Abstract
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents, and its treatment still needs to be improved. Here, we assessed the antitumor ability of CB-5083, an oral inhibitor of P97, in osteosarcoma. MTT, colony formation, sphere formation, cell cycle and apoptosis assays and animal studies showed that CB-5083 significantly inhibited osteosarcoma cell growth in vitro and in vivo. The inhibition of P97 also led to suppression of endoplasmic reticulum-associated degradation (ERAD), thereby resulted in activation of the apoptosis function of the unfolded protein response (UPR), and ultimately induced the death of osteosarcoma cells. Furthermore, an analysis of clinical patient samples confirmed that P97 can predict the outcomes of patients with osteosarcoma. Our studies showed that CB-5083 inhibited the growth and stem cell abilities of osteosarcoma cells both in vitro and in vivo and might be a promising drug for osteosarcoma treatment.
Keywords: P97, protein homeostasis, osteosarcoma and stem cell
Introduction
Osteosarcoma is the most common primary malignant bone tumor, and its annual incidence rate among people aged less than 24 years is approximately 4.4 cases per 1 million, which indicates that osteosarcoma accounts for approximately 5% of childhood cancers [1,2]. The treatment strategy for this cancer involves neoadjuvant chemotherapy combined with surgery, and the 5-year survival rate of osteosarcoma patients is approximately 70% and has not improved since the 1980s due to the existence of a population of therapy-resistant tumor cells with the features of cancer stem cells (CSCs). Furthermore, no advances in overcoming chemoresistance have been achieved over the past 2 decades [3]. The identification of new therapies or drugs that would yield improved therapeutic effects and eliminate therapy-resistant tumor cells is imperative for the treatment of osteosarcoma.
The endoplasmic reticulum (ER) is essential for the folding and trafficking of proteins that enter the secretory pathway, where numerous genetic and environmental insults impede the ability of cells to properly fold and post translationally modify secretory and transmembrane proteins [4]. Due to the complicated nature of the tumor microenvironment, tumor development is always accompanied by changes in protein homeostasis [5]. The gathering of unfolded or misfolded proteins leads to ER stress (ERS), which then initiates the unfolded protein response (UPR) to restore protein homeostasis [5]. The UPR pathway can not only restore protein homeostasis but also induce cell apoptosis when unfolded or misfolded proteins inside the cells cannot be repaired [6]. The targeting of pathways related to protein homeostasis has become a new direction in cancer treatment, which requires the development of inhibitors of other regulators of protein homeostasis.
P97, which is also known as valosin-containing protein (VCP) and cell division cycle protein 48 (CDC48), is a member of the AAA+ (ATPases associated with diverse cellular activities) ATPase family [7]. P97 has many cellular functions, such as DNA replication and repair and cell cycle regulation, and the most important of these functions is the regulation of protein homeostasis [8,9]. This protein is an important regulator of ER-associated degradation (ERAD), which drives the degradation of irreparable unfolded or misfolded proteins [10-12]. P97 remodels and releases ERAD substrates into the cytosol, and the substrates are then ubiquitinated by the ubiquitin ligase complex and degraded by proteasomal proteins [13,14]. The inhibition of P97 can induce the accumulation of unfolded and misfolded proteins, which then leads to ERS and UPR.
Here, we investigated the oral P97 inhibitor CB-5083, which can inhibit osteosarcoma growth in vivo and in vitro and suppresses the stem cell abilities of osteosarcoma cells, and the results showed that CB-5083 is a promising compound for the treatment of osteosarcoma patients.
Materials and methods
Cell culture
hFOB1.19, U2OS, HOS, MNNG/HOS, 143B, SJSA-1, SAOS-2, MG63, and G292 cells were obtained from the American Type Culture Collection (ATCC). U2R (U2OS/MTX), a methotrexate-resistant derivative of the U2OS cell line, was kindly gifted by Dr. M. Serra (Instituti Ortopedici Rizzoli, Bologna, Italy). ZOS and ZOSM were collected by our lab from human osteosarcoma patients with primary tumors and metastases. All the cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) with 10% fetal bovine serum (FBS, Invitrogen) at 37°C in 5% CO2. Short tandem repeat profiling was used for cell line authentication.
Antibodies
The following antibodies were used: P97 (Abcam, ab109240), β-actin (CST, #3700), BIP (CST, #3177), PERK (CST, #5683), XBP1-S (CST, #27901), p-eIF2α (CST, #3398), CHOP (CST, #2895), and GAPDH (CST, #2118).
Cell viability assay
Osteosarcoma cells were seeded in 96-well plates at 4000 cells/well. After cell attachment, the cells were treated with different concentrations of CB-5083 for 72 hours, and the cell viability was detected as described previously [15].
Colony formation assay
The cells were treated with 0 μM, 0.25 μM and 0.5 μM CB-5083 for 24 hours and then seeded in 6-well plates at 500 cells/well. After a 12-day culture period, the cells were washed, fixed and dyed with crystal violet, and the plates were dried. Photographs were obtained, and the colonies that contained more than 50 cells were counted.
Cell cycle and apoptosis assay
The cells were treated with 0 μM, 0.5 μM and 1 μM CB-5083 for 48 hours and then collected, and proteins were detected as described previously [16].
Sphere formation assay
The cells were treated with 0 µM, 0.25 µM, or 0.5 µM CB-5083 for 24 hours, counted and seeded in 24-well low-adhesion plates at 500 cells/well. The cells were cultured for 14 days, photographs were obtained, and the spheres were counted.
RNA extraction and qPCR
The cells were treated with 0 μM and 0.5 μM CB-5083 for 48 hours, and RNA was then extracted using the RNAprep Pure Cell/Bacteria Kit (DP430, TIANGEN). PrimeScriptTM Master Mix (Perfect Real Time) (RR036A, Takara) and TB GreenTM Premix Ex TaqTM II (Tli RNaseH Plus) (RR820A, Takara) were used for cDNA synthesis and qPCR.
The RT2 Profiler PCR Array (QIAGEN, PAHS-176Z) was used to identify the CSC-related gene profile of osteosarcoma cells treated with 0.5 μM CB-5083. Eighty-four CSC-related genes were tested, and ACTB, B2M, GAPDH, HPRT1, and RPLP0 were used as control genes.
Primers
BIP-Forward: GAAAGAAGGTTACCCATGCAGT, BIP-Reverse: CAGGCCATAAGCAATAGCAGC; ATF4-Forward: ATGACCGAAATGAGCTTCCTG, ATF4-Reverse: GCTGGAGAACCCATGAGGT; CHOP-Forward: GGAAACAGAGTGGTCATTCCC, CHOP-Reverse: CTGCTTGAGCCGTTCATTCTC; GADD34-Forward: AGCCACGGAGGATAAAAGAACA, GADD34-Reverse: CTGAACGATACTCCCAGGACC; DNAJB9-Forward: TCTTAGGTGTGCCAAAATCGG, DNAJB9-Reverse: TGTCAGGGTGGTACTTCATGG; GAPDH-Forward: ACAACTTTGGTATCGTGGAAGG, GAPDH-Reverse: GCCATCACGCCACAGTTTC.
Animal study
The animal study was approved by the Institutional Review Board of Sun Yat-sen University. Nude mice were purchased from Shanghai Slac Laboratory Animal Company Limited and injected with SJSA-1 cells near the scapula. Once the average tumor volume reached approximately 100 mm3, the mice were randomly divided into two groups and treated with vehicle (DMSO in normal saline) or 100 mg/kg CB-5083. The tumor volume and body weight were measured every 5 days. After 30 days, the mice were sacrificed, and the tumors were weighed.
Immunochemistry staining
From January 2008 to December 2011, tumor samples were obtained from the patients during surgery at the Department of Musculoskeletal Oncology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. H&E and IHC staining were performed as described previously [17]. The study of patient samples was approved by the Institutional Review Board of Sun Yat-sen University, and informed consent was obtained from the patients prior to sample collection.
Statistical analysis
All the data were analyzed using IBM SPSS Statistics 21. The differences between two groups were assessed using two-tailed Student’s t test or χ2 test, and P<0.05 was considered statistically significant (*P<0.05, **P<0.01, and ***P<0.005).
Results
The P97 inhibitor CB-5083 effectively suppressed osteosarcoma cell line proliferation
Based on the function of P97 in solid tumors, we first tested the expression level of P97 in 11 osteosarcoma cell lines and the human osteoblast cell line hFOB1.19 and found that P97 was overexpressed in most osteosarcoma cell lines with the exception of MG63 and G292 (Figure 1A, 1B). These findings revealed the possibility that P97 might play important roles in osteosarcoma. We also treated these cells with various doses of CB-5083 for 72 hours, and the calculated half-maximal inhibitory concentration (IC50) values ranged from 0.3286 to 1.032 μM (Figure 1C, 1D). A colony formation assay was also conducted to test the antiproliferative activity of CB-5083, and we found that the colony number and colony size were also reduced in the SJSA-1 and U2OS cell lines after treatment with CB-5083 (Figure 1E, 1F). These results indicated that P97 is a key regulator in osteosarcoma and that the P97 inhibitor CB-5083 exhibits significant antiproliferative activity in osteosarcoma cells.
Figure 1.
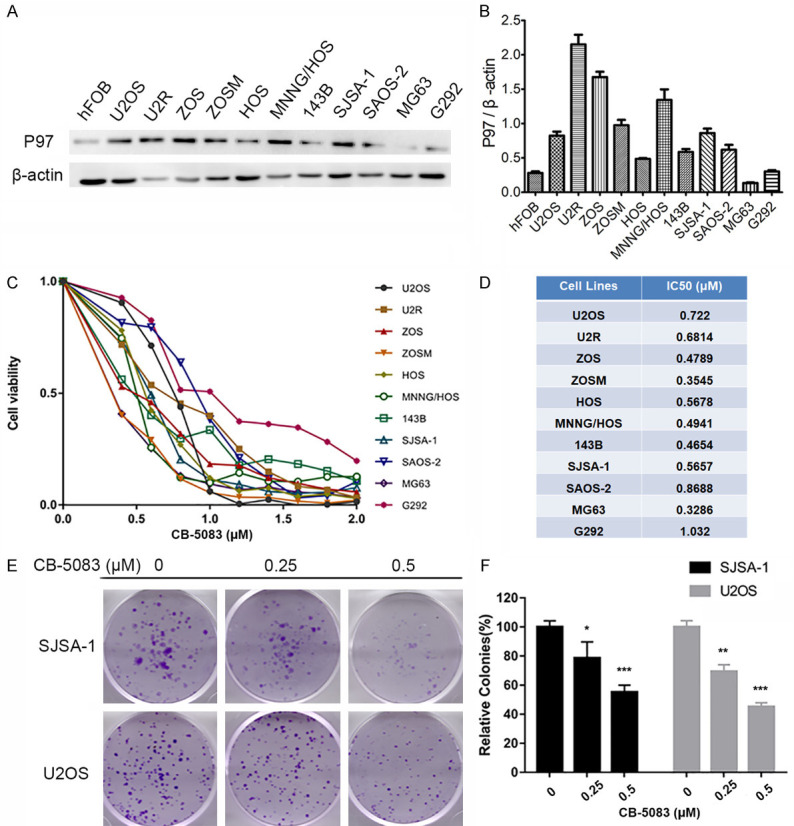
The P97 inhibitor CB-5083 suppressed osteosarcoma cell line proliferation. (A) P97 protein expression in 11 osteosarcoma cell lines (U2OS, U2R, ZOS, ZOSM, HOS, MNNG/HOS, 143B, SJSA-1, SAOS-2, MG63, and G292) and the human osteoblast cell line hFOB1.19. (B) Normalization of P97 protein expression by comparing the grayscale values of the Western blot bands. (C and D) Cell viability assay of 11 osteosarcoma cell lines treated with CB-5083. The cells were seeded in 96-well plates, cultured overnight and then treated with different concentrations of CB-5083 for 72 hours. The viable cells were detected using the MTT assay (C), and the IC50 values (D) were calculated. (E and F) Colony formation assay of SJSA-1 and U2OS cells treated with CB-5083. The cells were treated with CB-5083 for 24 hours, seeded at 500 cells/well in 6-well plates and cultured for 12 days. The cell colonies were the photographed, and the colonies that contained more than 50 cells were counted. *P<0.05, **P<0.01, and ***P<0.005 compared with the control group.
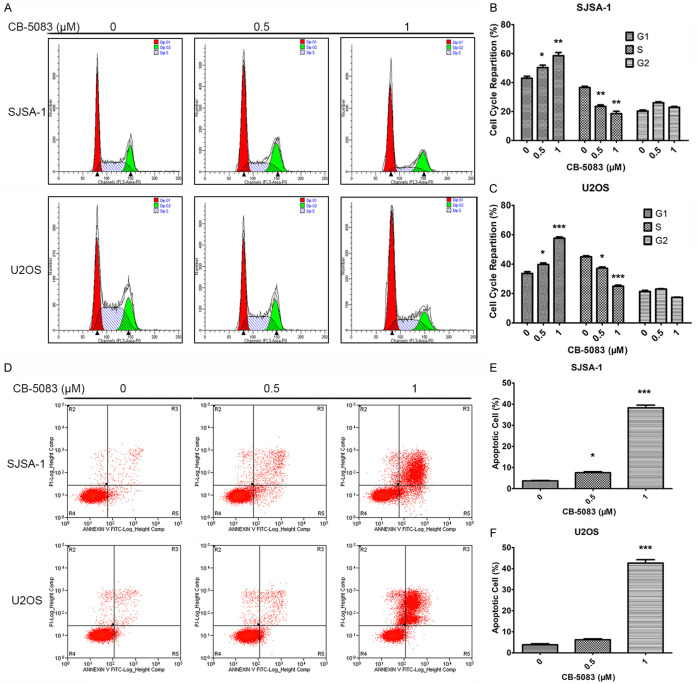
CB-5083 induced G1 cell cycle arrest and apoptosis in osteosarcoma cells
We wondered whether CB-5083 could affect cell cycle progression in osteosarcoma cells. As demonstrated by propidium iodide (PI) staining, the treatment increased the percentages of cells at the G0/G1 phase, decreased the percentages of cells at the S phase, and did not affect the percentages of cells at the G2/M phase, and this finding was obtained for both SJSA-1 and U2OS cells (Figure 2A-C). These results indicated that CB-5083 could induce G1 cell cycle arrest in osteosarcoma cells and thereby inhibit cell proliferation. The effect of the P97 inhibitor on cell apoptosis was also detected, and we found that CB-5083 could induce osteosarcoma cell apoptosis (Figure 2D-F). The above results showed that the P97 inhibitor affects both the cell cycle progression and proliferation of osteosarcoma cells.
Figure 2.
Cell cycle and apoptosis assays of SJSA-1 and U2OS cells treated with CB-5083. (A) Cell cycle assay of SJSA-1 and U2OS cells treated with CB-5083. The cells were treated with CB-5083 for 48 hours, and cell cycle assays were performed using PI staining and flow cytometry. (B and C) Lengths of the different cell cycle phases of CB-5083-treated (B) SJSA-1 and (C) U2OS cells. *P<0.05, **P<0.01, and ***P<0.005 compared with the control group. (D) Apoptosis assay of SJSA-1 and U2OS cells treated with CB-5083. The cells were treated for 48 hours, and cell apoptosis was detected by annexin V/PI staining and flow cytometry. (E and F) Percentages of apoptotic (E) SJSA-1 and (F) U2OS cells after CB-5083 treatment. *P<0.05, **P<0.01, and ***P<0.005 compared with the control group.
CB-5083 reduced osteosarcoma xenograft growth in nude mice
We discovered that the P97 inhibitor CB-5083 can inhibit osteosarcoma cell proliferation and effectively induce apoptosis, and these findings provide a potential avenue for the development of tumor therapies. Thus, the in vivo antitumor ability of the P97 inhibitor CB-5083 was investigated using a nude mouse xenograft model established with SJSA-1 cells. Once the tumor volume reached approximately 100 mm3, the mice were randomly divided into two groups (vehicle group and CB-5083 group): the CB-5083 group was treated with 100 mg/kg CB-5083 every day, whereas the vehicle group was treated with normal saline (with the same volume of DMSO). The P97 inhibitor CB-5083 inhibited tumor growth beginning 15 days after the first injection, and significant inhibition was detected through the end of the study (Figure 3A). The average tumor weight of the vehicle group was 823 mg, whereas that of the CB-5083 group was 416 mg, which also indicated an in vivo reduction in osteosarcoma growth (Figure 3B). The average body weights of the nude mice in the different groups showed no significant changes, which indicated that CB-5083 exhibits low toxicity (Figure 3C). Conclusively, these results demonstrated that the P97 inhibitor CB-5083 exhibits prominent antitumor properties and no distinct toxicity in vivo.
Figure 3.

CB-5083 inhibited osteosarcoma growth in vivo. Mice with osteosarcoma were divided into two groups and treated with vehicle or CB-5083. The tumor volume and body weight of each nude mouse were measured every 5 days. After 30 days, the mice were sacrificed, and the tumors were photographed and weighed. A. Volumes and photographs of tumors in nude mice with osteosarcoma treated with vehicle and CB-5083. **P<0.01. B. Weight of tumors in nude mice with osteosarcoma treated with vehicle and CB-5083. **P<0.01. C. Body weight of nude mice with osteosarcoma treated with vehicle and CB-5083.
CB-5083 suppressed CSC characteristics in osteosarcoma
CSCs possess the properties of self-renewal and maintenance of the tumor phenotype, which might lead to clinical treatment failure [18]. Subsequently, a sphere formation assay was conducted to test whether CB-5083 could suppress osteosarcoma CSC growth. After treatment with CB-5083 for 48 hours, the cells were counted and cultured in low-adhesion plates for 14 days. We found that CB-5083 significantly inhibited the sphere formation ability of SJSA-1 and U2OS cells (Figure 4A, 4B). Approximately 200 and 100 spheres were obtained from the analysis of the control cells and the cells treated with 0.5 μM, respectively (Figure 4B). To investigate the CB-5083 treatment-mediated changes in the expression of CSC-related genes, the RT2 Profiler PCR Array was used. The results showed that CB-5083 treatment induced a significant decline in the expression of 20 genes, and among these, DKK1, IL8 and MYC exhibited the most substantial changes in expression (Figure 4C, 4D).
Figure 4.

CB-5083 suppressed cancer stem cell characteristics in osteosarcoma. (A and B) Sphere formation assay of SJSA-1 and U2OS cells treated with CB-5083. The cells were treated with CB-5083 for 24 hours, seeded at 500 cells/well in 24-well low-adhesion plates, and cultured for 14 days. The spheres were the photographed (A) and counted (B). (C and D) Cancer stem cell-related gene profile of SJSA-1 and U2OS cells treated with CB-5083.
CB-5083 induced apoptosis by affecting ERS
Subsequently, we further investigated the mechanism through which CB-5083 affects osteosarcoma. P97 is an important regulator of protein homeostasis that can affect ER-associated degradation [9,19]. Due to changes in the tumor microenvironment, many unfolded or misfolded proteins accumulate in the ER, and this accumulation induces ER stress [20,21]. The UPR is initiated for the restoration of protein homeostasis and the resolution of ES stress, and apoptosis is induced if the unfolded or misfolded proteins cannot be repaired [6]. The UPR pathway can be activated through three different arms: protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6).
Our results revealed that CB-5083 could activate two of these pathways (Figure 5). First, CB-5083 induced the release of heat shock protein family A (HSP70) member 5 (BIP), which activated PERK; the activation of PERK resulted in the phosphorylation of eukaryotic translation initiation factor 2 subunit alpha (eIF2α) and subsequently the translation of activating transcription factor 4 (ATF4); and ATF4 upregulated the transcription of CCAAT/enhancer-binding protein homologous protein (CHOP) and thereby induced growth arrest and DNA damage-inducible 34 (GADD34)-mediated cell apoptosis in osteosarcoma (Figure 5). Second, CB-5083 activated the IRE1 pathway, which cut and spliced X-box binding protein 1 (XBP1) and led to the accumulation of CHOP transcripts and cell apoptosis (Figure 5). Furthermore, DnaJ heat short protein family (Hsp40) member B9 (DNAJB9), which is a gene involved in ER stress and the UPR, was also overexpressed in the cells treated with CB-5083 (Figure 5A-D). In summary, CB-5083 activates the UPR through the PERK and IRE1 pathways, which leads to cell apoptosis in osteosarcoma.
Figure 5.
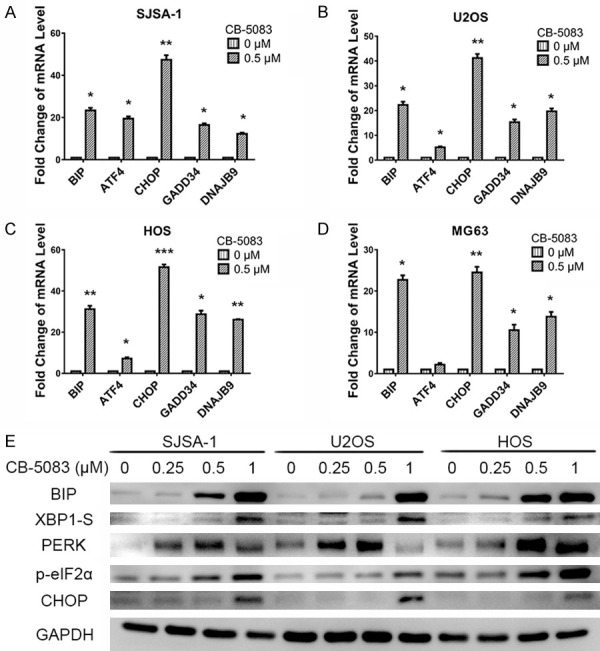
CB-5083 induced cancer cell apoptosis by affecting ER stress. (A-D) mRNA levels of UPR-related genes (BIP, ATF4, CHOP, GADD34, and DNAJB9) in (A) SJSA-1, (B) U2OS, (C) HOS, and (D) MG63 cells treated with CB-5083. (E) Western blotting of UPR-related genes (BIP, XBP1-S, PERK, p-elF2α, and CHOP) in osteosarcoma cells treated with CB-5083.
P97 predicted osteosarcoma patient prognosis
The P97 inhibitor CB-5083 could inhibit cell growth and induce cell apoptosis in osteosarcoma, which show potential clinical significance. We speculated that P97 might be a good marker for predicting the prognosis of osteosarcoma patients. By analyzing samples from 84 patients with osteosarcoma, we found that 38 samples (45.2%) could be regarded as positive for P97, whereas 46 samples (54.8%) could be regarded as negative for this marker (Supplementary Table 1 and Figure 6). A Kaplan-Meier survival analysis showed that the P97-positive groups had a worse prognosis, with a 5-year overall survival rate of 23.7%, compared with the P97-negative groups (Figure 6B). Furthermore, an analysis of the correlation between P97 gene expression and the metastasis rate revealed that the P97-positive group exhibited a metastasis rate of 73.7%, which was markedly higher than that of the P97-negative group (Table 1). The above-mentioned results show that the overexpression of P97 in osteosarcoma patients is correlated with a high risk of metastasis and poor prognosis.
Figure 6.
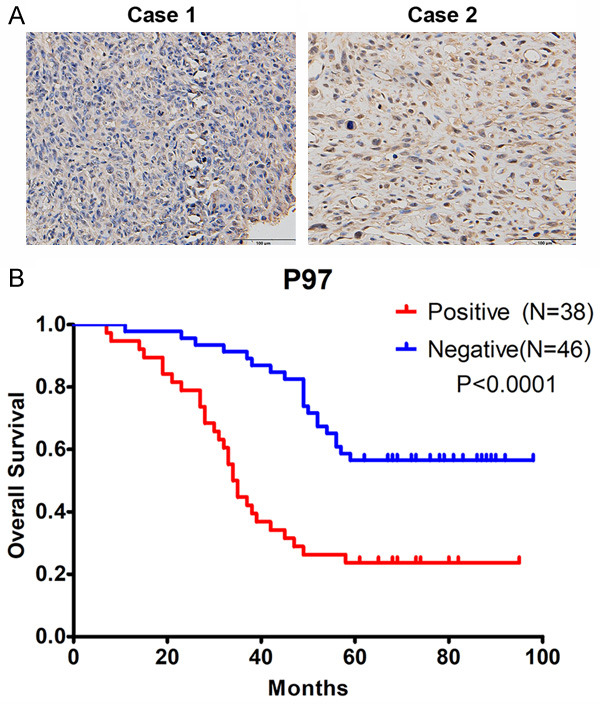
P97 overexpression was related to poor prognosis in osteosarcoma patients. A. Eighty-four surgical tumor samples were collected from January 2008 to December 2011. Representative images of P97 staining in the osteosarcoma patient samples are shown. B. Kaplan-Meier survival analysis of osteosarcoma patients based on P97 expression. P<0.0001.
Table 1.
Correlation between P97 gene expression and the metastasis rate in osteosarcoma
| Gene expression (number of patients) | Number of metastasis patients | Metastasis rate (%) | P value |
|---|---|---|---|
| P97-positive (38) | 28 | 73.7% | <0.0001 |
| P97-negative (46) | 12 | 26.1% |
Discussion
The clinical outcome of patients with osteosarcoma can be improved by chemotherapy, and the 5-year survival rate has reached 60% to 70%. However, numerous patients with osteosarcoma are either not sensitive to chemotherapy or develop drug resistance with the current chemotherapy regimens [22]. At present, effective agents that target resistant tumor cells and cause less severe side effects is needed. Our study found that CB-5083, an oral P97 inhibitor, can significantly inhibit osteosarcoma cell growth in vitro and in vivo and suppress the proprieties of CSCs, which indicates that CB-5083 might be a promising drug against osteosarcoma.
In this study, we discovered that most osteosarcoma cell lines have a higher P97 expression level than the human osteoblast cell line hFOB1.19. Treatment with the P97 inhibitor CB-5083 led to significant cell cytotoxicity in all osteosarcoma cell lines, and the IC50 values ranged from 0.3286 µM to 1.032 μM. Cell cycle arrest is an essential early event in the inhibition of cell proliferation, and our cell cycle assay results showed that CB-5083 could induce G1 cell cycle arrest in osteosarcoma cells. Myc was also inhibited by CB-5083, and this inhibition might have induced the observed G1 cell cycle arrest. In addition, CB-5083 inhibited the colony formation ability of osteosarcoma cells in vitro. We also found that CB-5083 exhibited antitumor ability in nude mice without significant side effects. All the above-mentioned results suggest that CB-5083 might be a helpful drug for osteosarcoma treatment in the future.
CSCs are known to play crucial roles in chemoresistance, tumor metastasis and recurrence. Osteosarcoma stem cells were first discovered by the Gibbs group, who detected that a subgroup of osteosarcoma cells has sphere formation ability [23]. We also found that CB-5083 could inhibit the sphere formation ability of osteosarcoma cells, which indicated that this P97 inhibitor has anti-CSC function. In addition, CB-5083 repressed CSC-related gene expression in osteosarcoma cells, which shows that this inhibitor has the potential to target CSCs in osteosarcoma. At present, there are no highly effective drugs that can target CSCs in osteosarcoma, which is the recurrent and metastatic root of this terrible disease [24]. Our results showed that the targeting of P97 and protein homeostasis might be a new direction for the treatment of osteosarcoma, which would specifically involve the targeting of osteosarcoma stem cells.
Tumors are always accompanied by a disorder in protein homeostasis, which indicates that tumor cells suffer severe ERS caused by large amounts of unfolded or misfolded proteins [5]. ERAD and the UPR are the two pathways that can alter protein homeostasis in cancer cells [20,21,25]. In the ERAD pathway, unfolded or misfolded proteins are transferred to the cytoplasm and degraded by the proteasome [13,14,26], and the UPR pathway can not only lead to protein degradation but can also induce cell death if the proteins become undegradable [6]. P97 is a key regulator of protein homeostasis, which controls ERAD [9,10]. To regain protein homeostasis, cancer cells overexpress P97 to drive the degradation of unfolded or misfolded proteins. Our results confirmed this speculation and revealed that P97 is overexpressed in osteosarcoma cells compared with hFOB1.19 human osteoblast cells. The inhibition of P97 by CB-5083 suppressed the ERAD pathway and led to the accumulation of unfolded or misfolded proteins, and the UPR pathway was then activated to drive protein degradation and cell apoptosis. The UPR pathway can be divided into three arms, which are activated by three different proteins: PERK, IRE1 and ATF6. We discovered that CB-5083 could activate both the PERK- and IRE1-related UPR pathways and ultimately induced CHOP transcription and cell apoptosis in osteosarcoma cells. This finding confirmed that CB-5083 could inhibit P97 and ERAD, which resulted in the accumulation of irreparable unfolded or misfolded proteins, and the PERK- and IRE1-related UPR is then activated, which ultimately leads to cancer cell apoptosis.
In summary, we have demonstrated that CB-5083 exerts strong antitumor effects against human osteosarcoma cells without noticeable side effects and that CB-5083 can reduce the sphere formation ability through the downregulation of CSC-related genes. The underlying mechanism through which CB-5083 exhibits anti-osteosarcoma activity appears to involve the alteration of protein homeostasis. Our results showed that P97 can act as both a therapeutic target and a prognostic marker for osteosarcoma patients. Thus, CB-5083 might be a promising inhibitor that can be used in osteosarcoma treatment.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81602356 and 81702943), the Medical Science and Technology Research Foundation of Guangdong, China (No. A2018543), and the Scientific Research Project of Traditional Chinese Medicine Bureau of Guangdong Province, China (20202042).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PDQ Cancer Information Summaries. Bethesda (MD): 2002. Osteosarcoma and malignant fibrous histiocytoma of bone treatment (PDQ(R)): health professional version. [Google Scholar]
- 3.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, Ayala AG, Shuster JJ, Abelson HT, Simone JV, Vietti TJ. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 4.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Drie JH. Protein folding, protein homeostasis, and cancer. Chin J Cancer. 2011;30:124–137. doi: 10.5732/cjc.010.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura G. ER stress: to live or let die. Nat Cell Biol. 2014;10:695. [Google Scholar]
- 7.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 8.Meyer H, Weihl CC. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014;127:3877–3883. doi: 10.1242/jcs.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14:117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 10.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb Perspect Biol. 2013;5:a013185. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vekaria PH, Home T, Weir S, Schoenen FJ, Rao R. Targeting p97 to disrupt protein homeostasis in cancer. Front Oncol. 2016;6:181. doi: 10.3389/fonc.2016.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemus L, Goder V. Regulation of endoplasmic reticulum-associated protein degradation (ERAD) by ubiquitin. Cells. 2014;3:824–847. doi: 10.3390/cells3030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushioda R, Hoseki J, Nagata K. Glycosylation-independent ERAD pathway serves as a backup system under ER stress. Mol Biol Cell. 2013;24:3155–3163. doi: 10.1091/mbc.E13-03-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Yin JQ, Wu MS, Song G, Xie XB, Zou C, Tang Q, Wu Y, Lu J, Wang Y, Wang J, Kang T, Jia Q, Shen J. Dihydromyricetin activates AMP-activated protein kinase and P38(MAPK) exerting antitumor potential in osteosarcoma. Cancer Prev Res (Phila) 2014;7:927–938. doi: 10.1158/1940-6207.CAPR-14-0067. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Jia Q, Wu MS, Xie X, Wang Y, Song G, Zou CY, Tang Q, Lu J, Huang G, Wang J, Lin DC, Koeffler HP, Yin JQ, Shen J. Degalactotigonin, a natural compound from solanum nigrum L., inhibits growth and metastasis of osteosarcoma through GSK3beta inactivation-mediated repression of the Hedgehog/Gli1 pathway. Clin Cancer Res. 2018;24:130–144. doi: 10.1158/1078-0432.CCR-17-0692. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, Wu Y, Yin J, Xie X, Shen J, Kang T, Wang J. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-kappaB pathway. Cancer Lett. 2014;342:150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 18.Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshaies RJ. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014;12:94. doi: 10.1186/s12915-014-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oromendia AB, Amon A. Aneuploidy: implications for protein homeostasis and disease. Dis Model Mech. 2014;7:15–20. doi: 10.1242/dmm.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heare T, Hensley MA, Dell’Orfano S. Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr. 2009;21:365–372. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs CP Jr, Levings PP, Ghivizzani SC. Evidence for the osteosarcoma stem cell. Curr Orthop Pract. 2011;22:322–326. doi: 10.1097/BCO.0b013e318221aee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189–195. doi: 10.1016/j.canlet.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 26.Stach L, Freemont PS. The AAA+ ATPase p97, a cellular multitool. Biochem J. 2017;474:2953–2976. doi: 10.1042/BCJ20160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.