Abstract
Spinal cord injury (SCI) is a severe traumatic disease of the central nervous system characterized by high incidence and disability rate. We aimed to investigate the therapeutic potential of Ezetimibe (Eze) in SCI and identify the underlying mechanisms. Acute SCI rat model was established by using the modified weight-drop method. Following administration with Eze, the neurological function was evaluated using the Basso, Beattie, and Bresnahan (BBB) locomotor scale score, and the motor neurons were stained with Nissl staining. The pathological changes of spinal cord tissues were tested using Hematoxylin and eosin staining. The presence of apoptotic cells was examined using Terminal dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. Moreover, the expression of main autophagy markers LC3II/I, Beclin1 and p62 and apoptosis-related proteins was tested using western blot analysis. The changes of PI3K/AKT/mTOR signaling-associated proteins were measured. Experimental results showed that Eze treatment obviously improved functional recovery, the neuronal survival and morphological characteristics of spinal cord. Additionally, Eze administration dramatically upregulated the expression of LC3II/I and Beclin1 whereas downregulated that of p62. Concurrently, significantly reduced apoptosis was observed following Eze intervention, accompanied by increased expression of anti-apoptotic protein Bcl-2 and decreased expression of pro-apoptotic proteins Bax, cleaved caspase-3 and cleaved caspase-9. Further results indicated that Eze treatment remarkably suppressed the expression of phospho-PI3K (p-PI3K), p-AKT and p-mTOR. These findings demonstrated that Eze could protect against SCI by activating autophagy and hindering apoptosis through regulating PI3K/AKT/mTOR signaling, suggesting a potential candidate for SCI therapy.
Keywords: Spinal cord injury, autophagy, apoptosis, Ezetimibe, PI3K, mTOR
Introduction
Spinal cord injury (SCI) is a devastating central nervous system disease which can result in severe and irreversible neurological deficits, and even lifelong paralysis [1]. Its high morbidity and disability rates are closely related to the serious complications that not only bring great pains to the patients, but also carry a heavy burden to the society [2]. It has been well reported that about 23 out of per million cases occur every year worldwide [3]. Recently, although a great deal of basic researches and clinical therapy studies about SCI have been carried out, no ideal curative effect has been achieved [4]. Therefore, it is imperative to thoroughly elucidate the mechanism of this disease and develop a novel therapy for the effective therapies.
Pathophysiologically, the progression of SCI is subcategorized into primary and secondary injuries [5]. The primary injury is typically caused by the initial mechanical change and occurs immediately after injury, which is irreversible physical injury. The secondary injury is reversible and is considered to have a more significant impact on neurofunctional recovery after SCI, which often incorporates apoptosis, autophagy, hypoxia and inflammation [6,7]. It is generally well known that apoptosis of neural cells is one of the most important causes in spinal cord dysfunction, and that apoptosis inhibition can notably improve the recovery of SCI [8]. Autophagy is a cellular response that sustains homeostasis of tissue structure and functions during development and under stress conditions [9]. Compelling evidence indicates that autophagy contributes to the apoptosis inhibition and activation of autophagy accelerates the recovery of neurological function through suppressing apoptosis in SCI [10].
Ezetimibe (Eze), an anti-hyperlipidemia drug by lowering cholesterol levels, is reported to improve neurological function and relieve inflammation in a rat SCI model by combination with Simvastatin [11]. It is worthy of note that Eze protects against steatohepatitis through activating autophagy and suppressing NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome [12]. However, whether Eze functions in SCI through activating autophagy and suppressing apoptosis has not be elucidated. In this study, an acute SCI rat model was established via applying the modified weight-drop method. The therapeutic potential of Eze in SCI and the underlying regulatory mechanism were investigated.
Materials and methods
Experimental animals
A total of fifty SPF grade adult male Sprague-Dawley (SD) rats (200-250 g) were purchased from Shanghai SLAC Laboratory Animal Company Ltd (Shanghai, China). Rats were maintained in a suitable environment with a 12-h light/dark cycle at 21±3°C (2 rats per IVC cage). All animals had free access to water and standard rat chow. They were housed for at least one week before the experimental performance. All of the experiment protocols used in this study were approved by the Ethics Committee on Animal Experiments of Union Hospital Affiliated to Fujian Medical University.
Establishment of SCI rat model
Following 8 h of fasting, rats were anesthetized by injecting pentobarbital sodium (50 mg/kg) into the peritoneum. The rat model of SCI was established by using the modified weight-drop method as previously described [13]. Briefly, the rats were placed in a prone position and a mid-line incision (about three centimeters in length) was made in the back to expose the T6-10 vertebra. Laminectomy was performed at the T8 level. After the spine was immobilized, 25 g cm (10 g×2.5 cm) to spinal cord was set as the injury gravity which could induce a moderate injury. The spinal cord hemorrhage, delayed extension of hind limbs and tail swing indicated the successful establishment of the SCI model. Subsequently, the incision was sutured layer by layer. Signs of successful modeling included spinal cord hemorrhage, convulsions in hind limbs and tail swing. Until spontaneous urination was resolved, the bladder of rats was evacuated manually. Seven days after the SCI, all animals were subjected to intraperitoneal anesthesia with 50 mg/kg pentobarbital sodium. The spinal cord specimens and blood samples were obtained for the follow-up experiments.
Drug administration
All animals were divided into five groups arbitrary: sham group, SCI group, SCI+ dimethylsulfoxide (DMSO) group, SCI+5 mg/kg Eze group and SCI+10 mg/kg Eze group. Eze was supplied by Merck Sharp and Dohme Corp. (Rahway, NJ, USA). At 1, 24, and 72 h after injury, the corresponding doses of drugs were administered by oral gavage. Rats in the SCI+DMSO group received an identical volume of DMSO solution. Rats with laminectomy only were used as the sham group. Rats in the sham (control) group underwent the same operation as the model group, but they did not receive spinal cord compression.
Neurological score
The neuronal function recovery after injury was scored in accordance with 21-point Basso-Beattie-Bresnahan (BBB) locomotor scale, which was divided into 0 to 21 representing complete paralysis to normal locomotion respectively [14]. All the following neurological tests were performed before injury and at 24 h, 48 h, 72 h, 96 h and 7 days after the operation. All of the rats were evaluated by three trained examiners in a double-blind manner.
Histological assessment
The spinal cord samples were first immobilized with 4% paraformaldehyde over night at 4°C. Subsequently, these tissues were dehydrated under different concentrations of ethyl alcohol solutions. Then spinal cords were embedded into paraffin, which were further cut into 5 μm-thick section slices by a rotary microtome. These slices were subsequently stained with hematoxylin & eosin (H&E) dye for conventional morphological evaluation. The pictures were obtained from an optic microscope (DP73; Olympus, Tokyo, Japan).
Nissl staining
Nissl staining was conducted to assess the neuronal survival of spinal cord. In brief, the tissue sections were cleared using xylene, rehydrated in graded concentrations of ethanol, treated with Nissl staining solution (Boster, China). Only cells with visible nuclei and typical neuronal morphology were counted. For each rat, every other continuous section was selected and a total of three sections were counted. The imagines were observed and photographed under an optical microscope (DP73; Olympus, Tokyo, Japan). The number of surviving neurons was counted for the quantitative statistics analysis by Image J software.
Terminal dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining
Apoptotic cells in spinal cord tissues were examined using a TUNEL apoptosis detection kit (Vazyme, Nanjing, China) following standard protocols. Spinal cord tissues were washed and permeabilized with Triton-X 100, and then washed with PBS. Spinal cord tissue sections were immersed in TUNEL assay reaction mixture and incubated at room temperature. The localized green fluorescence of apoptotic cells was observed under an optic microscope (DP73; Olympus, Tokyo, Japan).
Western blot analysis
The sample tissues were lysed using RIPA buffer and quantified using a Bio-Rad protein assay kit (Bio-Rad, USA). A total of 40 µg proteins in each group were separated by sodium dodecyl sulfate polyacrylamide (SDS-PAGE) using a 10% gel, and transferred to a polyvinylidene fluoride membranes (Merck Millipore, Billerica, USA). After soaking with 5% skim milk, these membranes were incubated with primary antibodies. Following incubation with secondary antibodies (Stanta Cruz Biotechnology, CA, USA), the bands were visualized using an Odyssey Infrared Imaging Scanner (LI-COR Biosciences). Band intensities were quantified using Image J software. β-actin or GAPDH was used as an endogenic control in all samples. Anti-NeuN (cat. no. 24307T), anti-Beclin1 (cat. no. 3495T), anti-p62 (cat. no. 23214S), anti-LC3II/I (cat. no. 12741T), anti-Bax (cat. no. 14796S), anti-cleaved caspase-3 (cat. no. 9661T), anti-cleaved caspase-9 (cat. no. 20750S), anti-phospho-AKT (p-AKT; cat. no. 4060T), anti-p-mTOR (cat. no. 5536T), anti-GAPDH (cat. no. 5174T) and anti-β-actin (cat. no. 4970T) was purchased from Cell Signaling Technology (Boston, MA, USA). Anti-Bcl-2 (cat. no. ab196495) and anti-p-PI3K (cat. no. ab182651) were the products of Abcam (Cambridge, USA).
Statistical analysis
All results were obtained from at least three independent experiments. Statistical analysis was performed using SPSS software (version 21.0, Chicago, IL, USA). All numerical data were presented as the mean ± standard deviation. Student’s t-test was used to compare the significant differences between the two groups. One way analysis of variance (ANOVA) followed by Tukey’s test was employed to compare multiple groups. The level of significance was set at P < 0.05.
Results
Eze improved functional recovery in SCI rats
To investigate the effect of Eze on the recovery of function, BBB locomotor scale score, a valid and predictive measure of locomotor recovery, was performed in the course of the experiment. We found that rats in the SCI and SCI+DMSO groups walked abnormally with bilateral hind limb paralysis, and the BBB score (Figure 1A) was significantly lower than that in the sham group. After treatment with 5 mg/kg Eze or 10 mg/kg Eze, SCI-induced descent of BBB scores was recovered with time in a dose-dependent manner. These observations indicated that Eze could concentration-dependently improve the behavioral performance of SCI rats.
Figure 1.
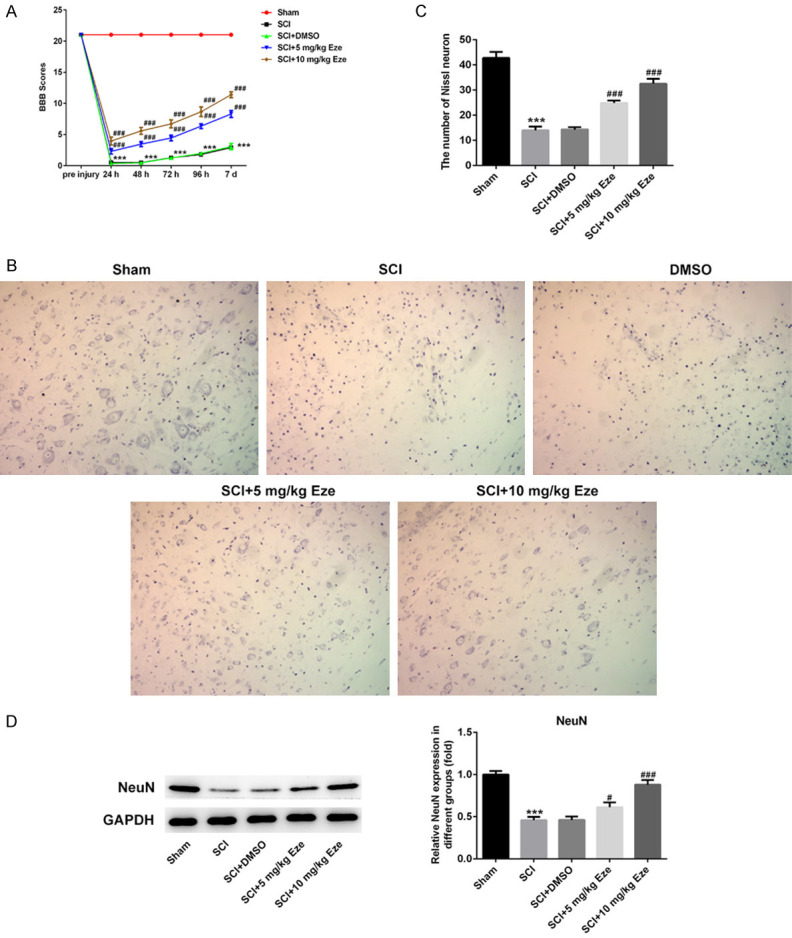
Eze improved functional recovery and neuronal damage in SCI rats. A. The recovery of function was determined using BBB locomotor scale score. B. The function of Eze on the loss of motor neurons was measured by Nissl staining. (magnification, 200×). C. The number of motor neurons was qualified. D. The expression of neuron-specific protein NeuN was evaluated using western blot analysis. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. ***P < 0.001 vs. sham; #P < 0.05, ###P < 0.001 vs. SCI+DMSO. Eze, Ezetimibe; SCI, spinal cord injury; BBB, Basso-Beattie-Bresnahan; DMSO, dimethylsulfoxide.
Eze reduced neuronal damage in spinal cord tissue of SCI rats
To study the function of Eze on the loss of motor neurons after SCI in rats, the number of motor neurons was stained with Nissl staining at day 7 after contusion. As displayed in Figure 1B and 1C, neurons in the sham group exhibited an integrative and granular-like morphology. Rats in the SCI and SCI+DMSO groups shown a great loss of large anterior horn cells compared with the sham group, whereas Eze intervention obviously enhanced the number of motor neurons in the anterior horns of rats. Moreover, the expression of the neuron-specific marker NeuN was remarkably upregulated in the Eze-treated groups as compared to the SCI group (Figure 1D). These results implicated that Eze attenuated neuronal damage in spinal cord tissue of SCI rats.
Eze promoted tissue recovery after SCI
The pathological changes after SCI were examined using H&E staining. As observed in Figure 2, SCI led to the disordered tissue structure, prominent edema, hemorrhage and neutrophil infiltration. Consistently, the pathologic features of tissues in the SCI+DMSO group were similar to those in the SCI group. As expected, the 5 mg/kg Eze and 10 mg/kg Eze groups displayed better histologic characteristics relative to the SCI group. These data provided evidence that Eze promoted the recovery of spinal cord tissue after SCI.
Figure 2.
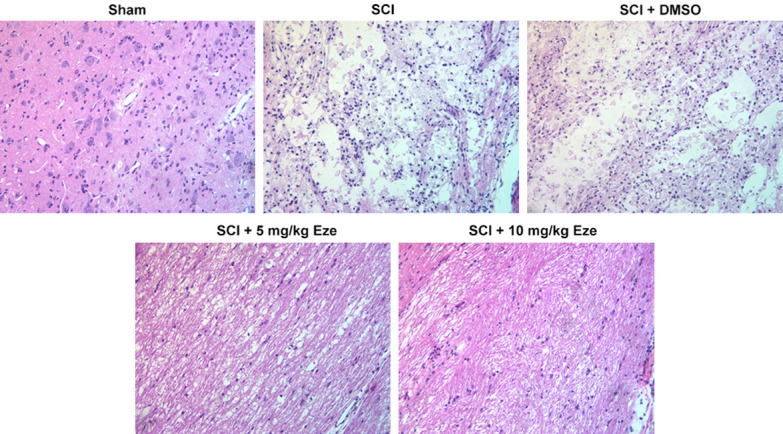
Eze promoted tissue recovery after SCI. The pathological changes after SCI were examined using H&E staining. (magnification, 200×). Eze, Ezetimibe; SCI, spinal cord injury; H&E, hematoxylin & eosin.
Eze enhanced autophagy in rats’ neurons after SCI
To explore whether Eze treatment affected autophagy in rats’ neurons after SCI, the expression of autophagy key markers LC3II/I, Beclin1 and p62 was tested using western blot analysis. As displayed in Figure 3, SCI and SCI+DMSO groups exhibited dramatic downregulation of LC3II/I and Beclin1 expression accompanied by marked upregulation of p62 expression. In contrast, Eze intervention significantly promoted the levels of LC3II/I and Beclin1 but decreased that of p62, especially in 10 mg/kg Eze treatment group. Through the above findings we proved that Eze could activate autophagy in neurons of SCI model rats.
Figure 3.
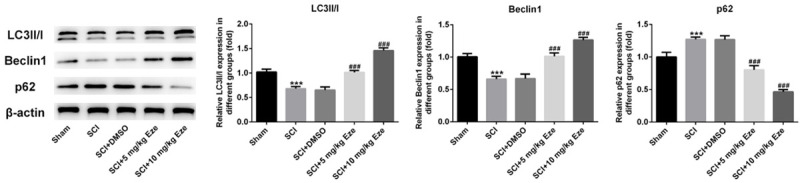
Eze enhanced autophagy in rats’ neurons after SCI. The expression of autophagy key proteins LC3II/I, Beclin1 and p62 was measured by western blot analysis. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. ***P < 0.001 vs. sham; ###P < 0.001 vs. SCI+DMSO. Eze, Ezetimibe; SCI, spinal cord injury; DMSO, dimethylsulfoxide.
Eze reduced the apoptosis in rats’ neurons after SCI
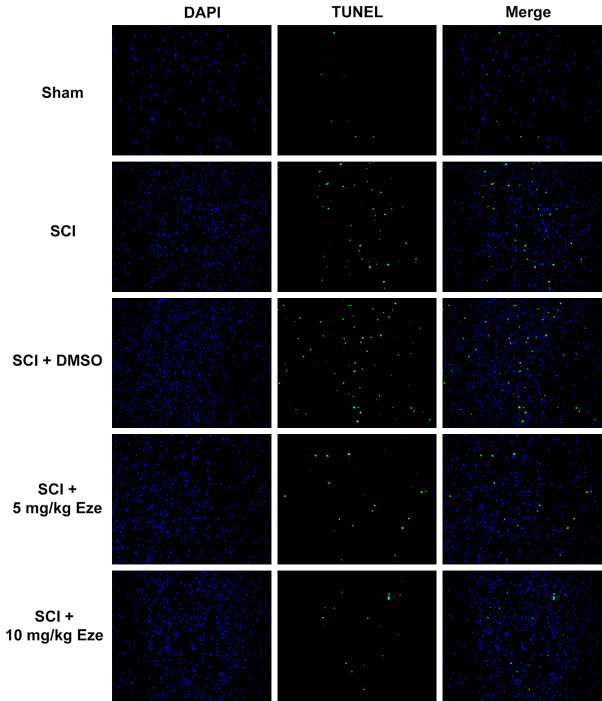
The effect of Eze on apoptosis of neurons after SCI induction was examined in the following experiments. The results of TUNEL staining indicated that the number of TUNEL-positive neurons was markedly elevated in SCI and SCI+DMSO groups as compared to the sham surgery group (Figure 4). Following treatment with Eze, apoptosis of cells was notably reduced and the results shown a certain level of dose-dependent efficacy. Subsequently, the expression of apoptosis-related proteins was detected using western blot analysis. As presented in Figure 5, the expression of Bax, activated caspase-3 and caspase-9 was notably enhanced whereas the level of Bcl-2 was decreased in both SCI and SCI+DMSO groups, in comparison to the sham group. Following administration with Eze, the expression levels of above-mentioned apoptosis-related proteins were markedly reversed. Collectively, these findings provided a clue that Eze reduced the apoptosis in rats’ neurons after SCI.
Figure 4.
Eze reduced the apoptosis in rats’ neurons after SCI. Apoptosis of rats’ neurons after SCI was detected using TUNEL assay. (magnification, 200×). Eze, Ezetimibe; SCI, spinal cord injury; TUNEL, Terminal dexynucleotidyl transferase-mediated dUTP nick end labeling.
Figure 5.
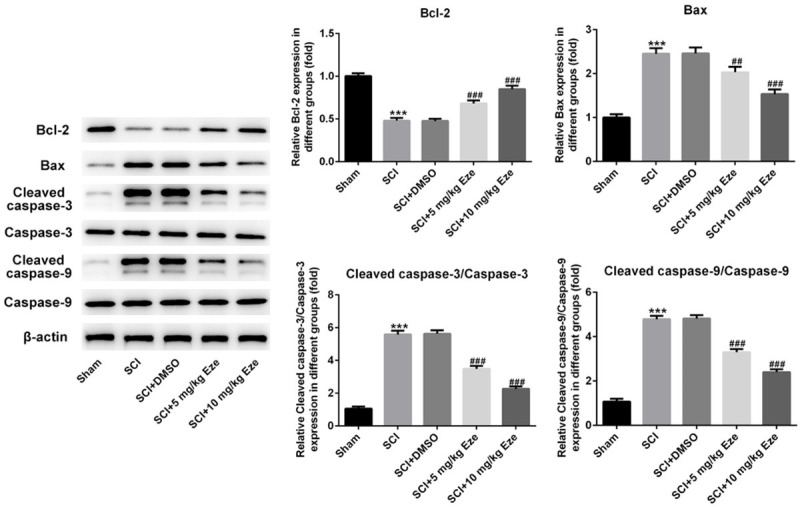
Eze affected the expression of apoptosis-associated proteins in spinal cord after SCI. The expression of Bcl-2, Bax, cleaved caspase-3 and cleaved caspase-9 was assessed by western blot analysis. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. ***P < 0.001 vs. sham; ##P < 0.01, ###P < 0.001 vs. SCI+DMSO. Eze, Ezetimibe; SCI, spinal cord injury; DMSO, dimethylsulfoxide.
Eze activated autophagy through inactivating PI3K/AKT/mTOR signaling in SCI rat model
In order to further probe the underlying mechanism of Eze in SCI, the expression of proteins in PI3K/AKT/mTOR signaling, a crucial pathway regulating autophagy, was evaluated using western blot analysis. Results of Figure 6 suggested that SCI induction significantly enhanced the expression of p-PI3K, p-AKT and p-mTOR compared with the sham group. Obviously reduced expression of above-mentioned PI3K/AKT/mTOR signaling proteins was observed after intervention with Eze. Higher dose of Eze presented a better inhibitory effect on these proteins. The above data revealed that Eze activated autophagy through restraining PI3K/AKT/mTOR signaling in SCI rat model.
Figure 6.
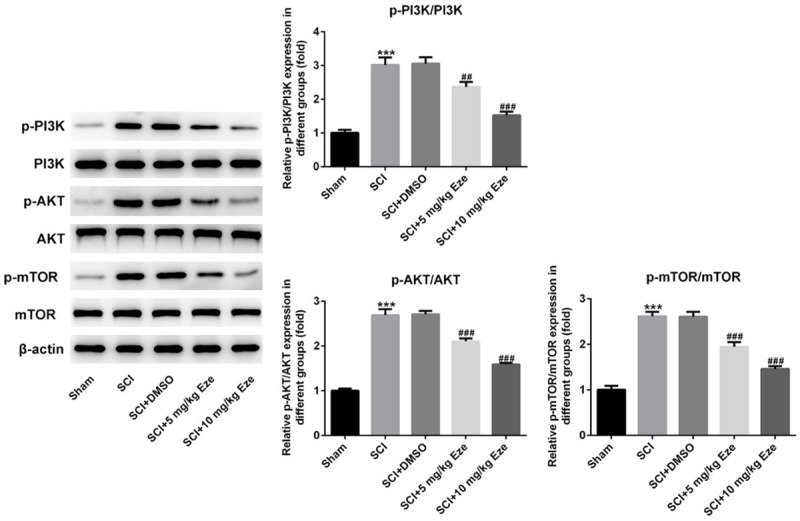
Eze inactivated the PI3K/AKT/mTOR signaling in SCI rat model. The expression of p-PI3K, p-AKT and p-mTOR was examined using western blot analysis. All results were obtained from at least three independent experiments. All numerical data were presented as the mean ± standard deviation. ***P < 0.001 vs. sham; ##P < 0.01, ###P < 0.001 vs. SCI+DMSO. Eze, Ezetimibe; SCI, spinal cord injury; p-PI3K, phosphp-PI3K; DMSO, dimethylsulfoxide.
Discussion
Acute SCI derives from direct or indirect damage to the spinal cord, with high morbidity and disability rate, bringing a great burden to families and society. The pathogenesis during SCI is complicated and has not been precisely illuminated. It has been well reported that a series of molecular and cellular events occur, including apoptosis, autophagy and inflammation, in the SCI development [15,16]. In the present study, a rat SCI model was established to explore the effects of Eze and the potential mechanism in SCI. We confirmed that Eze could protect against SCI by activating autophagy and repressing apoptosis through inhibition of PI3K/AKT/mTOR signaling pathway.
A growing body of literature has shown that apoptosis of neural cells is a main factor in neuronal loss and a primary difficulty in SCI treatment attributing to its critical role in physical and functional deficits [17]. Apoptosis is principally mediated by the caspase and Bcl-2 families. Autophagy is indispensable for normal neuronal homeostasis and its dysfunction in death of neuron [18]. Reports have demonstrated previously that neuronal autophagy exerts a neuroprotective effect in rats with SCI [19]. Moreover, it has been well reported that autophagy could attenuate neuronal damage and accelerate locomotor recovery via inhibiting apoptosis after SCI in rats [20]. LC3, is the most important and reliable indicator of autophagy induction in mammals. When autophagy is induced, LC3 can translocate to autophagosomal membranes from cytosol and convert from LC3I to LC3II [21]. Therefore, the ratio of LC3II/I is considered to be one symbol of autophagic induction. Beclin-1, a key regulator of autophagy, has been reported to induce autophagy by regulating PI3K signaling [22]. p62 can interact with LC3II and the accumulation of p62 is indicative of the autophagy inactivation [23]. Eze, an FDA-approved lipid-lowering agent, exhibits neuroprotective effects in rats with middle cerebral artery occlusion by attenuating neuronal apoptosis through autophagy activation [24]. Additionally, emerging evidence supports that Eze protects against steatohepatitis through activating autophagy and suppressing NLRP3 inflammasome [12]. Importantly, Eze has also been proved to restore neurological function and alleviate inflammatory responses in a rat SCI model by combination with Simvastatin [11]. Therefore, this study focused on the effects of Eze on autophagy and apoptosis following acute SCI. We demonstrated that Eze dramatically promoted autophagy and suppressed apoptosis coupled with expression changes of autophagy- and apoptosis-related proteins after SCI in rats.
It is generally well known that the PI3K/Akt/mTOR signaling participates in the monitor of autophagy, and activation of PI3K/Akt/mTOR signaling suppresses autophagy [25,26]. Studies have proven that melatonin promotes locomotor recovery in SCI model by activating autophagy and decreasing apoptosis via the PI3K/AKT/mTOR signaling pathway [27]. In addition, autophagy attenuates PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury [28]. In the present study, the expression of p-PI3K, p-AKT and p-mTOR was markedly downregulated after Eze innervation, in accordance with the previous study [29]. To sum up, above findings proved that Eze protected against SCI of rat by enhancing autophagy and decreasing apoptosis through inactivation of PI3K/AKT/mTOR signaling.
Taken together, from these results we conclude that Eze can improve functional and tissue recovery as well as neuronal damage in rats with SCI. Mechanically, Eze promotes autophagy and inhibits apoptosis through inactivation of PI3K/AKT/mTOR signaling. Our findings offer the theoretical basis of therapeutic effect of Eze in patients with SCI. The lack of studies of Eze in clinical patients with SCI are limitations of the present research and therefore, a comprehensive analysis is required in the future.
Acknowledgements
The present study was supported by the Fujian Provincial Health and Family Planning Youth Project (Grant No. 2018-1-44).
Disclosure of conflict of interest
None.
References
- 1.Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, Xia J, Xu H, Zhao Y, Xiao J, He Y, Ye Q. Effects of transplanted heparin-poloxamer hydrogel combining dental pulp stem cells and bFGF on spinal cord injury repair. Stem Cells Int. 2018;2018:2398521. doi: 10.1155/2018/2398521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28:202–210. doi: 10.1097/BSD.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 3.Hu W, Wang H, Liu Z, Liu Y, Wang R, Luo X, Huang Y. Neuroprotective effects of lycopene in spinal cord injury in rats via antioxidative and anti-apoptotic pathway. Neurosci Lett. 2017;642:107–112. doi: 10.1016/j.neulet.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr. 2017;75:387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 5.Shende P, Subedi M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed Pharmacother. 2017;91:693–706. doi: 10.1016/j.biopha.2017.04.126. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Dai W, Shi L, Teng H, Li X, Wang J, Geng W. Neuroprotection by paeoniflorin against nuclear factor Kappa B-induced neuroinflammation on spinal cord injury. Biomed Res Int. 2018;2018:9865403. doi: 10.1155/2018/9865403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang YJ, Li K, Yang CL, Huang K, Zhou J, Shi Y, Xie KG, Liu J. Bisperoxovanadium protects against spinal cord injury by regulating autophagy via activation of ERK1/2 signaling. Drug Des Devel Ther. 2019;13:513–521. doi: 10.2147/DDDT.S187878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varma AK, Das A, Wallace Gt, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Xiao H, Li J, Zhang J, Li Y, Zhang J, Wang X, Bai X, Tao K, Hu D, Guan H. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab Invest. 2018;98:1423–1437. doi: 10.1038/s41374-018-0099-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6:e1582. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang CL, Chen HJ, Liliang PC, Wang HK, Tsai YD, Cho CL, Lu K, Wang KW. Simvastatin and simvastatin-ezetimibe improve the neurological function and attenuate the endothelial inflammatory response after spinal cord injury in rat. Ann Clin Lab Sci. 2019;49:105–111. [PubMed] [Google Scholar]
- 12.Kim SH, Kim G, Han DH, Lee M, Kim I, Kim B, Kim KH, Song YM, Yoo JE, Wang HJ, Bae SH, Lee YH, Lee BW, Kang ES, Cha BS, Lee MS. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy. 2017;13:1767–1781. doi: 10.1080/15548627.2017.1356977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Peng L, Li J. The lipoxin A4 receptor agonist BML-111 alleviates inflammatory injury and oxidative stress in spinal cord injury. Med Sci Monit. 2020;26:e919883. doi: 10.12659/MSM.919883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cong Y, Wang CQ, Wang JY, Li HX, Li Q. NT-3 promotes oligodendrocyte proliferation and nerve function recovery after spinal cord injury by inhibiting autophagy pathway. J Surg Res. 2020;247:128–135. doi: 10.1016/j.jss.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Rong Y, Liu W, Zhou Z, Gong F, Bai J, Fan J, Li L, Luo Y, Zhou Z, Cai W. Harpagide inhibits neuronal apoptosis and promotes axonal regeneration after spinal cord injury in rats by activating the Wnt/beta-catenin signaling pathway. Brain Res Bull. 2019;148:91–99. doi: 10.1016/j.brainresbull.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhu N, Ruan JW, Yang XM, Huang Y, Jiang YT, Wang YC, Cai DY, Geng Y, Fang MR. Triptolide improves spinal cord injury by promoting autophagy and inhibiting apoptosis. Cell Biol Int. 2020;44:785–794. doi: 10.1002/cbin.11273. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Xia X, Zhu X, Cao J, Xu D, Ni Y, Liu Y, Yan S, Cheng X, Liu Y, Wang Y. Expression of SGTA correlates with neuronal apoptosis and reactive gliosis after spinal cord injury. Cell Tissue Res. 2014;358:277–288. doi: 10.1007/s00441-014-1946-1. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Wang F, Zhai X, Li XH, He XJ. Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen Res. 2018;13:2191–2199. doi: 10.4103/1673-5374.241473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Liu F, Zhang L, Cao Y, Shao Y, Wang X, Jiang X, Chen Z. Neuroserpin restores autophagy and promotes functional recovery after acute spinal cord injury in rats. Mol Med Rep. 2018;17:2957–2963. doi: 10.3892/mmr.2017.8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao J, Zheng K, Zhang X. Rosiglitazone exerts neuroprotective effects via the suppression of neuronal autophagy and apoptosis in the cortex following traumatic brain injury. Mol Med Rep. 2015;12:6591–6597. doi: 10.3892/mmr.2015.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Shi S, Wu B, Zhang J, Li Y, Wu X, Zhang J, Wang K, Zhao B, Cai W, Bai X, Hu D, Guan H. Autophagy protein LC3 regulates the fibrosis of hypertrophic scar by controlling Bcl-xL in dermal fibroblasts. Oncotarget. 2017;8:93757–93770. doi: 10.18632/oncotarget.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Y, Glover K, Su M, Sinha SC. Conformational flexibility of BECN1: Essential to its key role in autophagy and beyond. Protein Sci. 2016;25:1767–1785. doi: 10.1002/pro.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe F, Yone K, Kawabata N, Sakakima H, Matsuda F, Ishidou Y, Maeda S, Abematsu M, Komiya S, Setoguchi T. Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy. 2011;7:1462–1471. doi: 10.4161/auto.7.12.17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Li X, Matei N, McBride D, Tang J, Yan M, Zhang JH. Ezetimibe, a NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp Neurol. 2018;307:12–23. doi: 10.1016/j.expneurol.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Wang AL, Zhang JW, Li L, Wei JN, Li L, Chen HM, Han L, Lu CJ. PSORI-CM02 ameliorates psoriasis in vivo and in vitro by inducing autophagy via inhibition of the PI3K/Akt/mTOR pathway. Phytomedicine. 2019;64:153054. doi: 10.1016/j.phymed.2019.153054. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Zheng L, Liu X, Xing W, Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. 2018;12:2413–2421. doi: 10.2147/DDDT.S155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Guo Y, Fan Y, Tian H, Li K, Mei X. Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway. Neurochem Res. 2019;44:2007–2019. doi: 10.1007/s11064-019-02838-w. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Zhou L, Zheng X, Chen G, Pan R, Li J, Liu W. Autophagy protects against PI3K/Akt/mTOR-mediated apoptosis of spinal cord neurons after mechanical injury. Neurosci Lett. 2017;656:158–164. doi: 10.1016/j.neulet.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Yin H, Shen L, Xu C, Liu J. Lentivirus-mediated overexpression of miR-29a promotes axonal regeneration and functional recovery in experimental spinal cord injury via PI3K/Akt/mTOR pathway. Neurochem Res. 2018;43:2038–2046. doi: 10.1007/s11064-018-2625-5. [DOI] [PubMed] [Google Scholar]