Abstract
Endometrial carcinoma is the most common malignant tumors of the reproductive system, and fragile histidine triad (FHIT) plays an important role in multiple tumors. The purpose of this study was to investigate the expression of FHIT gene in endometrial carcinoma, and its effect on proliferation, invasion, and metastasis after upregulation. In vitro, the endometrial carcinoma cell lines were cultured. The FHIT-saRNA expression vector was constructed. The endometrial carcinoma cell line that upregulated the expression of FHIT was established, and whether the saRNA had a direct targeting regulation on the FHIT was verified. A difference of expression of FHIT in normal endometrial and endometrial carcinoma was detected. We detected the proliferation of endometrial carcinoma cell lines before and after activating FHIT. The endometrial carcinoma cell lines were compared with the corresponding transiently transfected cell lines in their capabilities of cell migration and invasion. The results showed that the expression of FHIT in endometrial carcinoma was significantly decreased or even deficient compared with normal endometrium. Upregulating the expression of FHIT is related to inhibiting the proliferation, invasion and metastasis of endometrial carcinoma. The possible mechanism is related to the regulation of cell cycle regulation, and plays a role in inhibiting tumor proliferation. The research on molecular mechanism in the development and progression of endometrial carcinoma has important theoretical significance for improving the diagnosis, treatment and prognosis of clinical tumors.
Keywords: Endometrial carcinoma, FHIT, RNA activation, proliferation, invasion and metastasis
Introduction
Endometrial carcinoma is one of the three major malignant tumors in gynecology, and it has become a serious threat to women’s health and life. Therefore, the specific mechanism of endometrial carcinoma development has become a research hotspot for scholars [1,2]. It is believed that tumor is a genetic disease, and activation of proto-oncogenes, loss of function of suppressor genes, and changes in the function of some modified genes lead to the occurrence and development of tumors [3,4]. The research on suppressor genes is a hot spot in the field of tumor research.
RNA activation (RNAa) was first discovered by Li et al. in 2006 [5], and the double-stranded RNA (dsRNA) molecule promoter region of the target is introduced into tumor cells to promote the expression of the target gene [6]. RNAa is mediated by dsRNA that targets specific gene promoter regions. These sequences directed against non-coding regions of dsRNAs are called antigene RNAs (agRNAs), and agRNAs that induce RNAa are called small activating RNAs (saRNAs) [7].
The FHIT gene is the first active fragile site of the tumor suppressor genes, and plays an important negative regulatory role in the occurrence and progression of tumors [8]. There are different degrees of expression loss and correlation in various tumors such as lung carcinoma and breast carcinoma [9]. This study set out to detect the expression of FHIT gene in endometrial carcinoma, to explore its correlation with clinicopathologic factors, and to further clarify their role in infiltration and metastasis of endometrial carcinoma. The expression of FHIT gene in human endometrial carcinoma cell lines, and its effect on the invasion and migration of endometrial carcinoma cells, may show a theoretical basis for the clinical treatment and prognosis of endometrial carcinoma.
Materials and methods
Clinical specimens
The endometrial carcinoma tissues were selected from 93 patients who were admitted to the department of pathology, the First Affiliated Hospital of Bengbu Medical College from January 2018 to July 2019. All patients before surgery did not receive any anticancer treatments such as chemotherapy or radiotherapy. The control group was normal endometrial tissues, which were selected from 35 patients with gynecological biopsy endometrial tissues or hysterectomy for other benign diseases. All patients were informed and agreed.
Immunohistochemical analysis
Immunohistochemical (IHC) staining was performed on paraffin-embedded endometrial carcinoma tissues and normal endometrial tissues sections to determine the expression of FHIT. Briefly, the sections were incubated with FHIT antibody (1:50, Proteintech) at 4°C overnight and detected by the HRP-conjugated secondary antibody (1:500, Thermo Fisher Scientific, Waltham, MA, USA) after incubated at 37°C for 60 min. Sections were incubated with peroxidase substrate DAB (Solarbio) for color development and counterstained with hematoxylin for 3 min. The slides were observed and photographed under the microscope (Olympus, Japan).
Cell culture and transfection
We chose 4 human endometrial carcinoma cells ISK, RL-952, HEC-1-B, AN3 CA (Cell Research Shanghai, China) were cultured in Dulbecco’s Modifies Eagle Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Hyclone, South Logan, UT, USA) in a humidified incubator at 37°C with 5% CO2. Briefly, cells were seeded in 6-well plates and cultured until they reached a density of approximately 70% to 80% confluent. The transfection step was carried out according to the lipofentamineTM2000 (Invitrogen, Carlsbad, CA, USA) reagent specification. The experiment was divided into a blank control group (not transfected), a negative control group (transfected unordered RNA), and an experimental group (transfected with dsFhit).
saRNA was synthesized according to the sequence known in the literature (Jima Pharmaceutical Technology Co. Ltd Shanghai, China). Sequences of FHIT-saRNA were as follows: forward: 5’-CCAACUCAUUCUCCAAGUA-3’; reverse: 5’-UACUUGGAGAAUGAGUUGG-3’; and the negative saRNA Control: forward: 5’-ACUACUGAGUGACAGUAGA-3’; reverse: 5’-UCUACUGUCACUCAGUAGU-3’. The FHIT-primers for PCR amplification were obtained from GenScript (Nanjing, China) and the sequences were as follows: FHIT Forward, 5’-CATCCTGGAAGCTTTGAAGCTCA-3’ at nucleotides -222 to -200 bp; FHIT Reverse, 5’-CATGCTGATTCAGTTCCTCTTGG-3’ at nucleotides 523 to 545 bp. After transfection, cells were incubated in DMEM medium with 10% FBS before subjecting them to the required experiments.
Western blot
Protein levels were determined by western blot. After 48 h of cells transfection, briefly, cells were lysed in RIPA buffer (Solarbio) containing 10 μl PMSF (Solarbio) and the protein concentrations were determined using BCA Protein Assay Kit (Solarbio). 10-20 μg equal amounts of protein were separated by SDS-PAGE and then transferred to the PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk in TBST buffer for 1 h and then incubated overnight at 4°C with primary antibodies: anti-FHIT (1:1000, Proteintect) and anti-β-actin (1:1000, Proteintech). After washing for 4 times with TBST, membranes were incubated with the HRP-conjugated (1:3000, Solarbio) at 37°C for 1 h and the signals were visualized using ECL reagent (Solarbio).
Quantitative real-time PCR (qRT-PCR)
The qRT-PCR was used to measure FHIT expression levels in transfected endometrial carcinoma cells. Briefly, total RNAs from cultured endometrial carcinoma cells were extracted by the RNA simple total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The cDNA of FHIT was reversely transcribed using a TIANSeq M-MLV (Tiangen) and expression levels of saRNA and FHIT were measured by qRT-PCR system containing 2 × Taq PCR MasterMix (Tiangen) and SYBR Green (Solarbio, Beijing, China) according to the manufacturer’s protocol. U6 and GAPDH were used as normalizing controls to normalize FHIT expression using the 2-ΔΔCT method. The DNA bands were observed under an ultraviolet lamp, photographed, and subjected to gray value analysis using a gel image processing system.
CCK-8 assay
Cell proliferation assay was measured with a Cell Counting Kit-8 (CCK-8) (Key Gen Biotech, Nanjing, China). Adjusted to the endometrial carcinoma cells concentration of 5 × 104 ml, seeded into 96-well plates with 5 replications per group, 150 μl per well, and incubated in a cell culture incubator. After 24 h, FHIT-saRNA or their controls were transfected to cells, then the cells were cultured subsequently for 0, 24, 48, 72, 96, and 120 h, respectively. The 10 μl CCK-8 solution was added to each well and the cells were incubated for 2 h at 37°C. The absorbance of each well was measured at 450 nm by a microplate reader (BioTek, Vermont, USA). The experiment was repeated three times.
Transwell assays
The migration and invasion of endometrial carcinoma cells were evaluated by transwell assays. The experiment used some 24-well plates and a polyvinyl-pyrrolidone-free polycarbonate filter (8 μm pore size). The lower chamber was filled with 500 μl medium containing 10% FBS, the upper chamber filled serum-free medium containing 2 × 104 cells. After incubation 24 h, cells were fixed and stained with 4% paraformaldehyde and 5% crystal violet. We counted the cells number in the surface of the lower chamber under microscope (Olympus, Japan), and the average value was set. The assay could be used to detect the ability of cells to invade and migrate. However, when it was used to detect the ability of cell invasion, the 8 microspore was coated with Matrigel (BD Biosciences, USA). Every chamber was set in parallel for each group, and the experiment was repeated three times.
Statistical analysis
All experiments were repeated at least three times and representative images are shown. Statistical analysis was performed using software Statistical Product and Service Solutions (SPSS) 23.0. The data were expressed as the mean ± standard deviation (SD). Graphpad software was used for Student T test, Chi-square, correlation analysis, and Kaplan-Meier analysis in this study, to evaluate the differences between groups. The variance analysis between groups was performed using a one-way ANOVA. P < 0.05 was considered significant.
Results
The expression level of FHIT is decreased in endometrial carcinoma tissues
To investigate the expression levels of FHIT in endometrial carcinoma tissues and normal endometrial tissues, we collected 93 pairs of endometrial carcinoma tissues and 35 pairs of normal endometrial tissues. We found that the expression of FHIT was positive in 11 cases of endometrial carcinoma tissues and 32 cases of normal endometrial tissues (Table 1). Immunohistochemistry showed statistical differences between the two groups (P < 0.01). Positive staining was observed in the cytoplasm of normal endometrial tissues. Compared with endometrial carcinoma tissues, the expression level of FHIT protein in normal endometrial tissue was significantly increased or even missing (Figure 1A, 1B).
Table 1.
Fhit expression in different tissues
| FHIT | ||||
|---|---|---|---|---|
| Histologic type | Cases | (-) | (+) | P value |
| Normal endometrial tissues | 35 | 3 | 32 | <0.01 |
| Endometrial carcinoma tissues | 93 | 82 | 11 |
Figure 1.
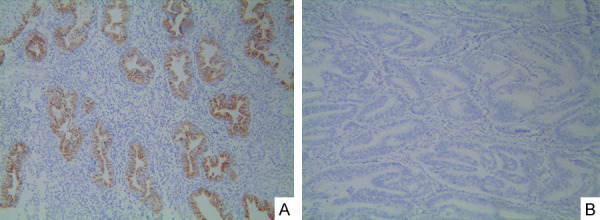
Fhit expression in different endometrial tissues. A. Fhit expression in endometrial tissue (IHC stain × 100); B. Fhit negative expression in endometrial carcinoma tissue (IHC stain × 100).
The relationship between the expression of FHIT and the clinicopathologic factors in endometrial carcinoma
The results showed that the expression of FHIT in endometrial carcinoma tissues was significantly correlated with histologic grade, FIGO stage, musculocutaneous invasion, and lymph node metastasis (P < 0.05), but has no significant correlation with age (Table 2).
Table 2.
The relationship between the expression of FHIT and clinicopathologic factors in endometrial carcinoma
| FHIT | ||||
|---|---|---|---|---|
| Factor | Cases | (-) | (+) | P value |
| Age | ||||
| <50 | 16 | 8 | 7 | 0.785 |
| ≥50 | 77 | 44 | 33 | |
| Histologic grade | ||||
| G1 | 23 | 13 | 10 | 0.036 |
| G2 | 45 | 28 | 17 | |
| G3 | 25 | 22 | 3 | |
| FIGO stage | ||||
| I + II | 50 | 32 | 18 | 0.032 |
| III + IV | 43 | 36 | 7 | |
| Muscular layer infiltration | ||||
| ≤1/2 | 47 | 27 | 20 | 0.032 |
| >1/2 | 46 | 36 | 10 | |
| Lymph node metastasis | ||||
| No | 69 | 39 | 30 | 0.019 |
| Yes | 24 | 20 | 4 |
RNA activation up-regulated the expression of FHIT in endometrial carcinoma
The results of western blot showed statistical differences among the groups after transfection 48 h (P < 0.05). Compared with the blank control group and the negative control group, the protein expression in the experimental group was significantly increased (experimental group vs. blank control group, P < 0.05; experimental group vs. negative control group, P < 0.05) (Figure 2A).
Figure 2.
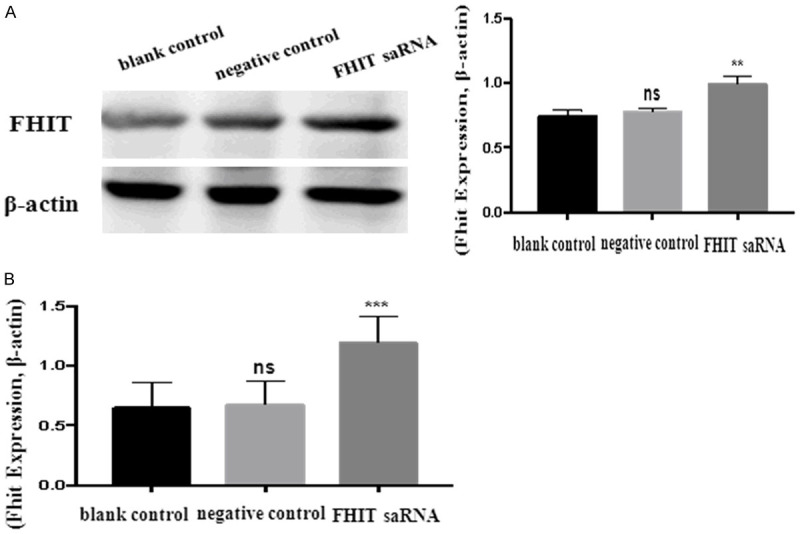
(A) Western blot and (B) qRT-PCR were performed to evaluate the effect of FHIT activating on the level of protein in endometrial carcinoma cells.
The results of RT-PCR showed that there were statistically significant differences between the groups after transfection 48 h (P < 0.05). The expression level of mRNA in the experimental group was significantly upregulated compared with that in the blank group and the negative control group (experimental group vs. blank group, P < 0.05; experimental group vs. negative control group, P < 0.05) (Figure 2B).
Activation of FHIT inhibited the proliferation of endometrial carcinoma cells
The CCK-8 assay was used to assess the effect of FHIT-saRNA on endometrial carcinoma cell proliferation. The results of CCK-8 showed that the inhibition rates of 24, 48, 72, 96, and 120 h were 38.8%, 30.8%, 24.7%, 30.0%, and 31.4%, respectively. Compared with the negative control group, the proliferation of endometrial carcinoma cells in the experimental group was slowed down, and the growth was significantly inhibited, with statistical differences (P < 0.05) (Figure 3). The results indicated that upregulation of FHIT-saRNA inhibits the endometrial carcinoma cell proliferation.
Figure 3.
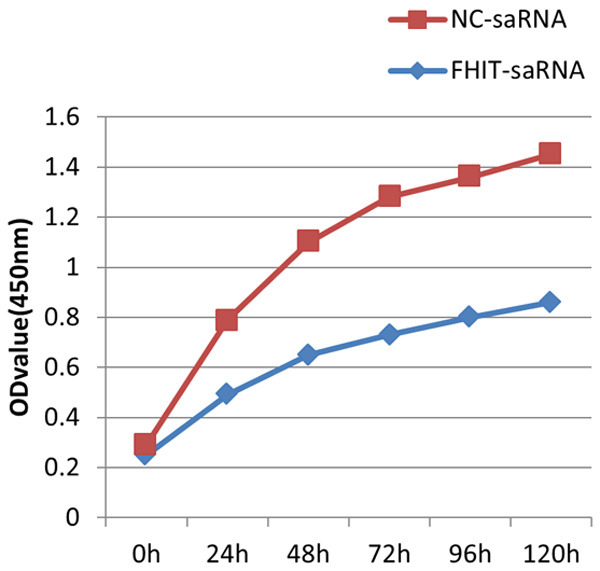
Activation of FHIT inhibited the proliferation of endometrial carcinoma cells that was detected by CCK-8 assay.
Activation of FHIT inhibited migration and invasion of endometrial carcinoma cells
To further explore the effects of FHIT upregulation on migration and invasion abilities of endometrial carcinoma cells, transwell assays were performed. The results of transwell chamber experiments showed that compared with the blank control group and the negative control group, dsFHIT experimental endometrial carcinoma cells’ invasion and migration ability had an obvious drop. The results of invasion experiment showed that the experimental group through the basement membrane for the number of cells (64.1 ± 6.5), and compared with the blank control group (98.1 ± 7.2) and the negative control group (92.0 ± 5.3) it was different (experimental group vs. blank group (P < 0.05; experimental group vs. control group P <0.05). The results of the migration experiment showed that the number of cells in the experimental group was (69.1 ± 6.5), and the number of cells crossing the membrane in the blank control group and the negative control group was (116.1.1 ± 4.3) and (98.3 ± 6.9), respectively (experimental group vs. blank control group (P < 0.05; experimental group vs. negative control group P < 0.05) (Figure 4).
Figure 4.
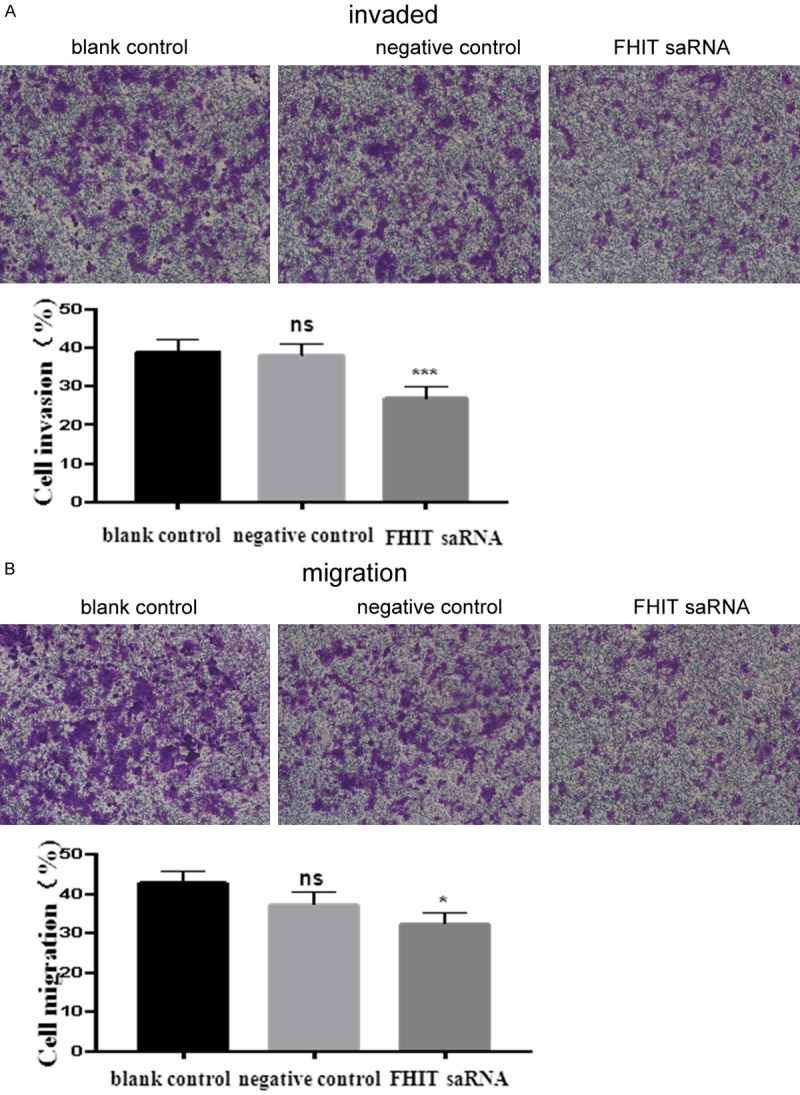
Activation of FHIT inhibited migration and invasion of endometrial carcinoma cells. A. Transwell invasion assay was measured, and the results were expressed as the number of invaded cells per field; B. Transwell migration assay was measured and the results were expressed as the number of invaded cells per field. *P < 0.05.
Discussion
Endometrial carcinoma has a high incidence and low cure rate. Scientists have also been working on the pathogenesis of this tumor, hoping to help its diagnosis, treatment, and prognosis at the molecular level. The occurrence and development of tumors are closely related to abnormal activation of oncogenes, inactivation of tumor suppressor genes, and abnormalities of DNA mismatch repair genes [10]. RNAa is one of the hottest technologies in the field of gene function and therapy in recent years. RNAa inhibits tumor growth by selectively activating or enhancing the expression of a tumor suppressor gene, without the need to find tumor-specific oncogenes. RNAa effects do not involve the degradation of any target sequence, but rather through the recruitment of transcriptional activators leads to the activation of the gene transcription process and the activation of chromatin modifications [11]. Therefore, RNAa has almost unlimited target genes, which can increase the total abundance of the target gene mRNA, while retaining the diversity of the natural splice isoforms of mRNA. It plays a role at the level of transcription and epigenetics, and does not alter the genome [12]. This discovery will pioneer the expansion of the treatment of tumor, metabolic and hereditary diseases [13].
Tumor suppressor genes are a large group of genes that can inhibit cells growth and potentially inhibit cancerization. The inactivation or deletion of these genes is related to cell cancerization [14]. FHIT gene is a tumor suppressor gene isolated and identified in the 3p14.2 region by differential display analysis probe and exon capture method from Ohta et al. in 1996 [15]. It is 1095 bp in length and consists of 10 exons. The number 5-9 exon constitute an open reading frame, encoding an 147 amino acid molecule with a molecular weight of 16.8 kD. The starting exon is located at the centromere side of the hetero break at t (3: 8), t (3: 8) can interfere with FHIT gene expression. FHIT gene exists in epithelial tissues, and it mainly plays a role by participating in cell cycle regulation and apoptosis [16]. It can block cells in the S phase of the proliferation cycle and activate the caspase-mediated apoptosis cascade [17]. Based on the current research, this gene has different levels of mRNA FHIT expression in normal tissues such as the endometrium, ovary, and lung [18]. It is reduced or deleted in some human epithelial tumors, and is closely related to poor tumor prognosis [19].
In this study, we applied immunohistochemistry to detect the expression of FHIT protein about 135 endometrial carcinoma tissues and 40 normal endometrial tissues. The results showed that the positive expression rate of FHIT protein in endometrial carcinoma was 11.8%. Compared with the normal endometrium which was 91.4%, the positive expression rate was significantly reduced, and the expression of FHIT protein in two different endometrial tissues was significantly different (P < 0.01). The positive expression rate of FHIT protein has been significantly reduced. FHIT protein has a significant correlation with the occurrence and development of endometrial carcinoma. FHIT protein expression in endometrial carcinoma is associated with tumor histologic grade, clinical stage, myometrial invasion, and lymph node metastasis significantly (P < 0.05). Complete loss of FHIT protein expression was significantly correlated with high tumor grade of endometrial carcinomas. Weakly positive FHIT immunostaining was also frequently observed in tumors of higher grade. These findings suggest the possibility that inactivation or down-regulation of FHIT gene expression in endometrial carcinoma is involved in the de-differentiation of the tumor cells.
The CCK-8 kit is used to detect the proliferation of cells after activation of FHIT gene RNA in human endometrial carcinoma cells. The results showed that the cell growth of the experimental group is significantly slower than that of the negative control group, indicating that endometrial carcinoma cell doubling time was significantly longer after activating the FHIT gene. The transwell chamber model experiment is a better method to study tumor cell invasion and migration, and can simulate the process of tumor cells digesting matrix and crossing the barrier for invasion and migration. The results showed that after activating the FHIT protein expression, endometrial carcinoma cells that can cross the barrier were significantly reduced compared with the negative control group. This indicates that the ability of endometrial carcinoma cells to invade and migrate is inhibited. It further suggests that Fhit gene is closely related to the proliferation, invasion, and metastasis of human endometrial cancer cells.
This study found that the loss of FHIT gene expression is closely related to the occurrence and development of endometrial carcinoma, and detecting its expression level has a certain reference value for the early diagnosis, clinical progression, and prognosis of endometrial carcinoma [20]. Using multi-factor analysis to explore the inhibitory effect of FHIT gene in endometrial carcinoma and the risk factors of endometrial carcinoma metastasis, may facilitate screening of high-risk populations. Its detection will help to improve the accuracy and objectivity of diagnosis, estimate prognosis, and design treatment for endometrial carcinoma [21].
We are undertaking follow-up experiments about the abnormal expression of FHIT gene and tumor prognosis. The mechanism of the FHIT gene on the occurrence and development of endometrial carcinoma and the cause of FHIT protein expression in endometrial carcinoma tissues needs to be further studied [22]. With the development of FHIT gene research, various gene detection and intervention methods around the FHIT gene will become a powerful biologic research tool, for diagnosis and treatment of tumors [23].
Conclusion
Our current study showed the frequent loss or reduction of FHIT protein expression in high-grade endometrial carcinomas. In cases with partial loss of FHIT expression, the absence was frequently observed in less-differentiated cells. In addition, loss of FHIT expression was also significantly correlated with advanced stage of the disease. These findings indicated that the loss or reduction of FHIT protein in endometrial carcinoma might contribute to the progression toward less differentiated and more aggrieve tumors. However, further studies are required to clarify the exact role of FHIT protein in endometrial carcinogenesis.
Acknowledgements
This study was supported by the National Innovation and Entrepreneurship Program for College Students (201810367025) and Natural Science Foundation of Bengbu Medical College (BYKY1822ZD).
Disclosure of conflict of interest
None.
References
- 1.Segawa T, Sasagawa T, Saijoh K, Inoue M. Clinicopathological significance of fragile histidine triad transcription protein expression in endometrial carcinomas. Clin Cancer Res. 2000;6:2341–2348. [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Waters CE, Saldivar JC, Hosseini SA, Huebner K. The FHIT gene product: tumor suppressor and genome “caretaker”. Cell Mol Life Sci. 2014;71:4577–4587. doi: 10.1007/s00018-014-1722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B, Ying X, Wang J, Piriyapongsa J, Jordan IK, Sheng J, Yu F, Zhao P, Li Y, Wang H, Ng WL, Hu S, Wang X, Wang C, Zheng X, Li W, Curran WJ, Wang Y. Identification of a tumor-suppressive human-specific microRNA within the FHIT tumor-suppressor gene. Cancer Res. 2014;74:2283–2294. doi: 10.1158/0008-5472.CAN-13-3279. [DOI] [PubMed] [Google Scholar]
- 5.Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, Enokida H, Dahiya R. Small ds RNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Che Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 7.Place RF, Noonan EJ, Földes-Papp Z, Li LC. Defining features and exploring chemical modifications to manipulate RNAa activity. Curr Pharm Biotechnol. 2010;11:518–526. doi: 10.2174/138920110791591463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri AR, Khan IA, Prasad V, Robinson AK, Ludueña RF, Barnes LD. The tumor suppressor protein Fhit. A novel interaction with tubulin. J Biol Chem. 1999;274:24378–24382. doi: 10.1074/jbc.274.34.24378. [DOI] [PubMed] [Google Scholar]
- 9.Zochbauer-Muller S, Fong KM, Maitra A, Lam S, Geradts J, Ashfaq R, Virmani AK, Milchgrub S, Gazdar AF, Minna JD. 5’ CpG island methylation of the FHIT gene is correlated with loss of gene expression in lung and breast cancer. Cancer Res. 2001;61:3581–3585. [PubMed] [Google Scholar]
- 10.Saldivar JC, Bene J, Hosseini SA, Miuma S, Horton S, Heerema NA, Huebner K. Characterization of the role of Fhit in suppression of DNA damage. Adv Biol Regul. 2013;53:77–85. doi: 10.1016/j.jbior.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiss DL, Baez WD, Huebner K, Bundschuh R, Schoenberg DR. Loss of fragile histidine triad (Fhit) protein expression alters the translation of cancer-associated mRNAs. BMC Res Notes. 2018;11:178. doi: 10.1186/s13104-018-3278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiss DL, Baez W, Huebner K, Bundschuh R, Schoenberg DR. Impact of FHIT loss on the translation of cancer-associated mRNAs. Mol Cancer. 2017;16:179. doi: 10.1186/s12943-017-0749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter targeted duplexRNAs. Nat Chem Biol. 2007;3:166–173. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 14.Kiss DL, Waters CE, Ouda IM, Saldivar JC, Karras JR, Amin ZA, Mahrous S, Druck T, Bundschuh RA, Schoenberg DR, Huebner K. Identification of Fhit as a post-transcriptional effector of Thymidine Kinase 1 expression. Biochim Biophys Acta Gene Regul Mech. 2017;1860:374–382. doi: 10.1016/j.bbagrm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 16.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo MP, Gramegna M, Croce CM, Pierotti MA, Sozzi G. The tumor-suppressor gene FHITis in the regulation of apoptosis and in cell cycle control. Cell Biol. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevignani C, Calin GA, Cesari R, Sarti M, Ishii H, Yendamuri S, Vecchione A, Trapasso F, Croce CM. Restoration of fragile histidine triad (FHIT) expression induces apoptosis and suppresses tumorigenicity in breast cancer cell lines. Cancer Res. 2003;63:1183–1187. [PubMed] [Google Scholar]
- 18.Kujan O, Abuderman A, Al-Shawaf AZ. Immunohistochemical characterization of FHIT expression in normal human tissues. Interv Med Appl Sci. 2016;8:7–13. doi: 10.1556/1646.8.2016.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pekarsky Y, Zanesi N, Palamarchuk A, Huebner K, Croce CM. FHIT: from gene discovery to cancer treatment and prevention. Lancet Oncol. 2002;12:48. doi: 10.1016/s1470-2045(02)00931-2. [DOI] [PubMed] [Google Scholar]
- 20.Hadaczek P, Gronwald J, Chosia M, Huebner K, Lubiński J. FHIT protein expression in endometrial cancers: no correlation with histological grade. Pol J Pathol. 2001;52:199–203. [PubMed] [Google Scholar]
- 21.Vural S, Simon R, Krushkal J. Correlation of gene expression and associated mutation profiles of APOBEC3A, APOBEC3B, REV1, UNG, and FHIT with chemo sensitivity of cancer cell lines to drug treatment. Hum Genomics. 2018;12:20. doi: 10.1186/s40246-018-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shu R, He J, Wu C, Gao J. The association between RARβ and FHIT promoter methylation and the carcinogenesis of patients with cervical carcinoma: a meta-analysis. Tumour Biol. 2017;39:1010428317709126. doi: 10.1177/1010428317709126. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Wu G, Yao X, Hou G, Jiang F. The clinicopathological significance and ethnic difference of FHIT hypermethylation in non-small-cell lung carcinoma: a meta-analysis and literature review. Drug Des Devel Ther. 2016;10:699–709. doi: 10.2147/DDDT.S85253. [DOI] [PMC free article] [PubMed] [Google Scholar]


