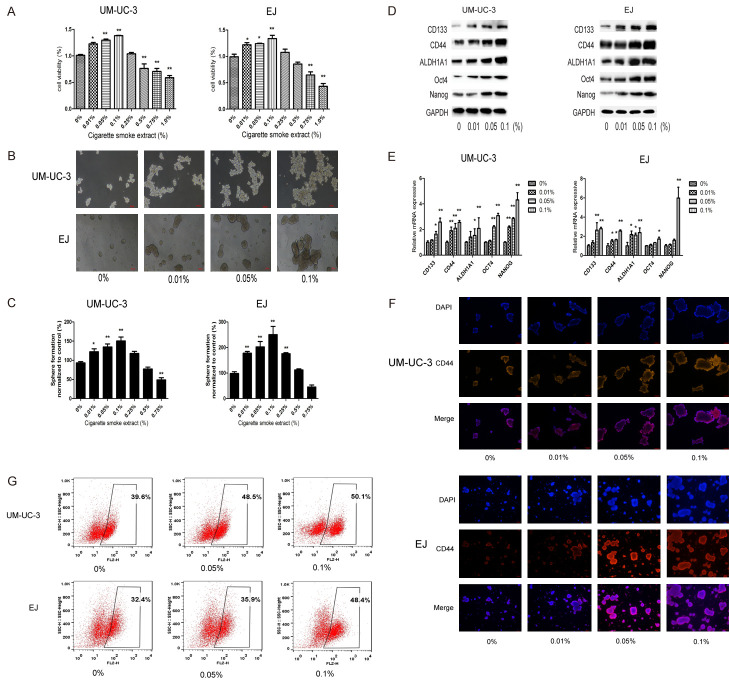
In order to investigate the effect of CSE on the viability of bladder CSCs, UM-UC-3 and EJ tumor spheres were treated with various concentrations of CSE for 7 days and cell viability was examined by CCK-8 assay as described above (Figure 2A). To examine the effects of CSE on bladder CSCs, UM-UC-3 and EJ tumor spheres were treated with various concentrations of CSE (0, 0.01, 0.05, or 0.1) for 7 days. Our data indicated that CSE increased the size and numbers of tumor spheres (Figure 2B and 2C). The protein and mRNA levels of bladder CSCs markers were also significantly increased (Figure 2D and 2E). Immunofluorescence staining also indicated that CSE increased the proportion of CD44 positive sphere-forming cells in a dose-dependent manner (Figure 2F). Moreover, flow cytometry analysis showed that CSE increased the percentage of CD44 positive cells in those sphere-forming cells (Figure 2G). These data suggested that CSE induced the stemness of bladder CSCs.
Figure 2.
CSE promoted the stemness of bladder CSCs. UM-UC-3 and EJ cell tumor spheres were treated with different concentrations of CSE for 7 days. (A) Cell viability was examined by CCK-8 assay. (B) Images of tumor spheres. Bar = 100 μm. (C) The number of tumor spheres was counted. (D) Western blotting and (E) qRT-PCR were used to analyze the protein and mRNA levels of bladder CSC markers. (F) Immunofluorescent staining of CD44 expression in UM-UC-3 and EJ spheres. Bar = 100 μm. (G) Percentage of CD44+ cells after CSE treatment. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with control group.
CSE promoted proliferation and the EMT on bladder CSCs
We further explored whether CSE affected the proliferation of bladder CSCs. As shown in Figure 3A and 3B, cell proliferation-associated proteins CyclinD1 and PCNA were markedly increased by CSE. Next, Transwell assay and western blot analysis were used to evaluate the effect of CSE on EMT of bladder CSCs. CSE treatment increased the invasive capacity of bladder CSCs. The protein expression of the epithelial markers such as E-cadherin and ZO-1 was decreased whereas the protein level of the mesenchymal markers such as Vimentin and N-cadherin were increased by CSE treatment (Figure 3C and 3D). Taken together, these data suggested that CSE promoted the EMT process and proliferation of bladder CSCs.
Figure 3.
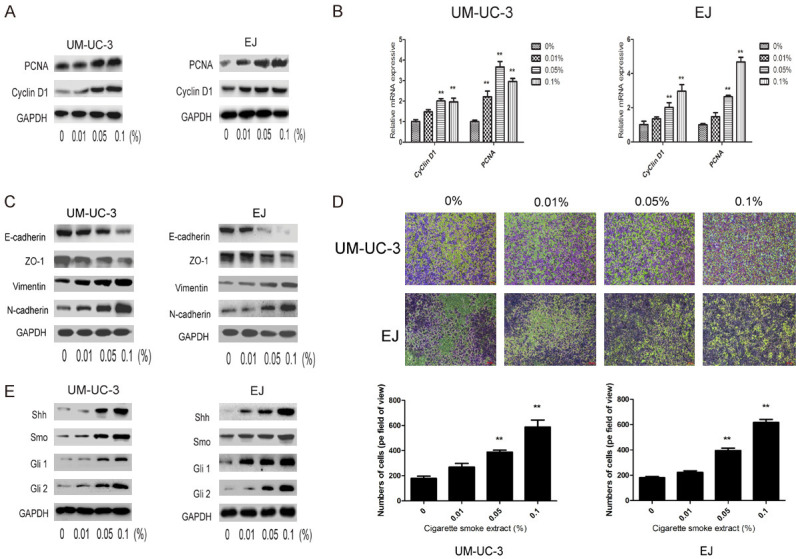
CSE promoted EMT and proliferation of bladder CSCs. A. Expression levels of cell proliferation related-proteins were determined by western blotting. B. Expression levels of cell proliferation related-genes were determined by qRT-PCR. C. Expression of EMT related-proteins was determined by western blotting. D. Transwell invasion assay was used to determine the invasive ability of bladder CSCs. E. Expression of SHH pathway related-proteins was measured by western blotting. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with control group.
SHH pathway mediated the promotive effects of CSE on bladder CSCs
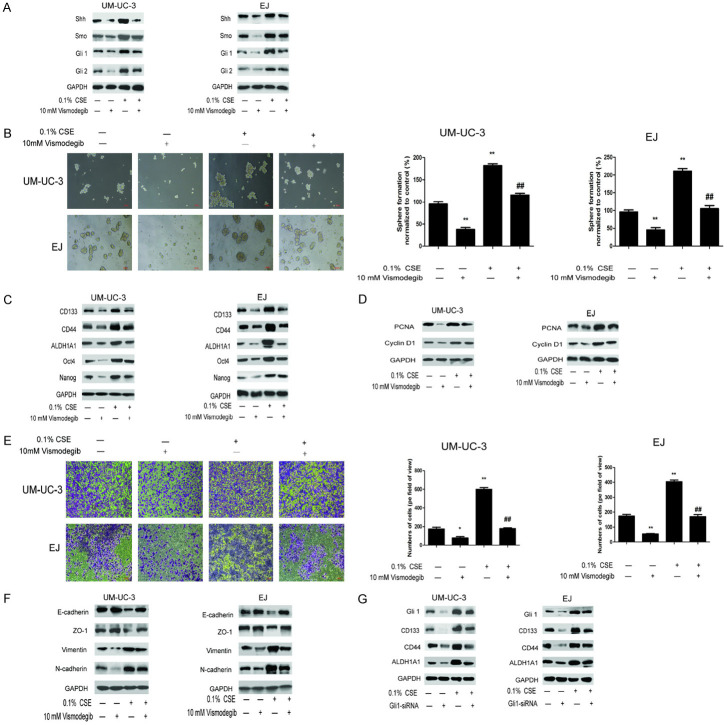
Next, we investigated the involvement of the SHH pathway in the promotive effects of CSE on bladder CSCs. As shown in Figure 3E, CSE treatment increased the expression of Shh, Smo, Gli1 and Gli2 in both UM-UC-3 and EJ tumor sphere-forming cells. In order to further examine the role of SHH pathway in the effects of CSE on bladder CSCs, Vismodegib, a specific inhibitor of SHH pathway, was used. Vismodegib treatment suppressed expression members in the SHH pathway as shown in Figure 4A, reduced the expression of bladder CSCs markers (Figure 4C) and suppressed tumor spheres formation in both UM-UC-3 and EJ cells (Figure 4B). Vismodegib treatment also diminished the effect of CSE on EMT and proliferation of bladder CSCs (Figure 4D-F). Additionally, induction of bladder CSCs markers by CSE was also abrogated by Gli1 siRNA (Figure 4G). These results suggested that CSE promoted the stemness and EMT of bladder CSCs through activation of the SHH pathway.
Figure 4.
SHH pathway regulates the promotive effects of CSE on bladder CSCs. The tumor spheres were treated with CSE and Vismodegib or Gli1-siRNA. A. SHH pathway related-proteins were detected by western blotting. B. Images of tumorspheres. C. Expression of bladder CSCs markers was measured by western blotting. D. Expression of cell proliferation related-proteins was determined by western blotting. E. The invasive ability was determined by Transwell assay. F. Expression of EMT related-proteins was determined by western blotting. G. Expression of bladder CSC markers after Gli1 siRNA treatment was measured by western blotting. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with control group. ## P < 0.01 compared with the 0.1% CSE group.
Clinical association of smoking and CSCs in bladder cancer patients
The clinical characteristics of a total of 38 bladder cancer patients are shown in Table 2, including 20 smoker patients and 18 non-smoker patients. qRT-PCR, western blotting, and immunohistochemistry (IHC) staining were used to examine the expression of bladder CSCs markers and SHH pathway-related proteins in the tumor tissues. qRT-PCR showed that the mRNA level of CD133 was upregulated in smoker tumor tissues (Figure 5A). Western blot analysis showed that the expression levels of CD133, Oct4, and Nanog were higher in smokers’ tumor specimens than in non-smokers’ tumor specimens (Figure 5B and 5C). Furthermore, the levels of Shh, Smo, and Gli1 were also increased in smokers’ tumor specimens (Figure 5D and 5E). Similar results were also revealed by IHC staining (Figure 5F and 5G). Collectively, these results suggested that bladder CSC markers and SHH pathway-related proteins were upregulated in the bladder cancer of smokers.
Table 2.
Clinical characteristics of patients (n = 38)
| Variable | Cases n (%) | Non-smoker n (%) | Smoker n (%) | P-value |
|---|---|---|---|---|
| Age (years) | 0.342 | |||
| ≥ 70 | 16 (42.11) | 6 (37.5) | 10 (62.5) | |
| < 70 | 22 (57.89) | 12 (54.55) | 10 (45.45) | |
| Gender | 0.521 | |||
| Male | 34 (89.47) | 14 (41.18) | 20 (58.82) | |
| Female | 4 (10.53) | 4 (100) | 0 (0) | |
| Tumor grade | 0.746 | |||
| G1/G2 | 19 (50) | 10 (52.63) | 9 (47.37) | |
| G3 | 19 (50) | 8 (42.11) | 11 (57.89) | |
| Tumor stage | 0.522 | |||
| T1/T2 | 23 (60.53) | 12 (52.17) | 11 (47.83) | |
| T3/T4 | 15 (39.47) | 6 (40) | 9 (60) | |
| Lymph node metastasis | 0.719 | |||
| No | 28 (73.68) | 14 (50) | 14 (50) | |
| Yes | 10 (26.32) | 4 (40) | 6 (60) | |
| Distant metastasis | 0.238 | |||
| No | 30 (78.95) | 16 (53.33) | 14 (46.67) | |
| Yes | 8 (21.05) | 2 (25) | 6 (75) |
Figure 5.
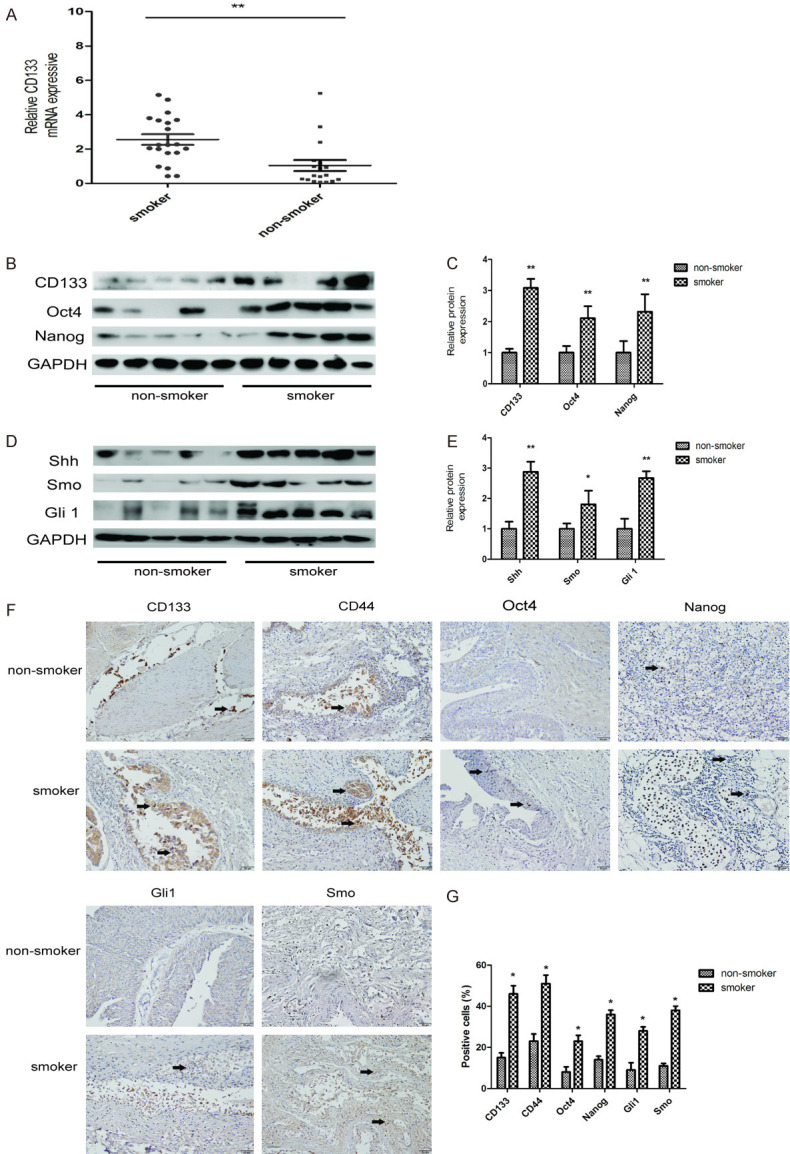
Analysis of clinical bladder cancer tissues. A. The mRNA level of CD133 in non-smoker and smoker bladder cancer patient tissues was measured by qRT-PCR. B, C. The protein levels of bladder CSCs markers were measured by western blotting. D, E. The expression levels of SHH pathway related-proteins were measured by western blotting. F, G. Immunohistochemical (IHC) staining of bladder CSCs markers and SHH pathway related-proteins, 200× magnification. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01 compared with control group.
Discussion
In the present study, we report that CSE supports bladder cancer progression by promoting the stemness of CSCs of bladder cancer cell lines, enhancing EMT, and proliferation of CSC. Mechanistically, SHH signaling is involved in the above phenomena. More importantly, in patients with bladder cancer, the above observations are reproducible.
CSCs play multiple roles in the development and progression of malignant tumors. Resistance to chemotherapy is correlated with a poor outcome of bladder cancer where CSCs are drastically implicated in drug resistance. Bladder CSCs were first identified in 2009 from primary human bladder cancer cells [26]. It has been acknowledged that bladder CSCs contribute to the tumor progression and recurrence. Studies have been focused on the regulatory mechanisms and therapeutics of bladder CSCs. Bladder CSCs markers such as CD133, CD44, ALDH1A1, Oct4, Nanog, Sox2, and CD47 have been identified [27].
In the present study, we used a series of markers to identify CSCs. It has been demonstrated that CD133+ cells possess higher tumorigenic features and capacities of chemoresistance compared to CD133- cells in bladder cancer cells [28]. CD44+ bladder cancer cells were first isolated from tissue specimens, which showed an enhanced capacity to form xenografts in immune-compromised mice in comparison with CD44- cells [26]. ALDH1A1+ bladder cancer cells have been confirmed to have the characteristics of CSCs with tumorigenicity and self-renewal potential [29,30]. Nanog and Oct4 are reported to be upregulated in tumor sphere formation in bladder cancer and have also been recognized as CSC markers [31,32]. In the present study, these markers have been used to identify CSCs of bladder cancer.
Serum-free medium culture is one of the most commonly used methods to enrich and isolate CSCs, which is based on the tumor sphere formation ability of CSCs under serum-free culture condition--a specific feature of CSCs [33,34]. In the previous study, we found that UM-UC-3 and EJ cells formed three-dimensional spheres with the serum-free medium. Compared to adherent cells cultured in the completed medium, significant increased expression levels of bladder CSCs markers (CD44, CD133, ALDH1A1, Oct4, Nanog) in tumor sphere-forming cells were observed. Meanwhile, flow cytometry analysis showed that the numbers of CD44 positive cells were also elevated. These data suggested the characteristics of bladder CSCs were encouraged through culture with serum-free medium. The in vivo tumorigenic potential of these bladder cancer cell lines used in our study agrees with previous publications [35-37].
EMT is highly related to functions of CSCs. Mani et al. have reported that EMT promotes the generation of CSCs [16]. Other studies have indicated that EMT is associated with the characteristics and development of CSCs [38,39]. Both stemness and EMT are extremely critical factors for cells to obtain more invasive and malignant biologic behaviors.
Environmental and genetic factors, such as aging, exposure to CS and occupational chemicals, are important in bladder carcinogenesis. It has been reported almost 50% male patients and 25% female patients of bladder cancer are associated with smoking in Americans [40]. CS is one of the leading risk factors for bladder cancer and exposure to smoke often persists after the diagnosis. Evidence suggests that CS is associated with the development of various cancer stem cells and is responsible for bladder cancer progression [41-44]. Thus it is speculated that the effects of CS on bladder CSCs may also be stimulatory. In the present study, we showed that CS significantly promoted the properties of bladder CSCs stemness by enhancing tumor sphere formation capacity, increasing the expression levels of bladder CSCs markers and elevating the population of CD44 positive cells. Transwell assay and western blotting also revealed that CS enhanced EMT process and proliferation of bladder CSCs. Moreover, we illustrated that the levels of bladder CSC markers were significantly upregulated in smokers’ bladder cancer specimens in comparison with non-smoker tumor specimens. Together, our data suggested promotor effects of CS on bladder CSC properties.
Hedgehog pathway plays a crucial role in regulating CSC generation. Hedgehog homologs in human beings, composed of SHH, Desert Hedgehog (DHH) and Indian Hedgehog (IHH), have been reported to be associated with CSCs and EMT progression [45,46]. It has been shown that the SHH signaling pathway is important for maintenance of CSCs [47]. Evidence has also illustrated that SHH is critically involved in the invasion and metastasis of bladder cancer. Fei et al. described an important role of SHH signaling in regulating the tumorigenicity of bladder cancer [48]. Islam et al. also showed that SHH promoted tumorigenicity, stemness, and EMT features in bladder cancer patient tumors and cell lines [49]. In addition to the above knowledge, we found that CS-promoted CSCs and EMT of bladder cancer cells by the activation of the SHH pathway. We also found that the levels of SHH related-proteins were higher in bladder tumor tissues of smokers compared to non-smokers. Moreover, we demonstrated that suppression of SHH pathway by Vismodegib and Gli1-siRNA diminished the stimulatory effects of CS on the stemness and EMT of bladder CSCs. Collectively, these results revealed that CS promoted the stemness and EMT of bladder CSCs through the SHH signaling pathway.
In conclusion, our data illustrated for the first time that the SHH pathway regulates CS-stimulated stemness and EMT features of bladder CSCs through the SHH pathway. Findings from this study could give new insight into the regulatory mechanisms of CS-triggered bladder CSCs as well as its being a target for intervention.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81573139 and No. 81773431).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Alguacil J, Kogevinas M, Silverman DT, Malats N, Real FX, García-Closas M, Tardón A, Rivas M, Torà M, García-Closas R, Serra C, Carrato A, Pfeiffer RM, Fortuny J, Samanic C, Rothman N. Urinary pH, cigarette smoking and bladder cancer risk. Carcinogenesis. 2011;32:843–847. doi: 10.1093/carcin/bgr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teleka S, Häggström C, Nagel G, Bjørge T, Manjer J, Ulmer H, Liedberg F, Ghaderi S, Lang A, Jonsson H, Jahnson S, Orho-Melander M, Tretli S, Stattin P, Stocks T. Risk of bladder cancer by disease severity in relation to metabolic factors and smoking; a prospective pooled cohort study of 800,000 men and women. Int J Cancer. 2018;143:3071–3082. doi: 10.1002/ijc.31597. [DOI] [PubMed] [Google Scholar]
- 5.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Lundin A, Driscoll B. Lung cancer stem cells: progress and prospects. Cancer Lett. 2013;338:89–93. doi: 10.1016/j.canlet.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiotaki R, Polioudaki H, Theodoropoulos PA. Cancer stem cells in solid and liquid tissues of breast cancer patients characterization and therapeutic perspectives. Curr Cancer Drug Targets. 2015;15:256–269. doi: 10.2174/1568009615666150211102503. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wang Z, Yu J, Shi JZ, Wang C, Fu WH, Chen ZW, Yang J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012;322:70–77. doi: 10.1016/j.canlet.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Shiozawa Y, Nie B, Pienta KJ, Morgan TM, Taichman RS. Cancer stem cells and their role in metastasis. Pharmacol Ther. 2013;138:285–293. doi: 10.1016/j.pharmthera.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell V, Copland M. Hedgehog signaling in cancer stem cells: a focus on hematological cancers. Stem Cells Cloning. 2015;8:27–38. doi: 10.2147/SCCAA.S58613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of Hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 13.Sun X, Zhang T, Deng Q, Zhou Q, Sun X, Li E, Yu D, Zhong C. Benzidine induces epithelial-mesenchymal transition of human bladder cancer cells through activation of ERK5 pathway. Mol Cells. 2018;41:188–197. doi: 10.14348/molcells.2018.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng H, Zhao L, Liang Z, Zhang Z, Xie D, Bi L, Wang Y, Zhang T, Cheng L, Yu D, Zhong C. ERK5 positively regulates cigarette smoke induced urocystic epithelial mesenchymal transition in SV40 immortalized human urothelial cells. Oncol Rep. 2015;34:1581–1588. doi: 10.3892/or.2015.4130. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Kitamura H. Cancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: possible pathways and potential therapeutic approaches. Int J Urol. 2018;25:7–17. doi: 10.1111/iju.13404. [DOI] [PubMed] [Google Scholar]
- 16.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Z, Lu L, Mao J, Li X, Qian H, Xu W. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/beta-catenin. Cell Death Dis. 2017;8:e3066. doi: 10.1038/cddis.2017.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T, Zhang J, Zhang J, Zhang N, Zeng Y, Tang S, Tao Z, Qu X, Jia J, Zhu W, Sun X, Chen H. Nicotine-enhanced stemness and epithelial-mesenchymal transition of human umbilical cord mesenchymal stem cells promote tumor formation and growth in nude mice. Oncotarget. 2017;9:591–606. doi: 10.18632/oncotarget.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Wadei MH, Banerjee J, Al-Wadei HA, Schuller HM. Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signaling. Eur J Cancer. 2016;52:188–196. doi: 10.1016/j.ejca.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng TK, Huang L, Cao D, Yip YW, Tsang WM, Yam GH, Pang CP, Cheung HS. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci Rep. 2015;5:7828. doi: 10.1038/srep07828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian W, Kong X, Zhang T, Wang D, Song J, Li Y, Li X, Geng H, Min J, Kong Q, Liu J, Liu Z, Wang D, Zhang Z, Yu D, Zhong C. Cigarette smoke stimulates the stemness of renal cancer stem cells via Sonic Hedgehog pathway. Oncogenesis. 2018;7:24. doi: 10.1038/s41389-018-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Luo F, Xu Y, Wang B, Zhao Y, Xu W, Shi L, Lu X, Liu Q. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol Appl Pharmacol. 2015;282:9–19. doi: 10.1016/j.taap.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Yu CC, Chang YC. Enhancement of cancer stem-like and epithelial-mesenchymal transdifferentiation property in oral epithelial cells with long-term nicotine exposure: reversal by targeting SNAIL. Toxicol Appl Pharmacol. 2013;266:459–469. doi: 10.1016/j.taap.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Tian D, Zhu M, Chen WS, Li JS, Wu RL, Wang X. Role of glycogen synthase kinase 3 in squamous differentiation induced by cigarette smoke in porcine tracheobronchial epithelial cells. Food Chem Toxicol. 2006;44:1590–1596. doi: 10.1016/j.fct.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Gál K, Cseh A, Szalay B, Rusai K, Vannay A, Lukácsovits J, Heemann U, Szabó AJ, Losonczy G, Tamási L, Müller V. Effect of cigarette smoke and dexamethasone on Hsp72 system of alveolar epithelial cells. Cell Stress Chaperones. 2011;16:369–378. doi: 10.1007/s12192-010-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. PNAS. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohishi T, Koga F, Migita T. Bladder cancer stem-like cells: their origin and therapeutic perspectives. Int J Mol Sci. 2015;29:17. doi: 10.3390/ijms17010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang P, Watanabe M, Kaku H, Ueki H, Noguchi H, Sugimoto M, Hirata T, Yamada H, Takei K, Zheng S, Xu K, Nasu Y, Fujii Y, Liu C, Kumon H. Cancer stem cell-like characteristics of a CD133(+) subpopulation in the J82 human bladder cancer cell line. Mol Clin Oncol. 2013;1:180–184. doi: 10.3892/mco.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F. ALDH1A1 positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falso MJ, Buchholz BA, White RW. Stem-like cells in bladder cancer cell lines with differential sensitivity to cisplatin. Anticancer Res. 2012;32:733–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120:1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 32.Amini S, Fathi F, Mobalegi J, Sofimajidpour H, Ghadimi T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol. 2014;47:1–11. doi: 10.5115/acb.2014.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu YT, Lei CY, Luo Y, Liu N, He CW, Chen W, Li F, Deng YJ, Tan WL. A modified method for isolation of bladder cancer stem cells from a MB49 murine cell line. BMC Uro. 2013;13:51–57. doi: 10.1186/1471-2490-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Huang X, Zheng X, Wang X, Li S, Zhang L, Yang Z, Xia Z. Enrichment of prostate cancer stem-like cells from human prostate cancer cell lines by culture in serum-free medium and chemoradiotherapy. Int J Biol Sci. 2013;9:472–479. doi: 10.7150/ijbs.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojha R, Jha V, Singh SK. Gemcitabine and mitomycin induced autophagy regulates cancer stem cell pool in urothelial carcinoma cells. Biochim Biophys Acta. 2016;1863:347–59. doi: 10.1016/j.bbamcr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhu YT, Pang SY, Luo Y, Chen W, Bao JM, Tan WL. A modified method by differential adhesion for enrichment of bladder cancer stem cells. Int Braz J Urol. 2016;42:817–24. doi: 10.1590/S1677-5538.IBJU.2015.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, He L, Lin K, Zhang Y, Deng A, Liang Y, Li C, Wen T. The KMT1A-GATA3-STAT3 circuit is a novel self-renewal signaling of human bladder cancer stem cells. Clin Cancer Res. 2017;23:6673–6685. doi: 10.1158/1078-0432.CCR-17-0882. [DOI] [PubMed] [Google Scholar]
- 38.Koren A, Rijavec M, Kern I, Sodja E, Korosec P, Cufer T. BMI1, ALDH1A1, and CD133 transcripts connect epithelial-mesenchymal transition to cancer stem cells in lung carcinoma. Stem Cells Int. 2016;2016:9714315. doi: 10.1155/2016/9714315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Ren D, Guo W, Huang S, Wang Z, Li Q, Du H, Song L, Peng X. N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. Int J Oncol. 2016;48:595–606. doi: 10.3892/ijo.2015.3270. [DOI] [PubMed] [Google Scholar]
- 40.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D, Geng H, Liu Z, Zhao L, Liang Z, Zhang Z, Xie D, Wang Y, Zhang T, Min J, Zhong C. Cigarette smoke induced urocystic epithelial mesenchymal transition via MAPK pathways. Oncotarget. 2017;8:8791–8800. doi: 10.18632/oncotarget.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, Deng Q, Liang Z, Liu Z, Geng H, Zhao L, Zhou Q, Liu J, Ma J, Wang D, Yu D, Zhong C. Cigarette smoke extract induces epithelial-mesenchymal transition of human bladder cancer T24 cells through activation of ERK1/2 pathway. Biomed Pharmacother. 2017;86:457–465. doi: 10.1016/j.biopha.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, Wu XR, Chen LC, Tang MS. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. PNAS. 2018;13:E1560–E1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An Y, Kiang A, Lopez JP, Kuo SZ, Yu MA, Abhold EL, Chen JS, Wang-Rodriguez J, Ongkeko WM. Cigarette smoke promotes drug resistance and expansion of cancer stem cell-like side population. PLoS One. 2012;7:e47919. doi: 10.1371/journal.pone.0047919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Syed IS, Pedram A, Farhat WA. Role of Sonic Hedgehog (Shh) signaling in bladder cancer stemness and tumorigenesis. Curr Urol Rep. 2016;17:11. doi: 10.1007/s11934-015-0568-9. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Kong X, Li Y, Qian W, Ma J, Wang D, Yu D, Zhong C. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem Biophys Res Commun. 2017;493:521–527. doi: 10.1016/j.bbrc.2017.08.158. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fei DL, Sanchez-Mejias A, Wang Z, Flaveny C, Long J, Singh S, Rodriguez-Blanco J, Tokhunts R, Giambelli C, Briegel KJ, Schulz WA, Gandolfi AJ, Karagas M, Zimmers TA, Jorda M, Bejarano P, Capobianco AJ, Robbins DJ. Hedgehog signaling regulates bladder cancer growth and tumorigenicity. Cancer Res. 2012;72:4449–58. doi: 10.1158/0008-5472.CAN-11-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam SS, Mokhtari RB, Noman AS, Uddin M, Rahman MZ, Azadi MA, Zlotta A, van der Kwast T, Yeger H, Farhat WA. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol Carcinog. 2016;55:537–551. doi: 10.1002/mc.22300. [DOI] [PubMed] [Google Scholar]