Abstract
Indocyanine green (ICG) is an amphiphilic dye, which has been used as a diagnostic agent for decades. It is becoming increasingly utilized for the diagnosis and treatment of several diseases. Primary liver cancer is a common malignancy, particularly in China. We review the published literature describing how ICG plays increasingly important roles in the diagnosis, surgical planning and treatment of hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma, indocyanine green, diagnosis and treatment, nanoparticles, photodynamic therapy, photothermic therapy
Hepatocellular carcinoma (HCC) is a the fifth most common malignant tumor and the second most common cause of cancer death worldwide [1]. In 2015, there were 854,000 people living with HCC. Over 810,000 patients died from the disease [2]. In China, HCC is the fourth and fifth most common malignant tumor in males and females, respectively [3]. Although the modalities used in the treatment of HCC, including surgery, interventional therapy, biotherapy and ablation are improving, long-term survival outcomes remain poor. HCC is prone to developing recurrence and metastasis, resulting in poor prognosis, high mortality and short patient survival. Thus, it is extremely important to explore novel diagnostic and treatment options. In this review of the published literature, we describe how indocyanine green (ICG) plays increasingly important roles in the diagnosis, surgical planning and treatment of HCC.
ICG
ICG is an intravenously administered amphiphilic cyanamide dye consisting of two polar sulfonates and tetragonal ammonium groups. Protein-bound ICG can emit near-infrared light with a wavelength of 840 nm when stimulated by external light with a wavelength ranging from 750 to 810 nm. In 1954, it was approved by the United States Food and Drug Administration for clinical use in the measurement of cardiac output, assessment of liver function and inspection of the retina and choroidal vascular systems of the eye [4-6]. After intravenous injection of 0.1-0.3 mg/kg, ICG can be examined in a healthy subject in eight minutes [7]. There are no known negative effects of intravenous administration. The incidence of allergic reaction with its use is less than 1% [8]. In the 1970s, ICG with protein binding was found to have a strong absorptive capability in the near infrared light (NIR) spectra. Due to the contrasting low NIR spectral absorption of biological tissue, ICG is suitable for biological imaging using a contrasting signal-to-background ratio [9].
With their minimally invasive properties, photodynamic therapy (PDT) and photothermal therapy (PTT) [10,11] are emerging diagnostic and therapeutic modalities of light therapy [12]. PDT can treat tumors with low toxicity and a noninvasive nature [13]. In the treatment process, light energy is absorbed by a photosensitizer. Through an interaction between the agent and the surrounding oxygen-rich environment, electrons are elevated from the ground state to the singlet and subsequent triplet state [14,15]. The embodiment of PDT is the resultant production of reactive oxygen species (ROS), particularly monomer oxygen species (1O2) [16]. ROS act as therapeutic mediums with a luminescent effect, killing cancer cells [17]. PTT is another attractive antineoplastic treatment that converts light energy into heat energy with high efficiency, high selectivity and a low side effect profile [18,19] (Figure 1).
Figure 1.
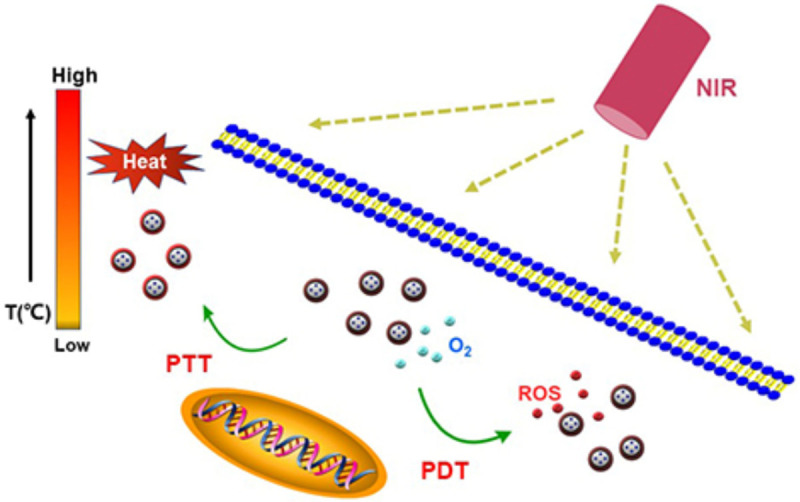
PDT and PTT treatment for tumor cells.
ICG in the diagnosis of HCC
Traditional methods of diagnosis of HCC include contrast enhanced ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI) [20,21]. Hypoechoic masses consistent with early stage HCC can be detected with a sensitivity of 63% using US [22]. The merits of US are its noninvasive and nonradiative properties; however, the drawbacks include limited visualization, large impedance in some patients and a variation in quality based on the operator’s skill level [23].
CT and MRI have also been widely used in HCC diagnosis. Enhanced MRI and CT are particularly useful in the detection of tumors less than 2 cm in size. Approximately 25%-30% of cases that are not visible on US can be detected using these modalities [21,23].
ICG is a hydrophilic organic anion with a high excretion rate and stability in hepatocytes [24]. The biliary excretion of ICG utilizes phospholipid microtubules and mixed lipid/bile salt micelles. ICG is rapidly absorbed in tissue using the anion transporting polypeptide 1B3 (OATP1B3) and sodium taurocholate-transporting polypeptide (NTCP) [25]. When stimulating light is applied, fluorescence appears. After a period of time, ICG is excreted into the biliary tract through multidrug resistance-associated protein 2 (MRP2), a key gene and protein involved in ICG metabolism [26] (Figure 2). ICG does not participate in the enterohepatic circulation. Therefore, fluorescence gradually weakens. ICG is acidophobic, with a good water solubility. As the hydrosolvent of ICG has no stability, ICG should no longer be present in circulation six to ten hours after dissolution [27].
Figure 2.
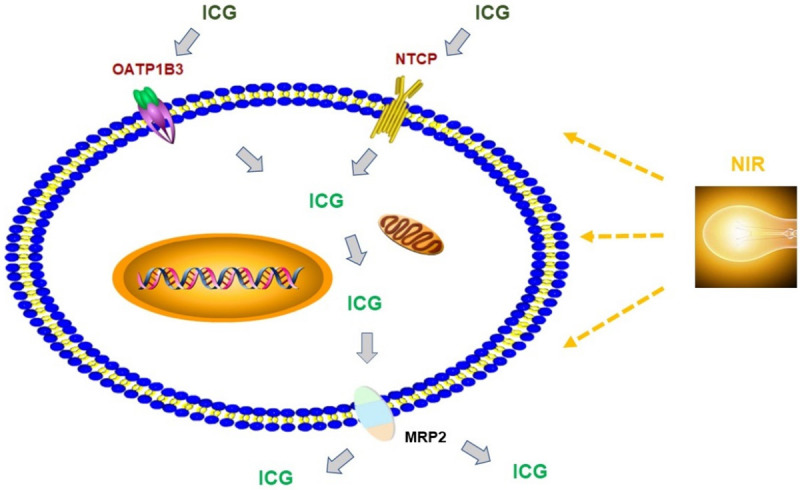
ICG is absorbed through OATP1B3 and NTCP in the liver cells. Once the cells are irrigated by NIR, ICG turns green; ICG is expelled from cells though MRP2.
There is a delay of ICG discharge in parts of a liver tumor or in cirrhotic nodules with impaired liver function, resulting in a delay of fluorescence regression [28]. A well differentiated HCC can be diagnosed by its strong and homogeneous fluorescence which is due to the ability of HCC cells to absorb ICG. A poorly differentiated HCC or metastatic liver tumor is surrounded by a circular fluorescence due to impaired metabolism of ICG by tumor cells, leading to the blockage of ICG release [28,29]. A clinical study, including 276 tumors in 170 HCC patients reported that ICG fluorescence occurred in 273 tumors. Thirty-five of these tumors were not detected prior to operation. Fourteen were found after liver resection. Twenty-one were found using ICG fluorescence in the gross resection specimens [30]. Thus, ICG can be used to diagnose small HCC at an early stage with the potential for a good cure rate [28,31].
The limited depth of NIR irradiation means ICG can only be used to detect tumors located in the superficial parts of the liver [32]. Also, liver tumors of less than 1 cm are difficult to detect. To overcome this, some researchers have attempted to detect small HCCs using a combination of photoacoustics and fluorescence. Au@liposome-ICG [33], ICG-loaded Au@SiO2 [34] and ICG-HAS-Au complex (ICG-human serum albumin-Au) [35] have been used in the early diagnosis of small HCCs either pre or intra-operatively (Table 1).
Table 1.
Characters of ICG and ICG-Au nano-particles for diagnosis
ICG evaluation of liver function
Methods for the assessment of liver function include static and dynamic tests. Static evaluation includes: serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels for the detection of hepatic parenchymal injury [36], serum lactate dehydrogenase [37] and plasma bilirubin concentrations for the assessment of synthetic activity [38], serum gamma-glutamyl transpeptidase (GGT) level to indicate cholestasis and serum bilirubin level to evaluate excretion [39]. Dynamic evaluation includes: ICG for clearance, galactose for elimination and lidocaine for metabolite formation [37].
ICG clearance was initially used to measure blood volume and cardiac output [40]. It is now widely used to assess liver function before resection and transplantation [41]. There are two indices for ICG assessment of liver function: 1) ICG plasma disappearance rate (ICGPDR) and 2) ICG retention rate at 15 min (ICGR15). ICGPDR measures the percentage decrease in ICG blood concentration from 100% (normal > 18%/min). ICGR15 measures the ratio of ICG blood concentration and the incipient concentration 15 min after the ICG injection (normal range, 0% to 10%) [40].
With a less adverse reaction rate, ICG fluorescence has the clinical advantages of safety and usability [42]. Thus, it is now used to assess liver function preoperatively. As assessment of liver function using ICGPDR is based on hepatic perfusion and hepatocyte metabolism, ICGPDR has also been used to estimate prognosis in chronic liver diseases and failure. The method of using ICG to evaluate liver function is based on the plasma clearance rate and plasma residual rate after intravenous administration of ICG [43]. The patient’s clearance is compared to the normal range of plasma clearance, which is between 18% and 24%/min [44]. ICGR15 correlates with Child’s grading and platelet count and interrelates with in-hospital mortality and long-term survival outcomes in patients with HCC [45].
ICG for surgical planning in liver cancer
Intraoperative diagnosis and localization of small HCCs is difficult, particularly for tumors without clear boundaries. The intraoperative application of ICG has improved the detection rate of these small lesions. It has also been used in the visualization of biliary tract anatomy [28] and mapping the lymphatic distribution [46]. The ICG fluorescence imaging system (FIS-ICG) was also used in the early 1970s for intraoperative retinography [47]. ICG is excreted into the biliary system making it a useful tool in hepatobiliary surgery [48]. A strong role in laparoscopic surgery is suggested because of the loss of tactile sensation to detect tumors [49]. FIS-ICG has been used in cholecystectomy, benign liver tumor operations and partial liver transplantation [50].
HCC and liver metastasis from colorectal cancer are the most common primary and secondary malignant tumors of the liver. ICG fluorescence in hepatectomy can help to define the edge of the tumor from the normal liver tissues prior to or during resection [51,52]. Studies have indicated that normal liver and tumor tissues can be distinguished by administering 7.5 mg of ICG. However, there has not been a consensus on the timing of administration [53,54]. Some researchers have shown that the time interval between injection and operation may even be longer after administration of ICG at a dosage of 0.5 mg/kg [55]. ICG secretion becomes dysfunctional once ICG enters the liver, leading to the development of fluorescence imaging. With current technology, fluorescence imaging cannot penetrate deeply enough to show tumors of more than 8 mm from the liver surface, limiting its use in deeper seated lesions [29].
Treatment of HCC by ICG nanosynthesis
Many nanoparticles have been developed for photoacoustic and fluorescence dual-mode imaging, including ICG liposomes [56], ICG liposome/gold nanoparticle hybrids [33] and ICG-loaded Au@SiO2 [34]. These could be used not only for pre-operative diagnosis, but also for intra-operation navigation.
The non-toxic characteristics of ICG have led researchers to also generate derivative compounds based on ICG to treat liver cancer. Kaneko et al. demonstrated after ICG uptake by HuH-7 and HepG2 HCC cell lines, followed by NIR irritation and photodynamic therapy, that growth of tumor cells was suppressed. Under microscopy, the nuclei of HCC cells became dense, the intercellular space increased and cell pyknosis followed [57]. In recent years, many nanoparticles have been developed for oncological treatment, including the various forms of nanoparticles for photothermal therapy (PTT), including carbon nanomaterials, organic NIR dyes and gold nanoparticles [58]. As ICG is a PTT agent with rapid degradation and clearance, nanomaterials with ICG have been increasingly used in malignant tumors, especially liver cancer.
Folic acid receptors (FR) are highly expressed on HCC cell membranes. As binding of folic acid (FA) to FR is highly effective, FA can be used to treat HCC [59]. Wang et al. generated FA-Janus Silver/Silica Nanoplatforms (FA-JNPs@ICG) and found FA-JNPs@ICG could especially kill HCC cells by FR-mediated endocytosis [58]. Hu et al. synthesized doxorubicin and indocyanine green@galactose-functionalized hydroxyethyl starch-polycaprolactone nanoparticles (DOX/ICG@Gal-HES-PCL NCs), which were used to kill liver cancer by PTT and chemotherapy based on asialoglycoprotein receptors (ASGPRs) endocytosis [60]. Inagaki et al. generated a conjugated compound of ICG with gemcitabine. This compound has the advantages of low toxicity to normal cells and superior anti-liver tumor action in HuH-7 and HepG2 cell lines, compared with gemcitabine alone [61] (Table 2). Recently, Huang et al. used a method of phosphatase-triggered self-assembly to form a tail to end structure of ICG which can be used to load drugs improving their biological characteristics [62].
Table 2.
Characters of ICG and ICG-Au NANO-particles for treatment
The above-mentioned ICG derivatives can potentially be used to treat liver cancer. Further development of these derivatives in liver cancer treatment requires more studies.
IR-780 and IR-808
IR-780, a photosensitizer both for PTT and PDT, is a small fluorescent molecule of heptamethylene cyanine. It has a similar structure to ICG with similar characteristics in tumor targeting and near-infrared light absorption [63]. IR-780 also shares the same process of hepatic metabolism and bile duct excretion [64].
IR-808, firstly synthesised by Chen et al., is an advanced cyanine with hydrophilic improvement and stability based on IR-708 [65]. It has an maximum absorption band at 808 nm. Chen et al. used murine models and demonstrated that, after caudal vein injection, IR-808 was first stored in the liver and heart. After 48 hours, almost all the IR-808 was expelled from the body. In the xenograft tumor modle, fluorescence could be still detected at 10 days after injecting IR-808 alone [66]. IR-808 could be used as oncotarget ligands to load anticancer drugs for tumor imaging and treatment [67] (Figure 3).
Figure 3.
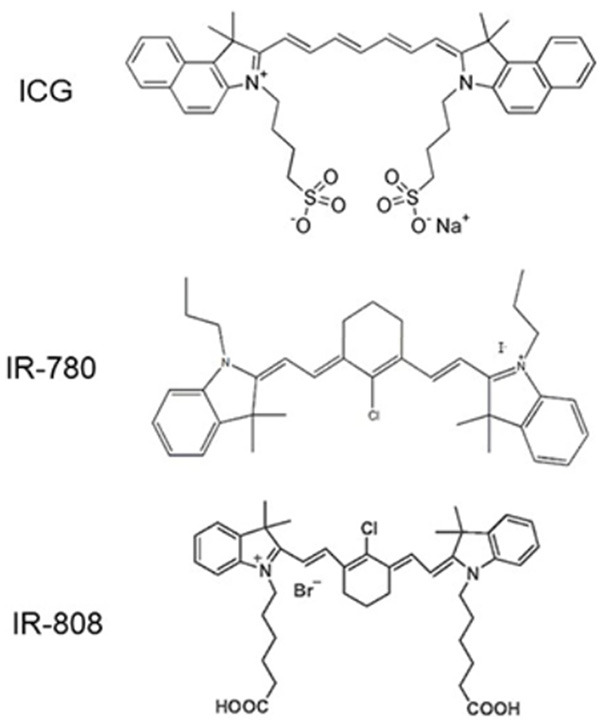
Chemical formula of ICG, IR-780 and IR-808.
Conclusions
ICG is a safe, increasingly utilized diagnostic reagent that can be used to outline HCC in situ and evaluate liver function. It plays a vital role in HCC surgical mapping. Some nanoparticles that have the potential to treat HCC have been fabricated based on ICG. Future research and subsquent development of nanometerials to specifically treat HCC are needed.
Acknowledgements
The National Natural Science Foundation of China, Grant/Award Numbers: 81701965, 81872255, 62041101; the Key Medical Talents Fund of Jiangsu Province, Grant/Award Numbers: 2016KJQWZDRC-03; the Natural Science Foundation of Liaoning Province, Grant/Award Number: 20180550116, 2019-MS-069; Southeast university - China pharmaceutical university Cooperative research project, Grant/Award Number: 2242019K3DZ06; the 333 High-level Talents Training Project of Jiangsu Province, Grant/Award Number: BRA2016508; and the Six Talents Peak Foundation of Jiangsu Province Project, Grant/Award Number: WSW041.
Disclosure of conflict of interest
None.
References
- 1.Lin Q, Huang X, Zhong C, Luo T, Zeng X, Chen S. Improved survival with radiotherapy in hepatocellular carcinoma with major vascular invasion: a propensity-matched analysis of surveillance, epidemiology, and end results database. Cancer Med. 2019;8:515–526. doi: 10.1002/cam4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Liver Cancer Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Furst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topor-Madry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Jing R, Zhou X, Zhao J, Wei Y, Zuo B, You A, Rao Q, Gao X, Yang R, Chen L, Lu Z, Zhou Q, Zhang N, Yin H. Fluorescent peptide highlights micronodules in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2018;68:1391–1411. doi: 10.1002/hep.29829. [DOI] [PubMed] [Google Scholar]
- 5.Lieto E, Galizia G, Cardella F, Mabilia A, Basile N, Castellano P, Orditura M, Auricchio A. Indocyanine green fluorescence imaging-guided surgery in primary and metastatic liver tumors. Surg Innov. 2018;25:62–68. doi: 10.1177/1553350617751451. [DOI] [PubMed] [Google Scholar]
- 6.Keller DS, Ishizawa T, Cohen R, Chand M. Indocyanine green fluorescence imaging in colorectal surgery: overview, applications, and future directions. Lancet Gastroenterol Hepatol. 2017;2:757–766. doi: 10.1016/S2468-1253(17)30216-9. [DOI] [PubMed] [Google Scholar]
- 7.Sakka SG. Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J Clin Monit Comput. 2018;32:787–796. doi: 10.1007/s10877-017-0073-4. [DOI] [PubMed] [Google Scholar]
- 8.Sugie T, Kinoshita T, Masuda N, Sawada T, Yamauchi A, Kuroi K, Taguchi T, Bando H, Yamashiro H, Lee T, Shinkura N, Kato H, Ikeda T, Yoshimura K, Ueyama H, Toi M. Evaluation of the clinical utility of the icg fluorescence method compared with the radioisotope method for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol. 2016;23:44–50. doi: 10.1245/s10434-015-4809-4. [DOI] [PubMed] [Google Scholar]
- 9.Miao Q, Pu K. Organic semiconducting agents for deep-tissue molecular imaging: second near-infrared fluorescence, self-luminescence, and photoacoustics. Adv Mater. 2018;30:e1801778. doi: 10.1002/adma.201801778. [DOI] [PubMed] [Google Scholar]
- 10.Fan W, Bu W, Shi J. On the latest three-stage development of nanomedicines based on upconversion nanoparticles. Adv Mater. 2016;28:3987–4011. doi: 10.1002/adma.201505678. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, You Q, Pang X, Tan X, Wang J, Liu L, Guo F, Tan F, Li N. A photoresponsive and rod-shape nanocarrier: single wavelength of light triggered photothermal and photodynamic therapy based on AuNRs-Capped & Ce6-Doped mesoporous silica nanorods. Biomaterials. 2017;122:188–200. doi: 10.1016/j.biomaterials.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Zou L, Wang H, He B, Zeng L, Tan T, Cao H, He X, Zhang Z, Guo S, Li Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics. 2016;6:762–772. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao S, Wang G, Qin Z, Wang X, Zhao G, Ma Q, Zhu L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials. 2017;112:324–335. doi: 10.1016/j.biomaterials.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Shi L, Hu F, Duan Y, Wu W, Dong J, Meng X, Zhu X, Liu B. Hybrid nanospheres to overcome hypoxia and intrinsic oxidative resistance for enhanced photodynamic therapy. ACS Nano. 2020;14:2183–2190. doi: 10.1021/acsnano.9b09032. [DOI] [PubMed] [Google Scholar]
- 15.Xie Y, Zheng W, Jiang X. Near-infrared light-activated phototherapy by gold nanoclusters for dispersing biofilms. ACS Appl Mater Interfaces. 2020;12:9041–9049. doi: 10.1021/acsami.9b21777. [DOI] [PubMed] [Google Scholar]
- 16.Fu X, Yang Z, Deng T, Chen J, Wen Y, Fu X, Zhou L, Zhu Z, Yu C. A natural polysaccharide mediated MOF-based Ce6 delivery system with improved biological properties for photodynamic therapy. J Mater Chem B. 2020;8:1481–1488. doi: 10.1039/c9tb02482d. [DOI] [PubMed] [Google Scholar]
- 17.Wan SS, Zeng JY, Cheng H, Zhang XZ. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials. 2018;185:51–62. doi: 10.1016/j.biomaterials.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Bacakova L, Pajorova J, Tomkova M, Matejka R, Broz A, Stepanovska J, Prazak S, Skogberg A, Siljander S, Kallio P. Applications of nanocellulose/nanocarbon composites: focus on biotechnology and medicine. Nanomaterials (Basel) 2020;10:196. doi: 10.3390/nano10020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Borg RE, Dow LP, Pruitt BL, Chen IA. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc Natl Acad Sci U S A. 2020;117:1951–1961. doi: 10.1073/pnas.1913234117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, Yang JD. Detection of circulating tumor cells and their implications as a novel biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2020 doi: 10.1002/hep.31165. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganeshan D, Pickhardt PJ, Morani AC, Javadi S, Lubner MG, Elmohr MM, Duran C, Elsayes KM. Hepatic hemangioendothelioma: CT, MR, and FDG-PET-CT in 67 patients-a bi-institutional comprehensive cancer center review. Eur Radiol. 2020;30:2435–2442. doi: 10.1007/s00330-019-06637-3. [DOI] [PubMed] [Google Scholar]
- 22.Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: an evidence-based approach. World J Gastroenterol. 2019;25:1550–1559. doi: 10.3748/wjg.v25.i13.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XH, Liang Q, Chen TW, Wang J, Zhang XM. Diagnostic value of imaging examinations in patients with primary hepatocellular carcinoma. World J Clin Cases. 2018;6:242–248. doi: 10.12998/wjcc.v6.i9.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue X, Fang T, Yin L, Jiang J, He Y, Dai Y, Wang D. Multistage delivery of CDs-DOX/ICG-loaded liposome for highly penetration and effective chemo-photothermal combination therapy. Drug Deliv. 2018;25:1826–1839. doi: 10.1080/10717544.2018.1482975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu MR, Huang YY, Hsiao JK. Use of Indocyanine Green (ICG), a medical near infrared dye, for enhanced fluorescent imaging-comparison of organic anion transporting polypeptide 1B3 (OATP1B3) and sodium-taurocholate cotransporting polypeptide (NTCP) reporter genes. Molecules. 2019;24:2295. doi: 10.3390/molecules24122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He K, Hong X, Chi C, Cai C, Wang K, Li P, Liu X, Li J, Shan H, Tian J. A new method of near-infrared fluorescence image-guided hepatectomy for patients with hepatolithiasis: a randomized controlled trial. Surg Endosc. 2020 doi: 10.1007/s00464-019-07290-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Alander JT, Kaartinen I, Laakso A, Patila T, Spillmann T, Tuchin VV, Venermo M, Valisuo P. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YM, Shi R, Hou JC, Liu ZR, Cui ZL, Li Y, Wu D, Shi Y, Shen ZY. Liver tumor boundaries identified intraoperatively using real-time indocyanine green fluorescence imaging. J Cancer Res Clin Oncol. 2017;143:51–58. doi: 10.1007/s00432-016-2267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakaseko Y, Ishizawa T, Saiura A. Fluorescence-guided surgery for liver tumors. J Surg Oncol. 2018;118:324–331. doi: 10.1002/jso.25128. [DOI] [PubMed] [Google Scholar]
- 30.Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M, Kokudo N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–2504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 31.Huang SW, Ou JJ, Wong HP. The use of indocyanine green imaging technique in patient with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2018;3:95. doi: 10.21037/tgh.2018.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Torres VC, Tichauer KM. Noninvasive detection of cancer spread to lymph nodes: a review of molecular imaging principles and protocols. J Surg Oncol. 2018;118:301–314. doi: 10.1002/jso.25124. [DOI] [PubMed] [Google Scholar]
- 33.Guan T, Shang W, Li H, Yang X, Fang C, Tian J, Wang K. From detection to resection: photoacoustic tomography and surgery guidance with indocyanine green loaded gold nanorod@liposome core-shell nanoparticles in liver cancer. Bioconjug Chem. 2017;28:1221–1228. doi: 10.1021/acs.bioconjchem.7b00065. [DOI] [PubMed] [Google Scholar]
- 34.Peng D, Du Y, Shi Y, Mao D, Jia X, Li H, Zhu Y, Wang K, Tian J. Precise diagnosis in different scenarios using photoacoustic and fluorescence imaging with dual-modality nanoparticles. Nanoscale. 2016;8:14480–14488. doi: 10.1039/c6nr03809c. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Lakowicz JR. A superior bright NIR luminescent nanoparticle preparation and indicating calcium signaling detection in cells and small animals. Cell Biosci. 2018;8:37. doi: 10.1186/s13578-018-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27–36. doi: 10.1097/SLA.0b013e31825d5d47. [DOI] [PubMed] [Google Scholar]
- 37.De Gasperi A, Mazza E, Prosperi M. Indocyanine green kinetics to assess liver function: ready for a clinical dynamic assessment in major liver surgery? World J Hepatol. 2016;8:355–367. doi: 10.4254/wjh.v8.i7.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabbia D, Roverso M, Guido M, Sacchi D, Scaffidi M, Carrara M, Orso G, Russo FP, Floreani A, Bogialli S, De Martin S. Western diet-induced metabolic alterations affect circulating markers of liver function before the development of steatosis. Nutrients. 2019;11:1602. doi: 10.3390/nu11071602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang CH, Ni XC, Chen BY, Qiu SJ, Zhu YM, Luo M. Combined preoperative albumin-bilirubin (ALBI) and serum gamma-glutamyl transpeptidase (GGT) predicts the outcome of hepatocellular carcinoma patients following hepatic resection. J Cancer. 2019;10:4836–4845. doi: 10.7150/jca.33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vos JJ, Wietasch JK, Absalom AR, Hendriks HG, Scheeren TW. Green light for liver function monitoring using indocyanine green? An overview of current clinical applications. Anaesthesia. 2014;69:1364–1376. doi: 10.1111/anae.12755. [DOI] [PubMed] [Google Scholar]
- 41.Kokudo T, Hasegawa K, Shirata C, Tanimoto M, Ishizawa T, Kaneko J, Akamatsu N, Arita J, Demartines N, Uldry E, Kokudo N, Halkic N. Assessment of preoperative liver function for surgical decision making in patients with hepatocellular carcinoma. Liver Cancer. 2019;8:447–456. doi: 10.1159/000501368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. 2017;31:1836–1840. doi: 10.1007/s00464-016-5181-6. [DOI] [PubMed] [Google Scholar]
- 43.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med. 2016;35:49–57. doi: 10.1016/j.accpm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Yang R, Hou M, Gao Y, Lu S, Zhang L, Xu Z, Li CM, Kang Y, Xue P. Biomineralization-inspired crystallization of manganese oxide on silk fibroin nanoparticles for in vivo MR/fluorescence imaging-assisted tri-modal therapy of cancer. Theranostics. 2019;9:6314–6333. doi: 10.7150/thno.36252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Au KP, Chan SC, Chok KS, Chan AC, Cheung TT, Ng KK, Lo CM. Child-Pugh parameters and platelet count as an alternative to ICG test for assessing liver function for major hepatectomy. HPB Surg. 2017;2017:2948030. doi: 10.1155/2017/2948030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraft JC, Treuting PM, Ho RJY. Indocyanine green nanoparticles undergo selective lymphatic uptake, distribution and retention and enable detailed mapping of lymph vessels, nodes and abnormalities. J Drug Target. 2018;26:494–504. doi: 10.1080/1061186X.2018.1433681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norat P, Soldozy S, Elsarrag M, Sokolowski J, Yagmurlu K, Park MS, Tvrdik P, Kalani MYS. Application of indocyanine green videoangiography in aneurysm surgery: evidence, techniques, practical tips. Front Surg. 2019;6:34. doi: 10.3389/fsurg.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alfano MS, Molfino S, Benedicenti S, Molteni B, Porsio P, Arici E, Gheza F, Botticini M, Portolani N, Baiocchi GL. Intraoperative ICG-based imaging of liver neoplasms: a simple yet powerful tool. Preliminary results. Surg Endosc. 2019;33:126–134. doi: 10.1007/s00464-018-6282-1. [DOI] [PubMed] [Google Scholar]
- 49.Kudo H, Ishizawa T, Tani K, Harada N, Ichida A, Shimizu A, Kaneko J, Aoki T, Sakamoto Y, Sugawara Y, Hasegawa K, Kokudo N. Visualization of subcapsular hepatic malignancy by indocyanine-green fluorescence imaging during laparoscopic hepatectomy. Surg Endosc. 2014;28:2504–2508. doi: 10.1007/s00464-014-3468-z. [DOI] [PubMed] [Google Scholar]
- 50.Majlesara A, Golriz M, Hafezi M, Saffari A, Stenau E, Maier-Hein L, Muller-Stich BP, Mehrabi A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn Ther. 2017;17:208–215. doi: 10.1016/j.pdpdt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, Fard N, Raisi H, Edalatpour A, Mehrabi A. Small for Size and Flow (SFSF) syndrome: an alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol. 2016;40:267–275. doi: 10.1016/j.clinre.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392. doi: 10.3748/wjg.v20.i39.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi H, Zaidi N, Berber E. An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumorss. J Surg Oncol. 2016;114:625–629. doi: 10.1002/jso.24363. [DOI] [PubMed] [Google Scholar]
- 54.Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, Frangioni JV, van de Velde CJ, Swijnenburg RJ, Vahrmeijer AL. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci. 2012;19:626–637. doi: 10.1007/s00534-012-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossi G, Tarasconi A, Baiocchi G, De’ Angelis GL, Gaiani F, Di Mario F, Catena F, Dalla Valle R. Fluorescence guided surgery in liver tumors: applications and advantages. Acta Biomed. 2018;89:135–140. doi: 10.23750/abm.v89i9-S.7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beziere N, Lozano N, Nunes A, Salichs J, Queiros D, Kostarelos K, Ntziachristos V. Dynamic imaging of PEGylated indocyanine green (ICG) liposomes within the tumor microenvironment using multi-spectral optoacoustic tomography (MSOT) Biomaterials. 2015;37:415–424. doi: 10.1016/j.biomaterials.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Kaneko J, Inagaki Y, Ishizawa T, Gao J, Tang W, Aoki T, Sakamoto Y, Hasegawa K, Sugawara Y, Kokudo N. Photodynamic therapy for human hepatoma-cell-line tumors utilizing biliary excretion properties of indocyanine green. J Gastroenterol. 2014;49:110–116. doi: 10.1007/s00535-013-0775-4. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Chang Z, Lu M, Shao D, Yue J, Yang D, Li M, Dong WF. Janus silver/silica nanoplatforms for light-activated liver cancer chemo/photothermal therapy. ACS Appl Mater Interfaces. 2017;9:30306–30317. doi: 10.1021/acsami.7b06446. [DOI] [PubMed] [Google Scholar]
- 59.Saroj S, Rajput SJ. Etoposide encased folic acid adorned mesoporous silica nanoparticles as potent nanovehicles for enhanced prostate cancer therapy: synthesis, characterization, cellular uptake and biodistribution. Artif Cells Nanomed Biotechnol. 2018;46:S1115–S1130. doi: 10.1080/21691401.2018.1533843. [DOI] [PubMed] [Google Scholar]
- 60.Hu H, Xiao C, Wu H, Li Y, Zhou Q, Tang Y, Yu C, Yang X, Li Z. Nanocolloidosomes with selective drug release for active tumor-targeted imaging-guided photothermal/chemo combination therapy. ACS Appl Mater Interfaces. 2017;9:42225–42238. doi: 10.1021/acsami.7b14796. [DOI] [PubMed] [Google Scholar]
- 61.Inagaki Y, Kokudo T, Kamiya M, Uno SN, Sato M, Kaneko J, Kokudo N, Urano Y, Hasegawa K. A novel liver-specific fluorescent anti-cancer drug delivery system using indocyanine green. Sci Rep. 2019;9:3044. doi: 10.1038/s41598-019-39269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang P, Gao Y, Lin J, Hu H, Liao HS, Yan X, Tang Y, Jin A, Song J, Niu G, Zhang G, Horkay F, Chen X. Tumor-specific formation of enzyme-instructed supramolecular self-assemblies as cancer theranostics. ACS Nano. 2015;9:9517–9527. doi: 10.1021/acsnano.5b03874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang D, Zhang S, Zhang T, Wan G, Chen B, Xiong Q, Zhang J, Zhang W, Wang Y. Pullulan-coated phospholipid and Pluronic F68 complex nanoparticles for carrying IR780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int J Nanomedicine. 2017;12:8649–8670. doi: 10.2147/IJN.S147591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uthaman S, Mathew AP, Park HJ, Lee BI, Kim HS, Huh KM, Park IK. IR 780-loaded hyaluronic acid micelles for enhanced tumor-targeted photothermal therapy. Carbohydr Polym. 2018;181:1–9. doi: 10.1016/j.carbpol.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Gulzar A, Liu Y, Bi H, Gai S, Liu B, Yang D, He F, Yang P. Integration of IR-808 sensitized upconversion nanostructure and MoS2 nanosheet for 808 nm NIR light triggered phototherapy and bioimaging. Small. 2017;13 doi: 10.1002/smll.201701841. [DOI] [PubMed] [Google Scholar]
- 66.Tan X, Luo S, Wang D, Su Y, Cheng T, Shi C. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials. 2012;33:2230–2239. doi: 10.1016/j.biomaterials.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 67.Luo S, Yang Z, Tan X, Wang Y, Zeng Y, Wang Y, Li C, Li R, Shi C. Multifunctional photosensitizer grafted on polyethylene glycol and polyethylenimine dual-functionalized nanographene oxide for cancer-targeted near-infrared imaging and synergistic phototherapy. ACS Appl Mater Interfaces. 2016;8:17176–17186. doi: 10.1021/acsami.6b05383. [DOI] [PubMed] [Google Scholar]


