Acute respiratory distress syndrome (ARDS) caused by the novel coronavirus SARS-CoV-2 is associated with a high rate of delirium resulting in encephalopathy, prominent agitation, and confusion [1]. Considering neurotropism of coronaviruses, a direct central nervous system invasion resulting in encephalopathy of SARS-CoV2 is discussed [2, 3]. Recent data reported an enhancement in leptomeningeal spaces and bilateral frontotemporal hypoperfusion in SARS-CoV-2 [1]. Since delirium however might also be caused by the systemic injury in critical illness [4], it remains debatable if the high rate of delirium is specifically associated with SARS-CoV-2 or rather a common complication of viral ARDS. We therefore compared delirium in ARDS patients caused by either SARS-CoV-2 or influenza A and B viruses.
We performed a single-center retrospective register analysis including invasive ventilated patients with ARDS and SARS-Cov-2 or influenza infection treated between 2015 and May 2020. We analyzed delirium by NuDesc (nursing delirium screening scale) score and RASS (Richmond agitation and sedation scale) score which are routinely assessed three times a day by especially trained nurses in all patients on our ICU. The NuDesc score is approved and shows a high sensitivity and specifity [5].
A total of 83 patients with ARDS were identified (44 and 39; with SARS-Cov-2 and influenza, respectively). Thirty-seven (22 and 15) died before extubation and 10 (2 and 8) were transferred with tracheotomia without the possibility of delirium evaluation using a verbal test. We therefore analyzed 36 (20 and 16) patients. Besides of age (patients with SARS-Cov-2 infection were significantly older), groups were homogenous (see Table 1).
Table 1.
Characteristics of patients with ARDS caused by SARS-CoV-2 or influenza A/B. For laboratory data, maximum values are shown. p value reported in bold if difference is significant (p < 0.05). Data are given as mean ± standard deviation or number of patients (percent of all patients in group). aStudent’s t test; bWelch t test, cchi-square test; dFisher’s exact test
| Influenza (N = 16) | COVID-19 (N = 20) | p | |
|---|---|---|---|
| Age | 54.31 ± 12.36 | 65.48 ± 10.99 | 0.007a |
| Female | 5 (31.3%) | 4 (20.0%) | 0.470d |
| ICU stay (days) | 19.85 ± 12.09 | 21.05 ± 11.77 | 0.765a |
| Death | 0 (0%) | 2 (10.0%) | 0.492d |
| Severe ARDS | 11 (68.8%) | 9 (45.0%) | 0.154c |
| Days of invasive ventilation | 18.28 ± 15.61 | 15.47 ± 10.34 | 0.522a |
| TISS 10 | 16.63 ± 5.73 | 15.25 ± 6.77 | 0.521a |
| SAPS 2 | 40.38 ± 9.88 | 44.70 ± 11.13 | 0.232 |
| Noradrenalin > 1 mg/h | 8 (50.0%) | 10 (50%) | 1.000 |
| Renal replacement therapy | 4 (25.0%) | 6 (30.0%) | 1.000 |
| Lactat mmol/l | 3.35 ± 1.82 | 3.07 ± 2.23 | 0.369 |
| CRP mg/dl | 302.99 ± 96.89 | 257.34 ± 84.46 | 0.140 |
| Procalcitonin ng/ml | 59.22 ± 106.43 | 17.19 ± 33.46 | 0.159 |
| Delirium | 12 (75.0%) | 13 (65.0%) | 0.718 |
| Delirium duration (days) | 2.83 ± 2.44 | 5.08 ± 4.29 | 0.126 |
| NuDesc score at maximum | 3.67 ± 1.78 | 5.15 ± 2.58 | 0.109 |
| Delirium onset after extubation (days) | 0.80 ± 1.55 | 0.50 ± 1.08 | 0.622 |
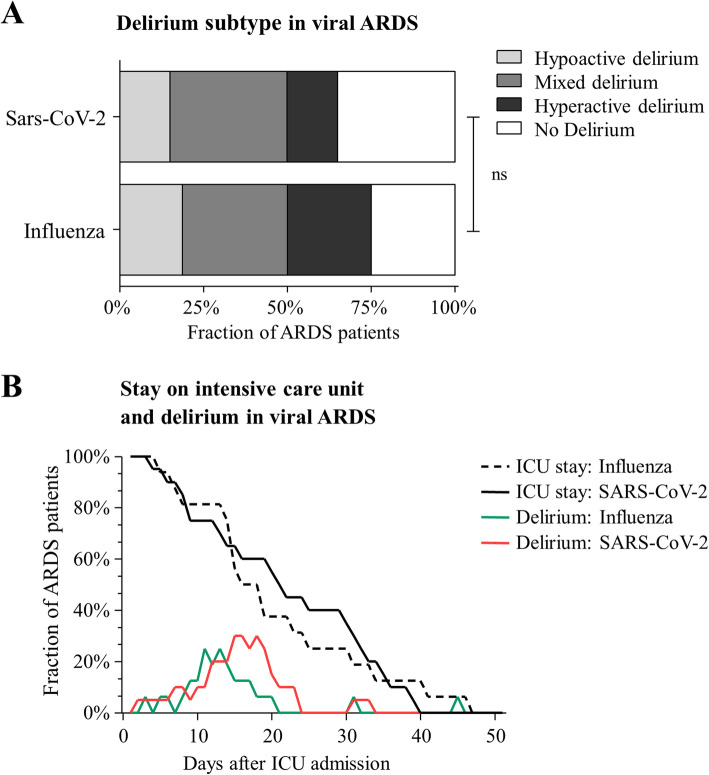
Of all analyzed patients 69.4% (65.0 and 75.0% with SARS-CoV-2 and influenza, respectively) were diagnosed with delirium at any time during the ICU stay. Delirium duration tended to be longer in patients with SARS-CoV-2 (5.1 ± 4.3 days vs. 2.8 ± 2.4 days, p = 0.13). Delirium severity, defined as maximum of NuDesc score, also tended to be more distinctive in SARS-Cov-2 patients (NuDesc score at maximum: 5.2 ± 2.6 vs. 3.7 ± 1.8, p = 0.11). The onset of delirium after extubation was similar (0.50 ± 1.08 days vs. 0.8 ± 1.6 days). For the delirium presentation, see Fig. 1.
Fig. 1.
Delirium presentation and duration. Graph shows delirium distribution of hyperactive/hypoactive/mixed delirium and no delirium shown in percent in patients with ARDS caused by SARS-CoV-2 or influenza A/B (a). Graph shows stay on the intensive care unit and fraction of delirium positive patients shown in percent in patients with ARDS caused by SARS-CoV-2 or influenza A/B (b)
In this registry study of delirium in viral ARDS, we found no statistical significant difference in delirium prevalence, intensity, or type of delirium comparing patients with SARS-CoV-2 to those with influenza. We therefore hypothesize that delirium observed in COVID-19 patients has to be considered a complication of ARDS rather than SARS-CoV-2 specific. Considering the retrospective nature of data presented here, our results have to be considered hypothesis generating and have to be confirmed in a larger patient collective.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- NuDesc
Nursing delirium screening scale
- RASS
Richmond agitation and sedation scale
- TISS
Therapeutic Intervention Scoring System
- SAPS
Simplified Acute Physiology Score
Authors’ contributions
MJ, PB, and DS carried out the data collection, design, and planning of this study. MJ and DS performed the statistical analysis and drafted the manuscript. All authors participated in the critical discussion of the study and interpretation of data. All authors read and approved the final manuscript.
Funding
The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and University of Freiburg in the funding programme Open Access Publishing.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the Albert Ludwigs University of Freiburg, file number 387/19.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, Anheim M, Meziani F. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020; 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed]
- 2.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24:176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larvie M, Lev MH, Hess CP. More on neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382 10.1056/NEJMc2015132. [DOI] [PubMed]
- 5.Gaudreau J-D, Gagnon P, Harel F, Tremblay A, Roy M-A. Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manag. 2005;29:368–375. doi: 10.1016/j.jpainsymman.2004.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.