Abstract
Objective: MicroRNAs (miRs) have been confirmed to be involved in the development of cardiovascular diseases, in spite of numerous studies elucidating the effect and mechanism of miRs in the progression of cardiac ischemia reperfusion injury (I/R), the understanding of their roles is still limited. Methods: All rats underwent the same I/R procedure, while sham group experienced the surgical procedure but without the ligation of left anterior descending coronary artery (LAD). Results: Here, we found miR-101a which was proved down-regulated significantly in myocardium and cariomyocytes subjected to I/R and H2O2 treatment respectively. In vivo and in vitro studies determined the protective role of miR-101a from I/R and oxidative stress injury. It attenuated the size of ischemia area and the cardiomycyte apoptosis under I/R and H2O2 treatment. Mechanically, BCL2L11 was predicted and then verified to be targeted by miR-101a. Moreover, rescue experiment and RNA pull down further verified the interaction between miR-101a and BCL2L11. Conclusions: Our findings revealed miR-101a may be a therapeutic target for the therapeutic target for ischemic heart diseases and expanded our understanding of the molecular mechanism underling the progression of I/R injury.
Keywords: miRs, I/R, cariomyocytes, H2O2
Introduction
Acute myocardial infarction (AMI), which is characterized by the block of blood supply to the heart keeps one of the leading causes of morbidity and mortality worldwide [1,2]. Restoration of blood flow has been demonstrated as one of the most effective therapeutic method to prevent the hearts from dysfunction or damage caused by imbalance of oxygen demand and supply [3,4]. However, the recovery of blood supply termed as reperfusion together with reoxygenation will result in exacerbation of tissue injury as well as inflammatory responses which is called ischemia reperfusion (I/R) injury. Apoptosis is well known to be the critical form of myocardial cells death induced by dysfunction of mitochondria and increase of lipid peroxides contributes mainly to I/R injury [5,6]. Deeper understanding of the mechanisms of cardiomyocyte apoptosis is critical to prevent heart injury and treat heart disease.
MicroRNAs (miRNAs) are a class of small non-coding RNAs that can legate messenger RNAs (mRNAs) at partially complementary binding locations and thus control the rate of protein synthesis by changing the stability of targeted mRNAs. They can control post-transcription gene expression negatively. Increasing evidence has shown that miRNAs engage in the modulation of cell differentiation, apoptosis, growth, and proliferation. MiRNAs can control cardiac function, including conduction of electrical signals, myocardial contraction, heart development, and morphogenesis.
Increasing evidence has demonstrated the crucial role of miRs in both the development and pathology of the cardiovascular system, including cardiac ischemia reperfusion injury [7,8]. MiR-206 mediates YAP-induced cardiac hypertrophy and survival [9]. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD [10]. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. However, the function and molecular mechanisms of cardiac miR-101a still remain limited [11].
In the present work, we focused on the function of a miR-101a on cardiac ischemic injury. Function studies revealed that miR-101a was capable of reducing infarct size and myocardial, moreover, decreasing the apoptosis of cardiomyocytes sujected to H2O2 treatment. In addition, we found that miR-101a targets BCL2L11 in H9c2 cell. These findings offer important insights into fundamental mechanisms underlying the function of miR-101a as well as a novel therapy venue for cardiac ischemic injury and its underlying pathology.
Materials and methods
Animals
Wistar rats were obtained from Charles River Laboratories and maintained in the specific pathogen free (SPF) environment with free access to water and food. All animal procedures were performed in accordance with institutional guidelines and approved by the Animal Studies Committee of The First Hospital of Jiaxing.
Plasmids construction and treatment
Vector overexpressing BCL2L11 and the negative control are designed and synthesized by Genepharma (Shanghai, China). The wild and mutant region of BCL2L11 targeted by miR-101a were synthesized by Genepharma (Shanghai, China) and cloned into pGL3 luciferase reporter vectors (Promega, CA, USA). Chemically modified oligonucleotides (agomiR) have been used to overexpress miR-101a in vivo and in vitro correspondingly and was proved to successfully increase the miR-101a expression. We treated rats with an agomiR-101a via three consecutive daily tail vein injection of antagomiR-101a (100 mg/kg).
I/R model establishment
All rats underwent the same I/R procedure, while sham group experienced the surgical procedure but without the ligation of left anterior descending coronary artery (LAD). The LAD was ligated using a 6-0 silk suture. Successful ligation of the LAD was confirmed by immediate cyanosis in the anterior ventricular wall and continuous electrocardiography monitoring. I/R procedure included 30 min ischemia and 2 h reperfusion. The heart tissue and blood samples were collected for further experiment after I/R injury.
TTC staining
For TTC staining, 2% Evans blue were injected to the heart from femoral vein after the ligation of LAD. Heart tissues were collected immediately after the injection and rinsed with ice-cold NS. The heart was frozen at -20°C for 30 min and then transversely cut into 1 mm-thick slices. The tissues were incubated with TTC at 37°C for 15 min. After fixation with formaldehyde for 24 hours, heart slices can be visualized. The white area was considered as infarct area, and the red area was considered as area at risk (AAR).
Cell culture
H9c2 cardiomyocytes were obtained from Cell bank of Chinese Academy of Sciences and cultured in DMEM (gibco) with 10% FBS (fetal bovine serum, gibco), 100 U/mL penicillin, 100 μg/mL streptomycin and 110 mg/mL sodium pyruvate under 5% CO2 at 37°C in a humidified atmosphere.
Flow cytometry
The cultured H9c2 cells were digested with trypsin, washed with cold PBS and dualstained with AnnexinV FITC/propidium iodide according to the manufacturer’s instructions. Cell apoptosis was detected by flow cytometry on a BD FACSCalibur (Becton Dickinson, NJ, USA).
Reverse transcription and quantitative real-time PCR
RNA extraction was performed with TriZOL reagent (invitrogen), precipitated with isopropanol, washed with 75% ethanol and dissolved in RNase free water. Reverse transcription was performed with 1 μg RNA using the cDNA transcription kit (transgen). 20 ng cDNA was used for qPCR to validate the expression of relative mRNA. It was determined by using SYBR green mix (Yisheng, Shanghai).
Pull-down assay with biotinylated miRNA
Biotin labeled miR-101a probe and control probe were synthesized by Sangon Biotech (Shanghai, China). Probe-coated beads were generated by coincubating the probe with streptavidin-coated beads (Invitrogen, CA, USA) at 25°C for 2 h. Cardiomyocytes were collected and lysed and incubated with miR-101a probes overnight at 4°C. Thereafter, the beads were eluted and the complex was purified with TRIzol (Takara, Dalian, China). Then the abundance of BCL2L11 was analyzed by qRT-PCR.
Luciferase activity assay
The wild-type or mutant seed sequence at the predicted region of BCL2L11 was synthesized and cloned into the pGL3 Luciferase Reporter vectors (Promega, CA, USA) at the KpnI and BamHI sites. Papillary thyroid carcinoma cells were co-transfected with miR-199a mimics or mimic control, together with pGL3 vectors, which contained the WT or mut predicted binding region of BCL2L11. TRL-SV40 plasmid (Promega, CA, USA) was also transfected as a normalizing control. The cells were harvested for the detection of the activity of luciferase with the use of the Dual-Luciferase Assay (Promega, WI, USA) at 48 h following the transfection.
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) were used to analyze all data for statistical significance. All the data are presented as the means ± SD. One-way ANOVA was used to assess the difference between multiple groups. Differences between two groups were analyzed by the Student’s t-test. P<0.05 was considered as statistical significance.
Results
MiR-101a is downregulated in the cardiomyocytes subjected to H2O2
First, we treated H9c2 cell with different concentrations of H2O2 to establish oxidative damage model. It was showed in the results that 50, 100 and 200 μM H2O2 significantly inhibited the cell viability of H9c2 cells. We chose 100 μM H2O2 for the further study and found that treatment of 100 μM H2O2 for 6, 12, 24 h in H9c2 cell notably reduced the expression level of miR-101a (Figure 1).
Figure 1.
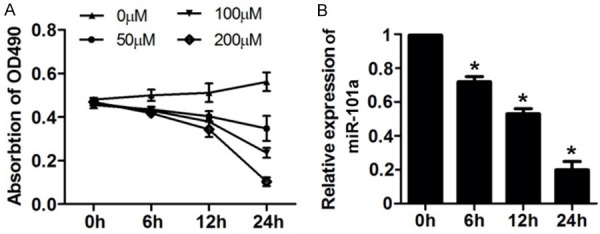
MiR-101a was up-regulated in H9c2 cell after H2O2 treatment. A. After different concentration and time of H2O2 treatment, MTT assay was used to detect the cell viability of H9c2 cell. B. qPCR was performed to evaluate the expression level of miR-101a. Data are expressed as mean ± SD; n=8 *P<0.05.
Protective effects of miR-101a on H2O2 induced injury of cardiomyocytes
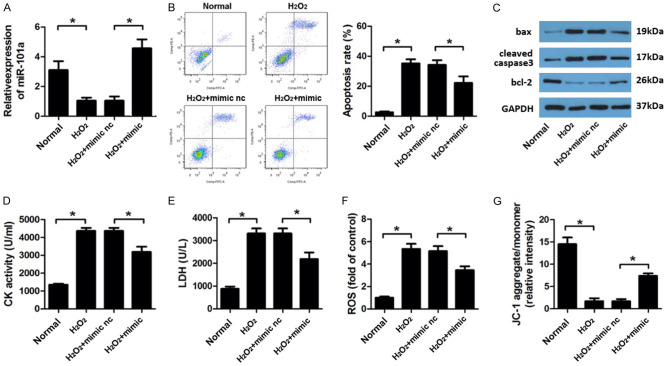
In order to investigate the effect of miR-101a in I/R injury. We treated the H9c2 cell with miR-101a mimic. First, the efficient of the mimic was evaluated by qPCR indicated a good overexpressing effect of it (Figure 2A). Flow cytometry results revealed that miR-101a overexpression significantly reduced the apoptosis of cardiomyocyte induced by H2O2 (Figure 2B). Moreover, we assessed the apoptotic protein expression. Western blot assay indicated that miR-101a mimic transfection reduced the level of bax, cleaved caspase3 and elevated that of bcl-2 compare to the H2O2 group (Figure 2C). Thus, we evaluated the activity change of CK, LDH and ROS in cardiomyocytes. Obviously, H2O2 notably promoted the amount of MDA, LDH and ROS, while miR-101a remarkably reversed this elevation (Figure 2D-F). We further detected the mitochondrial membrane potential (MMP) of H9c2 cell. Again, miR-101a overexpression reversed the effect of H2O2 on decreasing the MMP of H9c2 cell (Figure 2G).
Figure 2.
miR-101a knock down protect H9c2 cell form oxidative injury. (A) qPCR was performed to evaluate the expression level of miR-101a. (B) Flow cytometry was applied to investigate the apoptosis rate. (C) Western blot was used to evaluate the expression of apoptotic proteins such as bax, cleaved caspase3 and bcl-2. (D) CK, (E) LDH, (F) ROS was evaluated by commercial kits. (G) JC-1 staining was performed to detect the mitochondrial membrane potential (MMP) of H9c2 cell. Data are expressed as mean ± SD; n=8 *P<0.05.
MiR-101a directly target BCL2L11 in cardiomyocyte
Targetscan databases were used to predict the targets of miR-101a, among which BCL2L11 was selected as a potential one. Figure 3A showed the target region between miR-101a and BCL2L11. Luciferase activity assay in cardiomyocytes were carried out. As indicated by findings, miR-101a evidently down-regulated the luciferase activity co-transfected with pGL3-3’UTR of BCL2L11, but not the pGL3-3’UTR-mut of BCL2L11 (Figure 3B). The qPCR analyses further confirmed that miR-101a overexpression decreased the mRNA level of BCL2L11 while knock down of miR-101a promoted that of BCL2L11 (Figure 3C). Accordingly, western blot results indicated that miR-101a overexpression decreased the protein level of BCL2L11 while knock down of miR-101a promoted that of BCL2L11 (Figure 3D). Again, RNA pull down was carried out and confirmed that miR-101a directly binds BCL2L11 (Figure 3E). Finally, we performed Pearson analysis to detect the correlation between miR-101a and BCL2L11. There exerts a negative correlation between miR-101a and BCL2L11 (Figure 3F).
Figure 3.
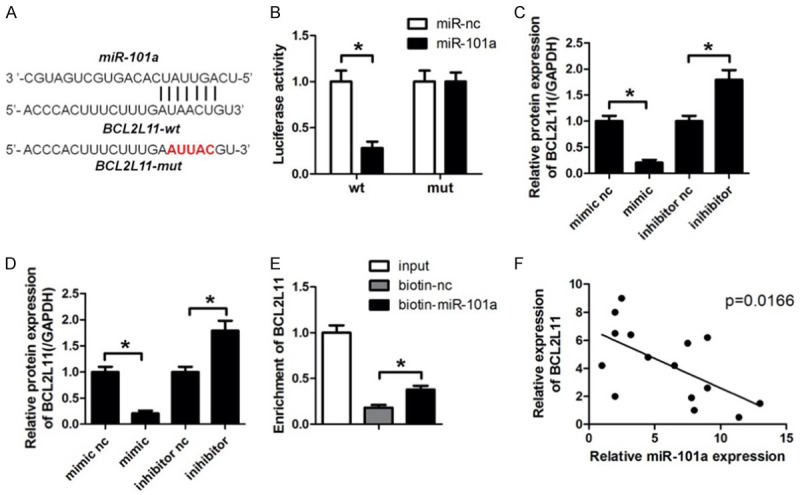
MiR-101a directly targets BCL2L11. (A) Sequence alignment between miR-101a and the 3’UTR of BCL2L11 in H9c2 cell. (B) Luciferase activity assay was performed to investigate whether miR-101a target BCL2L11. (C) qPCR and (D) Western blot analysis of protein level of HGF with miR-26b mimic or inhibitor transfected into H9c2 cells. (E) RNA pull down using specific probe was carried out to detect the interaction of miR-101a and BCL2L11. (F) Pearson analysis was performed to analysis the correlation between miR-101a and BCL2L11. Data are expressed as mean ± SD; n=8 *P<0.05.
BCL2L11 reversed the effect of MIR-101a induced apoptosis of cardiomyocytes
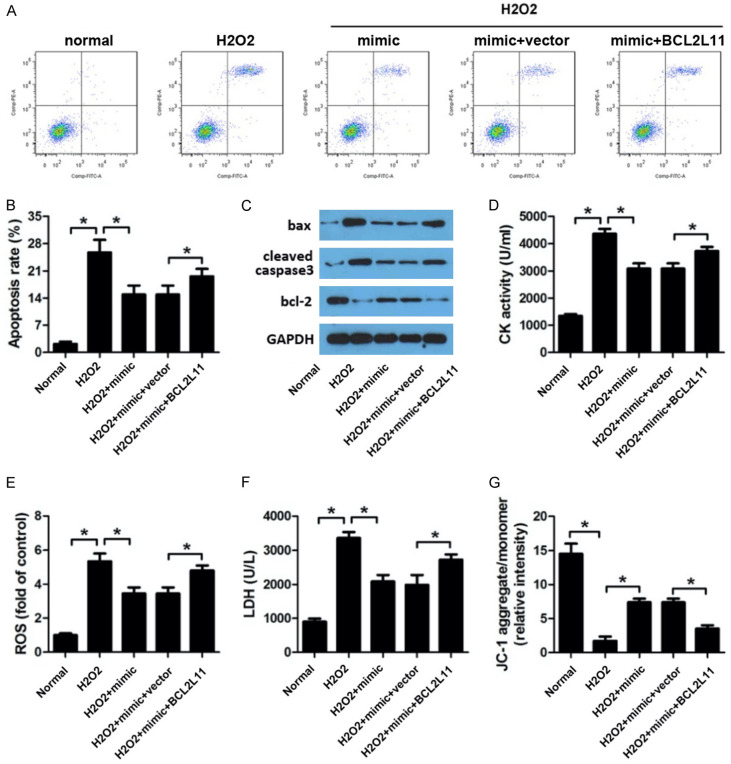
MiR-101a works as a sponge of BCL2L11 in cardiomyocytes. For the purpose of investigating the role of BCL2L11 in I/R injury. We carried out rescue experiment. Cardiomyocytes were transfected with miR-101a mimic and vector overexpressing BCL2L11 along with their negative control respectively. Flow cytometry were performed to evaluate the cell apoptosis. We observed that miR-101a significantly reduced the apoptosis rate induced by H2O2. BCL2L11 remarkably reversed this alteration (Figure 4A, 4B). In addition, we evaluated the expression of proptotic proteins. The results revealed that BCL2L11 reversed the effect of miR-191a on the expression of proptotic proteins such as bax, cleaved caspase3 and bcl-2 (Figure 4C). Again, we performed experiment to evaluate the activity of CK, LDH, ROS and MMP. As expected, BCL2L11 was capable of reversing the effect of miR-101a on elevating the activity of CK, LDH, ROS and the reduction of MMP (Figure 4D-F). The MMP was detected by JC-1 staining. We found that BCL2L11 reversed the inhibitory effect of BCL2L11 on the MMP (Figure 4G).
Figure 4.
BCL2L11 knock down reversed the effect of miR-101a inhibition on myocardiomyocyte oxdative stress injury. (A, B) Flow cytometry was applied to investigate the apoptosis rate after different kinds of treatment. (C) Western blot was used to evaluate the expression of apoptotic proteins such as bax, cleaved caspase3 and bcl-2. (D) CK, (E) ROS and (F) LDH was evaluated by commercial kits. (G) JC-1 staining was performed to detect the mitochondrial membrane potential (MMP) of H9c2 cell. Data are expressed as mean ± SD; n=8 *P<0.05.
Protective effects of miR-101a on I/R induced injury of rats
Finally, we established in vivo I/R model to investigate the effect of miR-101a on I/R injury. We used agomiR to overexpressing miR-101a and the qPCR results indicated a good efficient of agomiR (Figure 5A). TTC staining was performed to evaluate the infarct area of the hearts. We found that miR-101a reduced the infarct area notably (Figure 5B, 5C). The activity of CK, MDA, ROS and MMP were also detected in the myocardium. Accordingly, the results showed that miR-101a significantly decreased the activity of CK, MDA and ROS while increased the MMP of H9c2 cells (Figure 5D-G). IHC was performed to evaluate the expression of BCL2L11 in the myocardium. We found that BCL2L11 expression was notably promoted by I/R. MiR-101a significantly inhibited the level of BCL2L11 (Figure 5H).
Figure 5.
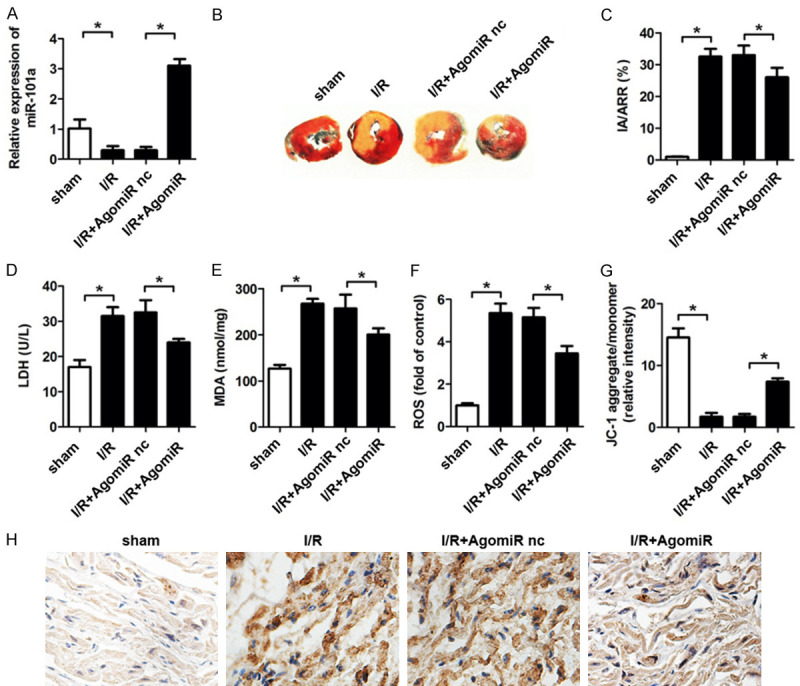
MiR-23a protect the hearts form I/R induced injury in rats. (A) qPCR was used to detect the expression of miR-23a in myocardium of rats subjected to ischemia/reperfusion and antagomiR treatment. (B, C) TTC staining was used to detect the infarct area of hearts. (D) LDH, (E) MDA, (F) ROS was evaluated by commercial kits. (G) JC-1 staining was performed to detect the mitochondrial membrane potential (MMP) of myocardium. (H) IHC was performed to evaluate the expression of BCL2L11 in the myocardium. Data are expressed as mean ± SD; n=8 *P<0.05.
Discussion
Reactive oxygen species will be produced in the process of cardiovascular diseases, which will lead to oxidative stress damage and eventually lead to myocardial cell apoptosis and necrosis [12]. H2O2 is an active oxygen that can induce oxidative stress injury of myocardial cells [13]. Therefore, it is most commonly used to simulate myocardial ischemia reperfusion injury and oxidative stress injury. In this study, it was found that H2O2 of 50, 100 and 200 μm can significantly induce apoptosis of H9c2 cells. We chose this concentration of 100 microns for the following experiment, which is similar to previous studies.
We evaluated the expression of miR-101a under H2O2 treatment and found it significantly down-regulated during different time of reperfusion which has not been reported before. The in vivo and in vitro function studies indicated the protective role of miR-101a on ischemia reperfusion injury. MiR-101a is a novel miR which has not been well studied. In cardiovascular system, miR-101a was reported to miR-101a ameliorates AngII-mediated hypertensive nephropathy by blockade of TGFβ/Smad3 and NF-κB signaling in a mouse model of hypertension [14]. MicroRNA-101a inhibits cardiac fibrosis induced by hypoxia via targeting TGFβ-RI on cardiac fibroblasts [15]. These findings indicated the critical role of miR-101a in preventing heart disease.
The main mechanism of miRNAs inhibiting target gene expression is to induce mRNA degradation or inhibit mRNA translation by matching 3’-UTR of target gene [16-18]. Here, we use bioinformatics analysis to search for potential targets of miR-101a and select BCL2L11 as the potential target. The following luciferase activity assay confirms this prediction. BCL2L11 is a BH3-only protein located in the outer membrane of mitochondria which can inhibit BCL2 expression and activate BAX-BAK1 proteins [19,20].
It was reported that apoptosis is promoted in the blood sample and heart tissue of patients with acute and myocardial ischemia [21,22]. Moreover, a previous study has shown that apoptosis is the early and predominant cell death form of cardiomyocyte mainly locate at the borders of the infracted region [23]. Cardiomyocyte apoptosis induced by I/R process contribute to cellular injury and cardiovascular dysfunction [24,25]. It’s evidenced that upregulation of cardiac specific caspase-3 accelerates infarct size and increases risk of die after I/R injury [26,27]. Thus, attenuating apoptosis of cardiomyocytes was crucial for the therapy of I/R injury.
BCL2L11 was indicated to be capable of interacting with BECN1 and inhibits autophagy [28]. BCL2L11 is the first identified molecule possessing both anti-autophagy and pro-apoptotic effects [29]. We speculated that miR-101a may be involved in the autophagy via regulating BCL2L22. It is the further study we plan to carry out.
In conclusion, in this study, we extended the understanding of the role of miR-101a in cardiac ischemia/reperfusion injury and provided a novel regulatory mechanism underlying the effect of BCL2L11 and MiR-101a. Our findings suggest novel biomarkers or potential therapeutic targets to treat ischemic heart diseases. Elucidation of the deep regulatory relationship between these molecules requires further studies.
Acknowledgements
This work was supported in part by The study was supported by the 2015 Zhejiang Province Medical and Health Science and Technology Research Fund (2015KYB387), the 2015 Zhejiang Province Traditional Chinese Medicine Scientific Research Fund (2015ZA203), the 2016 Zhejiang Province Traditional Chinese Medicine Scientific Research Fund (2016ZA191), the 2016 Zhejiang Province Medical and Health Science and Technology Research Fund (2016KYB287), the 2016 Jiaxing science and Technology Bureau Project (2016AY23038), the 2017 Jiaxing science and Technology Bureau Project (2017AY33021), Provincial-Municipal Joint Construction of Key Medical Disciplines In Zhejiang Province (2019-ss-xxgbx) and Jiaxing Key Innovation Team Fund (2015-CX1).
Disclosure of conflict of interest
None.
References
- 1.Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarction and multivessel disease. Nat Rev Cardiol. 2017;14:665–678. doi: 10.1038/nrcardio.2017.88. [DOI] [PubMed] [Google Scholar]
- 2.Chang HC, Shapiro JS, Ardehali H. Getting to the “heart” of cardiac disease by decreasing mitochondrial iron. Circ Res. 2016;119:1164–1166. doi: 10.1161/CIRCRESAHA.116.309746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenhofer-Murray AE, Gossen M, Pak DT, Botchan MR, Rine J. Separation of origin recognition complex functions by cross-species complementation. Science. 1995;270:1671–1674. doi: 10.1126/science.270.5242.1671. [DOI] [PubMed] [Google Scholar]
- 4.Boon RA, Jae N, Holdt L, Dimmeler S. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104:150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng CS, Wan S, Yim AP. Pulmonary ischaemia-reperfusion injury: role of apoptosis. Eur Respir J. 2005;25:356–363. doi: 10.1183/09031936.05.00030304. [DOI] [PubMed] [Google Scholar]
- 7.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Del Re DP, Nakano N, Sciarretta S, Zhai P, Park J, Sayed D, Shirakabe A, Matsushima S, Park Y, Tian B, Abdellatif M, Sadoshima J. miR-206 mediates YAP-induced cardiac hypertrophy and survival. Circ Res. 2015;117:891–904. doi: 10.1161/CIRCRESAHA.115.306624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, Zhou ZX, Liu J, Wang JL, Li PF. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117:352–363. doi: 10.1161/CIRCRESAHA.117.305781. [DOI] [PubMed] [Google Scholar]
- 11.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Zhou Y, Huang H. MiR-101a ameliorates AngII-mediated hypertensive nephropathy by blockade of TGFbeta/Smad3 and NF-kappaB signalling in a mouse model of hypertension. Clin Exp Pharmacol Physiol. 2019;46:246–254. doi: 10.1111/1440-1681.13042. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Wang K, Liao Y, Zeng Q, Li Y, Hu F, Liu Y, Meng K, Qian C, Zhang Q, Guan H, Feng K, Zhou Y, Du Y, Chen Z. MicroRNA-101a inhibits cardiac fibrosis induced by hypoxia via targeting TGFbetaRI on cardiac fibroblasts. Cell Physiol Biochem. 2015;35:213–226. doi: 10.1159/000369689. [DOI] [PubMed] [Google Scholar]
- 16.Juzwik CA, S Drake S, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, Douglas C, Morquette B, Moore CS, Fournier AE. microRNA dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol. 2019:101664. doi: 10.1016/j.pneurobio.2019.101664. [DOI] [PubMed] [Google Scholar]
- 17.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunderland N, Skroblin P, Barwari T, Huntley RP, Lu R, Joshi A, Lovering RC, Mayr M. MicroRNA biomarkers and platelet reactivity: the clot thickens. Circ Res. 2017;120:418–435. doi: 10.1161/CIRCRESAHA.116.309303. [DOI] [PubMed] [Google Scholar]
- 19.Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamm I, Schriever F, Dorken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol. 2001;2:33–42. doi: 10.1016/S1470-2045(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 21.Chimenti MS, Sunzini F, Fiorucci L, Botti E, Fonti GL, Conigliaro P, Triggianese P, Costa L, Caso F, Giunta A, Esposito M, Bianchi L, Santucci R, Perricone R. Potential role of cytochrome c and tryptase in psoriasis and psoriatic arthritis pathogenesis: focus on resistance to apoptosis and oxidative stress. Front Immunol. 2018;9:2363. doi: 10.3389/fimmu.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broughton KM, Wang BJ, Firouzi F, Khalafalla F, Dimmeler S, Fernandez-Aviles F, Sussman MA. Mechanisms of cardiac repair and regeneration. Circ Res. 2018;122:1151–1163. doi: 10.1161/CIRCRESAHA.117.312586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 24.Webster KA. Programmed death as a therapeutic target to reduce myocardial infarction. Trends Pharmacol Sci. 2007;28:492–499. doi: 10.1016/j.tips.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhardt SU, Weiss JB, Smolka C, Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt Y, Bjoern Stark G, Moser M, Bode C, Grundmann S. MicroRNA-155 aggravates ischemia-reperfusion injury by modulation of inflammatory cell recruitment and the respiratory oxidative burst. Basic Res Cardiol. 2015;110:32. doi: 10.1007/s00395-015-0490-9. [DOI] [PubMed] [Google Scholar]
- 27.Duan W, Yang Y, Yan J, Yu S, Liu J, Zhou J, Zhang J, Jin Z, Yi D. The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res Cardiol. 2012;107:263. doi: 10.1007/s00395-012-0263-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Zhang Y, Zhou L, Leng Y, Lin H, Kmieciak M, Pei XY, Jones R, Orlowski RZ, Dai Y, Grant S. A Bim-targeting strategy overcomes adaptive bortezomib resistance in myeloma through a novel link between autophagy and apoptosis. Blood. 2014;124:2687–2697. doi: 10.1182/blood-2014-03-564534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo S, Garcia-Arencibia M, Zhao R, Puri C, Toh PP, Sadiq O, Rubinsztein DC. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol Cell. 2012;47:359–370. doi: 10.1016/j.molcel.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]