Abstract
Osteoarthritis (OA) is a chronic degenerative joint disease and a leading cause of disability. It involves articular cartilage destruction and a whole joint inflammation. In spite of OA pathogenesis is still unclear, new studies on the OA pathophysiological aetiology and immunomodulation therapy continuously achieve significant advances with new concepts. Here, we focus on the indoleamine-2,3-dioxygenase1 (IDO1) activity in the osteoarthritis (OA), which is one of the noticeable enzymes in the synovial fluid of arthritis patients. It was recognized as an essential mediator of autoreactive B and T cell responses in rheumatoid arthritis (RA) and an interesting therapeutic target against RA. However, the role IDO1 plays in the OA pathogenesis hasn’t been discussed. The new OA experimental analysis evidenced IDO1 overexpression in the synovial fluid of OA patients, and recent studies reported that IDO1 metabolites were found higher in the OA synovial fluid than RA and spondyloarthropathies (SpA) patients. Moreover, the positive relation of IDO1 metabolites with OA pain and joint stiffness has been confirmed. Thus, the IDO1 plays a pivotal role in the pathogenesis of OA. In this review, the role IDO1 plays in the OA pathogenesis has been deeply discussed. It could be a promising target in the immunotherapy of OA disease.
Keywords: Osteoarthritis; indoleamine 2,3 dioxygenase 1; MSCs; chondrocytes; synovitis
Introduction
Osteoarthritis (OA) pathophysiology remains a serious challenge that hinders therapeutic researches. Clinically it is referred to severe joint cartilage degeneration [1-4]. Different reports described OA as an inflammatory disease according to the high levels of proinflammatory factors such as IL-1β, TNF-α, IFN-γ, IL-6 and IL-13 [5-7] as seen in Table 1. However, anti-inflammatory mediators such as TGF-β1 and regulatory T cells have been studied in OA pathophysiology as well [8-10]. Recently, Merlo and colleagues reported that indoleamine 2,3 dioxygenase1/2 (IDO1/2) are candidate therapeutic targets to treat inflammations in the autoimmune diseases [11,12]. IDO2 works as an essential mediator of autoreactive B and T cell responses in the rheumatoid arthritis (RA) [12]. He developed a novel monoclonal antibody (mAb)-based approach to target IDO2 in the preclinical arthritis models. Results showed an efficient treatment against RA in mice. Moreover, using of 1-Methyl-d-tryptophan (IDO inhibitor) blocked autoreactive B cell activation and recurrence of arthritis in mice [11]. In our hospital, 52 synovial fluid samples were obtained from OA patients in comparison to 5 synovial fluid samples were obtained from non- OA patients (during surgeries of joint replacement). By ELISA kits all the OA SF samples showed overexpression of IDO1 in comparison to non-OA samples as seen in the Table 2. Suggesting a possible role of IDO1 in the OA pathogenesis. Furthermore, the role IDO1 plays in the autoimmune arthritis is unclear yet. Some studies suggesting a regulatory function of IDO1 in the autoimmune RA [13] while others reported a proinflammatory role [14,15], or no role [16].
Table 1.
List of cytokines and chemokines involving osteoarthritis pathophysiology
| Cytokine/chemokine | Function | Situ in The Joint | Reference |
|---|---|---|---|
| IL-10, IL13, IL-4, IL-19, IL-32a, IL-32b, IL-32g and IL-32d, TGF-β | It has a chondroprotective role. Other literature suggesting an inflammatory enhancing role | Synovial fluid/subchondral bone/articular cartilage | [19,36,49,51,56,58,59,60,82] |
| TNF-α, IL-1β, IL-1α, IFN-γ, IL-6 | It increases inflammation and cartilage degradation by inducing synovitis and mmp13 in the chondrocytes | Synovial fluid/subchondral bone/articular cartilage | [49,50,51,53,58,101] |
| IL-8 | It induces inflammatory responses in the synovium | Synovial fluid | [19,51] |
| IL-15, IL-17 | It has inflammatory influence and some anti-inflammatory functions | Synovial fluid | [50,52] |
Table 2.
The obtained synovial fluid from OA patients and non-OA patients
| Patients No | Gender | Age | OA grade | Samples | IDO1 average level |
|---|---|---|---|---|---|
| 33 | Female* | > 67±13 | 3-4 | SF | 25.23±6.5 IU/mL |
| 19 | Male | > 65±11 | 3/4 | SF | 22.75±8.2 IU/mL |
| 5 | Male | > 42±9 | Negative | SF | 2.26±1.4 IU/ml |
OA: Osteoarthritis, SF: synovial fluid;
P<0.05.
In the ido deficient mice, no skeletal defects were observed [17], but it showed increased Th1/Th17 cells in arthritis joints which suggest a protective role of IDO1 in the joints [18]. A cross sectional studies reported that IDO1 increases peripheral inflammatory cells in the RA patients [19,20]. Altogether suggesting an imbalance of IDO1 in the synovium may seriously lead to arthritis development. However, the role of IDO in the OA hasn’t been searched. As known, mesenchymal stem cells (MSCs) produce IDO1 in response to inflammatory factors in the joint [21]. High levels of proinflammatory mediators such IL-1β, TNF-α, and IFN-γ in the OA [22] are supposed to induce DCs, Monocytes, and even MSCs release IDO1 in the synovial fluid in response to inflammation [23] as presented in the Figure 1. High inflammatory levels make inflammatory stress on the synovial MSCs that induce high regulatory mediators to rebalance immune response but the overexpression of regulatory mediators could also affect the cartilage and chondrogenesis by inducing hypertrophy and mmp-13 leading to induce cartilage degradation, see Figure 2 [23-25]. As reported, a metabolite of IDO controls the TNF-stimulated gene 6 (TSG-6)-mediated anti-inflammatory effects of human MSCs [26]. Wang and colleagues [26] concluded that kynurenine activates aryl hydrocarbon receptor (AhR) in MSCs that directly binds to the TSG-6 promoter that leads to induce TSG-6 expression. TSG-6 has anti-inflammatory role and chondroprotective effects in various models of inflammation and arthritis [27]. However, in recent few years the association between TSG-6 activities and osteoarthritis progression was determined at 3-year of follow-up [28]. Thus, the activation of TSG-6 in the synovial fluid of OA patients is strongly supposed to be stimulated by IDO1 metabolite (Kynurenic acid) which stimulates MSCs to release TSG-6 via activation of AhR receptor as presented in Figure 3.
Figure 1.
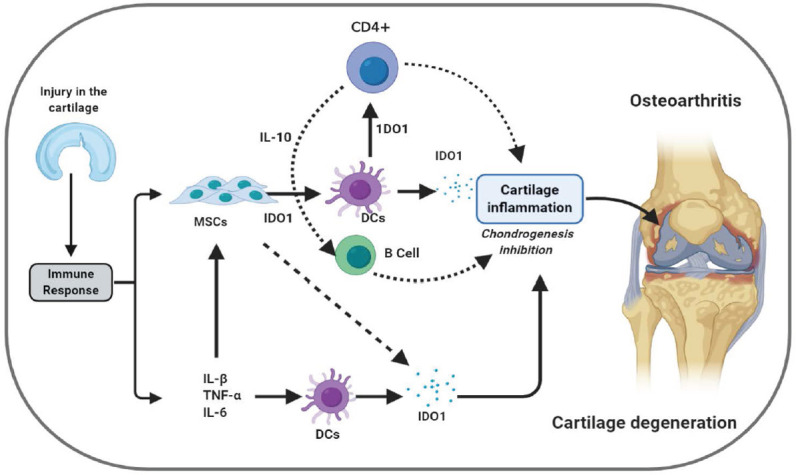
The potential role IDO could play in the pathogenesis of OA disease. As seen, in response As seen in response to cartilage injury proinflammatory cytokines such as IL-1β, TNF-α, and IFN-γ and inflammatory MSCs stimulates DCs to produce IDO1 to rebalance immune status. However, high IDO1 levels produced by DCs and MSCs inhibits chondrogenesis and increase the risk of cartilage erosion by enhancing cartilage inflammations.
Figure 2.
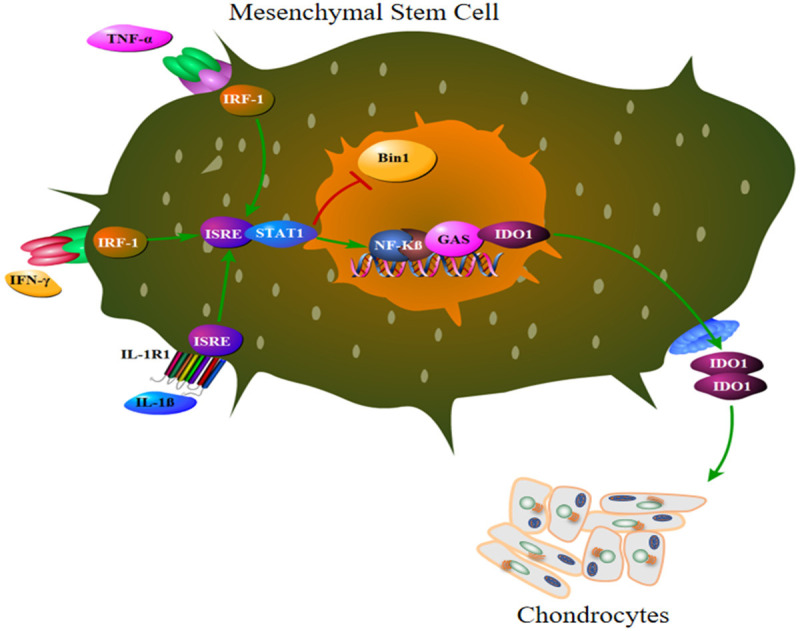
The mechanism by which proinflammatory cytokines in the OA knee joint stimulates MSCs to produce IDO1. IL-1β directly activates ISRE, while TNF-α, and IFN-γ activate ISRE via IRF1 molecule. ISRE signaling stimulates STAT1 which block IDO1 suppressor gene Bin1 and stimulate IDO1 production via NF-κβ pathway. High levels of IDO1 affects chondrocytes biology and inhibits chondrogenesis.
Figure 3.
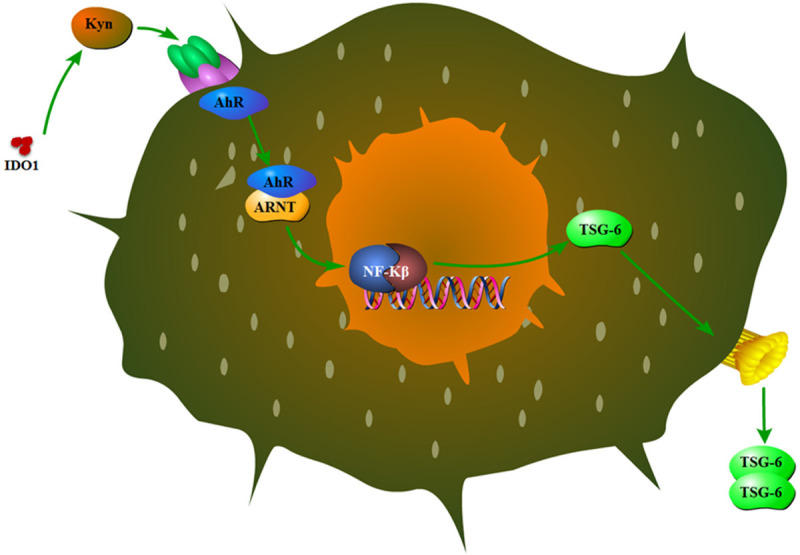
The mechanism by which IDO1 metabolites (kynurenine) impacts MSCs function in the OA pathogenesis. Kynurenine activates AhR receptor that induces TSG-6 production.
Osteoarthritis and indoleamine 2,3 dioxygenase activity
As known, during OA development the joints functional units comprising cartilage and bone undergo decontrolled catabolic and anabolic remodeling processes to adapt to local biochemical and biological signals [29]. Changes in cartilage, synovial fluid, and subchondral bone directly contribute to the OA virulence [30,31]. Increased vascularization and formation of micro-cracks in joints during OA have suggested the facilitation of molecules such as cytokines or enzymes from cartilage to bone and vice versa. The further investigation reported TGF-β as a key cytokine involved osteoarthritis fibrosis [32,33]. Zhen and colleagues [34] reported that inhibition of TGF-β activity attenuated degeneration of osteoarthritic articular cartilage. In contrast, TGF-β1 initiates pathological changes of osteoarthritis and may increase disease severity [35,36]. However, the role of TGF-β1 mechanism in the OA pathogenesis remain controversial, and its upstream molecules are unknown. There is a specific promoter initiates upstream mechanisms hasn’t been discussed. IDO1 is supposed to be the responsible of TGF-β1 upregulation through smad2/4 and subsequently IL-6 [37,38]. Moreover, IDO1 has the potential to activate pDCs and other immune cells to sustain TGF-β1 functions and other regulatory mediators in the synovial [39].
Although, the effect of IDO1 on chondrocytes is unknown, it has been reported that IDO1 could support chondroprotective potential in the RA by reducing functional autoreactive Th1/Th17 in the joints and draining lymph nodes [40]. Inhibition of IDO activity, or knockout of the gene encoding IDO in the RA animal model caused an increase in the severity of collagen-induced arthritis [40]. However, the overexpression of IDO1 in the RA synovial patients was a serious challenge suggesting a pathogenic role of IDO1 in the RA. In the OA patients IDO1 overexpression has been found in all collected samples as presented in Table 1. Thus, maybe it has a role in the inhibition of chondrogenic differentiation by stimulating TGS-6 overexpression that induces chondrocytes to produce mmp13 leading to break down collagen type II and degrade extracellular matrix. Furthermore, the therapeutic effect of IDO1 down regulation in the RA animal model [12] importantly suggest IDO1 involves inflammation mechanisms which suppose the same role in the synovial of OA patients. So, the study of IDO molecular mechanism in the OA pathogenesis will open new intellectual perspectives for OA novel treatment strategy. Moreover, the determination of IDO1 release and molecular mechanism in early stage of OA disease could also represent immunomodulatory effects of IDO1 involving the synovitis of OA disease.
Furthermore, as known human MSCs release IDO to reduce high inflammatory responses especially in the bone [21,41]. In OA disease, MSCs positively express nestin to modulate subchondral bone structure [42]. Increases in nestin is partially regulated by vascular endothelial growth factor (VEGF) in the OA [43]. Since VEGF increases the expression and activity of IDO1 in the DCs [44], we wonder whether the nestin+MSCs could depend on IDO pathway to develop changes leading to initiate OA disease. OA-nestin+MSCs could use IDO pathway long-term immune tolerance to modulate synovial immune status because plasmacytoid dendritic cells (pDCs) that were confirmed in the OA synovium efficiently enhance immune long-term tolerance in the presence of IDO1 [45,46]. Moreover, genomic and experimental studies evidenced that VEGF is associated with OA development, and its severity [47,48], while VEGF high expression increases IDO1 overexpression and activity, which importantly suggests a role played by IDO1 in the OA development.
The association of osteoarthritic cytokines and chemokines with IDO1 pathway
The most reported cytokines in the osteoarthritis are proinflammatory such as IL-1β, IFN-γ, IL-6, TNF-α, IL-8, and IL-17 [49-55]. However, regulatory chemokines also have been documented such as TGF-β, IL-10, IL-4, IL-13 and IL-19 [36,51,56-62] see Table 1. These inconsistent data presented incomprehensible OA mechanism [63]. The most interesting point here is that these proinflammatory cytokines have a pivotal role in the pathogenesis of RA (inflammatory disease) with significant expression but its expression in the OA was shown less than RA [63,64]. Moreover, recent reports revealed that ani-inflammatory cytokines and Treg cells showed significant increasing in the OA [61,64], suggesting a secondary role of proinflammatory cytokines in the OA. Also, its worthy mention that IFN-γ, TNF-α in combination with IL-1β could actively induce IDO enzyme secretion by MSCs, APCs or stromal cells to enhance underlying immunosuppressive responses [21,65]. However, high inflammatory responses induce IDO1 activity which contribute in the production of other mediators affecting chondrogenesis and induce chondrocyte apoptosis as seen in Figure 4. As well as IL-32 has a potential to induce IDO1 secretion [66]. Thus, the OA inflammatory agents are suggested to promote IDO pathway and subsequently TGF-β, but whether IL-1β is the essential factor to initiate IDO1 pathway or IFN-γ and TNF-α is still uncertain. Therefore, we suggest a deep investigation for these proinflammatory agents regarding IDO1 upregulation and consequently chondrocytes responses in the OA. Therefore, we provide overview on the major pathophysiological cytokines involving OA and a possible relation with IDO1 pathway as the following.
Figure 4.
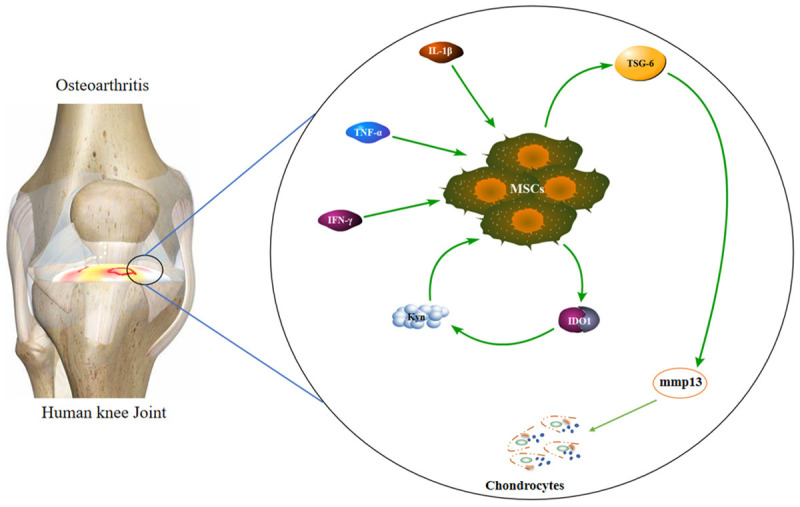
Schematic diagram shows the potential role IDO1 coud play in the cartilage. Proinflammatory cytokines in the cartilage in response to injury can elicit MSCs to producing IDO1. High levels of IDO1 me-tabolites modulate MSCs to produce TSG-6 which has a chondrogenic inhibition potential. It stimulates cleavage of casepase3 in the chondrocytes that induces chondrocyte apoptosis and extracellular matrix (ECM) degradation by mmp13.
Interleukin-1 beta (IL-1β)
IL-1β is one of the main proinflammatory and catabolic cytokines in the pathophysiology of OA disease [55,67]. Long monitoring of OA patients synovial fluids presented rapid increasing of IL-1β with OA early development [68]. Later, it was classified as an important biochemical marker of OA prognosis. IL-1β works to suppress type II collagen and aggrecan synthesis in the articular chondrocytes [69]. It could also contribute in the production of other inflammatory interleukins such as IL-6, IL-8 which also showed overexpression in the OA [70,71]. The blockage of IL-1β formation showed significant elimination in the OA severity but the results of treatment with these drugs were not entirely satisfactory this is suggesting a secondary role of IL-1β in the OA pathophysiology. However, recently kynurenine (IDO1 metabolite) exhibited capability to stimulate bioactive IL-1β secretion from myeloid cells through caspase 1 induction, while IDO-/- mice field to induce IL-1β cleavage and production [72]. So, IDO1 could has a role to initiate synovitis through promoting proinflammatory IL-1β.
Tumor necrosis factor alpha (TNF-α)
TNF-α is a kind of protein classified as proinflammatory factor that produced by antigen presenting cells (APCs) to stimulate inflammatory responses [73]. The increasing levels of TNF-α in the OA patients serum specimens showed significant association with joint stiffness and pain [62]. Many literatures reported that TNF-α works to recruit monocytes to increase IDO1 activity in the brain and some other organs [74-76]. Importantly, Pantsulaia and his colleagues revealed that there is no association between hand OA and plasma TNF-α levels [77]. Moreover, TNF-α in canine models did not show an association with mild osteoarthritic developments when increased in articular cartilage [78], these evidence suggest a secondary role of TNF-α in the OA pathogenesis that strongly expect its role to stimulate IDO pathway to stimulate synovitis.
Interleukin-6 (IL-6)
IL-6 is an amino acid residue that mostly works as proinflammatory factor [79,80]. Usually, osteoblasts secrete IL-6 to stimulate osteoclast formation [81]. In addition, normal chondrocytes secretes IL-6 with low expression to enhance chondrocytes proliferation [62]. However, high levels of IL-6 have been reported in the RA and OA synovial which being induced by TNF-α or IL-1β [82,83]. The overexpression of IL-6 inhibits collagen II expression in the chondrocytes [84,85]. Also, the dysregulated overproduction of IL-6 is responsible for the systemic inflammatory manifestations in RA patients [86]. Furthermore, IL-6 upregulates mmP13 expression in human and bovine cartilage explant cultures. In addition, IL-6 knock out mice displayed a low number of in the inflammatory cells in knee joints and a limited response to collagen induced arthritis [87]. Rübenhagen and his colleagues revealed that synovial IL-6 levels haven’t any correlation with OA severity in a study of 82 knee OA patients [88], since Van and his colleagues conducted that IL-6 joint cavity injection in IL6 deficient mice reduces cartilage destruction [89]. Worthy mention, IL-6 induces IDO1 expression through the JAK/STAT pathway [90]. This is suggesting that IL-6 could enhance OA synovitis through stimulation of IDO1 production that actively contributes to reduce collagen II production, as well as induces capspase3 cleavage.
Interferon-γ (IFN-γ)
IFN-γ is a soluble proinflammatory cytokine that rapidly induces inflammation responses [91]. It is secreted by T cells, natural killer (NK) cells and macrophages [92]. IFN-γ is a homodimer protein that binds to the interferon γ receptor that triggers a cellular response to viral, microbial or acute inflammation stimulation. It was early mentioned that IFN-γ actively participates in the RA synovitis [93,94]. Later, IFN-γ was reported with increasing levels in the OA patients synovial fluids [95]. It was suggested that IFN-γ stimulates inflammatory cells CD4 in the synovium that actively inhibits chondrocytes proliferation [64,96]. Others reported that IFN-γ induces the production of matrix metalloproteinases-13 (mmp-13) that inhibits collagen II production in the chondrocytes [97,98]. Besides, IFN-γ and TNF-α induce IL-6 production in the synovial fluid [70]. Importantly, IFN-γ induces IDO1 upregulation in the endothelial cells and tumor tissues [74,99,100]. Moreover, the induction of IDO1 in the RA and OA is associating with IFN-γ overexpression and other inflammatory factors [101]. Also, IFN-γ KO mice displayed increased levels of IL-17 producing T cells and the exacerbation of arthritis. Indeed, splenocytes of the IFN-γ KO mice increased IL-17 production when cultured with type II collagen [102]. Furthermore, the addition of IFN-γ to the culture of APCs from IFN-γ KO mice significantly reduced IL-17, while the inhibition of IDO1 by 1-methyl-DL-tryptophan abolished the inhibitory effects of IFN-γ. These results illustrate that IFN-γ regulates IL-17 production through IDO1 in the arthritis, since IFN-γ presented strong relation with IL-1β in the OA pathophysiology, which represent a strong relation of IDO1 with OA initiation.
Interleukin-10 (IL-10)
IL-10 is a pleiotropic anti-inflammatory cytokine that inhibits proinflammatory cytokines production [103]. It elicits diverse host defense mechanisms and maintaining the integrity and homeostasis of tissue epithelial layers [104]. Although, IL-10 classified as inhibitory cytokine it could block the activity of catabolic cytokines leading to joint stiffness and destruction [105]. High levels of IL-10 has been detected in the serum of OA patients [106] but it was suggested to provide a chondroprotective function [107,108]. Moreover, LPS stimulation induces low ex vivo production of IL-10 that associates increased risks of familial OA [109]. Meanwhile, recombinant human IL-10 is being tested in clinical trials to treat RA and other inflammatory diseases [110]. Van and colleagues [111] suggested that in the osteoarthritic synovium and cartilage explant culture MSCs could produce inhibitory factors such as IL-10, TGF-β, and IDO to reduce inflammation in short term. Recently, IL-10 mediates the inhibitory effect of umbilical cord-derived mesenchymal stem cells (UCMSC) that regulate Cadherin-11 (CDH11) expression by fibroblast-like synoviocytes (FLS) in RA by inducing IDO1 production [112]. In the OA there is no reports confirmed the role of IDO1 yet, since IL-10 high levels have been reported. This review reports the high expression of IDO1 in the synovial fluid of OA patients. So, the expected role of IDO1 in the OA could has a relation with IL-10 chondroprotective function which suggesting deep investigation of this point in the future.
Transforming growth factor beta (TGF-β)
TGF-β is a multifunctional regulatory cytokine that mostly enhances immunosuppressive process [113]. It appears in three different mammalian isoforms (TGF-β1, TGF-β2, and TGF-β3) and other signaling proteins [114,115]. TGF-β plays a critical role in the development and maintenance of the skeletal tissues [116]. Osteoblasts has the potential to secret the three isoforms of TGF-β which acts as anabolic factor that promotes osteoclasts differentiation and proliferation [114]. Moreover, the effect of TGF-β on the osteoblast is controversial, it can enhance osteoblasts differentiation, induce bone formation, and extracellular bone matrix production [117]. In contrast, osteoblasts showed decreased expression of TGF-β receptor I and receptor II in human, murine, and rat during differentiation, which indicates less sensitivity of osteoblasts to TGF-β1 in the differentiation late phase. Furthermore, TGF-β1 induces osteoclasts maturation and osteoclastogenesis of hematopoietic precursor [116,118]. However, TGF-β1 high levels could attenuates osteoclastogenesis through osteoproteogerin as well as down regulating RANKL pathway [116]. In addition, inflammatory factors such TNF-α and IL-1β increase the expression of RANKL in the chondrocytes leading to reduce collagen II expression. Altogether, TGF-β1 plays a role to reduce cartilage and bone degeneration by reducing RNAKL pathway [119]. In contrast, recently noticed that TGF-β1 modulate SMAD receptor signaling that affects chondrocyte differentiation and potentially induces osteoarthritis development [60]. Besides, alterations in TGF-β1 molecular activity actively contributes in the OA progression [120]. Importantly, TGF-β1 increasing levels associated the overexpression of IDO in many diseases that contribute in the immune tolerance enhancement [121,122]. In the antigen induced arthritis IDO/TGF-β1 mediates a protective effect of IFN-α [37]. However, the role of TGF-β1 in the OA is still controversial but the detection of IDO high levels in the OA patients synovial could largely help to understand TGF-β1 functions. Therefore, IDO mechanism in the OA needs an urgent study to provide clear interpretation for many inflammatory and regulatory factors involving OA pathogenesis.
Indoleamine 2,3 dioxygenase and possible activity in the joint
IDO is an intracellular immunomodulatory enzyme [123,124]. It engages immunotolerance mechanisms such as kynurenine pathway that catabolizing L-tryptophan through O2 dependent oxidation to enhance immunosuppressive processes [38,125]. It is secreted by many cell types such as antigen presenting cells (APCs), stromal cells, mesenchymal stem cells (MSCs), tumour cells, endothelial cells and some of the alternatively activated macrophages [126-128]. By 2018 the function of IDO in different diseases was a focus of research and drug discovery efforts. As well as, the efforts to use IDO as a biomarker for tumor prognosis [129]. Also, it associates with some neurodegenerative pathogenesis [130,131]. It has the potential to alter the immune status from inflammatory to regulatory and orchestrate intracellular mechanisms to elicit suppressive cytokines and signals [38,132]. Pathogenesis of OA and RA present high association with NF-κβ, RANKL, TGF-β, COX and IL-6 pathways that essentially has a strong relation with IDO1 [38,133]. In addition, many literatures evidenced the involvement of IDO in the pathogenesis of RA and some of autoimmune diseases [20,134,135]. The activity of IDO1 in the OA pathogenesis is the current hot topic in the new projects because promising results in the RA importantly suggest possible role of IDO1 in the OA pathogenesis. As presented in Figure 4 IDO1 could efficiently affects the chondrogenic functions of MSCs by different mechanisms, one of the proposed mechanisms by stimulating TSG-6 as described above.
The possible theories about the effect of IDO1 on chondrogenic functions of synovial MSCs as seen in Figure 5 could be summarized as the following 1) may be IDO1 induce Wnt signaling as reported in the cancer cell [136] which has been proved to regulate Sox9 activity [137]. 2) IDO1 could attenuates CCR2, Hifα, or PKA [38], which directly inhibits Sox9 activity [138,139]. 3) the activation of ERK1/2 could inhibited by IDO1 that leads to inactivate Sox9 in the MSCs [140]. All these theories suggest an indirect effect of IDO1 on the chondrogenic signaling Sox9 which directly mediates collagen type II and 9 production as well as aggrecan.
Figure 5.
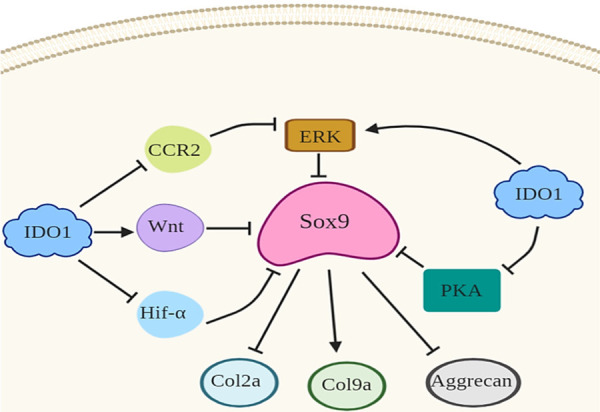
Schematic diagram displays the possible theories of IDO1 mechanisms by which IDO effects Sox9 signaling in the MSCs leading to inhibit chondrogenic genes. As known IDO1 induces Wnt signaling that directly inhibits Sox9 and consequently Col2a, Col9a, and aggrecan. Also, IDO has the capability to inhibit PKA, CCR2, or Hif-α which work as Sox9 upstream molecules. Indeed, IDO1 could directly elicits ERK1/2 signaling that directly inhibits Sox9 and subsequently inhibits chondrogenic genes.
On the other hand, IDO enzyme works on the tryptophan to produce kynurenic acid [141], which mainly stimulates calcium mobilization and inositol phosphate production in a GPR35-dependent manner in the presence of Gqi/o chimeric G proteins [142,143]. In the autoimmune diseases, the accumulation of kynurenic acid showed significant relation with mechanistic target of rapamycin (mTOR) activity [144]. Dai and his colleagues [145] reported that mTOR signaling is regulating runx2 in the skeletogenesis in the mice. Also, runx2 activates p13k/akt signaling in the skeletogenesis [146]. These lines of evidence strongly support the expected role of IDO in the chondrogenesis inhibition because the IDO deficient mice didn’t display any skeletal defects that supports the idea of IDO involves OA pathogenesis. Consequently, runx2 cooperates with mmp13 to degrade cartilage [147]. Further analysis showed that DNA methylation of runx2 P1 mediates mmp13 transaction in the chondrocytes [148]. So, the molecular activity of IDO to engage OA pathogenesis is extremely expected which encourage the deep investigation of IDO molecular activity in the chondrocytes. Upon the previous evidence it is supposed that activation of runx2 and mmp13 in the chondrocytes could be stimulated by IDO high levels in the synovial fluid which lead to initiate chondrocytes apoptosis and inhibition of chondrogenic proliferation but these suggestions need deep research.
Cellular populations in the OA synovitis and possible relation with IDO
Although, there are many evidence have consistently presented immunological changes in the subchondral bone and synovium before and during OA initiation [149-151] yet the role of infiltrating immune cells and molecular signals remain largely uncertain [152-155]. Furthermore, OA synovial fluid and bone marrow MSCs, osteoblasts, osteoclasts, and chondrocytes were investigated deeply, it presents changes in the function in comparison to the same normal cells [156,157]. However, the reason for these changes and its immune cellular motives remains a big challenge. Many literatures have discussed the immune cell involvement in the OA pathophysiology [154,158-161] as seen in Table 3. CD4+ T cells were significantly described in the synovium and subchondral bone of OA [162,163], but T cell subsets population haven’t being discussed enough, which makes T cells role in OA is very complex. Moreover, there are various immune cells have been reported in the OA such as NK, Mast cells, Macrophage, mDCs, and pDCs etc164 [164-166]. However, there are no clear roles for these cells in the pathogenesis process. Also, reported cytokines or secreted chemokines didn’t figure out a clear working mechanism, which also suggests a role for other molecules haven’t been searched yet. As the population of MSCs and pDCs has been massively described in the OA patients synovial. Thus, it’s supposed that IDO1 has a potential role to orchestrate immune cell functions in the synovium cavity. Moreover, IDO1 overexpression has been detected in the RA synovial [12,14], as well as in OA synovial as shown in Table 2. Furthermore, recent reports described the active role of regulatory cells in the OA pathogenesis such as Treg cells (CD4+CD25+FOXP3+) [30,155,167], which strongly supports that regulatory pathways could has a key secret in the OA pathophysiology. Nevertheless, interesting evidence of IDO1 in the OA specimens is encouraging deep searching of IDO1 accumulation rate at OA different grades.
Table 3.
List of the Immune Cells in the Osteoarthritis which have a relation with IDO1 immune activity
| OA-Immune cell | Activity in the OA-Synovial fluid | Reference |
|---|---|---|
| CD19+CD20+ | Low activity | [166] |
| CD3+CD8+ | Low activity | [160,166] |
| T cells/B Cells CD4+/CD8+ | High activity, it could stimulate synovitis by production of proinflammatory cytokines | [152,161,162] |
| CD4+ T Cells/Active Macrophage | Med activity by secretion of TNF-α | [153,154] |
| Treg CD4+CD25+ | High activity, it could contribute to increase TGF-β1 and IL-10 that increase pain and joint stiffness. It increases activated effector memory cells (CD62L-CD69+) | [8,167] |
| CD4+CD25+ Tim-3+ | Significant levels in the OA more than RA that enhance regulatory responses | [155] |
| Macrophage CD68+/B Cell CD20+ | Low expression in the OA synoviits | [161] |
| Macrophage CD68+ | Significantly high in RA but limited increasing in the OA | [164,165] |
| Mast Cells | It was noticed in the OA synovial but with low populations | [64,168] |
| CD56+CD16-/Low cytotoxicity NK Cells/Neutrophils | Recently it was reported but with low numbers | [169,170] |
| plasmacytoid DCs/Myeloid DCs | These cells were significantly noticed in the synovial and bone marrow of OA patients but its function isn’t clear | [45,171] |
Moreover, as presented in Table 3 recent references reported an interesting population of mast cells, NKs, and neutrophils in the synovium of OA patients [64,168-170]. Interestingly, others reported the role of plasmacytoid DCs in the OA [45,171], which is supposed to produce IDO enzyme under the effect of proinflammatory mediators. So, this is confirming a possible role of IDO pathway in the OA pathophysiology because IDO1 expression showed direct effect on chondrocytes proliferation and collagen II in the matrix that suggesting a possible effect on the metalloproteinases. In addition, all previous literature searched OA have considered the same cell populations of rheumatoid arthritis (RA), so they concluded an inflammatory status but less than RA, while OA is distinctly different from RA. Therefore, there are some critical questions need to be answered; why the same immune populations were determined in the RA also have been reported in the OA? We speculate that OA infiltrating cells yet haven’t clear interpretation specify their mechanisms in the OA pathogenesis. Also, high expressed cytokines in OA has been ascribed to the same reasons of RA, which is a real confusing point. Immune cells mentioned in the OA synovial showed proinflammatory and sometimes regulatory functions as seen in Table 3. This contradiction presents serious obstacles hinder OA pathology understanding. Moreover, several reports concluded that T cell’s specific functions aren’t clear in the pathophysiology of OA [64,172]. A thorough screening for active immune cells in the subchondral bone or synovium of OA still need deep understanding. Most active cells, signals, and overexpressed molecules leading to subchondral bone thickness, sclerotic and inflammations haven’t been investigated well because the amount of literature reporting OA and disease mediating immune cells showed massive conflicts in their functions [82]. Hence, our experimental findings open a new route to study OA pathophysiology regarding IDO1 activity that importantly could contribute to solve OA pathogenesis secrete.
Signaling pathways in the OA and possible relation with IDO1
There are many signaling pathways have been reported in the OA presented in Table 4, but the main pathway orchestrating pathological mechanism is undetermined. Thus, deep understanding of signaling is needed to provide superior strategy to treat OA. Virous pathways play important roles involving chondrocyte metabolism, differentiation, proliferation, apoptosis, synthesis, and degradation of extracellular matrix (ECM) in the OA pathological stages, see in Table 4 such as SRY-related protein 9 (Sox9) [173], insulin-like growth factor (IGF), nuclear factor-κappa beta (NF-κβ), bone morphogenetic protein (BMP), transforming growth factor β (TGF-β) that make osteoarthritis pathogenesis understanding is too difficult [174,175], because of paradox functions, some of these pathways are proinflammatory and others are anti-inflammatory [176]. This ambiguity hinders therapeutic strategies advancement. Therefore, the discussion of OA reported pathways regarding their association with IDO could help to declare many important points because IDO pathway works as a bridge between inflammatory and anti-inflammatory mechanisms. In the following some of the most important signaling pathways in the AO pathogenesis with brief description of possible relation with IDO.
Table 4.
List of OA signaling pathways that has a relation with IDO activity
| Pathway | Function | Situ in The Joint | IDO Relation | Reference |
|---|---|---|---|---|
| RNAKL/NF-KBP65/IGFBP5/GDF5/BMP-7/PPRA/ACVR2B/SMAD2/IRAK1/iNOS2/TRFA6/CHRD1/TIR/CCN2 | Signaling | Synovial fluid/subchondral bone | These pathways induce IDO secretion and function | [177,178,179] |
| RALA | Sox9 regulation | Synovial | Inhibits IDO | [173] |
| MMP13 /MMP-1/2/3/9/ADAMTS4 | Matrix degradation | Synovial fluid/subchondral bone | These pathways could be stimulated by IDO1 | [7,59,176] |
| COX2/TNFα/JAK2/STAT3 | Induce inflammations | Synovial fluid | These factors have a potential to induce IDO production | [59,68,102,201,207] |
| COLA1/COL2A1/ACAN | Intracellular matrix | Articular cartilage | IDO1 regulates COAL2A1 production while enhances COLA1 | [87,97,188] |
| SOX9/NF-κB | Transcription | Synovial fluid/articular cartilage | IDO1 regulates soxy9 expression | [198,200] |
| TGF-β | Regulatory | Synovial fluid/subchondral bone/articular cartilage | Induces IDO activity and massively expressed in the presence of IDO | [32,60,120,140] |
Receptor activator of nuclear factor-κβ ligand (RANKL)
RANKL is one of TNF family that located on the osteoblasts. It triggers osteoclastogenesis by interaction with RANK receptor on the osteoclasts [177]. Its early expression after fracture suggests that RANKL plays a pivotal role to regulate immune response [177,178]. So, it has been widely studied in the RA and OA pathophysiology [179]. Scientists suggest that RANKL is induced by vascular endothelial growth factor (VEGF) in the RA synovitis [180,181]. Besides, RANKL levels in the synovial of RA patients was correlated with VEGF concentration [182]. They play unique role to induce osteoclastogenesis and inhibition of chondrogenesis. In the murine and chicken, chondrocytes regulate osteoclastogenesis by producing RANKL [183]. Moreover, RANKL has the potential to regulate osteoclasts differentiation [184]. In the OA RANKL was significantly associated with osteoproteogerin (OPG) that produced by osteoblasts [185]. Recent evidence suggested that OPG/RANKL may be implicated in the subchondral bone changes that lead to develop articular cartilage degeneration [185]. In addition, the functional consequence of RANKL expression by the articular chondrocytes remains unknown. It’s thought that RANKL reduced function participates in the induction of chondrocytes apoptosis [186]. Few years ago, by the study of New Zealand (NZ) rabbits, articular cartilage in response to synovitis diffuses RANKL to subchondral bone which extremely induces subchondral bone changes and mineralization that reduce cartilage nourishment leading to degeneration [187]. Recent reports revealed that RANKL/OPG affects chondrocytes to produce high levels of matrix metalloproteinase-13 leading to inhibit collagen II production and induce cartilage degradation [188]. On the other hand, regulatory function of RANKL has been noticed [189]. In addition, two studies reported immunosuppressive role of RANKL in the regulatory DCs and Treg cells by inducing IL-10 expression [190,191]. As known, regulatory DCs and IL-10 strongly increase IDO1 production, as well as regulatory DCs produces high levels of IDO [192,193]. This is suggesting that RANKL could has a role to induce IDO1 production in the synovial fluid and subchondral bone area that could enhance IDO related factors to promote OA development. This point needs more investigation because no direct relation has been reported between RANKL and IDO in the RA or any other arthritis.
Nuclear factor kappa-BP65 (NF-κβ P65)
NF-κβ is a transcriptional pathway that play critical role to regulate the expression of many genes. It contains five family inducible transcription factors (p65/RelA, RelB, cRel, p50, and p52), which form homodimers or heterodimers that bind DNA differentially [194,195]. Moreover, NF-κβ has a potential to enhance inflammatory responses and regulate immunosuppressive mechanisms through phosphorylation and release NF-κβ from its inhibitor to bind DNA [196]. Moreover, NF-κβ p65 phosphorylation has not yet been fully characterized, it mainly depends on the stimulus and cell type [197]. Recently, it was reported that NF-κβ p65 is induced in chondrocytes by IL-1β [198]. Importantly, in 2008 Chen and colleagues [199] suppressed the experimental OA by targeting NF-κβ p65 expression. They used adenoviral vector-mediated nuclear factor-κBp65 in the rat model to reduce OA. Results showed that downregulation of NF-κβ p65 significantly reduced early OA development in the rat model. Additional studies revealed that NF-κβ p65 mediates cellular proliferation, inflammatory cytokines, and apoptosis mediators [200]. Many researchers suggest that NF-κβ p65 works to induce many cytokines such as TNF-α, IL-6, IL-1β and IL-2, as well as induces enzymes such as cyclooxygenase (COX)-2, and nitric oxide synthase (iNOS) [201-203] that actively contribute in the OA pathophysiology. This is strongly suggesting that NF-κβ p65 could stimulate IDO secretion in the synovial fluid, because most of the factors being induced by NF-κβ p65 have the potential to stimulate IDO1 expression. Furthermore, targeting NF-κβ p65 in the OA was reported as potential therapeutic strategy [204,205] but because the association of this pathway with many biological activities makes this choice unsatisfied. Moreover, it was reported that NF-κβ p65 facilitates chondrogenic differentiation by early transient activation as well as it determines Sox9 early expression and other chondrogenic pathways [206]. It means targeting NF-κβ p65 to treat OA isn’t a good choice. There are another interesting targets have been reported recently such as stat3 [207]. However, the investigation of IDO pathway in OA pathogenesis could explore a novel therapeutic option.
Conclusion
This review is the first article discusses the role of IDO1 in the OA pathogenesis according to the previous literature and new experimental evidence from synovial fluid of OA patients. IDO1 could play an important role in the early stage of OA pathogenesis and participates in the immune modulations that inhibits chondrogenesis and enhance cartilage degeneration. Therefore, we recommend a deep investigation of IDO1 molecular mechanism in the OA disease.
Acknowledgements
This work has been supported by National Natural Science Foundation of China (No. 81972116; No. 81972085; No. 81772394; No. 81572198; Key Program of Natural Science Foundation of Gungdong Province (No. 2018B0303110003); Shenzhen Peacock Project (KQTD20170331100838136); Shenzhen Science and Technology Projects (No. JCYJ20170817172023838; No. JCYJ20170306092215436; No. JCYJ20170412150609690; JCYJ20170413161649437; No. JCYJ20170413161800287; No. SGLH20161209105517753); Special Funds for the Construction of High level Hospitals in Guangdong Province; International Science and Technology Cooperation Project 2019 (1035043).
Disclosure of conflict of interest
None.
Abbreviations
- OA
Osteoarthritis
- RA
Rheumatoid Arthritis
- IDO1
Indoleamine 2,3 dioxygenase 1
- SF
Synovial fluid
- SCB
Subchondral bone
- KO
knock out
- APC
Antigen presenting cell
- Ihh gene
Indian hedgehog gene
- MSCs
Mesenchymal Stem Cells
- ECM
Extracellular matrix
- OPG
Osteoproteogerin
- VEGF
Vascular endothelial growth factor
- mDCs
myeloid dendritic cells
- pDCs
plasmacytoid dendritic cells
- LPS
Lipopolysaccharides
- CDH11
Cadherin-11
- FLS
Fibroblast-like synoviocytes
- TDO
Tryptophan-2,3-dioxygenase
References
- 1.Kraus VB, Kilfoil TM, Hash TW, Mcdaniel G, Renner JB, Carrino JA, Adams SB. Atlas of radiographic features of osteoarthritis of the ankle and hindfoot. Osteoarthritis Cartilage. 2015;23:2059–2085. doi: 10.1016/j.joca.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villafañe JH, Valdes K, Pedersini P, Berjano P. Osteoarthritis: a call for research on central pain mechanism and personalized prevention strategies. Clin Rheumatol. 2019;38:583–584. doi: 10.1007/s10067-018-4270-4. [DOI] [PubMed] [Google Scholar]
- 3.Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, Haugen IK, Herrero-Beaumont G, Jonsson H, Kjeken I, Maheu E, Ramonda R, Ritt MJ, Smeets W, Smolen JS, Stamm TA, Szekanecz Z, Wittoek R, Carmona L. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16–24. doi: 10.1136/annrheumdis-2018-213826. [DOI] [PubMed] [Google Scholar]
- 4.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 5.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 7.Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moradi B, Schnatzer P, Hagmann S, Rosshirt N, Gotterbarm T, Lorenz HM, Tretter T, Zeifang F. Accumulation of CD4+CD25+/highCD127low/- regulatory T cells in osteoarthritis joints - analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Osteoarthritis Cartilage. 2014;22:S449. doi: 10.1186/ar4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moradi B, Schnatzer P, Hagmann S, Rosshirt N, Gotterbarm T, Kretzer JP, Thomsen M, Lorenz H, Zeifang F, Tretter T. CD4+CD25+/highCD127low/- regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints-analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16:1–13. doi: 10.1186/ar4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Silverman RM, Shen J, O’Keefe RJ. Distinct metabolic programs induced by TGF-β1 and BMP2 in human articular chondrocytes with osteoarthritis. J Orthop Translat. 2018;12:66–73. doi: 10.1016/j.jot.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigott E, Duhadaway JB, Muller AJ, Gilmour SK, Prendergast GC, Mandiknayak L. 1-Methyl-tryptophan synergizes with methotrexate to alleviate arthritis in a mouse model of arthritis. Autoimmunity. 2014;47:409–418. doi: 10.3109/08916934.2014.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merlo LMF, Grabler S, DuHadaway JB, Pigott E, Manley K, Prendergast GC, Laury-Kleintop LD, Mandik-Nayak L. Therapeutic antibody targeting of indoleamine-2,3-dioxygenase (IDO2) inhibits autoimmune arthritis. Clin Immunol. 2017;179:8–16. doi: 10.1016/j.clim.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res Ther. 2007;9:1–7. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz R, Smith C, Duhadaway JB, Chandler P, Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubnoff D, Bieber T. The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy. 2012;67:718–725. doi: 10.1111/j.1398-9995.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 16.Put K, Brisse E, Avau A, Imbrechts M, Mitera T, Janssens R, Proost P, Fallarino F, Wouters C, Matthys P. IDO1 deficiency does not affect disease in mouse models of systemic juvenile idiopathic arthritis and secondary hemophagocytic lymphohistiocytosis. PLoS One. 2016;11:e0150075. doi: 10.1371/journal.pone.0150075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bult CJ, Smith CL, Kadin JA, Richardson JE The Mouse Genome Database Group. Mouse genome database (MGD) Nucleic Acids Res. 2019;47:D801–D806. doi: 10.1093/nar/gky1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Criado G, Šimelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- 19.Furuzawacarballeda J, Fonsecacamarillo G, Lima G, Yamamotofurusho JK. Indoleamine 2,3-dioxygenase: expressing cells in inflammatory bowel disease-a cross-sectional study. Clin Dev Immunold. 2013;2013:278035–278035. doi: 10.1155/2013/278035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuzawa-Carballeda J, Lima G, Jakez-Ocampo J, Llorente L. Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: a cross-sectional study. Eur J Clin Invest. 2011;41:1037–1046. doi: 10.1111/j.1365-2362.2011.02491.x. [DOI] [PubMed] [Google Scholar]
- 21.Mbongue JC, Nicholas DA, Torrez TW, Kim N, Firek A, Langridge WH. The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel) 2015;3:703–729. doi: 10.3390/vaccines3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW, Cho SG. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep. 2016;6:26851. doi: 10.1038/srep26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh YH, Lee G, Song WH, Koh JT, Ryu JH. Crosstalk between FLS and chondrocytes is regulated by HIF-2α-mediated cytokines in arthritis. Exp Mol Med. 2015;47:e197. doi: 10.1038/emm.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husa M, Liubryan R, Terkeltaub R. Shifting HIFs in osteoarthritis. Nat Med. 2010;16:641–644. doi: 10.1038/nm0610-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediatedimmunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 28.Wisniewski H, Colon E, Liublinska V, Karia R, Stabler T, Attur M, Abramson SB, Band PA, Kraus VB. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:235–241. doi: 10.1016/j.joca.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malek N, Starowicz K. Joint problems arising from lack of repair mechanisms: can cannabinoids help? Br J Pharmacol. 2019;176:1412–1420. doi: 10.1111/bph.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15:223–223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adebayo OO, Ko FC, Wan PT, Goldring SR, Goldring MB, Wright TM, van der Meulen MCH. Role of subchondral bone properties and changes in development of load-induced osteoarthritis in mice. Osteoarthritis Cartilage. 2017;25:2108–2118. doi: 10.1016/j.joca.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remst DF, Blaney Davidson EN, Vitters EL, Blom AB, Stoop R, Snabel JM, Bank RA, van den Berg WB, van der Kraan PM. Osteoarthritis-related fibrosis is associated with both elevated pyridinoline cross-link formation and lysyl hydroxylase 2b expression. Osteoarthritis Cartilage. 2012;21:157–164. doi: 10.1016/j.joca.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, Richards CD, van den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 36.Chu CQ, Field M, Abney E, Zheng RQ, Allard S, Feldmann M, Maini RN. Transforming growth factor-beta 1 in rheumatoid synovial membrane and cartilage/pannus junction. Clin Exp Immunol. 1991;86:380–386. doi: 10.1111/j.1365-2249.1991.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalise JP, Pallotta MT, Narendra SC, Carlsson B, Iacono A, Namale J, Boon L, Grohmann U, Magnusson M. IDO1 and TGF-β mediate protective effects of IFN-α in antigen-induced arthritis. J Immunol. 2016;197:3142–3151. doi: 10.4049/jimmunol.1502125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alahdal M, Xing Y, Tang T, Liang J. 1-Methyl-D-tryptophan Reduces tumor CD133(+) cells, Wnt/β-catenin and NF-κβ p65 while enhances lymphocytes NF-κβ2, STAT3, and STAT4 pathways in murine pancreatic adenocarcinoma. Sci Rep. 2018;8:9869–9869. doi: 10.1038/s41598-018-28238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillot X, Rullé S, Mussard J, Falgarone G. Implication of IDO in RA patients treated by biologics. Ann Rheum Dis. 2011;70:A49–A49. [Google Scholar]
- 40.Williams RO. Exploitation of the IDO pathway in the therapy of rheumatoid arthritis. Int J Tryptophan Res. 2013;6:67–73. doi: 10.4137/IJTR.S11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren G, Su J, Zhang L, Zhao X, Ling W, Lhuillie A, Zhang J, Lu Y, Roberts AI, Ji W. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 42.Kong L, Zheng L, Qin L, Ho KKW. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong A, Ghassemi E, Yellowley CE. Nestin expression in mesenchymal stromal cells: regulation by hypoxia and osteogenesis. BMC Vet Res. 2014;10:173. doi: 10.1186/s12917-014-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marti LC, Pavon L, Severino P, Sibov T, Guilhen D, Moreira-Filho CA. Vascular endothelial growth factor-A enhances indoleamine 2,3-dioxygenase expression by dendritic cells and subsequently impacts lymphocyte proliferation. Mem Inst Oswaldo Cruz. 2014;109:70–79. doi: 10.1590/0074-0276130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirohata S, Yanagida T, Tomita T, Yoshikawa H. Increased generation of pre-plasmacytoid dendritic cells in bone marrow of rheumatoid arthritis. Mod Rheumatol. 2014;24:443–447. doi: 10.3109/14397595.2013.843759. [DOI] [PubMed] [Google Scholar]
- 46.Chen W. IDO: more than an enzyme. Nat Immunol. 2011;12:809. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- 47.Nagao M, Hamilton JL, Kc R, Berendsen AD, Duan X, Cheong CW, Li X, Im HJ, Olsen BR. Vascular endothelial growth factor in cartilage development and osteoarthritis. Sci Rep. 2017;7:13027. doi: 10.1038/s41598-017-13417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen P, Jiao Z, Zheng JS, Xu WF, Zhang SY, Qin A, Yang C. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep. 2015;5:16244–16244. doi: 10.1038/srep16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ning L, Ishijima M, Kaneko H, Kurihara H, Arikawa-Hirasawa E, Kubota M, Liu L, Xu Z, Futami I, Yusup A, Miyahara K, Xu S, Kaneko K, Kurosawa H. Correlations between both the expression levels of inflammatory mediators and growth factor in medial perimeniscal synovial tissue and the severity of medial knee osteoarthritis. Int Orthop. 2011;35:831–838. doi: 10.1007/s00264-010-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scanzello CR, Umoh E, Pessler F, Diaztorne C, Miles T, Dicarlo EF, Potter HG, Mandl LA, Marx RG, Rodeo SA. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17:1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Hulejova H, Baresova V, Klezl Z, Polanska M, Adam M, Senolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38:151–156. doi: 10.1016/j.cyto.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Sun L, Liu J, Su B, Shi L. Serum interleukin-15 levels are associated with severity of pain in patients with knee osteoarthritis. Dis Markers. 2013;35:203–206. doi: 10.1155/2013/176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage. 2002;10:277–281. doi: 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- 54.Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Walter H, Sevdalina L, Ulf ML. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14:108–116. doi: 10.2174/1573397113666170829155149. [DOI] [PubMed] [Google Scholar]
- 56.Alanara T, Karstila K, Moilanen T, Silvennoinen O, Isomaki P. Expression of IL-10 family cytokines in rheumatoid arthritis: elevated levels of IL-19 in the joints. Scand J Rheumatol. 2010;39:118–126. doi: 10.3109/03009740903170823. [DOI] [PubMed] [Google Scholar]
- 57.Furuzawa-Carballeda J, Alcocer-Varela J. Interleukin-8, Interleukin-10, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression levels are higher in synovial tissue from patients with rheumatoid arthritis than in osteoarthritis. Scand J Immunol. 1999;50:215–222. doi: 10.1046/j.1365-3083.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 58.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter RW, Wei X, Xu D. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finnson KW, Parker WL, Chi Y, Hoemann CD, Goldring MB, Antoniou J, Philip A. Endoglin differentially regulates TGF-β-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthritis Cartilage. 2010;18:1518–1527. doi: 10.1016/j.joca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Der Kraan PMV, Davidson ENB, Blom AB, Den Berg WBV. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis Cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6:95–105. doi: 10.5312/wjo.v6.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond AM, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Delarosa O, Lombardo E, Beraza A, Manchenocorvo P, Ramirez C, Menta R, Rico L, Camarillo E, Garcia L, Abad JL. Requirement of IFN-γ-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Engineering Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 66.Ohmatsu H, Humme D, Gonzalez J, Gulati N, Möbs M, Sterry W, Krueger JG. IL-32 induces indoleamine 2,3-dioxygenase+CD1c+ dendritic cells and indoleamine 2,3-dioxygenase+CD163+ macrophages: relevance to mycosis fungoides progression. OncoImmunology. 2017;6:e1181237. doi: 10.1080/2162402X.2016.1181237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Role of interleukin-1 inhibitors in osteoarthritis. Drugs Aging. 2012;29:343–358. doi: 10.2165/11599350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Choi YS, Park JK, Kang EH, Lee Y, Kim TK, Chung J, Zimmerer JM, Carson WE, Song YW, Lee YJ. Cytokine signaling-1 suppressor is inducible by IL-1beta and inhibits the catabolic effects of IL-1beta in chondrocytes: its implication in the paradoxical joint-protective role of IL-1beta. Arthritis Res Ther. 2013;15:1–11. doi: 10.1186/ar4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chadjichristos C, Ghayor C, Kypriotou M, Martin G, Renard E, Ala-Kokko L, Suske G, de Crombrugghe B, Pujol JP, Galéra P. Sp1 and Sp3 transcription factors mediate interleukin-1β down-regulation of human type ii collagen gene expression in articular chondrocytes. J Biol Chem. 2003;278:39762–39772. doi: 10.1074/jbc.M303541200. [DOI] [PubMed] [Google Scholar]
- 70.Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990;144:499–505. [PubMed] [Google Scholar]
- 71.Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol. 1992;148:466–473. [PubMed] [Google Scholar]
- 72.Elzaatari M, Chang Y, Zhang M, Shreiner AB, Kao JY. 101 Indoleamine-2,3-Dioxygenase 1 (IDO1) mediates gastric metaplasia via the induction of caspase 1 and the production of bioactive IL-1β. Gastroenterology. 2014:146. [Google Scholar]
- 73.Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610. doi: 10.1053/j.gastro.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 74.Oconnor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-γ and Tumor Necrosis Factor-α Mediate the Upregulation of Indoleamine 2,3-Dioxygenase and the induction of depressive-like behavior in mice in response to bacillus calmette-guérin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie W, Cai L, Yu Y, Gao L, Xiao L, He Q, Ren Z, Liu Y. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflammation. 2014;11:41–41. doi: 10.1186/1742-2094-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Peng Y, Liu L, Wu T, Zhang Y, Lian Y, Yang Y, Kelley KW, Jiang C, Wang Y. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw. 2015;26:15–25. doi: 10.1684/ecn.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pantsulaia I, Kalichman L, Kobyliansky E. Association between radiographic hand osteoarthritis and RANKL, OPG and inflammatory markers. Osteoarthritis Cartilage. 2010;18:1448–1453. doi: 10.1016/j.joca.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Kammermann JR, Kincaid SA, Rumph PF, Baird DK, Visco DM. Tumor necrosis factor-α (TNF-α) in canine osteoarthritis: immunolocalization of TNF-α, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthritis Cartilage. 1996;4:23–34. doi: 10.1016/s1063-4584(96)80004-5. [DOI] [PubMed] [Google Scholar]
- 79.Hammacher A, Ward LD, Weinstock J, Treutlein HR, Yasukawa K, Simpson RJ. Structure-function analysis of human IL-6: identification of two distinct regions that are important for receptor binding. Protein Sci. 1994;3:2280–2293. doi: 10.1002/pro.5560031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamamoto Y, Yamasuge W, Imai S, Kunisawa K, Hoshi M, Fujigaki H, Mouri A, Nabeshima T, Saito K. Lipopolysaccharide shock reveals the immune function of indoleamine 2,3-dioxygenase 2 through the regulation of IL-6/stat3 signalling. Sci Rep. 2018;8:15917. doi: 10.1038/s41598-018-34166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 84.Porée B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ, Malléin-Gerin F, Pujol JP, Boumediene K, Galéra P. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human Type II collagen gene expression in articular chondrocytes requires a decrease of Sp1·Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2007;283:4850–4865. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 85.Emma A, Paolo Matteo A, Domenico P, Rossella De A. Synovial fluid and serum concentrations of inflammatory markers in rheumatoid arthritis, psoriatic arthritis and osteoarthitis: a systematic review. Curr Rheumatol Rev. 2017;13:170–179. doi: 10.2174/1573397113666170427125918. [DOI] [PubMed] [Google Scholar]
- 86.Wang P, Zhu F, Konstantopoulos K. Interleukin-6 synthesis in human chondrocytes is regulated via the antagonistic actions of prostaglandin (PG)E2 and 15-deoxy-Δ12,14-PGJ2. PLoS One. 2011;6:e27630. doi: 10.1371/journal.pone.0027630. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, De Benedetti F, Poli V, Ciliberto G. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rübenhagen R, Schüttrumpf JP, Stürmer KM, Frosch KH. Interleukin-7 levels in synovial fluid increase with age and MMP-1 levels decrease with progression of osteoarthritis. Acta Orthop. 2012;83:59–64. doi: 10.3109/17453674.2011.645195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van de Loo FA, Kuiper S, van Enckevort FH, Arntz OJ, van den Berg WB. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol. 1997;151:177–191. [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H, Chen L, Lim G, Sung B, Wang S, Mccabe MF, Rusanescu G, Yang L, Tian Y, Mao J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–2954. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 92.Zhu H, Stybayeva G, Macal M, Ramanculov E, George MD, Dandekar S, Revzin A. A microdevice for multiplexed detection of T-cell-secreted cytokines. Lab Chip. 2008;8:2197–2205. doi: 10.1039/b810244a. [DOI] [PubMed] [Google Scholar]
- 93.Zangerle PF. Direct stimulation of cytokines(Il-1β, TNF-α, Il-6, Il-2, IFNγ, and GM-CSF)in whole blood: II. application to rheumatoid arthritis and osteoartheritis. Cytokine. 1992;4:568–575. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 94.Li S, Yin H, Zhang K, Wang T, Yang Y, Liu X, Chang X, Zhang M, Yan X, Ren Y. Effector T helper cell populations are elevated in the bone marrow of rheumatoid arthritis patients and correlate with disease severity. Sci Rep. 2017;7:4776–4776. doi: 10.1038/s41598-017-05014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li S, Ren Y, Peng D, Yuan Z, Shan S, Sun H, Yan X, Xiao H, Li G, Song H. TIM-3 genetic variations affect susceptibility to osteoarthritis by interfering with interferon gamma in CD4+ T cells. Inflammation. 2015;38:1857–1863. doi: 10.1007/s10753-015-0164-7. [DOI] [PubMed] [Google Scholar]
- 96.Wang H, Zeng Y, Zhang M, Ma H, Xu B, Jiang H, Wang J, Li G. CD56 bright CD16- natural killer cells are shifted toward an IFN-γ-promoting phenotype with reduced regulatory capacity in osteoarthritis. Hum Immunol. 2019;80:871–877. doi: 10.1016/j.humimm.2019.07.283. [DOI] [PubMed] [Google Scholar]
- 97.Ahmad R, Mabrouk ME, Sylvester J, Zafarullah M. Human osteoarthritic chondrocytes are impaired in matrix metalloproteinase-13 inhibition by IFN-γ due to reduced IFN-γ receptor levels. Osteoarthritis Cartilage. 2009;17:1049–1055. doi: 10.1016/j.joca.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 98.Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11:681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 99.Nagaraj RH, Mailankot M. Interferon-gamma induces indoleamine 2,3-dioxygenase and causes apoptosis in lens epithelial cells. Int J Biochem Cell Biol. 2010;51:2618–2618. doi: 10.1016/j.biocel.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarkar SA, Wong R, Hackl SI, Moua O, Gill RG, Wiseman A, Davidson HW, Hutton JC. Induction of indoleamine 2,3-dioxygenase by interferon-γ in human islets. Diabetes. 2007;56:72–79. doi: 10.2337/db06-0617. [DOI] [PubMed] [Google Scholar]
- 101.Dolhain RJ, ter Haar NT, Hoefakker S, Tak PP, de Ley M, Claassen E, Breedveld FC, Miltenburg AM. Increased expression of interferon (ifn)-gamma together with ifn-gamma receptor in the rheumatoid synovial membrane compared with synovium of patients with osteoarthritis. Rheumatology. 1996;35:24–32. doi: 10.1093/rheumatology/35.1.24. [DOI] [PubMed] [Google Scholar]
- 102.Lee J, Lee J, Park MK, Lim MA, Park EM, Kim EK, Yang EJ, Lee SY, Jhun JY, Park SH, Kim HY, Cho ML. Interferon gamma suppresses collagen-induced arthritis by regulation of Th17 through the induction of indoleamine-2,3-deoxygenase. PLoS One. 2013;8:e60900–e60900. doi: 10.1371/journal.pone.0060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang W, Ogarra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 105.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 106.Ding J, Niu X, Su Y, Li X. Expression of synovial fluid biomarkers in patients with knee osteoarthritis and meniscus injury. Exp Ther Med. 2017;14:1609–1613. doi: 10.3892/etm.2017.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waly NE, Refaiy A, Aborehab NM. IL-10 and TGF-β: roles in chondroprotective effects of glucosamine in experimental osteoarthritis? Pathophysiology. 2017;24:45–49. doi: 10.1016/j.pathophys.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Cicuttini FM, Byron KA, Maher D, Wootton AM, Muirden KD, Hamilton JA. Serum IL-4, IL-10 and IL-6 levels in inflammatory arthritis. Rheumatol Int. 1995;14:201–206. doi: 10.1007/BF00262298. [DOI] [PubMed] [Google Scholar]
- 109.Riyazi N, Slagboom E, de Craen AJ, Meulenbelt I, Houwing-Duistermaat JJ, Kroon HM, van Schaardenburg D, Rosendaal FR, Breedveld FC, Huizinga TW, Kloppenburg M. Association of the risk of osteoarthritis with high innate production of interleukin-1β and low innate production of interleukin-10 ex vivo, upon lipopolysaccharide stimulation. Arthritis Rheum. 2005;52:1443–1450. doi: 10.1002/art.21014. [DOI] [PubMed] [Google Scholar]
- 110.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy-review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 111.van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JAN, Bernsen MR, van Osch GJVM. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186–1196. doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Zhao C, Zhang L, Kong W, Liang J, Xu X, Wu H, Feng X, Hua B, Wang H, Sun L. Umbilical cord-derived mesenchymal stem cells inhibit cadherin-11 expression by fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol Res. 2015;2015:10. doi: 10.1155/2015/137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 114.Luo XH, Liao E, Su X. Progesterone upregulates TGF-b isoforms (b1, b2, and b3) expression in normal human osteoblast-like cells. Calcif Tissue Int. 2002;71:329–334. doi: 10.1007/s00223-001-2129-0. [DOI] [PubMed] [Google Scholar]
- 115.Singh VK, Arora D, Singh A. Antioxidant potential and in-silico analysis of compounds from Citrus sinensis peel extracts against TGF-B isoforms. Biochem Biophys Res Commun. 2016;9:567–575. [Google Scholar]
- 116.Kasagi S, Chen W. TGF-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013;3:4. doi: 10.1186/2045-3701-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pilkington MF, Sims SM, Dixon SJ. Transforming growth factor-β induces osteoclast ruffling and chemotaxis: potential role in osteoclast recruitment. J Bone Miner Res. 2001;16:1237–1247. doi: 10.1359/jbmr.2001.16.7.1237. [DOI] [PubMed] [Google Scholar]
- 118.Dieudonné SC, Foo P, Van Zoelen EJJ, Burger EH. Inhibiting and stimulating effects of TGF-β1 on osteoclastic bone resorption in fetal mouse bone organ cultures. J Bone Miner Res. 1991;6:479–487. doi: 10.1002/jbmr.5650060509. [DOI] [PubMed] [Google Scholar]
- 119.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Finnson KW, Chi Y, Bougharios G, Leask A, Philip A. TGF-beta signaling in cartilage homeostasis and osteoarthritis. Front Biosci (Schol Ed) 2012;4:251–268. doi: 10.2741/S266. [DOI] [PubMed] [Google Scholar]
- 121.Loubaki L, Chabot D, Bazin R. Involvement of the TNF-α/TGF-β/IDO axis in IVIg-induced immune tolerance. Cytokine. 2015;71:181–187. doi: 10.1016/j.cyto.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 122.Nouel A, Pochard P, Simon Q, Segalen I, Meur YL, Pers J, Hillion S. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J Autoimmun. 2015;59:53–60. doi: 10.1016/j.jaut.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 123.Coletti A, Greco FA, Dolciami D, Camaioni E, Sardella R, Pallotta MT, Volpi C, Orabona C, Grohmann U, Macchiarulo A. Advances in indoleamine 2,3-dioxygenase 1 medicinal chemistry. Med Chem Comm. 2017;8:1378–1392. doi: 10.1039/c7md00109f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 125.Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci. 2017;3:52–56. [Google Scholar]
- 126.Romani R, Pirisinu I, Calvitti M, Pallotta MT, Gargaro M, Bistoni G, Vacca C, Michele AD, Orabona C, Rosati J. Stem cells from human amniotic fluid exert immunoregulatory function via secreted indoleamine 2,3-dioxygenase1. J Cell Mol Med. 2015;19:1593–1605. doi: 10.1111/jcmm.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I. Indoleamine 2,3-dioxygenase depletes tryptophan, activates general control non-derepressible 2 kinase and down-regulates key enzymes involved in fatty acid synthesis in primary human CD4+ T cells. Immunology. 2015;146:292–300. doi: 10.1111/imm.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salazar F, Awuah D, Negm OH, Shakib F, Ghaemmaghami AM. The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep. 2017;7:43337. doi: 10.1038/srep43337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. 2018;96:21–33. doi: 10.1111/imcb.1003. [DOI] [PubMed] [Google Scholar]
- 130.Dounay AB, Tuttle JB, Verhoest PR. Challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J Med Chem. 2015;58:8762–8782. doi: 10.1021/acs.jmedchem.5b00461. [DOI] [PubMed] [Google Scholar]
- 131.Jiang X, Xu L, Tang L, Liu F, Chen Z, Zhang J, Chen L, Pang C, Yu X. Role of the indoleamine-2,3-dioxygenase/kynurenine pathway of tryptophan metabolism in behavioral alterations in a hepatic encephalopathy rat model. J Neuroinflammation. 2018;15:3. doi: 10.1186/s12974-017-1037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bocelli-Tyndall C, Trella E, Frachet A, Zajac P, Pfaff D, Geurts J, Heiler S, Barbero A, Mumme M, Resink TJ, Schaeren S, Spagnoli GC, Tyndall A. FGF2 induces RANKL gene expression as well as IL1β regulated MHC class II in human bone marrow-derived mesenchymal progenitor stromal cells. Ann Rheum Dis. 2015;74:260–266. doi: 10.1136/annrheumdis-2013-204235. [DOI] [PubMed] [Google Scholar]
- 134.Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin Rheumatol. 2006;25:334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 135.Pertovaara M, Hasan T, Raitala A, Oja SS, Ylikerttula U, Korpela M, Hurme M. Indoleamine 2,3-dioxygenase activity is increased in patients with systemic lupus erythematosus and predicts disease activation in the sunny season. Clin Exp Immunol. 2007;150:274–278. doi: 10.1111/j.1365-2249.2007.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thaker AI, Rao MS, Bishnupuri KS, Kerr T, Foster L, Marinshaw JM, Newberry RD, Stenson WF, Ciorba MA. IDO1 metabolites activate β-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416–425. doi: 10.1053/j.gastro.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakai D, Suzuki T, Osumi N, Wakamatsu Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development. 2006;133:1323–1333. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- 139.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chang Y, Ueng SW, Linchao S, Chao CCK. Involvement of Gas7 along the ERK1/2 MAP kinase and SOX9 pathway in chondrogenesis of human marrow-derived mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16:1403–1412. doi: 10.1016/j.joca.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 141.Kanth VR, Lavanya K, Srinivas J. Elevated expression of indoleamine 2,3-dioxygenase (IDO) and accumulation of kynurenic acid in the pathogenesis of STZ-induced diabetic cataract in wistar rats. Curr Eye Res. 2009;34:274–281. doi: 10.1080/02713680902725954. [DOI] [PubMed] [Google Scholar]
- 142.Cosi C, Mannaioni G, Cozzi A, Carla V, Sili M, Cavone L, Maratea D, Moroni F. G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology. 2011;60:1227–1231. doi: 10.1016/j.neuropharm.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 143.Agudelo LZ, Ferreira DMS, Cervenka I, Bryzgalova G, Dadvar S, Jannig PR, Pettersson-Klein AT, Lakshmikanth T, Sustarsic EG, Porsmyr-Palmertz M, Correia JC, Izadi M, Martínez-Redondo V, Ueland PM, Midttun Ø, Gerhart-Hines Z, Brodin P, Pereira T, Berggren PO, Ruas JL. Kynurenic acid and Gpr35 Regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27:378–392. e5. doi: 10.1016/j.cmet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 144.Perl A, Hanczko R, Lai Z, Oaks Z, Kelly R, Borsuk R, Asara JM, Phillips PE. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics. 2015;11:1157–1174. doi: 10.1007/s11306-015-0772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dai Q, Xu Z, Ma X, Niu N, Zhou S, Xie F, Jiang L, Wang J, Zou W. mTOR/Raptor signaling is critical for skeletogenesis in mice through the regulation of Runx2 expression. Cell Death Differ. 2017;24:1886–1899. doi: 10.1038/cdd.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tandon M, Chen Z, Pratap J. Runx2 activates PI3K/Akt signaling via mTORC2 regulation in invasive breast cancer cells. Breast Cancer Res. 2014;16:R16. doi: 10.1186/bcr3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirata M, Kugimiya F, Fukai A, Saito T, Yano F, Ikeda T, Mabuchi A, Sapkota BR, Akune T, Nishida N, Yoshimura N, Nakagawa T, Tokunaga K, Nakamura K, Chung UI, Kawaguchi H. C/EBPβ and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2α as the inducer in chondrocytes. Hum Mol Genet. 2012;21:1111–1123. doi: 10.1093/hmg/ddr540. [DOI] [PubMed] [Google Scholar]
- 148.Takahashi A, De Andres MC, Hashimoto K, Itoi E, Otero M, Goldring MB, Oreffo ROC. DNA methylation of the RUNX2 P1 promoter mediates MMP13 transcription in chondrocytes. Sci Rep. 2017;7:7771. doi: 10.1038/s41598-017-08418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fang H, Huang L, Welch I, Norley C, Holdsworth DW, Beier F, Cai D. Early changes of articular cartilage and subchondral bone in the dmm mouse model of osteoarthritis. Sci Rep. 2018;8:2855. doi: 10.1038/s41598-018-21184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zamli Z, Robson Brown K, Sharif M. Subchondral bone plate changes more rapidly than trabecular bone in osteoarthritis. Int J Mol Sci. 2016;17:1496. doi: 10.3390/ijms17091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sniekers YH, Intema F, Lafeber FP, van Osch GJ, van Leeuwen JP, Weinans H, Mastbergen SC. A role for subchondral bone changes in the process of osteoarthritis; a micro-CT study of two canine models. BMC Musculoskelet Disord. 2008;9:20. doi: 10.1186/1471-2474-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ginaldi L, De Martinis M. Osteoimmunology and beyond. Curr Med Chem. 2016;23:3754–3774. doi: 10.2174/0929867323666160907162546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shen PC, Wu CL, Jou IM, Lee CH, Juan HY, Lee PJ, Chen SH, Hsieh JL. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19:728–736. doi: 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 154.Klein-Wieringa IR, de Lange-Brokaar BJ, Yusuf E, Andersen SN, Kwekkeboom JC, Kroon HM, van Osch GJ, Zuurmond AM, Stojanovic-Susulic V, Nelissen RG, Toes RE, Kloppenburg M, Ioan-Facsinay A. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43:771–778. doi: 10.3899/jrheum.151068. [DOI] [PubMed] [Google Scholar]
- 155.Li S, Wan J, Anderson W, Sun H, Zhang H, Peng X, Yu Z, Wang T, Yan X, Smith W. Downregulation of IL-10 secretion by Treg cells in osteoarthritis is associated with a reduction in Tim-3 expression. Biomed Pharmacother. 2016;79:159–165. doi: 10.1016/j.biopha.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 156.Hagmann S, Gotterbarm T, Müller T, Baesig A-M, Gantz S, Dreher T, Kämmerer PW, Frank S, Zeifang F, Moradi B. The influence of bone marrow- and synovium-derived mesenchymal stromal cells from osteoarthritis patients on regulatory T cells in co-culture. Clin Exp Immunol. 2013;173:454–462. doi: 10.1111/cei.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davatchi F, Abdollahi BS, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- 158.Hügle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology. 2016;56:1461–1471. doi: 10.1093/rheumatology/kew389. [DOI] [PubMed] [Google Scholar]
- 159.Könnecke I, Serra A, El Khassawna T, Schlundt C, Schell H, Hauser A, Ellinghaus A, Volk HD, Radbruch A, Duda GN, Schmidt-Bleek K. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone. 2014;64:155–165. doi: 10.1016/j.bone.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 160.Kraan MC, Haringman JJ, Post WJ, Versendaal J, Breedveld FC, Tak PP. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology. 1999;38:1074–1080. doi: 10.1093/rheumatology/38.11.1074. [DOI] [PubMed] [Google Scholar]
- 161.Geurts J, Patel A, Hirschmann MT, Pagenstert G, Mullergerbl M, Valderrabano V, Hugle T. Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J Orthop Res. 2016;34:262–269. doi: 10.1002/jor.23009. [DOI] [PubMed] [Google Scholar]
- 162.Ponchel F, Burska AN, Hensor EM, Raja R, Campbell M, Emery P, Conaghan PG. Changes in peripheral blood immune cell composition in, osteoarthritis. Osteoarthritis Cartilage. 2015;23:1870–1878. doi: 10.1016/j.joca.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li YS, Luo W, Zhu SA, Lei GH. T cells in osteoarthritis: alterations and beyond. Front Immunol. 2017;8:356. doi: 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 165.Netzer C, Urech K, Hugle T, Benz RM, Geurts J, Scharen S. Characterization of subchondral bone histopathology of facet joint osteoarthritis in lumbar spinal stenosis. J Orthop Res. 2016;34:1475–1480. doi: 10.1002/jor.23281. [DOI] [PubMed] [Google Scholar]
- 166.Zhu W, Zhang X, Jiang Y, Liu X, Huang L, Wei Q, Huang Y, Wu W, Gu J. Alterations in peripheral T cell and B cell subsets in patients with osteoarthritis. Clin Rheumatol. 2020;39:523–532. doi: 10.1007/s10067-019-04768-y. [DOI] [PubMed] [Google Scholar]
- 167.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kroner J, Kovtun A, Kemmler J, Messmann JJ, Strauss G, Seitz S, Schinke T, Amling M, Kotrba J, Froebel J. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J Bone Miner Res. 2017;32:2431–2444. doi: 10.1002/jbmr.3234. [DOI] [PubMed] [Google Scholar]
- 169.Jaime P, Garciaguerrero N, Estella R, Pardo J, Garciaalvarez F, Martinezlostao L. CD56+/CD16- natural killer cells expressing the inflammatory protease granzyme a are enriched in synovial fluid from patients with osteoarthritis. Osteoarthritis Cartilage. 2017;25:1708–1718. doi: 10.1016/j.joca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 170.Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom AB, Van Lent PLEM, Morrone S, Santoni A, Bernardini G. CXCR3/CXCL10 axis regulates neutrophil-NK cell cross-talk determining the severity of experimental osteoarthritis. J Immunol. 2017;198:2115–2124. doi: 10.4049/jimmunol.1601359. [DOI] [PubMed] [Google Scholar]
- 171.Hirohata S, Nagai T, Asako K, Tomita T, Yoshikawa H. Induction of type B synoviocyte-like cells from plasmacytoid dendritic cells of the bone marrow in rheumatoid arthritis and osteoarthritis. Clin Immunol. 2011;140:276–283. doi: 10.1016/j.clim.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 172.de Lange-Brokaar BJE, Ioan-Facsinay A, Yusuf E, Kroon HM, Zuurmond AM, Stojanovic-Susulic V, Nelissen RGHH, Bloem JL, Kloppenburg M. Evolution of synovitis in osteoarthritic knees and its association with clinical features. Osteoarthritis Cartilage. 2016;24:1867–1874. doi: 10.1016/j.joca.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 173.Karlsen TA, Jakobsen RB, Mikkelsen TS, Brinchmann JE. microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells Dev. 2014;23:290–304. doi: 10.1089/scd.2013.0209. [DOI] [PubMed] [Google Scholar]
- 174.Bailey AJ, Mansell JP, Sims TJ, Banse X. Biochemical and mechanical properties of subchondral bone in osteoarthritis. Biorheology. 2004;41:349–358. [PubMed] [Google Scholar]
- 175.Sanchez C, Deberg M, Bellahcene A, Castronovo V, Msika P, Delcour J, Crielaard J, Henrotin Y. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 2008;58:442–455. doi: 10.1002/art.23159. [DOI] [PubMed] [Google Scholar]
- 176.Matsukawa T, Sakai T, Yonezawa T, Hiraiwa H, Hamada T, Nakashima M, Ono Y, Ishizuka S, Nakahara H, Lotz MK, Asahara H, Ishiguro N. MicroRNA-125b regulates the expression of aggrecanase-1 (ADAMTS-4) in human osteoarthritic chondrocytes. Arthritis Res Ther. 2013;15:R28. doi: 10.1186/ar4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ito S, Hata T. Vitamins & Hormones. Academic Press; 2004. Crystal structure of RANK ligand involved in bone metabolism; pp. 19–33. [DOI] [PubMed] [Google Scholar]
- 178.Steen BM, Gerstenfeld LC, Einhorn TA. Chapter 17 - The Role of the Immune System in Fracture Healing. In: Lorenzo J, Horowitz MC, Choi Y, Takayanagi H, Schett G, editors. Osteoimmunology. 2nd Edition. San Diego: Academic Press; 2016. pp. 297–310. [Google Scholar]
- 179.Haerim K, Kyoungwoon K, Bomi K, Mila C, Sangheon L. The expression of VEGF and RANKL in the synovial fluid, serum, and synovial tissues of RA patients. PLoS One. 2015 [Google Scholar]
- 180.Haerim K, Kyoungwoon K, Bomi K, Mila C, Sangheon L. VEGF-induced RANKL expression in RA synovial fibroblasts. PLoS One. 2015;10:e0124909. [Google Scholar]
- 181.Henriksen K, Karsdal MA, Delaisse J, Engsig MT. RANKL and Vascular endothelial growth factor (vegf) Induce osteoclast chemotaxis through an ERK1/2-dependent mechanism. J Biol Chem. 2003;278:48745–48753. doi: 10.1074/jbc.M309193200. [DOI] [PubMed] [Google Scholar]
- 182.Kim HR, Kim KW, Kim BM, Cho ML, Lee SH. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PLoS One. 2015;10:e0124909. doi: 10.1371/journal.pone.0124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Usui M, Xing L, Drissi H, Zuscik MJ, Okeefe RJ, Chen D, Boyce BF. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2007;23:314–325. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xing L, Xiu Y, Boyce BF. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J Orthop. 2012;3:212–222. doi: 10.5312/wjo.v3.i12.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tat SK, Pelletier J, Velasco CR, Padrines M, Martelpelletier J. New perspective in osteoarthritis: the OPG and RANKL system as a potential therapeutic target? Keio J Med. 2009;58:29–40. doi: 10.2302/kjm.58.29. [DOI] [PubMed] [Google Scholar]
- 186.Shimizu S, Asou Y, Itoh S, Chung U, Kawaguchi H, Shinomiya K, Muneta T. Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheum. 2007;56:3358–3365. doi: 10.1002/art.22941. [DOI] [PubMed] [Google Scholar]
- 187.Martinezcalatrava MJ, Prietopotin I, Romanblas JA, Tardio L, Largo R, Herrerobeaumont G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res Ther. 2012;14:1–13. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zeng JZ, Wang ZZ, Ma LF, Meng H, Yu HM, Cheng WH, Zhang YK, Guo A. Increased receptor activator of nuclear factor κβ ligand/osteoprotegerin ratio exacerbates cartilage destruction in osteoarthritis in vitro. Exp Ther Med. 2016;12:2778–2782. doi: 10.3892/etm.2016.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 190.Williamson E, Bilsborough J, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-kappa B (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol. 2002;169:3606–3612. doi: 10.4049/jimmunol.169.7.3606. [DOI] [PubMed] [Google Scholar]
- 191.Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Akiba H, Okumura K. Rank-rankl signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol. 2009;182:6079–6087. doi: 10.4049/jimmunol.0711823. [DOI] [PubMed] [Google Scholar]
- 192.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 193.Wang Y, Yang BH, Li H, Cao S, Ren XB, Yu JP. IDO-DCs and signalling pathways. Curr Cancer Drug Targets. 2013;13:278–288. doi: 10.2174/15680096113139990073. [DOI] [PubMed] [Google Scholar]
- 194.Patel H, Zaghloul N, Lin K, Liu SF, Miller EJ, Ahmed M. Hypoxia-induced activation of specific members of the NF-kB family and its relevance to pulmonary vascular remodeling. Int J Biochem Cell Biol. 2017;92:141–147. doi: 10.1016/j.biocel.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 195.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li J, Chen S, Chen W, Ye Q, Dou Y, Xiao Y, Zhang L, Minze LJ, Li XC, Xiao X. Role of the NF-κB family member relb in regulation of foxp3+ regulatory t cells in vivo. J Immunol. 2018;200:1325–1334. doi: 10.4049/jimmunol.1701310. [DOI] [PubMed] [Google Scholar]
- 197.Vermeulen L, De Wilde G, Notebaert S, Berghe WV, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-κB p65 subunit. Biochem Pharmacol. 2002;64:963–970. doi: 10.1016/s0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 198.Haseeb A, Chen D, Haqqi TM. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1Ser376 in human articular chondrocytes. Rheumatology. 2013;52:998–1008. doi: 10.1093/rheumatology/kes363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen L, Lin L, Wang H, Wei X, Fu X, Zhang J, Yu C. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-κBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16:174–184. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 200.Giridharan S, Srinivasan M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J Inflamm Res. 2018;11:407–419. doi: 10.2147/JIR.S140188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 202.Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res Ther. 2017;19:94. doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 204.Romanblas JA, Jimenez SA. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 205.Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1:e000061. doi: 10.1136/rmdopen-2015-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Caron MM, Emans PJ, Surtel DA, Cremers A, Voncken JW, Welting TJM, Van Rhijn LW. Activation of NF-κB/p65 facilitates early chondrogenic differentiation during endochondral ossification. PLoS One. 2012;7:e33467. doi: 10.1371/journal.pone.0033467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lee SY, Lee SH, Na HS, Kwon JY, Kim GY, Jung K, Cho KH, Kim SA, Go EJ, Park MJ, Baek JA, Choi SY, Jhun J, Park SH, Kim SJ, Cho ML. The therapeutic effect of STAT3 signaling-suppressed MSC on pain and articular cartilage damage in a rat model of monosodium iodoacetate-induced osteoarthritis. Front Immunol. 2018;9:2881. doi: 10.3389/fimmu.2018.02881. [DOI] [PMC free article] [PubMed] [Google Scholar]


