Abstract
Chondrocytes from microtia patients are a valuable cell source for the tissue-engineering of auricles. However, dedifferentiation of microtia chondrocytes remains an obstacle for clinical translation. Strategies, such as three-dimensional (3D) culture systems, and the use of chondrogenic growth factors, have successfully induced redifferentiation of dedifferentiated chondrocytes from healthy individuals. However, it remains unknown whether these strategies are similarly effective for microtia patient-derived chondrocytes, which may carry genomic defects. To address this issue, dedifferentiated microtia chondrocytes (DMCs) were cultured in a 3D chondrogenic culture system for 4-8 weeks to investigate their redifferentiated properties and to generate redifferentiated microtia chondrocytes (RMCs). To predict the degree and course of redifferentiation, RMCs at different time points were harvested and examined for cell morphology, cell proliferation, type II collagen expression at passaging, and chondrogenic capacity. We show that a 3D chondrogenic culture system can effectively induce DMCs to become redifferentiated, functional chondrocytes, enabling them to regenerate mature cartilage. Furthermore, RMCs achieved their full original function after culture in the chondrogenic culture system for 6-8 weeks. Interestingly, redifferentiation of microtia chondrocytes exhibited a time-dependent trend. Although the primary mechanism by which the 3D chondrogenic culture system regulated the transition of DMCs into RMCs remains unknown, the current study provides deeper insight into microtia chondrocytes and promotes clinical translation of tissue-engineered auricles.
Keywords: Microtia chondrocytes, tissue engineering, dedifferentiation, redifferentiation, 3D chondrogenic culture system
Introduction
Microtia is a congenital abnormality of the external ear with a prevalence rate of 0.83 to 17.4 per 10,000 live births. Patients with microtia suffer psychologically and have a financial disadvantage [1,2]. The most common treatment for severe damage of the auricle is ear reconstruction with autologous costal cartilage grafts, which can result in severe donor site morbidity of rib cartilage and a high risk of thoracic deformation [3,4]. Tissue engineering of auricles, which can exactly replicate patient-specific auricles, provides a promising approach for the treatment of patients with microtia. In recent decades, many important advances have been made in tissue engineering technologies, including clinical translation of engineered bone, cartilage, skin, and bladder [5-7]. Several groups have demonstrated the feasibility of constructing three dimensional (3D) cartilaginous tissue using tissue engineering techniques [8-10]. Cell sources applied in previous studies were mainly obtained from normal healthy cartilage, which is inevitably associated with impairment of the donor site. For microtia patients, the remnant auricular cartilage might be an ideal cell source because healthy rib cartilage would not be needed and it may regenerate the desired cartilage tissue with similar function to the native auricular cartilage. Many researchers have successfully obtained sufficient numbers of microtia chondrocytes for tissue engineering through in vitro expansion and subculture. More importantly, Kamil et al. [11], Yanaga et al. [12], and Zhang et al. [13] reported that microtia chondrocytes can generate robust cartilage tissue with similar anatomical features, cartilage-specific composition and mechanical properties as auricular cartilage. These results indicate that microtia chondrocytes are candidates for clinical application.
Unfortunately, microtia chondrocytes usually lose their phenotype (dedifferentiate), become fibroblast-like chondrocytes, and are unable to form cartilage tissue during subculture [13]. Therefore, obtaining a sufficient number of cells with robust chondrogenic ability from microtia chondrocytes remains a primary obstacle for engineering normally sized human ear-shaped cartilage (approximately 150 × 106 cells are needed according to our previous studies). Notably, dedifferentiation is frequently observed in healthy normal chondrocytes [14-16]. To functionally reverse the dedifferentiation of chondrocytes, several strategies have been reported to successfully prevent the dedifferentiation process or to induce redifferentiation of dedifferentiated chondrocytes. Indeed, numerous investigations have demonstrated that 3D culture systems [17,18], growth factor regulation [19-21], and varying the density of cultured cells can reverse the dedifferentiation phenotype and lead to redifferentiation of dedifferentiated chondrocytes [22,23]. Thus, generating sufficient quantities of functional chondrocytes might be enabled by these redifferentiation regulatory systems. However, most reports are based on healthy normal chondrocytes (such as articular, costal, and auricular), whereas little is known about microtia chondrocytes.
Microtia is a congenital disorder; therefore, microtia chondrocytes may carry a genomic defect and/or be unable to redifferentiate [24-26], thereby limiting their clinical application. It is therefore important to clarify whether dedifferentiated microtia chondrocytes (DMCs) can regain chondrogenic function and regenerate mature cartilage tissue after modulation in a 3D chondrogenic culture system (most commonly used for tissue engineered cartilage), and whether these DMCs can regain their original phenotype. Moreover, characterizing the redifferentiation and dedifferentiation processes of microtia chondrocytes is also important.
To address these issues, we harvested microtia cartilage from microtia patients (n = 3) for chondrocyte isolation. Type II collagen (COL II) protein expression, and mRNA levels of collagen type II (COL 2A1) and aggrecan were investigated to evaluate the dedifferentiation process of native microtia chondrocytes (NMCs) during monolayer subculture. Then, completely dedifferentiated cells (passage five, P5) were harvested and cultured in a 3D chondrogenic culture system for 4-8 weeks to generate redifferentiated microtia chondrocytes (RMCs).
Simultaneously, to evaluate the redifferentiation process and the degree of RMC redifferentiation, these chondrocytes were evaluated for cell morphology, cell proliferation, and COL II expression, as well as chondrogenic capacity during passaging. Their original chondrocytes were employed as a native control group. This study focused on the key issue of phenotypic redifferentiation of DMCs and provides detailed insights and guidance for clinical application of microtia chondrocytes.
Materials and methods
General experimental design
This study was conducted in three stages to evaluate the redifferentiation potential and dedifferentiation/redifferentiation processes of microtia chondrocytes (Figure 1). First, NMCs were passaged five times to enable the harvest of completely dedifferentiated chondrocytes (P5 DMC group). Next, a 3D chondrogenic culture system was employed to regulate redifferentiation of these DMCs for 4-8 weeks. The resulting RMCs and their original NMCs were harvested to evaluate their characteristics, including cell morphology, proliferation, expression of COL II, and chondrogenic properties.
Figure 1.
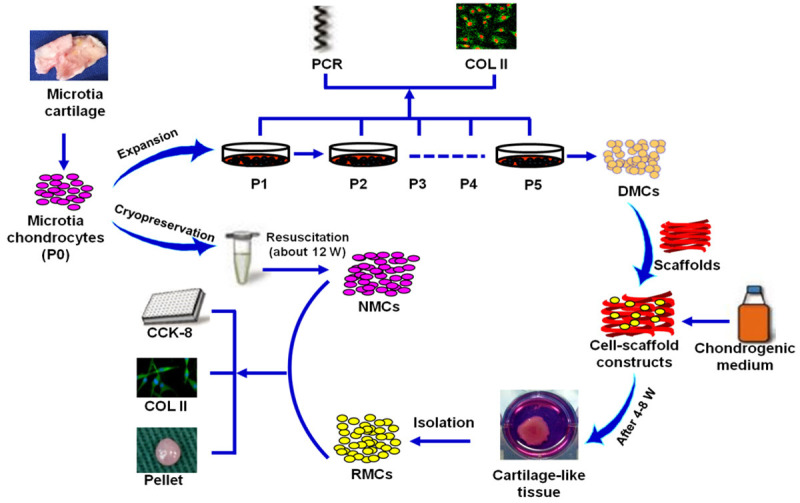
Overview of the experimental design. DMCs: dedifferentiated microtia chondrocytes; NMCs: native microtia chondrocytes; RMCs: redifferentiated microtia chondrocytes; COL II: type II collagen.
Isolation, culture, expansion and cryopreservation of microtia chondrocytes
The acquisition of human cells and all experimental protocols were approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine (Shanghai, P. R. China). Informed consent was obtained from all participants. Microtia auricular cartilage specimens were obtained from three microtia patients (9-12 years of age) attending the Eye & ENT Hospital of Fudan University (Shanghai, P.R. China). Cartilage tissue in each specimen was dissected to remove fibrous tissue. Then, to isolate chondrocytes, cartilage specimens were minced and digested with 0.15% collagenase (Sigma-Aldrich, St. Louis, MO, USA) as described previously [13,27]. After cell quantification, half of the primary cells were cryopreservated in liquid nitrogen for later experiments, and half were cultured. Cells were cultured and expanded in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone Laboratories, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories, Victoria, Australia, regular medium). Chondrocytes at passages 1-5 (P1-P5) were used for the following experiments.
Fluorescent staining and transmission electron microscopy analysis of NMCs
NMCs (P1-P5) were fixed and washed with phosphate-buffered saline (PBS) for fluorescent staining. A mouse anti-human COL II monoclonal antibody (1:200 in PBS; Santa Cruz Biotechnology, Dallas, TX, USA) and a goat anti-mouse IgG conjugated to Alexa Fluor 488 (A11001, Invitrogen, Carlsbad, CA, USA) was used to detect COL II in NMCs. Propidium iodide was used to stain nuclei as previously described [13]. For transmission electron microscopy (TEM) analysis, dedifferentiated chondrocytes (P5) and non-differentiated chondrocytes (P1) were collected and fixed in 2.5% glutaraldehyde for 24 h and then in 1% osmic acid for 2 h. Samples were then dehydrated in ethanol and embedded in epoxy resin, and ultrathin sections were then cut. Specimens were stained with 0.1% lead citrate and 10% uranyl acetate, and examined using a transmission electron microscope (100CX II, JEOL, Peabody, MA, USA).
Real-time PCR analysis of NMCs
Microtia chondrocytes at different passages (P1-P5; 2 × 106 cells for each passage) were collected, and then RNA was extracted. Total RNA was reverse transcribed into single-stranded cDNA as previously described [13,28]. Real-time PCR was performed using a continuous fluorescence detector (MX3000P, Stratagene, San Diego, CA, USA) and a SYBR green kit (SYBR Green PCR master mix; Applied Biosystems, Foster City, CA, USA). To normalize mRNA levels, GAPDH was quantified as an internal control. Forward and reverse primer pairs are listed in Supplementary Table 1.
Chondrogenic redifferentiation of DMCs
Chondrogenic redifferentiation was performed using P5 microtia chondrocytes from three individuals, which were completely dedifferentiated. A 3D chondrogenic culture system including polyglycolic acid/polylactic acid (PGA/PLA) scaffolds and chondrogenic medium was employed to regulate DMCs. Briefly, PGA/PLA scaffolds were prepare as previously reported [13,29]. DMCs were collected, seeded into PGA/PLA scaffolds (60 × 106 cells per 0.1 mL in each scaffold), and cultured in chondrogenic medium [DMEM supplemented with 10 ng/mL transforming growth factor β1 (TGFβ1, HumanZyme, Chicago, IL, USA), 100 ng/mL insulin-like growth factor 1 (IGF1, R&D Systems, Minneapolis, MN, USA), 40 ng/mL dexamethasone (Sigma-Aldrich), insulin-transferrin-selenium (ITS, R&D Systems, Minneapolis, MN, USA)] for 4-8 weeks.
Histological and biochemical evaluation of chondrocyte-PGA/PLA constructs
After 4, 6, and 8 weeks of chondrogenic culture, the chondrocyte-PGA/PLA constructs that formed tissue-engineered cartilage were examined, weighed, and then volume measured. All harvested samples were fixed, embedded in paraffin, and 5-μm sections cut. These sections were stained with hematoxylin and eosin (HE), a COL II monoclonal antibody (ab34712, 1:100; Abcam, Cambridge, UK), and Safranin O (SO) to evaluate anatomical features, and the expression of COL II and sulfated glycosaminoglycan (GAG) in protein [13,30]. Samples were also collected for quantitative evaluation of DNA, GAG, and COL II. Briefly, a Quant-iT Pico-Green dsDNA kit (Invitrogen) was employed to detect the DNA content in all samples. GAG content was determined with a dimethylmethylene blue chloride (Sigma Aldrich) method. COL II content was quantified using an enzyme-linked immunosorbent assay (ELISA; monoclonal antibody ab34712, 1:100; Abcam) as previously described [13,31].
Isolation of RMCs and resuscitation of cryopreserved primary microtia chondrocytes
At 4, 6, and 8 weeks, PGA-chondrocyte constructs that formed tissue-engineered cartilage were harvested to isolate RMCs. All samples were collected, minced, and treated with 0.1% collagenase using the procedures described above. Primary RMCs of different time point groups were harvested, seeded on culture dishes at a density of 1.5 × 104 cells/cm2, and subcultured to P5 in regular medium. RMCs at 4, 6, and 8 weeks were designated as RMC-4W, RMC-6W, and RMC-8W groups, respectively. For use as NMC controls, cryopreserved primary microtia chondrocytes were rapidly resuscitated and cultured in regular medium and subcultured to P5. P1, P3, and P5 cells of different RMC groups and NMCs were used for subsequent experiments.
Cell counting kit-8 (CCK-8) assay of RMC and NMC proliferation
Cell proliferation of NMCs and RMCs at P1, P3, and P5 was tested using a CCK-8 assay (Beyotime, Beijing, China). Briefly, as previously described [13,27], chondrocytes were plated at a density of 2000 cells per well in 96-well plates, and the absorbance at 450 nm of each well was measured on days 1, 3, 5, 7, and 9 using an automated ELISA reader (BioTek Instruments, Winooski, VT, USA).
Fluorescent staining of RMCs and NMCs
RMCs and NMCs from each patient at P1, P3, and P5 were collected for evaluation of cell morphology and COL II expression. COL II expression was qualitatively examined using a mouse anti-human COL II monoclonal antibody, with nuclei identified by diamidino-2-phenylindole (DAPI), as previously described [30].
Pellet culture and cartilage sheet regeneration
For pellet culture, P1 NMCs and RMCs of different groups were harvested. Aliquots of 4 × 105 cells in 1 mL of regular culture medium were centrifuged to form pellets as previously described [13,29]. The resulting pellets were cultured in chondrogenic medium for 3 weeks, and then processed for gross observation, histology, and immunohistochemical analysis. For large-scale cartilage regeneration, a cartilage sheet was prepared by high-density seeding of P1 NMCs, RMC-6W and RMC-8W cells (2.2 × 106 cells/cm2) into six-well plates, according to a previous study [27]. Cells were cultured in regular medium for 7 days and in chondrogenic medium for another 5 weeks. After 6 weeks of in vitro culture, newly formed cartilage sheets were collected and used for subsequent experiments.
Gross observation and histological evaluation of engineered cartilage
For each group, pellets and cartilage-sheet specimens were harvested, rinsed in PBS, fixed in buffered formalin, embedded in paraffin, and 5-μm sections cut for histological and immunohistochemical analyses. To evaluate engineered cartilage for histological structure and the presence of cartilage-specific extracellular matrix (ECM), sections were stained with HE, SO, and COL II using the procedures described above.
Statistical analysis
All quantitative data are presented as the mean ± standard deviation. Statistical significance between groups and within groups for mRNA levels and biochemical quantification was determined with one-way analysis of variance (ANOVA) and Tukey’s post-hoc test using SPSS Statistics 17.0 software. To identify significant differences in cell proliferation between NMC and RMC groups, the paired-samples T test was applied. P < 0.05 was considered to indicate statistical significance between groups.
Results
Dedifferentiation process of NMCs
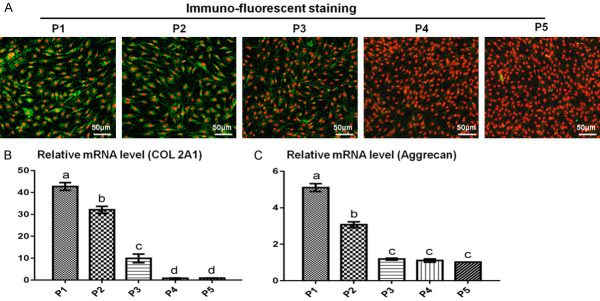
Loss of chondrogenic phenotypes are vital indicators for the dedifferentiation of chondrocytes. Changes in chondrogenic phenotypes during expansion were investigated to evaluate the dedifferentiation process and chondrogenic ability. As shown in Figure 2, substantially decreased COL II expression at both protein and mRNA levels (COL 2A1) was observed with subculture. Similarly, aggrecan mRNA levels also substantially decreased with passaging. Generally, expression of COL 2A1 and aggrecan were significantly decreased at P3, and no expression was observed at P5. These results demonstrated that NMCs began to lose their chondrogenic phenotype at P3 and completely lost their chondrogenic phenotype by P5, similar to healthy normal auricular chondrocytes. To observe the ultrastructural features of dedifferentiated and non-differentiated chondrocytes, we performed TEM analysis. Dedifferentiated chondrocytes showed segmented nuclei with smaller nucleoli, fewer secretory vesicles, but a greater amount of smooth endoplasmic reticulum compared with non-dedifferentiated chondrocytes, which showed larger nuclei and nucleoli and abundant secretory vesicles in the cytoplasm. These results indicated that non-dedifferentiated chondrocytes might have a greater capacity to secrete ECM compared with dedifferentiated chondrocytes (Supplementary Figure 1).
Figure 2.
Dedifferentiation process of microtia chondrocytes. COL II immuno-fluorescence staining showed strong COL II expression in microtia chondrocytes at P1 and P2 but significantly decreased expression at P3 (A). No expression was observed at P5 (A). The mRNA levels of COL 2A1 (B) and aggrecan (C) also decreased significantly within three passages.
Redifferentiation potential of DMCs
To engineer robust cartilage, a strong redifferentiation potential is very important for candidate cell sources. Therefore, a classical and stable 3D chondrogenic culture system (PGA/PLA scaffold and chondrogenic medium) was employed to regulate the dedifferentiation of microtia chondrocytes and to confirm their redifferentiation abilities. After 4-8 weeks of in vitro culture, all samples in different groups formed ivory-white cartilage-like tissue with typical lacuna structures, abundant GAG deposition and strong collagen II expression (Figure 3). Quantitative biochemical evaluations further confirmed these observations (Supplementary Figure 2A-F). As shown in Supplementary Figure 1D-F, total GAG, total collagen, and COL II were highly expressed. These results demonstrated that the 3D chondrogenic culture system can induce DMCs to adopt a redifferentiation phenotype, thereby indicating DMCs to be a suitable candidate cell source for cartilage regeneration. Furthermore, with increasing in vitro culture time, constructs showed increasing cartilage-specific ECM content (Supplementary Figure 2D-F), indicating that functional recovery of DMCs might be significantly influenced by in vitro chondrogenic culture time.
Figure 3.
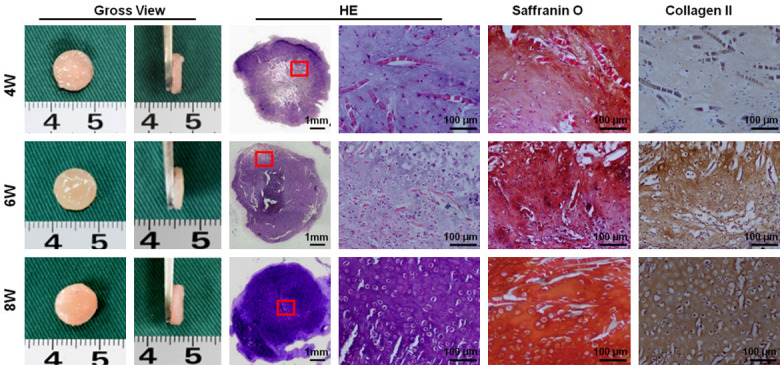
Chondrogenic properties of DMCs. DMCs at 4W, 6W and 8W all formed milky-white cartilage-like tissue, similar to native cartilage tissue, and mature lacuna structures and abundant extracellular matrix (ECM) deposition were observed by hematoxylin-eosin evaluation. Furthermore, strong positive staining for cartilage-specific ECM (COL II and GAG) was observed in all groups. No significant differences in overall appearance was observed among samples at different time points, whereas the level of cartilage-specific ECM exhibited an increasing trend with in vitro culture time.
Characterization of chondrogenic phenotype, cell proliferation, and chondrogenic potential of RMCs
Cell morphology of RMCs at different passages
To investigate the degree of RMC redifferentiation, cell morphology was evaluated at different passages using NMCs as a control group. As shown in Figure 4, most NMCs exhibited round or polygonal morphologies with a clean background by light microscopy, while RMCs (RMC-4W, RMC-6W, and RMC-8W groups) primarily exhibited fibroblast-like morphologies with spindle shapes. Moreover, RMCs were relatively smaller than most NMCs, suggesting that RMCs might have different biological behaviors compared with their original chondrocytes. Nevertheless, neither RMCs nor NMCs exhibited significant changes in cell size or shape, or presented signs of aging during passaging, indicating that these cells might maintain a robust proliferation capacity.
Figure 4.
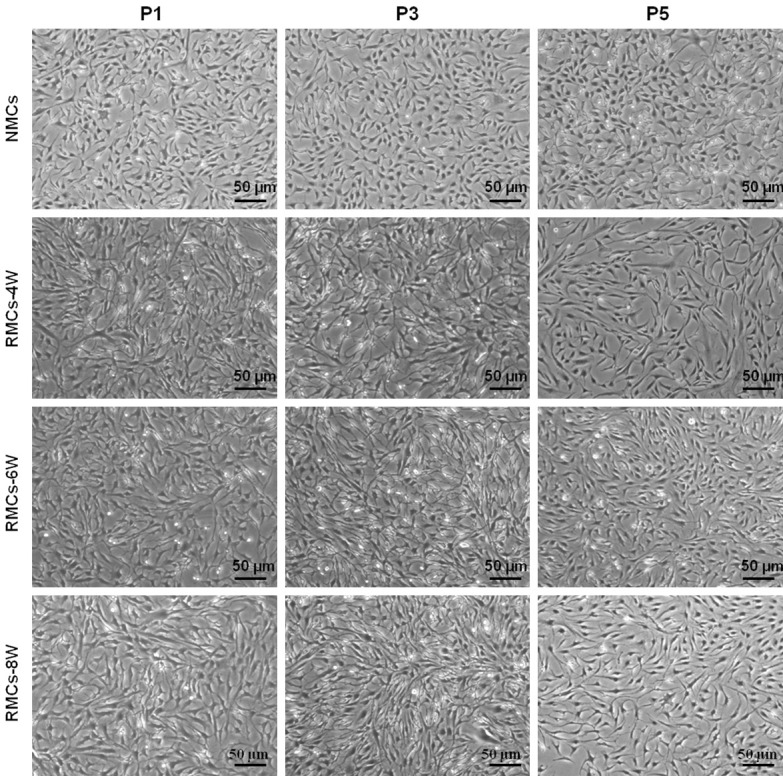
Cell morphology of RMCs and NMCs during subculture. NMCs mainly exhibited polygonal or triangular shapes, while RMCs mainly exhibited a spindle-like morphology. However, chondrocytes in both RMC and NMC groups did not exhibit significant changes in size or morphology during subculture (P1-P5). RMCs in different groups (4W, 6W and 8W) did not show obvious morphological differences by light microscopy evaluation.
Collagen II expression in RMCs at different passages
Expression of COL II is a vital indicator for the level of redifferentiation and functional evaluation of dedifferentiated chondrocytes. Therefore, expression of COL II was investigated in RMC-4W, RMC-6W, and RMC-8W groups to predict their redifferentiation level. As shown in Figure 5, although RMCs in all groups showed positive COL II expression, the RMC-4W group had far weaker COL II immunofluorescence staining compared with RMC-6W and RMC-8W groups. Moreover, no significant difference was found between RMC-6W and RMC-8W groups, and these two groups showed similar COL II expression to that of the NMC group. These results indicated that 4 weeks of chondrogenic culture might not be enough to induce complete redifferentiation of DMCs. Furthermore, RMCs in all groups exhibited a noticeable dedifferentiation trend during passaging, and the dedifferentiation process of RMCs was consistent with that of NMCs, indicating that RMCs may have regained their original biological behavior.
Figure 5.
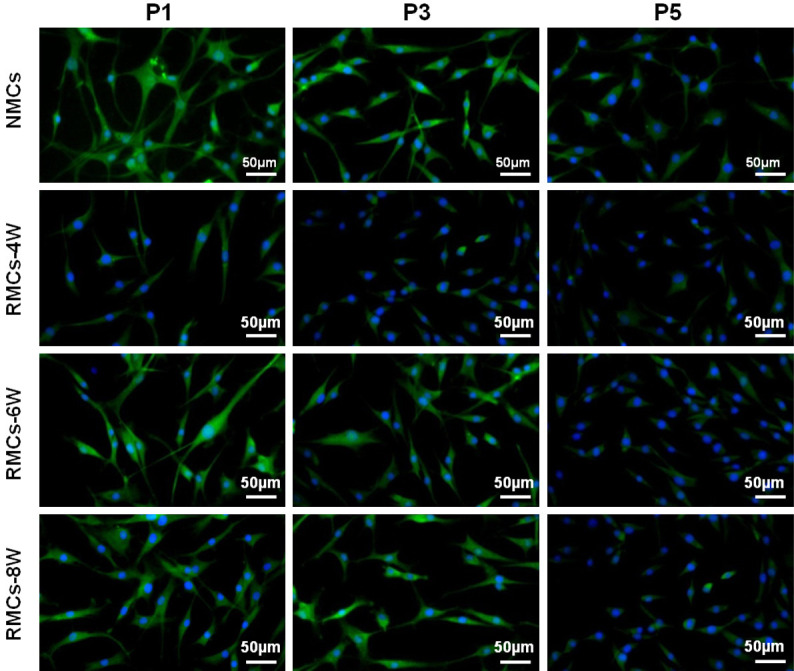
Type II collagen expression in RMCs and NMCs during subculture. Chondrocytes in NMC and different RMC groups all presented a substantial decrease in collagen II expression with passaging. Generally, COL II staining was strong at P1, decreased at P3, and absent at P5. However, cells in the RMC-4W group presented much weaker COL II staining compared with the NMC group. In contrast, chondrocytes in RMC-6W and RMC-8W groups showed similar levels of collagen II expression compared with their original chondrocytes.
Proliferation of RMCs
Cell proliferation is another important characteristic of redifferentiated chondrocytes. The proliferation of RMCs and NMCs was evaluated at P1, P3, and P5 by cell-growth curves. In contrast to the evaluation of COL II expression, proliferation curves of NMC and RMC groups did not reveal any significant differences (Figure 6A-I). Notably, although chondrocytes in the RMC-4W group did not achieve complete recovery of COL II expression, these cells exhibited robust proliferation potential similar to NMCs (Figure 6A-C). These results indicated that the 3D chondrogenic system could induce DMCs to redifferentiate into chondrocytes, and that these resulting RMCs exhibited similar proliferation potential to their native chondrocytes.
Figure 6.
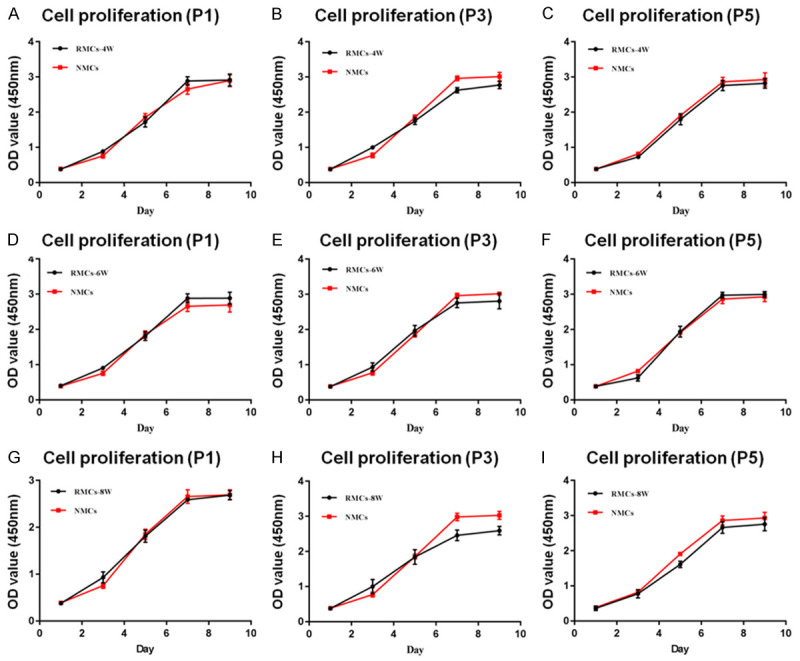
Cell proliferation of RMCs and NMCs. RMCs in 4W (A-C), 6W (D-F) and 8W (G-I) groups exhibited robust proliferation potential after eight passages (black curve), and no significant differences in proliferation rate were observed between RMC groups and their original chondrocytes (red curve).
Chondrogenic potential of RMCs
Chondrogenic potential is the most important characteristic of a functional chondrocyte. Therefore, the chondrogenic ability of RMCs was evaluated in pellet and cell-sheet culture systems. Pellet culture was first performed to confirm the ability of RMCs to form 3D cartilage. P1 chondrocytes from RMC-4W, RMC-6W, and RMC-8W groups all formed cartilage-like pellets with an ivory-white appearance, mature lacunae, and abundant GAG and COL II deposition on histological evaluation (Figure 7). In addition, no significant differences were observed between the RMC and NMC groups (Figure 7), indicating a strong chondrogenic potential of RMCs. To further investigate chondrogenic potential, a scaffold-free cartilage sheet model, which allows engineering of large cartilage grafts [27], was also employed. P1 chondrocytes in RMC-4W and RMC-8W groups all generated mature cartilage-sheet after 6 weeks of in vitro culture (Supplementary Figure 3), further indicating the strong chondrogenic potential of RMCs. In addition, cell-sheet cartilage of RMC-4W and RMC-8W groups did not exhibit significant differences in gross appearance or histological evaluation compared with the NMC group. Collectively, the evidence described above indicates that the 3D chondrogenic system can induce DMCs to regain their original function, and that these redifferentiated chondrocytes behave similarly to their native chondrocytes.
Figure 7.
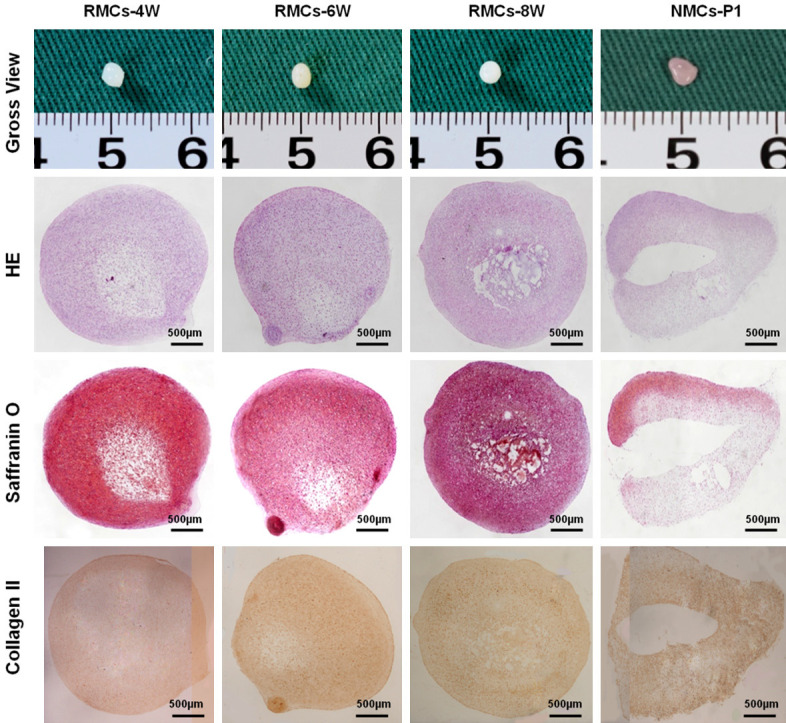
Gross view and cartilage-specific staining of pellets formed by RMCs. P1 chondrocytes from RMC-4W, RMC-6W, and RMC-8W groups all formed cartilage-like pellets with an ivory-white appearance after in vitro culture for 3 weeks. Histologically, all samples exhibited mature lacunae structures and abundant GAG and COL II deposition, which was similar to the pellets formed by P1 NMCs.
Discussion
The tissue-engineering of cartilage is a promising approach for functional ear reconstruction [12,32]. However, there has not been a significant breakthrough in clinical translation because the dedifferentiation of chondrocytes remains a substantial obstacle for the generation of tissue-engineered auricles [13,24]. The current study revealed that a 3D chondrogenic culture system can induce DMCs to redifferentiate into functional chondrocytes, thus enabling them to regenerate mature cartilage. After culture in this system for 4-8 weeks, RMCs achieved biological behaviors and chondrogenic functions similar to their original chondrocytes. In addition, the degree of redifferentiation of dedifferentiated chondrocytes was time-dependent. Collectively, these results demonstrate that microtia chondrocytes are an appropriate cell candidate for the regeneration of tissue-engineered auricles.
Obtaining sufficient numbers of microtia chondrocytes is critical for engineering normal-sized human ear cartilage grafts. Successive passaging is essential to achieve this; however, it induces chondrocyte dedifferentiation. For healthy normal chondrocytes, techniques such as co-culture, 3D-culture regulation, and cytokine induction are often conducted to maintain the chondrogenic phenotype or to protect chondrocytes from dedifferentiation [13,17,19]. For example, Zhang et al. successfully regenerated functional human ear-shaped cartilage by co-culturing human microtia chondrocytes with bone marrow mesenchymal stem cells (BMSCs) [13]; however, harvesting BMSCs is inevitably associated with donor site impairment. Therefore, it is difficult for patients to accept this technique. Caron et al. found that a 3D alginate-bead chondrocyte culture system could induce dedifferentiated articular chondrocytes to adopt a redifferentiation phenotype [18]. In addition, Bauge et al. and Das et al. confirmed that TGFβ can modulate the phenotype of dedifferentiated chondrocytes [20,33]. However, as the abovementioned techniques were mainly based on healthy normal chondrocytes, it remains unknown whether these regulatory techniques are also effective for microtia chondrocytes, which reportedly carry single-gene defects [24-26]. In the current study, a 3D chondrogenic culture system consisting of a PGA/PLA scaffold and growth factors for chondrogenic induction (including TGFβ, IGF1, and ITS, which are the most commonly used growth factors in tissue-engineered cartilage [34,35]) was employed to modulate the phenotype of DMCs. We found that DMCs formed mature cartilage tissue with robust expression of cartilage-specific ECM and appropriate mechanical strength, indicating that dedifferentiated cells can regain their original chondrogenic properties making them an ideal candidate source of cells. Based on these results, dedifferentiation of microtia chondrocytes may no longer be the main obstacle for the tissue-engineering of auricles because functional human ear-shaped cartilage can be generated using DMCs and an appropriate 3D chondrogenic culture system.
Another important issue is whether DMCs can completely regain their original biological behavior in the 3D chondrogenic culture system. The current results revealed that redifferentiated chondrocytes exhibited similar characteristics to their original cells, especially with respect to proliferation, COL II expression, and chondrogenic properties. Moreover, these RMCs exhibited a dedifferentiation trend during multiple monolayer passages. Most importantly, the dedifferentiation process of RMCs was consistent with that of their original chondrocytes, suggesting that these RMCs might have completely regained their original cell function. This achievement confirms stable in vivo cartilage regeneration by the dedifferentiated chondrocytes (fully functional microtia chondrocytes could regenerate stable mature cartilage tissues both in vitro and in vivo). Although the current study demonstrated that DMCs can regain their original function in a 3D chondrogenic culture system, the key factors in this system for regulating the DMCs’ redifferentiation phenotype and how those factors work remain unknown. According to previous reports, many pathways play important roles in the redifferentiation of healthy chondrocytes. For example, Wang X et al. found that increased MEK-ERK1/2 pathway activity resulted in articular chondrocyte dedifferentiation [14]; whereas, Öztürk et al. demonstrated that inhibited Wnt/β-catenin signaling could significantly enhance the expression of chondrogenic markers [36]. As such, the involvement and potential roles of these pathways in the redifferentiation of microtia chondrocytes needs further investigation. Although the main molecular mechanism for the redifferentiation of microtia chondrocytes remains unknown, we revealed that DMCs can regain their original characteristics and become fully functional chondrocytes after modulation in the 3D chondrogenic culture system, which has not been previously reported in the literature. Thus, our findings provide a deeper understanding of microtia chondrocytes.
Interestingly, the current study also found that DMCs redifferentiate and functionally recovery to different degrees at different time points of in vitro chondrogenic culture. The current results showed that complete functional recovery and relatively satisfactory redifferentiation were only achieved in RMC-6W and RMC-8W groups, but not in the RMC-4W group (chondrocytes showed much weaker expression of COL II in the RMC-4W group compared with RMC-6W and RMC-8W groups, as well as their original chondrocytes), indicating a time-dependent redifferentiation process for DMCs. Several reasons might account for this phenomenon. Indeed, after losing their differentiation characteristics, microtia chondrocytes might revert back to a progenitor-like cell state in a two-dimensional monolayer culture system, which can be speculated from our previous study. We found that these dedifferentiated chondrocytes (including healthy and microtia auricular chondrocytes) exhibited a plastic phenotype and mesenchymal stem cell-like differentiation properties, as they showed obvious potential for differentiation into lineage phenotypes such as osteoblasts and adipose cells (data not shown). Furthermore, we and others found that BMSCs present a gradually maturing trend with increased in vitro induction time [29,37]. Therefore, according to the results described above, it is reasonable that DMCs exhibit a time-dependent redifferentiation course, as they need sufficient time to achieve a differentiated phenotype after dedifferentiation. These results indicate that appropriate prolongation of in vitro culture time is beneficial for full functional recovery of dedifferentiated chondrocytes.
Conclusions
The current study demonstrated that a 3D chondrogenic culture system can effectively induce DMCs to become functionally redifferentiated chondrocytes, thus enabling them to regenerate mature cartilage. Furthermore, this redifferentiation is time-dependent, with redifferentiated chondrocytes achieving their original function after culture in the chondrogenic culture system for 6-8 weeks.
Acknowledgements
We appreciate the technical support from the Shanghai Key Laboratory of Tissue Engineering of China. This work was supported by the National Natural Science Foundation of China (31700837 and 81571823) and the Shanghai science and technology commission (18411969300). We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bly RA, Bhrany AD, Murakami CS, Sie KC. Microtia reconstruction. Facial Plast Surg Clin North Am. 2016;24:577–591. doi: 10.1016/j.fsc.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luquetti DV, Leoncini E, Mastroiacovo P. Microtia-anotia: a global review of prevalence rates. Birth Defects Res A Clin Mol Teratol. 2011;91:813–822. doi: 10.1002/bdra.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen A, Klaiber S, Katzbach R, Nitsch S, Konig IR, Frenzel H. The psychosocial consequences of reconstruction of severe ear defects or third-degree microtia with rib cartilage. Aesthet Surg J. 2008;28:404–411. doi: 10.1016/j.asj.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Brent B. Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases. Clin Plast Surg. 2002;29:257–271. vii. doi: 10.1016/s0094-1298(01)00013-x. [DOI] [PubMed] [Google Scholar]
- 5.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 6.Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, Wolf F, Jundt G, Marsano A, Farhadi J, Heberer M, Jakob M, Schaefer DJ, Martin I. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384:337–346. doi: 10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- 7.Muhart M, McFalls S, Kirsner R, Kerdel F, Eaglstein WH. Bioengineered skin. Lancet. 1997;350:1142. doi: 10.1016/S0140-6736(05)63788-9. [DOI] [PubMed] [Google Scholar]
- 8.Liao HT, Zheng R, Liu W, Zhang WJ, Cao Y, Zhou G. Prefabricated, ear-shaped cartilage tissue engineering by scaffold-free porcine chondrocyte membrane. Plast Reconstr Surg. 2015;135:313e–321e. doi: 10.1097/PRS.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 9.Pomerantseva I, Bichara DA, Tseng A, Cronce MJ, Cervantes TM, Kimura AM, Neville CM, Roscioli N, Vacanti JP, Randolph MA, Sundback CA. Ear-shaped stable auricular cartilage engineered from extensively expanded chondrocytes in an immunocompetent experimental animal model. Tissue Eng Part A. 2016;22:197–207. doi: 10.1089/ten.tea.2015.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, Lowder E, Landis WJ. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691–703. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 11.Kamil SH, Vacanti MP, Vacanti CA, Eavey RD. Microtia chondrocytes as a donor source for tissue-engineered cartilage. Laryngoscope. 2004;114:2187–2190. doi: 10.1097/01.mlg.0000149455.68135.de. [DOI] [PubMed] [Google Scholar]
- 12.Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg. 2009;124:817–825. doi: 10.1097/PRS.0b013e3181b17c0e. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, He A, Yin Z, Yu Z, Luo X, Liu W, Zhang W, Cao Y, Liu Y, Zhou G. Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials. 2014;35:4878–4887. doi: 10.1016/j.biomaterials.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Xue Y, Ye W, Pang J, Liu Z, Cao Y, Zheng Y, Ding D. The MEK-ERK1/2 signaling pathway regulates hyaline cartilage formation and the redifferentiation of dedifferentiated chondrocytes in vitro. Am J Transl Res. 2018;10:3068–3085. [PMC free article] [PubMed] [Google Scholar]
- 15.Wuest SL, Calio M, Wernas T, Tanner S, Giger-Lange C, Wyss F, Ille F, Gantenbein B, Egli M. Influence of mechanical unloading on articular chondrocyte dedifferentiation. Int J Mol Sci. 2018;19:1289. doi: 10.3390/ijms19051289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan L, Ma B, Liang Y, Chen J, Zhu W, Li M, Wang D. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am J Transl Res. 2015;7:194–208. [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng L, Chen X, Zhang Q, Yu F, Li Y, Yao Y. Redifferentiation of dedifferentiated chondrocytes in a novel three-dimensional microcavitary hydrogel. J Biomed Mater Res A. 2015;103:1693–1702. doi: 10.1002/jbm.a.35309. [DOI] [PubMed] [Google Scholar]
- 18.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, van Rhijn LW, Welting TJ. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, van Osch GJ. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231–241. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Das R, Timur UT, Edip S, Haak E, Wruck C, Weinans H, Jahr H. TGF-beta2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann Anat. 2015;198:1–10. doi: 10.1016/j.aanat.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cell Mater. 2005;9:58–67. doi: 10.22203/ecm.v009a08. discussion 67. [DOI] [PubMed] [Google Scholar]
- 22.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng. 2004;10:109–118. doi: 10.1089/107632704322791754. [DOI] [PubMed] [Google Scholar]
- 23.Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89:373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Kang N, Dong P, Liu X, Wang Q, Fu X, Yan L, Jiang H, Cao Y, Xiao R. Chondrocytes from congenital microtia possess an inferior capacity for in vivo cartilage regeneration to healthy ear chondrocytes. J Tissue Eng Regen Med. 2018;12:e1737–e1746. doi: 10.1002/term.2359. [DOI] [PubMed] [Google Scholar]
- 25.Li CL, Chen Y, Shan J, Hao SJ, Jin L, Qing FH, Zhang TY. Phenotypic characterization and risk factors for microtia in East China, a case-control study. Int J Pediatr Otorhinolaryngol. 2014;78:2060–2063. doi: 10.1016/j.ijporl.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Wei G. Bioinformatics analysis of microRNA comprehensive regulatory network in congenital microtia. Int J Pediatr Otorhinolaryngol. 2015;79:1727–1731. doi: 10.1016/j.ijporl.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 27.He A, Xia H, Xiao K, Wang T, Liu Y, Xue J, Li D, Tang S, Liu F, Wang X, Zhang W, Liu W, Cao Y, Zhou G. Cell yield, chondrogenic potential, and regenerated cartilage type of chondrocytes derived from ear, nasoseptal, and costal cartilage. J Tissue Eng Regen Med. 2018;12:1123–1132. doi: 10.1002/term.2613. [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu Y, Zhang WJ, Cao Y, Zhou G. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31:3564–3571. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 29.He A, Liu L, Luo X, Liu Y, Liu Y, Liu F, Wang X, Zhang Z, Zhang W, Liu W, Cao Y, Zhou G. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci Rep. 2017;7:40489. doi: 10.1038/srep40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, Zhang W, Liu W, Cao Y, Zhou G. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 31.Luo X, Zhou G, Liu W, Zhang WJ, Cen L, Cui L, Cao Y. In vitro precultivation alleviates post-implantation inflammation and enhances development of tissue-engineered tubular cartilage. Biomed Mater. 2009;4:025006. doi: 10.1088/1748-6041/4/2/025006. [DOI] [PubMed] [Google Scholar]
- 32.Melgarejo-Ramirez Y, Sanchez-Sanchez R, Garcia-Lopez J, Brena-Molina AM, Gutierrez-Gomez C, Ibarra C, Velasquillo C. Characterization of pediatric microtia cartilage: a reservoir of chondrocytes for auricular reconstruction using tissue engineering strategies. Cell Tissue Bank. 2016;17:481–489. doi: 10.1007/s10561-016-9574-5. [DOI] [PubMed] [Google Scholar]
- 33.Bauge C, Duval E, Ollitrault D, Girard N, Leclercq S, Galera P, Boumediene K. Type II TGFbeta receptor modulates chondrocyte phenotype. Age (Dordr) 2013;35:1105–1116. doi: 10.1007/s11357-012-9433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Jiang H, Yin Z, Liu Y, Zhang Q, Zhang C, Pan B, Zhou J, Zhou X, Sun H, Li D, He A, Zhang Z, Zhang W, Liu W, Cao Y. In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine. 2018;28:287–302. doi: 10.1016/j.ebiom.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–302. doi: 10.1097/00006534-199708000-00001. discussion 303-294. [DOI] [PubMed] [Google Scholar]
- 36.Ozturk E, Hobiger S, Despot-Slade E, Pichler M, Zenobi-Wong M. Hypoxia regulates RhoA and Wnt/beta-catenin signaling in a context-dependent way to control re-differentiation of chondrocytes. Sci Rep. 2017;7:9032. doi: 10.1038/s41598-017-09505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, Liu TY, Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–2192. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.