Abstract
Purpose: To detect the expression of AGO1 in cholangiocarcinoma and explore its role and significance in the progression of cholangiocarcinoma. Patients and methods: Using immunohistochemistry, Western blotting and qPCR, we examined the expression of AGO1 in cholangiocarcinoma tissues. Through the analysis of clinical case data, the relationship between AGO1 and clinical prognosis was explored. The effect of AGO1 on cholangiocarcinoma was verified by cell functional experiments. Finally, we examined the effects of AGO1 on EMT-related proteins and signaling pathways. Results: AGO1 is significantly upregulated in cholangiocarcinoma and is associated with the prognosis of cholangiocarcinoma. AGO1 can significantly increase the proliferation, migration and invasion of cholangiocarcinoma cell lines. AGO1 affects the prognosis of cholangiocarcinoma by affecting epithelial-mesenchymal transition (EMT)-related TGF-β-PI3K-AKT signaling pathways. Conclusion: AGO1 is an independent predictor of cholangiocarcinoma prognosis and a potential target for the treatment of cholangiocarcinoma.
Keywords: Cholangiocarcinoma (CCA), AGO1, epithelial-mesenchymal transition (EMT), prognosis
Introduction
Cholangiocarcinoma is a malignant tumor originating from the bile duct epithelium. It is anatomically divided into intrahepatic, extrahepatic and hilar cholangiocarcinomas. It is one of the most invasive and poorly prognostic tumors in malignant diseases of the digestive tract [1]. Since the 21st century, the incidence and mortality of cholangiocarcinoma have been increasing worldwide [2-4], and it has become the second most common hepatobiliary tumor after liver cancer [5].
Because conventional chemoradiotherapy provides limited long-term survival, radical surgery is still the only opportunity for CCA patients to achieve long-term survival. Unfortunately, many patients have missed the best time for radical surgery when diagnosed with CCA due to severe invasion and metastasis [6,7]. Therefore, studying the mechanism of tumor cell metastasis in patients with cholangiocarcinoma is essential for the selection of therapeutic targets for cholangiocarcinoma and prevention of recurrence.
Multiple miRNAs are regulated by the AGO family (AGO1, AGO2, AGO3 and AGO4) and are essential protein components of the RNAi machinery. The RNA-induced silencing complex (RISC) contains AGO family proteins [8]. AGO family proteins are required for the production of mature miRNAs [9]. Among the AGO family members, AGO1 is the main component of RISC and has been the most studied. Recently, researchers have discovered the role of AGO1 in cancer. Li et al. [10] found that AGO1 was a new early diagnostic marker that was associated with the development of colon cancer. AGO1 has also been found to play an important role in lung cancer and breast cancer [11,12]. However, the role of AGO in cholangiocarcinoma is still unclear. Therefore, exploring the role of AGO in cholangiocarcinoma and its use in clinical treatment is crucial.
Material and methods
Cell lines and culture
The Key Laboratory of General Surgery of Anhui Province provided the RBE, Hucct-1, Hccc9810, and QBC939 cell lines for this study. These cell lines were cultured in RPMI 1640 (Gibco, USA) medium containing 10% fetal bovine serum (FBS, Gibco, USA) in a 37°C incubator under 5% CO2.
Clinical information
This study used the paraffin sections of 110 patients with CCA diagnosed by pathological diagnosis at Anhui Provincial Hospital affiliated with the Anhui Medical University from January 2010 to January 2015. All patients underwent radical surgery, and the pathological diagnosis was CCA. No chemotherapy or radiation therapy was performed before surgery. Paraffin-embedded tissue samples, including tumor tissue specimens and their corresponding paracancerous tissue specimens, were obtained from the pathology department. CCA tissues 2 cm above the tumor margin were defined as adjacent tissues. The clinicopathological data were obtained from retrospective medical histories, including age, sex, tumor location, tumor size, organization academic grade, lymph node metastasis (LNM), CA-199, and tumor TNM staging. Details are provided in Table 1. This study was approved by the Ethics Committee of Anhui Provincial Hospital.
Table 1.
Correlation between AGO1 expression and clinicopathologic characteristics of extrahepatic cholangiocarcinoma
| Parameter | n | AGO1 Expression | p-value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (years) | ||||
| <60 | 39 | 12 | 27 | 0.446 |
| >60 | 71 | 27 | 44 | |
| Gender | ||||
| Male | 53 | 23 | 30 | 0.093 |
| Female | 57 | 16 | 41 | |
| Tumor size | ||||
| <3 cm | 66 | 21 | 45 | 0.329 |
| >3 cm | 44 | 18 | 26 | |
| Tumor location | ||||
| Perihilar | 71 | 22 | 49 | 0.186 |
| Distal | 39 | 17 | 22 | |
| Differentiation | ||||
| Well + moderate | 79 | 33 | 46 | 0.027 |
| Poor | 31 | 6 | 25 | |
| lymph invasion | ||||
| Absent | 82 | 35 | 47 | 0.007 |
| Present | 28 | 4 | 24 | |
| CA-199 | ||||
| <37 | 19 | 12 | 7 | 0.006 |
| >37 | 91 | 27 | 64 | |
| TNM stage | ||||
| I-II | 76 | 34 | 42 | 0.002 |
| III-IV | 34 | 5 | 29 | |
Immunohistochemistry
The paraffin sections were dewaxed by heating in a 60°C oven for 20 min, dehydrated by xylene, hydrated in a graded concentration of ethanol (100%, 95%, and 75%), and then rinsed with PBS solution. Each slice was placed in a citrate buffer (Thermo, USA) and then transferred into a pressure cooker inside a container, heated and boiled, and the pressure valve was closed 5 min before the pressure was released. The endogenous peroxidase activity was blocked by 3% H2O2; the blocking time was 10 min, and the slides were washed in PBS 3 times for 3 min each time. An antibody diluted at 1:100 was added and incubated overnight at 4°C. DAB (GK6007, Gentech, Shanghai, China) was employed to visualize the signal, and color development was monitored under a microscope. Finally, hematoxylin was used as a counterstain, alcohol was used to dehydrate the samples, xylene was applied to clear the sample, the slide was sealed with gum and examined under a microscope, and photographs were captured.
Immunohistochemistry result analysis
According to the percentage of positive cells in 5 randomly selected 400× fields of 100 cells were scored as follows: greater than 60%: 3 points, 31% to 60%: 2 points, 11% to 30%: 1 point; less than 10%: 0 points. Cells were also scored according to staining intensity: 0 points for unstained, 1 point for light yellow, 2 points for yellow, and 3 points for brown. The scores were calculated by the same pathologist, and each samples that had a score that was >3 points for after the combination of both scores was identified as having positive expression.
Western blotting
A RIPA kit (Beyotime, Shanghai, China) was used to extract total protein from cholangiocarcinoma and paracancerous tissues, and the total protein concentration was determined using a BCA kit (Beyotime, Shanghai, China). Next, 10% SDS-PAGE products were transferred in a semi-dry manner to PVDF membranes, and the membranes were blocked in 5% skim milk for 1 h. The primary antibody (1:1,000) was incubated with the membranes overnight at 4°C. The membrane was washed 3 times with TBST for 5 min each time. The secondary antibody (1:5,000) was added and incubated for 1 h at room temperature on a shaker. The membranes were then washed with TBST 3 times for 5 min each time. The blots were captured and visualized using the Tanon Imaging System (Tanon, USA). Grayscale values of the target proteins and the internal reference proteins were analyzed using ImageJ software. The relative expression was calculated according to the ratio of the gray value of the target protein to the gray value of the internal reference protein.
qRT-PCR
Total RNA was isolated from tissues and cell lines using a TRIzol kit (Vazyme, Nanjing, China).
Total RNA samples were reverse transcribed into cDNA using SuperScriptTM III reverse transcriptase (Invitrogen, USA). A SYBR-based qPCR reaction was performed using an ABI 7500 FAST Real-Time PCR system (Applied Biosystems, Carlsbad). The relevant primer sequences are shown in the Antibodies and related sequences section. The expression level was analyzed by the 2-ΔΔCt method.
AGO1 silencing and overexpression
For AGO1 silencing, four shRNAs against AGO1 were designed. The target shRNA sequences are shown in the Antibodies and related sequences section. The full-length AGO1 cDNA was cloned into the pCMV6/AC/GFP vector for AGO1 overexpression. Silencing and overexpression of AGO1 were performed using a Takara Transfection Kit (Takara, USA) according to the manufacturer’s instructions.
Cell function experiments
The proliferation of cholangiocarcinoma cell lines was examined using a CCK-8 kit (Selleck, USA). The absorbance at 490 nm was measured at 0, 24, 48, and 72 h. Wound healing experiments were used to detect the ability of cancer cells to migrate. The following formula was used: (average wound area at 24 h - average wound area at 0 h)/average wound area at 0 h. Transwell experiments were used to compare the invasiveness of cancer cells. Using an 8-well Transwell chamber (Corning, USA), highly invasive cells passed through the chamber membrane. These cells were fixed with methanol and stained with crystal violet.
Xenograft model in nude mice
Healthy five-week-old BALB/c mice (Charles River, Beijing, China) weighing 20 g were used for the xenograft assay. The animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA), and the protocol was approved by the Ethics Committee of the Affiliated Provincial Hospital of Anhui Medical University.
Twelve nude mice were randomly divided into 4 groups and injected with Hucct-1/QBC939 cells (5×106 cells per mouse) with stable AGO1 knockdown or stably overexpressing AGO1 in the right underarm. The length (L) and width (W) of the tumor were measured every four days. The tumor volume formula is as follows: TV=(L×W2)/2. The nude mice were sacrificed 20 days later.
Antibodies and related sequences
AGO1 (5053, CST, Danvers); N-cadherin (13116, CST, Danvers); Vimentin (5741, CST, Danvers); Snail (ab108714, Abcam, UK); mTOR (2972, CST, Danvers); PI3K (4255, CST, Danvers); AKT (9272, CST, Danvers); TFG-β (3711, CST, Danvers); AGO1 siRNA-1 sense 5’-CCCAGAUACUCCACUAUGATT-3’; AGO1 siRNA-1 antisense 5’-UCAUAGUGGAGUAUCUGGGTT-3’; AGO1 siRNA-2 sense 5’-CCCACCCAUUUGAGUUUGATT-3’; AGO1 siRNA-2 antisense 5’-UCAAACUCAAAUGGGUGGGTT-3’; AGO1 siRNA-3 sense 5’-CCCACACAUUUGAGUCAGATT-3’; AGO1 siRNA-3 antisense 5’-UCAAACUCCCCUGCGUGGGTT-3’; AGO1 siRNA-4 sense 5’-CCCACCCAUGGGAGUUUGATT-3’; AGO1 siRNA-4 antisense 5’-UCAAACUAACCUGUGUGGGTT-3’; AGO1 sense 5’-GTTTCCCTCGTCAATGCCACGCTCTC-3’; AGO1 antisense 5’-GCAGCAGCGCCGCCAACTCCCTTAA-3’.
Statistics
Statistical analysis was performed using SPSS 17. 0 (SPSS, Inc., Chicago, IL, USA) and R3.2.3 (http://www.R-project.org). Categorical patient characteristics were compared using the χ2 test. Associations between AGO1, N-cadherin, Snail and vimentin were evaluated by Spearman’s rank correlation analysis. The Kaplan-Meier method was used to analyze CCA patient OS and DFS. Differences were assessed by a log-rank test. In the univariate and multivariate analyses, a Cox proportional hazard regression model was used to calculate the unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for survival. P<0.05 was considered statistically significant.
Results
Expression of AGO1 in cholangiocarcinoma and normal bile duct tissues
According to the immunohistochemistry results, the expression of AGO1 in histopathological sections of cholangiocarcinoma (64.5%, 71/110) was significantly higher than that in adjacent normal tissues (35.5%, 39/110) (P<0.05, Figure 1A; Tables 1, 3). Then, we examined the expression of AGO1 in 24 pairs of human specimens by Western blotting and qPCR. As shown in Figure 1B, 1C, the expression of AGO1 protein and mRNA in cholangiocarcinoma tissues was significantly higher than that in normal bile duct tissues (P<0.05).
Figure 1.
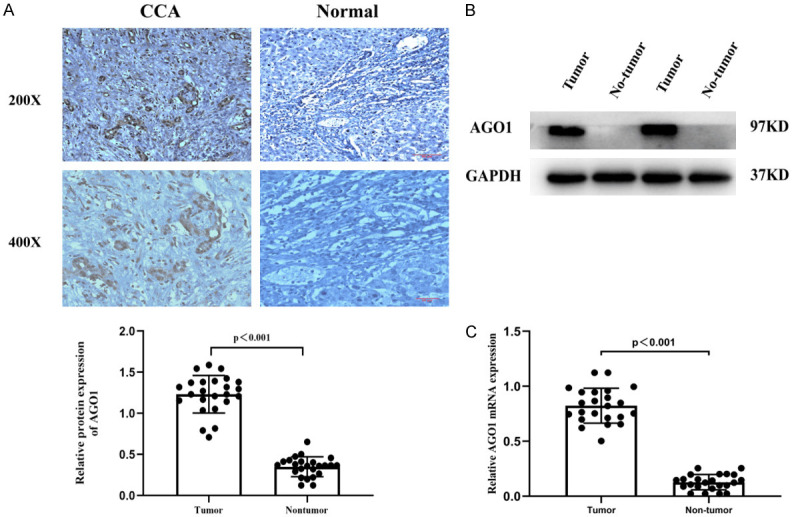
We examined the expression of AGO1 in cholangiocarcinoma tissues by immunohistochemistry, WB and qPCR. AGO1 was confirmed to be overexpressed in cholangiocarcinoma. A. Representative images of IHC staining for AGO1 with paraffin-embedded sections. Magnification 200×, 400×. B. Western blot analysis of AGO1 expression in 24 pairs of CCA tissues and matched adjacent normal tissues. C. qPCR analysis of AGO1 expression in 24 pairs of CCA tissues and matched adjacent normal tissues. Abbreviations: CCA, cholangiocarcinoma; IHC, immunohistochemistry; qPCR, quantitative real-time polymerase chain reaction.
Table 3.
Differential expression of AGO1 in CCA tissues and corresponding paracarcinoma tissues
| Tissue | AGO1 expression | P-value | |
|---|---|---|---|
|
| |||
| Low | High | ||
| CCA tissues | 39 | 71 | <0.001 |
| Paracarcinoma tissues | 81 | 29 | |
Expression of AGO1 in different cholangiocarcinoma cell lines and siRNA
In this study, we examined the expression of AGO1 in different cholangiocarcinoma cell lines. Consistent with the mRNA expression level, the AGO1 protein was highly expressed in the Hucct-1 and RBE cell lines and was expressed at low levels in the HCCC9810 and QBC939 cell lines. Therefore, we selected the Hucct-1 and QBC939 cell lines for subsequent experimental validation (Figure 2A, 2B).
Figure 2.
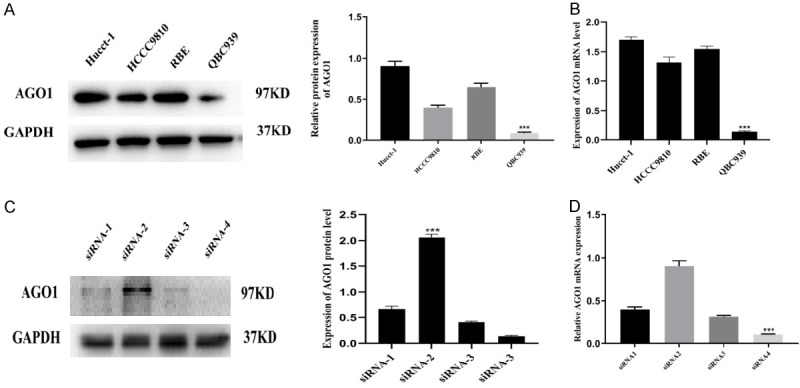
We examined the expression of AGO1 in four cholangiocarcinoma cell lines and tested the silencing efficiency of four small interfering RNAs. QBC939, Hucct-1 and siRNA-4 were used for subsequent experimental validation. A, B. The relative protein and mRNA expression of AGO1 in four CCA cell lines. C, D. The relative protein and mRNA expression of four small interfering RNA in AGO1 transfected cells.
We then used four small interfering RNAs to silence AGO1 in Hucct-1 cells. As shown in Figure 2C, 2D, siRNA-4 had the highest silencing efficiency and thus was used for subsequent experimental validation.
Relationship between AGO1 and epithelial-mesenchymal transition (EMT)-related proteins
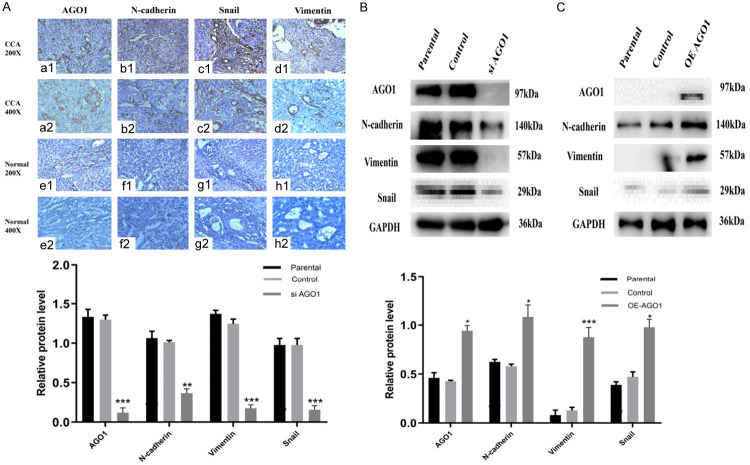
As shown in Table 2, among 110 patients with cholangiocarcinoma, 60 patients with cholangiocarcinoma had high expression of N-cadherin (60/71, 85.7%, Figure 3b1, 3b2, 3f1, 3f2), 65 patients with cholangiocarcinoma had high expression of Snail (65/71, 91.5%, Figure 3c1, 3c2, 3g1, 3g2), and 56 patients with cholangiocarcinoma had high expression of vimentin (56/71, 78.8%, Figure 3d1, 3d2, 3h1, 3h2). The expression of AGO1 (Figure 3a1, 3a2, 3e1, 3e2) in cholangiocarcinoma patients was positively correlated with the expression of N-cadherin (r=0.473, P<0.001; Table 2), Snail (r=0.261, P<0.05; Table 2) and vimentin (r=0.357, P<0.001; Table 2).
Table 2.
Correlations of AGO1 expression with E-cadherin, vimentin, Snail-1 in extrahepatic CCA
| Immunoreactivity | AGO expression | r-value | P-value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| N-cadherin expression | 0.473 | <0.001 | ||
| Low | 24 | 11 | ||
| High | 15 | 60 | ||
| Vimentin expression | ||||
| Low | 22 | 15 | 0.357 | <0.001 |
| High | 17 | 56 | ||
| Snail-1 expression | 0.006 | |||
| Low | 11 | 6 | 0.261 | |
| High | 28 | 65 | ||
Figure 3.
Immunohistochemical staining and Western blotting for AGO1, N-cadherin, Snail and vimentin. We examined the expression of EMT-related proteins in tissues and cell lines. The expression of AGO1 is positively correlated with the expression of EMT-related proteins. A. a1, a2: Positive AGO1 expression in CCA tissue. e1, e2: Negative AGO1 expression in adjacent tissue. c1, c2: Positive N-cadherin expression in CCA tissue. f1, f2: Negative N-cadherin expression in adjacent tissue. c1, c2: Positive Snail expression in CCA tissue. g1, g2: Negative Snail expression in adjacent tissue. d1, d2: Positive Vimentin expression in CCA tissue. h1, h2: Negative Vimentin expression in adjacent tissue. (magnification: ×200, ×400). B. Western blot analysis of EMT signaling molecules (N-cadherin, Snail and Vimentin) in AGO1 silenced Hucct1 cell line. C. Western blot analysis of EMT signaling molecules (N-cadherin, Snail and Vimentin) in AGO1 overexpressed QBC939 cell line. Representative of three independent experiments.
We then examined the expression of EMT-related proteins after knocking out and overexpressing AGO1 in cholangiocarcinoma cell lines. As shown in Figure 3B, the expression of N-cadherin, vimentin, and Snail decreased after silencing AGO1. After overexpression of AGO1, the expression of N-cadherin, vimentin, and Snail was elevated (Figure 3C).
Relationship between AGO1 and clinicopathological factors and prognosis
As shown in Table 1, the expression of AGO1 in cholangiocarcinoma was significantly associated with histological grade, lymph node invasion, advanced TNM staging, and preoperative serum CA19-9 (P<0.05). AGO1 had no relationship with age, sex, tumor size or tumor location.
Next, we assessed the relationship between AGO1 and the prognosis of cholangiocarcinoma by Kaplan-Meier analyses. As shown by the survival curve in Figure 4, patients with high AGO1 expression had shorter DFS (P<0.05) and OS (P<0.05) than patients with low expression.
Figure 4.
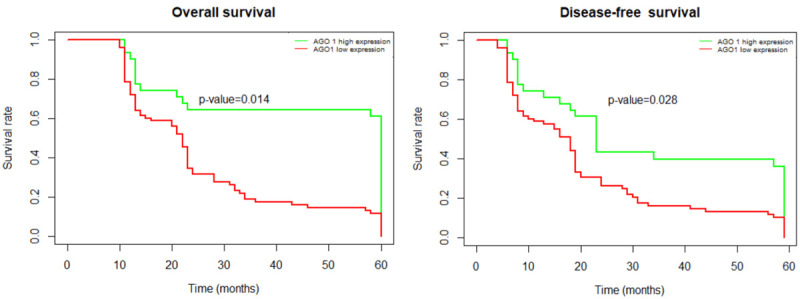
Kaplan-Meier survival analysis of association between AGO1 expression and prognosis of patients with cholangiocarcinoma. Patients with high AGO1 expression has a shorter OS compared with patients with low AGO1 expression (P<0.05). Patients with high AGO1 expression has shorter DFS compared with patients with low AGO1 expression (P<0.05). Abbreviations: DFS, disease-free survival; OS, overall survival.
By univariate analysis, AGO1 expression, histological grade, TNM staging and lymph node invasion had a significant effect on DFS and OS (Table 4).
Table 4.
Univariate and multivariate analysis of factors associated with OS and DFS
| OS | Univariate analysis | Multivariate analysis | ||
|
|
|
|||
| HR | P | HR | P | |
|
| ||||
| AGO1 Expression | 1.343 (1.231-2.457) | 0.014 | 1.467 (1.383-2.765) | 0.002 |
| Age | 0.753 (0.562-1.381) | 0.093 | ||
| Sex | 1.254 (0.736-1.678) | 0.435 | ||
| Grade | 2.136 (1.883-3.419) | 0.039 | 1.764 (1.231-3.513) | 0.004 |
| TNM | 1.645 (1.231-2.879) | 0.012 | 1.923 (1.733-3.897) | 0.032 |
| CA-199 | 1.212 (0.851-1.617) | 0.579 | ||
| Lymph invasion | 2.343 (1.921-3.764) | 0.011 | 2.213 (1.867-4.123) | 0.009 |
|
| ||||
| DFS | Univariate analysis | Multivariate analysis | ||
|
|
|
|||
| HR | P | HR | P | |
|
| ||||
| AGO1 Expression | 1.788 (1.456-2.745) | 0.028 | 1.289 (1.196-3.157) | 0.009 |
| Age | 0.967 (0.625-1.169) | 0.551 | ||
| Sex | 1.452 (0.366-1.834) | 0.544 | ||
| Grade | 1.636 (1.429-3.018) | 0.016 | 1.472 (1.109-3.374) | 0.017 |
| TNM | 1.993 (1.459-3.977) | 0.004 | 1.816 (1.045-2.798) | 0.042 |
| CA-199 | 1.749 (0.923-2.716) | 0.884 | ||
| Lymph invasion | 2.479 (1.743-3.845) | 0.007 | 2.495 (1.653-4.328) | 0.017 |
After the multivariate analysis, we found that AGO1 expression [DFS hazard ratio (HR) 1.289; 95% confidence interval (CI) 1.196-3.157; P<0.05; OS hazard ratio (HR) 01.467; 95% confidence interval (CI) 1.383-2.765; P<0.05], histological grade [DFS hazard ratio (HR) 1.472; 95% confidence interval (CI) 1.109-3.374; P<0.05; OS hazard ratio (HR) 1.764; 95% confidence interval (CI) 1.231-3.513; P<0.05], TNM stage [DFS hazard ratio (HR) 1.816; 95% confidence interval (CI) 1.045-2.798; P<0.05; OS hazard ratio (HR) 1.923; 95% confidence interval (CI) 1.733-3.897; P<0.05] and lymph node invasion [DFS hazard ratio (HR) 2.495; 95% confidence interval (CI) 1.653-4.328; P<0.05; OS hazard ratio (HR) 2.213; 95% confidence interval (CI) 1.867-4.123; P<0.05] were independent predictors of prognosis.
AGO1 can affect the proliferation, migration and invasion of cholangiocarcinoma cells
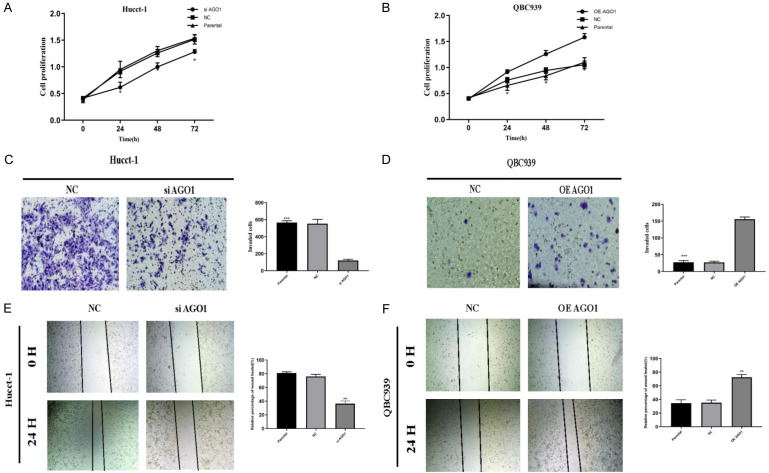
Using a CCK8 assay, we examined the effect of AGO1 on the proliferation of cholangiocarcinoma cell lines. After silencing AGO1, the proliferative capacity of the Hucct-1 cell line was significantly reduced (Figure 5A). After overexpression of AGO1, the proliferative capacity of the QBC939 cell line was significantly increased (Figure 5B).
Figure 5.
AGO1 enhances the proliferation, metastasis and invasion of cholangiocarcinoma in vitro. A. Silencing by siRNA reduced cell proliferation by CCK-8 assay in Hucct-1 cell line. B. Overexpression of AGO1 increased cell proliferation by CCK-8 assay in QBC939 cell line. C. Silencing AGO1 by siRNA reduced cell invasion by tanswell assay in Hucct-1 cell line. D. Overexpression of AGO1 increased cell invasion by transwell assay in QBC939 cell line. E. Silencing AGO1 by siRNA reduced cell migrate by wound healing assay in Hucct-1 cell line. F. Overexpression of AGO1 increased cell migrate by wound healing assay in QBC939 cell line.
Then, we examined the effect of AGO1 on the invasive ability of cholangiocarcinoma cells by cell invasion assay. After silencing AGO1, the invasive ability of the cell line was reduced (Figure 5C). After overexpression of AGO1, the invasive ability of the cell line was increased (Figure 5D).
Finally, we examined the effect of AGO1 on the migration of cholangiocarcinoma cells by wound healing experiments. As shown in Figure 5E, 5F, after silencing AGO1, the migration ability of the cell line was decreased, and after overexpression of AGO1, the migration ability of the cell line was increased.
Effect of AGO1 on the EMT-related non-Smad signaling pathway
Finally, we examined the effect of AGO1 on the EMT-related non-Smad signaling pathway by silencing and overexpressing AGO1 in cholangiocarcinoma cell lines. After silencing AGO1 in the Hucct-1 cell line, the expression of the components of the TGF-β-PI3K-AKT-mTOR pathway was significantly decreased (Figure 6A). After overexpression of AGO1 in the QBC939 cell line, the expression of the components of the TGF-β-PI3K-AKT-mTOR pathway was significantly increased (Figure 6B). Therefore, AGO1 may affect the epithelial-mesenchymal transition process by affecting the EMT-related non-Smad signaling pathway.
Figure 6.
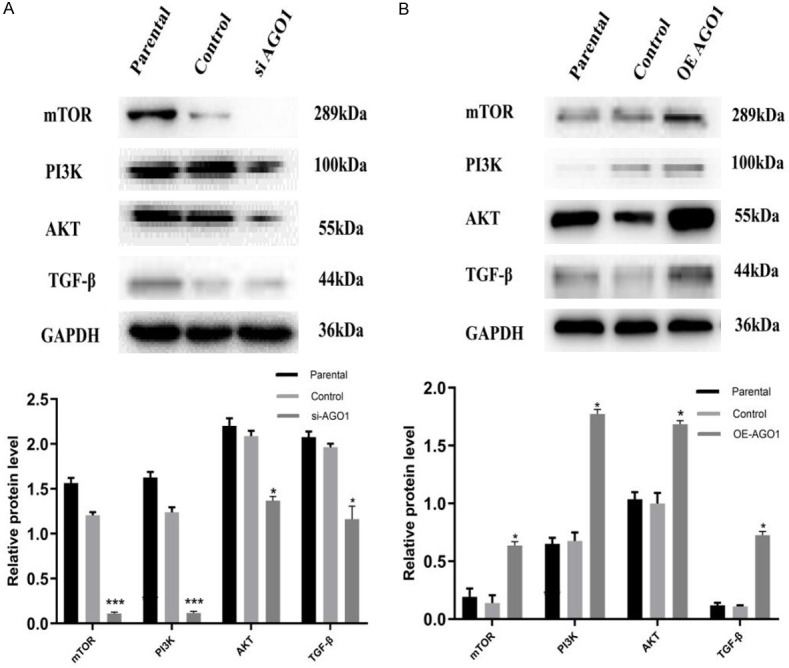
Regulation of EMTsignaling molecules by AGO1 expression in CCA cell lines. A. The relative protein expression of EMT signaling molecules (TGF-β, PI3K, AKT, and mTOR) in AGO1 silenced Huctt-1 cell line. B. The relative protein expression of EMT signaling molecules (TGF-β, PI3K, AKT, and mTOR) in AGO1 overexpressed QBC939 cell line.
The role of AGO1 in CCA according to xenograft models
Clinical data and in vitro results demonstrate that AGO1 affects the proliferation of cholangiocarcinoma. To further confirm the role of AGO1 in CCA, we examined the effect of AGO1 on tumor growth using a nude mouse subcutaneous xenograft model (Figure 7A). Tumor size and weight were significantly lower in the AGO1-silenced group than in the control group (P<0.001). Tumor size and weight were significantly increased in the AGO1-overexpressing group (P<0.001) (Figure 7B, 7C). Western blotting detected the expression of AGO1 in tumor tissues (Figure 7D).
Figure 7.
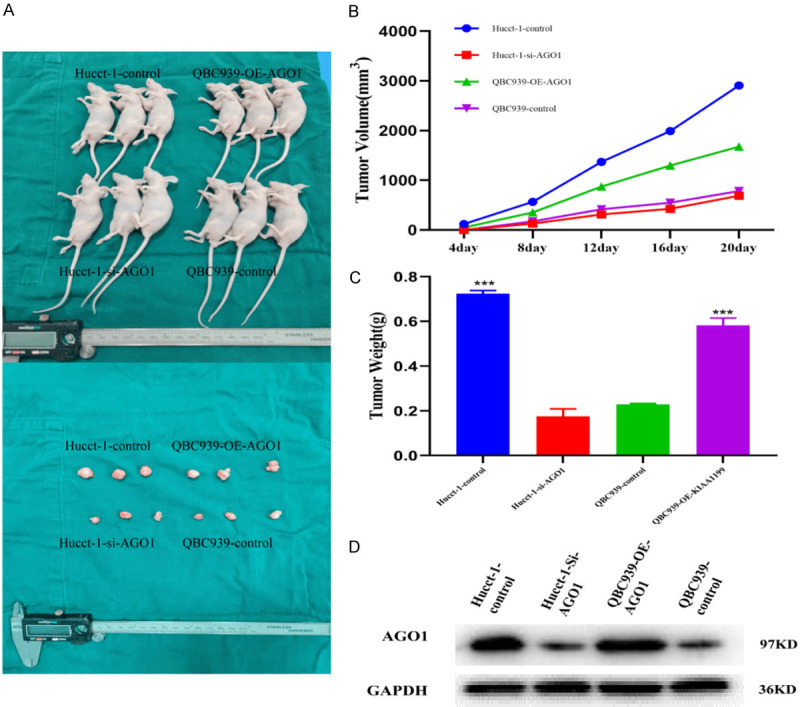
AGO1 promoted CCA growth in vivo. A. AGO1 knockdown reduced Hucct-1 cell xenograft tumor growth in nude mice while AGO1 upregulation enhanced QBC939 growth. B. Tumor volume was measured in every 4 day. C. Tumor weight in each group was measured on day 20. D. Expression of AGO1 in each group of tumor tissues.
Discussion
Early diagnosis of cholangiocarcinoma is difficult because of the lack of typical signs and symptoms. When bile duct cancer is diagnosed, many patients have entered an advanced stage. The early treatment of cholangiocarcinoma mainly includes surgery. Radical surgical resection is the only definitive treatment for cholangiocarcinoma. However, many patients have local and distant recurrence after tumor resection [13]. Chemotherapy is the main method for advanced cholangiocarcinoma, but its efficacy is poor, and it cannot effectively control the metastasis of cholangiocarcinoma. Therefore, finding a reliable biomarker associated with cholangiocarcinoma recurrence and metastasis is critical.
The Argonaute (AGO) family protein AGO1, an miRNA maturation regulator, is a core component of the miRISC complex. It stabilizes mature miRNA strands and plays an important role in degrading mRNA and impairing mRNA translation [14]. At present, an increasing number of studies are beginning to pay attention to the significance of miRNA maturation regulators in the progression of cancer. Argonaute proteins, as an important component of miRNA maturation regulators, have attracted the attention of some researchers. The tumor-specific expression of AGO1 has been reported in studies in several cancers, for example, clear cell renal cell carcinoma [15], prostate cancer [16], bladder cancer [17,18] and ovarian cancer [19,20]. The AGO1 protein is highly expressed in genitourinary tract tumors and has potential for diagnosis and prognosis. AGO1 expression is upregulated in epithelial skin cancer, estrogen receptor alpha-negative breast cancer cell lines [21], lung cancer [22], colon cancer [23], and esophageal squamous cell carcinoma [24], and it plays a key role in tumorigenesis. Wang et al. [25] also found that elevated levels of AGO1 expression were significantly associated with histological grade, lymph node metastasis, and distant metastasis in hepatocellular carcinoma. However, the role of AGO1 in the diagnosis and clinicopathological behavior of cholangiocarcinoma remains unknown.
In this study, we found that AGO1 was overexpressed in cholangiocarcinoma tissues. Through the analysis of follow-up data from a large number of patients, the expression of AGO1 was found to be associated with the prognosis of cholangiocarcinoma and tumor metastasis and recurrence. Tumor recurrence and metastasis characteristics are important factors affecting the prognosis of cholangiocarcinoma, and thus, these findings led us to further study the role of AGO1 in tumor metastasis. By silencing and upregulating the expression of AGO1 in cholangiocarcinoma cell lines, we found that AGO1 could enhance the proliferation, migration and invasion of cholangiocarcinoma cells. This result strongly supports the conclusions drawn from clinical data. The expression of AGO1 can enhance the metastasis and invasion of cholangiocarcinoma.
In the study of the mechanism of tumor recurrence and metastasis, epithelial-mesenchymal transition (EMT) is undoubtedly the most concern occurrence [26]. The process of EMT includes 1. loss of polarity between cells in the epithelial cell layer; 2. loss of cell adhesion; 3. marked cytoskeletal plasticity; and 4. increased cell motility and invasiveness. 5. After epithelial-mesenchymal transition in cholangiocarcinoma, the expression of the interstitial cell markers N-cadherin, Snail and vimentin was upregulated [27]. In the present study, we examined the expression of EMT-associated marker proteins after upregulating and silencing the expression of AGO1 in cholangiocarcinoma cell lines. The upregulation of N-cadherin, Snail and vimentin after the overexpression of AGO1 demonstrated that AGO1 regulates EMT in cholangiocarcinoma. We can also conclude that AGO1 affects the invasion and metastasis of cholangiocarcinoma by regulating EMT.
Tumor cells activate multiple signaling pathways during EMT, including the TGF-β signaling pathway, Wnt signaling pathway, nuclear transcription factor NF-κB signaling pathway and Notch signaling pathway [28]. In digestive tract tumors, Wang at el found that AGO1 regulated the EMT process in hepatocellular carcinoma by affecting the TGF-β signaling pathway [25]. In this study, we further explored the mechanism by which AGO1 regulates EMT. By detecting TGF-β signaling-mediated expression of non-Smad signaling pathway proteins, we found that AGO1 activates PI3K-AKT-mTOR signaling to participate in transcriptional regulation, thereby achieving EMT-triggered effects [29].
Conclusion
AGO1 may be a predictive biomarker for long-term follow-up of cholangiocarcinoma. Our data also suggest that, as a potential therapeutic target, we can control the growth and metastasis of cholangiocarcinoma by targeting AGO1 and affecting the TGF-β pathway.
Disclosure of conflict of interest
None.
References
- 1.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667–1674. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 5.Capussotti L, Vigano L, Ferrero A, Muratore A. Local surgical resection of hilar cholangiocarcinoma: is there still a place? HPB (Oxford) 2008;10:174–178. doi: 10.1080/13651820801992534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faehnle CR, Joshua-Tor L. Argonautes confront new small RNAs. Curr Opin Chem Biol. 2007;11:569–577. doi: 10.1016/j.cbpa.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosse GD, Simard MJ. A new twist in the microRNA pathway: not Dicer but Argonaute is required for a microRNA production. Cell Res. 2010;20:735–737. doi: 10.1038/cr.2010.83. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Yu C, Gao H, Li Y. Argonaute proteins: potential biomarkers for human colon cancer. BMC Cancer. 2010;10:38. doi: 10.1186/1471-2407-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JS, Choi YY, Jin G, Kang HG, Choi JE, Jeon HS, Lee WK, Kim DS, Kim CH, Kim YJ, Son JW, Jung TH, Park JY. Association of a common AGO1 variant with lung cancer risk: a two-stage case-control study. Mol Carcinog. 2010;49:913–921. doi: 10.1002/mc.20672. [DOI] [PubMed] [Google Scholar]
- 12.Sung H, Zhang B, Choi JY, Long J, Park SK, Yoo KY, Noh DY, Ahn SH, Zheng W, Kang D. Common genetic variants in the microRNA biogenesis pathway are not associated with breast cancer risk in Asian women. Cancer Epidemiol Biomarkers Prev. 2012;21:1385–1387. doi: 10.1158/1055-9965.EPI-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Liu M, Feng Y, Xu YF, Che JP, Wang GC, Zheng JH, Gao HJ. Evaluation of Argonaute protein as a predictive marker for human clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2013;6:1086–1094. [PMC free article] [PubMed] [Google Scholar]
- 16.Bian XJ, Zhang GM, Gu CY, Cai Y, Wang CF, Shen YJ, Zhu Y, Zhang HL, Dai B, Ye DW. Down-regulation of Dicer and Ago2 is associated with cell proliferation and apoptosis in prostate cancer. Tumour Biol. 2014;35:11571–11578. doi: 10.1007/s13277-014-2462-3. [DOI] [PubMed] [Google Scholar]
- 17.Yang FQ, Huang JH, Liu M, Yang FP, Li W, Wang GC, Che JP, Zheng JH. Argonaute 2 is up-regulated in tissues of urothelial carcinoma of bladder. Int J Clin Exp Pathol. 2014;7:340–347. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang G, Kong C, Bi J, Gong D, Yu X, Shi D, Zhan B, Ye P. EIF2C, Dicer, and Drosha are up-regulated along tumor progression and associated with poor prognosis in bladder carcinoma. Tumour Biol. 2015;36:5071–5079. doi: 10.1007/s13277-015-3158-z. [DOI] [PubMed] [Google Scholar]
- 19.Sand M, Skrygan M, Georgas D, Arenz C, Gambichler T, Sand D, Altmeyer P, Bechara FG. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2012;51:916–922. doi: 10.1002/mc.20861. [DOI] [PubMed] [Google Scholar]
- 20.Vaksman O, Hetland TE, Trope CG, Reich R, Davidson B. Argonaute, Dicer, and Drosha are up-regulated along tumor progression in serous ovarian carcinoma. Hum Pathol. 2012;43:2062–2069. doi: 10.1016/j.humpath.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Adams BD, Claffey KP, White BA. Argonaute-2 expression is regulated by epidermal growth factor receptor and mitogen-activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology. 2009;150:14–23. doi: 10.1210/en.2008-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang X, Yin Z, Li X, Xia L, Zhou B. Polymorphisms in GEMIN4 and AGO1 genes are associated with the risk of lung cancer: a case-control study in chinese female non-smokers. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanematsu S, Tanimoto K, Suzuki Y, Sugano S. Screening for possible miRNA-mRNA associations in a colon cancer cell line. Gene. 2014;533:520–531. doi: 10.1016/j.gene.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Yoo NJ, Hur SY, Kim MS, Lee JY, Lee SH. Immunohistochemical analysis of RNA-induced silencing complex-related proteins AGO2 and TNRC6A in prostate and esophageal cancers. APMIS. 2010;118:271–276. doi: 10.1111/j.1600-0463.2010.02588.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Zhang L, Liu Z, Zhou J, Pan Q, Fan J, Zang R, Wang L. AGO1 may influence the prognosis of hepatocellular carcinoma through TGF-beta pathway. Cell Death Dis. 2018;9:324. doi: 10.1038/s41419-018-0338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606–613. doi: 10.1007/s00534-012-0542-6. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci. 2010;67:2605–2618. doi: 10.1007/s00018-010-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]