Abstract
Hepatic macrophages play pivotal roles in tolerance induction after liver transplantation (LT). However, macrophages possess functional heterogeneities, and the protective role of M2c macrophages, a macrophage subtype characterized by the surface marker CD163 that secretes interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1), in acute rejection following LT, has not been addressed. The aim of this study was to determine whether polarized macrophages of the M2c subtype could improve outcomes after LT for rats, including survival rate, liver function, and inflammatory infiltration. In our study, the numbers of CD163-positive cells were found to be increased in tolerant liver grafts. Immediately following the surgery, M2c macrophages induced from rat bone marrow-derived cells were infused into recipients; this significantly improved survival rate and liver function. The expression levels of IL-10 and TGF-β1 were markedly increased in these rats compared to those in the control group. Furthermore, CD8+ T-cell infiltration was reduced, whereas the numbers of apoptotic cells increased, in rats treated with M2c. To explore the mechanisms of the protective role of M2c, the numbers of major histocompatibility complex (MHC) class II positive cells were found to be decreased and the expression of N-acetylglucosaminyltransferase V (MGAT5) was up-regulated in M2c infusion groups. Together, these findings demonstrate that polarization of macrophages towards the M2c phenotype ameliorated acute rejection in a rat LT model and may provide a novel and effective therapeutic approach for AR after transplantation.
Keywords: Acute rejection, liver transplantation, M2c, interleukin-10
Introduction
Liver transplantation (LT) remains the only effective treatment for chronic end-stage liver disease and acute liver failure [1]. Though traditional immunosuppressive agents greatly attenuate acute rejection (AR) of the liver allograft, they also cause a series of complications including tumor recurrence, severe infection, metabolic diseases, and drug-induced liver injury [2,3]. Therefore, new therapeutic strategies are essential to achieve both the therapeutic effect and the minimization or withdrawal of immunosuppression.
Recently, treatments with immunomodulatory cells have appeared to be effective strategies to prolong recipients’ survival time by preventing AR. For instance, pre-treatment of immature dendritic cells (imDCs), particularly imDCs that have been co-transfected with interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1), can prolong the survival time of liver allografts for rats [4,5]. The delayed infusion of donor bone marrow cells as well as regular T-cells (Treg) can induce donor-specific tolerance following LT [6-8]. Besides, adoptive transfer of bone marrow mesenchymal stem cells leads to the generation of Treg in liver grafts, and thereby improves the outcomes of allogeneic liver transplantation [9-11].
As important members of the innate immune systems, hepatic macrophages, also called Kupffer cells (KCs), are generally thought to mediate inflammatory responses that lead to liver injury [12]. However, our understanding of KCs has been revolutionized with research on heterogeneous subsets of KCs [13].
Macrophages are generally classified into two groups: pro-inflammatory M1 macrophages, and anti-inflammatory M2 macrophages that secrete high levels of anti-inflammatory cytokines and participate in the Th2 response [14]. Further studies have demonstrated that tolerant liver allografts contain a higher proportion of M2 macrophages, which secrete a high level of the immunomodulatory cytokine IL-10 and inhibit the proliferation of T lymphocytes through the Fas/FasL pathway [15]. In addition, a switch from the M1 to the M2 phenotype has been shown to significantly inhibit AR following liver transplantation in rats [16,17].
M2 macrophages can be further divided into several subsets: M2a, characterized by high expression levels of Fizz1, promotes type II immune responses and fibrogenesis; M2b, induced by immune complexes, secretes both IL-10 and inflammatory cytokines; and M2c, identified by the surface marker CD163, expresses high levels of IL-10 and TGF-β1 [18]. M2c macrophages play protective roles in many diseases such as in acute lung injury, adriamycin nephrosis, and systemic lupus erythematosus [19-21]. One of the best-characterized functions of M2c macrophages is to limit the duration and intensity of immune and inflammatory reactions in animal disease models [18,22]. However, whether M2c macrophages can alleviate AR in transplantation requires further elucidation. Thus, the purpose of the present study is to investigate the protective role of M2c macrophages in a rat orthotopic LT model, providing a novel strategy to prevent acute rejection.
Materials and methods
Animals
Inbred male Lewis and Brown Norway (BN) rats (8 weeks old, body weight 180-220 g) were purchased from Vital River, Inc. (Beijing, China) and maintained under specific pathogen-free conditions. All experiments were approved by the Animal Experiment Administration Committee of the Fourth Military Medical University and in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Rats orthotopic liver transplantation (OLT)
Rats orthotopic liver transplantation was performed according to the modified two-cuff method described by Kamada [23,24]. The AR model was established using the Lewis rats as donors and the BN rats as recipients. The tolerance model, on the other hand, was established using the BN rats as donors and the Lewis rats as recipients [25]. No immunosuppression agent was given to all recipients in this study.
Adoptive transfer protocol
In the adoptive transfer experiment, macrophages (3×106 cells per rat) were transferred into the recipients of AR model through portal vein by Braun Omnican Insulin Syringes, immediately following OLT. Recipients were divided into 4 groups (n = 13 per group) including AR+ phosphate buffered saline (PBS), AR+ M0 macrophages, AR+ Lewis-M2c macrophages, and AR+ BN-M2c macrophages. At day 7, five recipients of each group were sacrificed for collections of liver grafts and blood samples. The rest were monitored for survival.
Macrophage isolation and polarization
Bone marrow derived macrophages (BMDMs) were isolated and cultured from rats’ bone marrow as described preciously [26,27]. In brief, whole bone marrow cells were flushed from tibias and femurs of the BN or Lewis rats and dispersed mechanically using syringes. After centrifuging for 5 minutes at 300×g, Red Blood Cell Lysis Buffer (Solarbio) was used to lysis red blood cells. The remaining cells were resuspended and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. These bone marrow derived cells were stimulated with macrophage colony-stimulating factor (MCSF, Bioworld Technology, Inc) (20 ng/mL) for 7 days to become M0 macrophages. To further obtain M2c macrophages, the M0 macrophages were stimulated with dexamethasone (50 ng/ml) for 24 hours.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA of the BMDMs was isolated using RNAiso Plus reagent (Takara, Japan) and then reverse-transcribed by PrimScriptTM RT Master Mix (Takara, Japan). qRT-PCR was carried out with SYBR Premix EX TaqTM II kit (Takara, Japan). All primer sequences were summarized in Table 1.
Table 1.
Primers used for qRT-PCR
| Name | Sequence | |
|---|---|---|
| IL-10 | F | 5’-CACTTCCCAGTCAGCCAGA |
| R | 5’-GTCAGCAGTATGTTGTCCAGC | |
| TGF-β1 | F | 5’-TTGCTTCAGCTCCACAGAGA |
| R | 5’-TGGTTGTAGAGGGCAAGGAC | |
| CD163 | F | 5’-TGAGCCTGAGACTGGTGGATGG |
| R | 5’-CACAGAAGCGTCACGGCGATC | |
| ACTB | F | 5’-TGTCACCAACTGGGACGATA |
| R | 5’-GGGGTGTTGAAGGTCTCAAA | |
Enzyme-linked immunosorbent assay (ELISA)
The rat serum samples were collected 7 days after liver transplantations from the peripheral blood. The concentration of IL-10 and TGF-β1 in serum was examined following the manufacturer’s instructions of ELISA Kits (mlbio, China). All samples were measured in triplicates.
Histological changes
The tissues collected from the recipients were fixed in 10% paraformaldehyde, embedded in paraffin wax, sectioned, and stained with hematoxylin-eosin (HE). Allograft rejection was evaluated by rejection activity index (RAI) according to Banff criteria including portal inflammation, bile duct inflammation damage and venous endothelial inflammation [28].
Liver functions
The serum samples of recipients were obtained through posterior orbital vein on day 3, 7, and 10 after liver transplantations. The level of alanine transaminase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) was detected using an automatic biochemical analyzer (Chemray 240, Rayto) according to the manufacturers’ instructions.
Tissue immunohistochemistry (IHC)
Immunohistochemistry of CD163 and major histocompatibility complex (MHC) class II was carried out as described. Primary anti-rat CD163 antibody (NBP2-39099, Novus Biologicals Europe) was used at a dilution of 1:100; primary anti-rat MHC-II antibody (ab23990, Abcam) was used at a dilution of 1:300, whereas the PBS was used as a negative control. The results of immunohistochemistry were analyzed independently by two pathologists blinded to the samples. The CD163-positive cells were counted under ×400 magnification in 5 different portal areas. The MHC-II positive cells were counted under ×200 magnification in 5 different portal areas.
Immunofluorescence (IF)
The immunofluorescence of CD68 and CD8 was performed as described. Primary antibodies were diluted at 1:400 for CD68 proteins (ab125212, Abcam) and 1:300 for CD8 proteins (ab33786, Abcam). Secondary antibody was applied at a 1:400 dilution for both. Nuclei of all cells was counter-stained with EasyProbes™ DAPI Fixed Cell Stain (GeneCopoeia). All photographs were taken under a fluorescence microscope (U-TV0.5XC-3, Olympus, Japan). The CD68 or CD8 positive cells from 5 random fields of each slide were counted.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Liver allografts were examined 7 days after transplantation for apoptosis using TUNEL Assay Kit (C1088, Beyotime) according to the manufacturer’s instructions. Results were analyzed independently by two pathologists blinded to the samples. The TUNEL-positive cells were counted under ×400 magnification in 5 different portal areas.
Western blot analysis
The protein lysate was isolated in the lysis buffer (50 mM of Tris pH 7.4, 150 mM of NaCl, 1% sodium deoxycholate, 1% TritonX-100, 0.1% SDS, 1 mM EDTA and 2 mM of sodium pyrophosphate; Beyotime, China) and then separated using sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) onto the 10% gels. Proteins were then transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocked for 1 hour at room temperature, these membranes were incubated with anti-β-actin monoclonal antibody and anti-N-acetylglucosaminyltransferase V (MGAT5) monoclonal antibody at 4°C overnight and with secondary antibody for 2 hours at room temperature. Signals were detected by the chemiluminescent reaction using a gel imaging system (ChemiDoc MP, BIO-RAD, China). The antibodies are presented as following: β-actin (1:1,000; cat. no. 3700; Cell Signaling Technology, Inc.), MGAT5 (1:1000; cat. no. bs-5841R; Bioss).
Statistical analysis
SPSS version 12 (IBM, Chicago, IL) Analytical Software was used for statistical analysis. Results are presented as mean ± standard deviation (SD). Unpaired student t test was used to determine differences between two groups. Differences among multiple groups were analyzed by one-way analysis of variance (ANOVA) post hoc test of Tukey’s. Survival rate was examined using the Breslow-Gehan-Wilcoxon test. Differences of at least P < 0.05 were considered significant.
Results
The Infiltration of CD163-positive cells increased in the tolerant liver grafts
Rats orthotopic liver transplantation model was successfully established. The mean of anhepatic phase was 17.2 ± 3.2 minutes; the mean time of recipient surgeries was 58.4 ± 7.1 minutes; the ratio of successful surgeries was 91.3% (95/104).
In the AR group, portal inflammatory cell infiltration, bile duct damage and endothelial inflammation were more severe than those in the tolerance group (Figure 1A). Besides, the expression level of ALT and AST in the AR group increased compared with the tolerance group (Figure 1B). In addition, the concentration of anti-inflammatory cytokines, IL-10 and TGF-β1, was 10.8 ± 2.3 ng/ml and 22.8 ± 4.7 pg/ml in the AR group; however, the concentration of IL-10 and TGF-β1 in tolerance group was 17.1 ± 4.0 ng/ml and 41.0 ± 5.7 pg/ml, which significantly increased when compared with the AR group (Figure 1C, 1D). Then the surface marker of M2c macrophages, CD163, was examined by IHC (Figure 1E). The numbers of CD163-positive cells increased in tolerant liver grafts compared with that in the AR group (20.2 ± 2.6 versus 6.0 ± 1.2, P < 0.01), which indicated that M2c macrophages might play important roles in immune tolerance after rats liver transplantation (Figure 1F).
Figure 1.
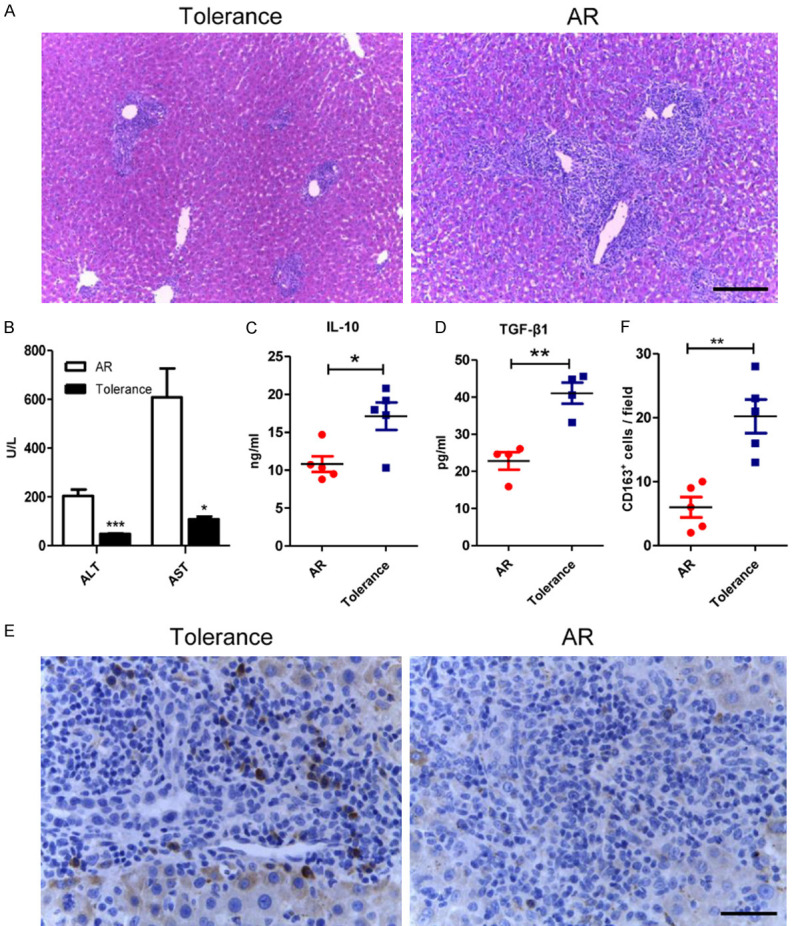
The infiltration of the CD163-positive cells increased in the tolerant liver grafts. A. Representative images of HE staining of liver allografts in the tolerance and the AR group 7 days following transplantation with original magnifications of ×100. Characteristics like portal inflammatory cell infiltration, bile duct damage and endothelial inflammation significantly reduced in the tolerance group. Scale bar in right lower corner represents 100 µm. B. Liver functions were assessed on day 7 after transplantation. Both ALT and AST were significantly lowered in tolerance group. C, D. ELISA was used to detect serum IL-10 and TGF-β1 levels of the recipients in both groups. Both anti-inflammatory cytokines were significantly increased in the tolerant recipients. E. Illustrating IHC microscopic finding for identification of CD163 positive cells (brown color) with original magnifications of ×400. Scale bar in right lower corner represents 25 µm. F. Analytical results of the numbers of CD163 positive cells. The numbers of CD163 positive cells in the AR group were less than that in the tolerance group. All statistical analyses were performed by an unpaired t-test. Data are presented as the mean ± SD. (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
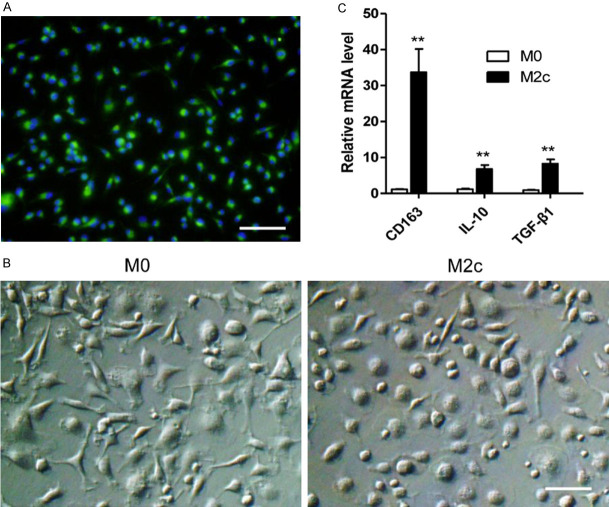
Macrophages of M2c subtype were induced and identified in vitro
To study the effects of M2c macrophages in acute rejection, the total bone marrow cells were stimulated with MCSF for 7 days to induce mature macrophages. The BMDMs were tested by immunofluorescence staining with FITC-labeled anti-CD68 antibody (Figure 2A). Nearly all of the BMDMs were CD68-positive. The BMDMs were then stimulated with PBS or dexamethasone for 24 hours to become M0 macrophages or M2c macrophages (Figure 2B). The polarized phenotypes of BMDMs were assessed using qRT-PCR. In comparison with M0 macrophages, M2c macrophages expressed higher levels of CD163, IL-10 and TGF-β1 (Figure 2C).
Figure 2.
M2c BMDMs were successfully induced in vitro. A. The bone marrow derived cells were examined by immunofluorescence staining with anti-CD68 antibody after being stimulated by MCSF for 7 days. Nearly all cells expressed CD68, the specific rat macrophage marker (Magnification, 200). Scale bars in right lower corner represents 50 µm. These cells were then stimulated by PBS or dexamethasone for 24 h for M0 or M2c polarization. B. Representative images of the M0 and the M2c with original magnifications of ×400. Scale bar in right lower corner represents 25 µm. C. M2c polarization markers expression determined by qRT-PCR. The expression levels of CD163, IL-10, TGF-β1 in the M2c macrophages were significantly higher than those of the M0 macrophages. The statistical analyses were performed by an unpaired t-test. Data are presented as the mean ± SD. (n = 3, **P < 0.01).
Infusion of the M2c BMDMs alleviated rat liver acute rejection
The expression levels of ALT, AST, and TBIL were 31.3 ± 2.2 U/L, 65.2 ± 5.1 U/L and 13.0 ± 2.3 μmol/L, respectively, in the normal rats. The expression levels of ALT, AST, and TBIL on day 3 after liver transplantations were slightly increased compared with the normal rats. However, no significant differences were observed among the four groups. On day 7 and day 10 following OLT, the expression levels of serum ALT, AST, and TBIL significantly increased compared with those on day 3. Besides, serum ALT, AST, and TBIL improved in the recipients with the transfusions of the M2c macrophages compared with those of the recipients received transfusions of the M0 macrophages and PBS. Furthermore, the M2c from BN rats showed no differences of improving liver function compared with the M2c from Lewis rats (Figure 3C).
Figure 3.
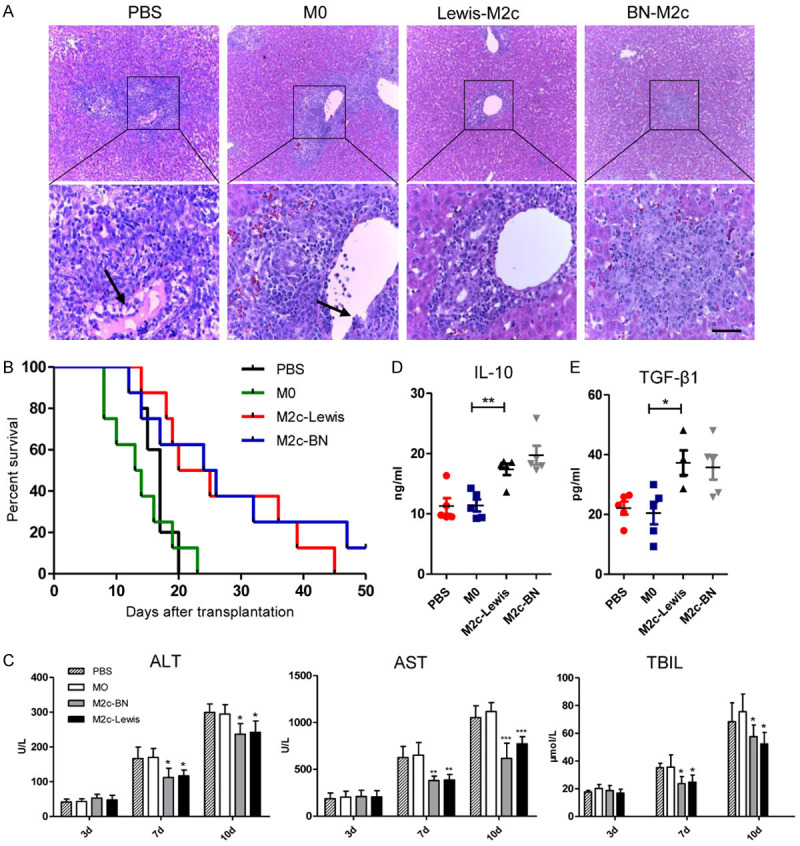
Infusion of the M2c BMDMs alleviated acute rejection in rat liver transplantation. The M0, Lewis-M2c, BN-M2c macrophages (3×106 cells per rat) or PBS were infused into recipients through portal vain immediately following OLT. A. Representative images of HE staining of liver allografts 7 days after liver transplantation with original magnifications of ×100 (upper panel) and ×400 (lower panel). The lymphocytes infiltration, bile duct damage and endothelial inflammation significantly reduced in recipients received the M2c macrophages, while no significant differences were observed between the M0 group and the PBS group. The black arrows show endothelial inflammation. Scale bar in right lower corner represents 25 µm. B. The survival analysis of the recipients was monitored and performed by the Breslow-Gehan-Wilcoxon test. The survival time of the recipients was significantly prolonged in the M2c infusion groups compared with that in the M0 and the PBS infusion group. C. Liver functions on day 3, 7, and 10 following liver transplantation. In comparison with the M0 and the PBS infusion group, ALT, AST, and TBIL in the M2c infusion groups were significantly improved. D, E. Serum IL-10 and TGF-β1 levels were quantified by ELISA and were significantly reduced in the M2c infusion groups compared with those in the M0 and the PBS infusion groups. No significant differences were observed between the M0 and the PBS infusion groups. All statistical analyses except survival rate were performed by one-way ANOVA, followed by post hoc test of Tukey’s (n = 5 for each group). Data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs M0 group.
The recipient rats in four groups were executed 7 days after liver transplantations for histological assessment of donor liver grafts (Figure 3A). The portal inflammatory cell infiltration, bile duct damage and endothelial inflammation all reduced in the recipients of M2c infusion groups compared with recipients of the M0 or the PBS infusion group. The data indicated that RAI values were higher in the groups of PBS and M0 infusion compared with those of M2c infusion groups. Besides, no differences of RAI values were observed between the BN-M2c group and the Lewis-M2c group (Table 2).
Table 2.
Assessment of rejection activity index after liver transplantation
Values are means ± SD.
P < 0.01 versus M0 group.
The mean values of survival time of the BN-M2c group and the Lewis-M2c group were 25.0 ± 12.2 days and 26.0 ± 12.4 days, which were significantly increased compared with the M0 group (13.9 ± 5.3 days) and the PBS group (16.6 ± 2.3 days). No statistical significance was observed between the M0 group and the PBS group. In other word, the data indicated that the M2c macrophages from both BN and Lewis rats, rather than M0 macrophages, could prolong recipients’ survival time (Figure 3B).
The expression levels of serum anti-inflammatory cytokines (IL-10, TGF-β1) on day 7 after OLT were chosen for assessment of tolerance after liver transplantations. In accordance with liver functions, the expression levels of serum IL-10 and TGF-β1 significantly increased in the M2c groups compared with the PBS group and the M0 group (Figure 3D, 3E). The results indicated that M2c macrophages can alleviate acute rejection and induce tolerance after OLT.
The M2c BMDM reduced the number of CD8+ T cell and promoted apoptosis of lymphocytes
To study the effects of the M2c BMDMs on lymphocytes after liver transplantations, the numbers of CD8-positive T-cells in four groups were counted. The infiltration of CD8+ T-cells in the M2c infusion groups significantly reduced in comparison with the PBS group and the M0 group (Figure 4A and 4B). However, the infusion of the M0 macrophages did not reduce the infiltration of CD8+ T cells in liver grafts. Then TUNEL assay was used to assess the apoptosis of lymphocytes around the portal area (Figure 5A). The numbers of apoptic lymphocytes were significantly increased in the M2c infusion groups compared with the M0 and the PBS group (Figure 5B), while no significant differences were observed between the M0 and the PBS group. Our results indicated that adoptive transfer of M2c macrophages might induce tolerance by reducing lymphocytes infiltration and promoting lymphocytes apoptosis.
Figure 4.
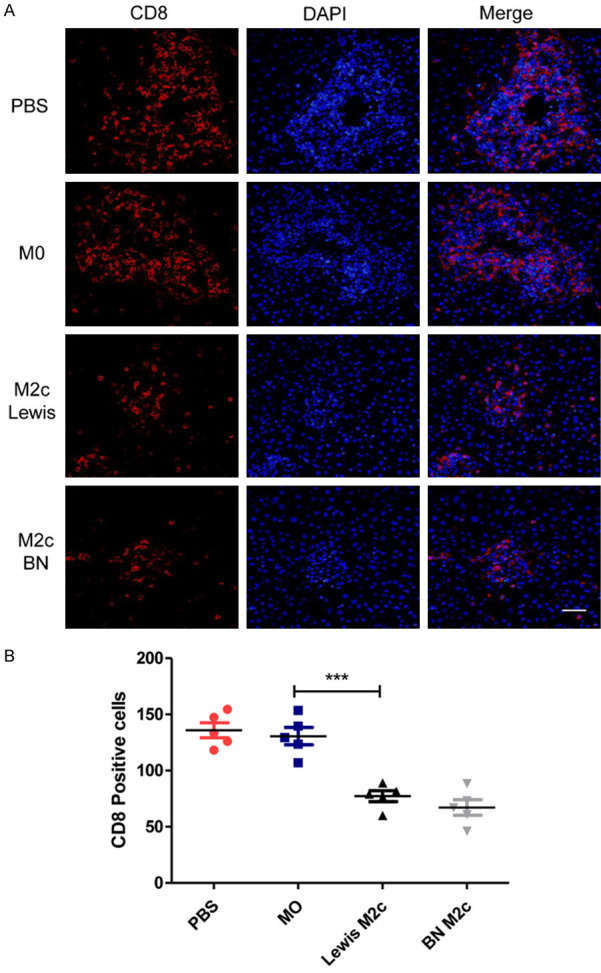
The numbers of CD8+ T cells were reduced in the M2c infusion groups. (A) Immunofluorescence microscopic analysis for identification of CD8 positive T cells 7 days following liver transplantation with original magnifications of ×400. CD8 was immunostained with Cy3 (left graph) and nucleus were stained with DAPI (middle graph). The right graph shows the merge of the Cy3 and the DAPI. Scale bar in right lower corner represents 25 µm. (B) The numbers of the CD8+ T cells in (A) were counted and quantitatively compared. The CD8+ T cells were significantly reduced in the M2c infusion groups. No significant differences were observed between the M0 and the PBS group. Data were analyzed by one-way ANOVA, followed by post hoc test of Tukey’s (n = 5 for each group). Data are presented as the mean ± SD. ***P < 0.001 vs M0 group.
Figure 5.
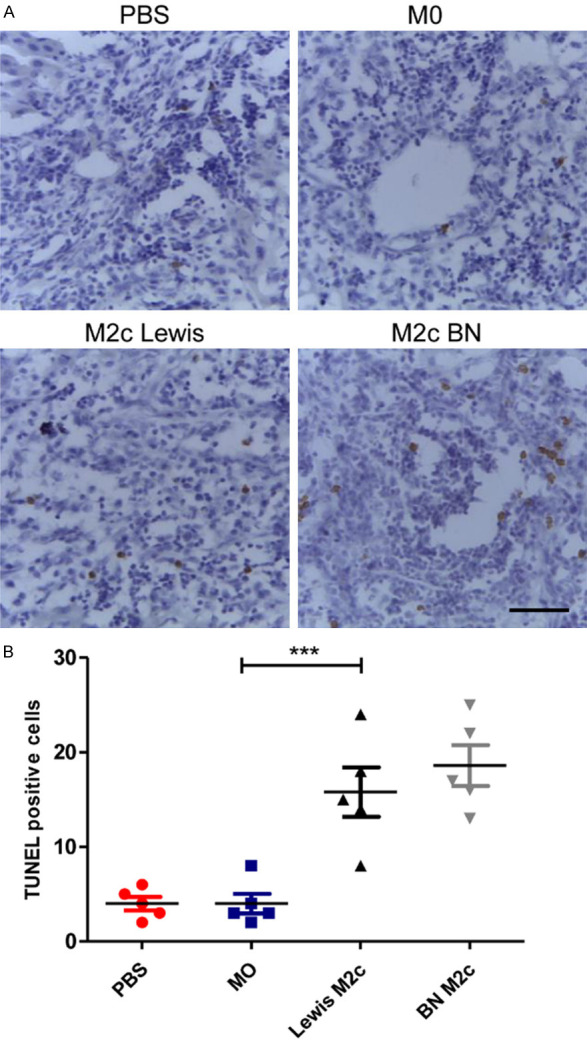
The M2c BMDMs promote apoptosis of lymphocytes. A. Representative images of TUNEL staining of liver allografts in four groups 7 days following transplantation with original magnifications of ×400. TUNEL positive cells are in brown color. Scale bar in right lower corner represents 25 µm. B. The numbers of the apoptic cells in recipients with the transfusion of the M2c macrophages were significantly increased compared with the recipients received the M0 macrophages. Statistical analysis was performed by one-way ANOVA, followed by post hoc test of Tukey’s (n = 5 for each group). Data are presented as the mean ± SD. ***P < 0.001 vs M0 group.
The M2c BMDM change the expression of MHC-II and MGAT5
The potential mechanisms underlying the protective role M2c macrophages against liver acute rejection were explored. MHC-II, which plays a critical role in the events leading to the rejection or acceptance of allografts, was detected by IHC [29] (Figure 6A). The numbers of MHC-II positive cells in M2c infusion groups significantly reduced compared with the M0 infusion group. However, no statistical significance was observed between PBS and M0 infusion group (Figure 6B). Previous researches reported that IL-10 can directly restrict CD8+ T cell activation by upregulating MGAT5 [30,31]. The expression of MGAT5 in liver grafts was detected by western blot analysis, revealing the high-expression of MGAT5 in M2c infusion groups (Figure 6C). Therefore, our data indicated that M2c might protect liver grafts from AR by inhibiting MHC-II and upregulating the expression MGAT5.
Figure 6.
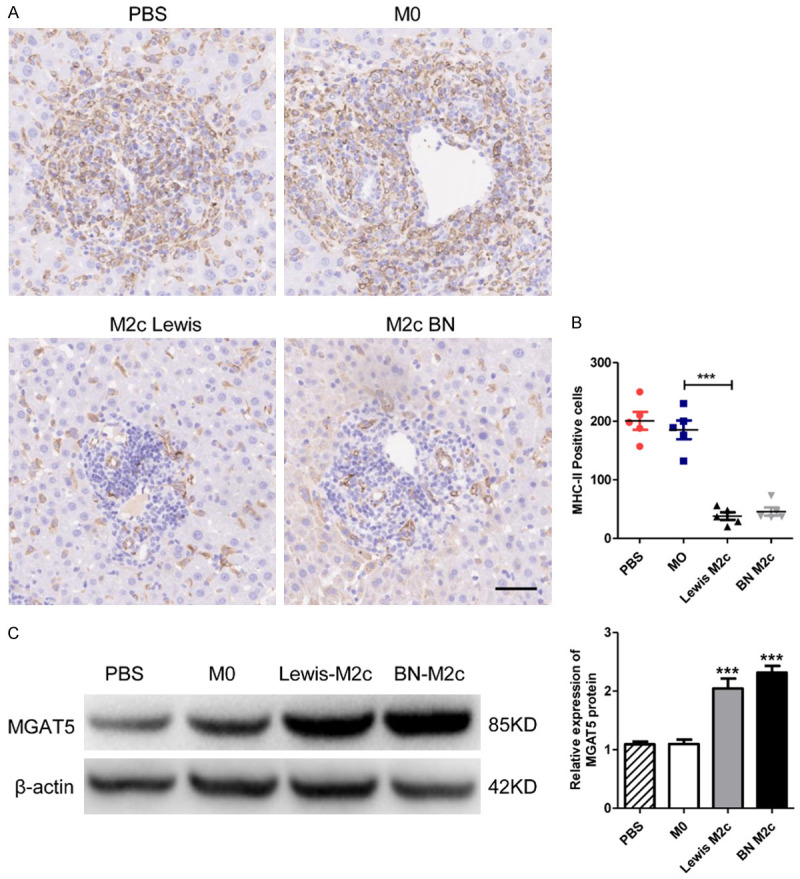
The expression of MHC-II and MGAT5 in liver grafts. (A) Representative images of IHC staining of MHC-II positive cells in liver grafts 7 days following liver transplantation with original magnifications of ×200. The MHC-II positive cells are in brown color. Scale bar in right lower corner represents 50 µm. (B) The numbers of the MHC-II positive cells in (A) were counted and quantitatively compared. MHC-II positive cells significantly reduced in the M2c infusion groups. No significant differences were observed between the M0 and the PBS group. Data were analyzed by one-way ANOVA, followed by post hoc test of Tukey’s (n = 5 for each group). Data are presented as the mean ± SD. ***P < 0.001 vs M0 group. (C) MGAT5 and β-actin protein expression in liver grafts were detected by western blot analysis. The expression of MGAT5 was significantly upregulated in the M2c infusion groups. No significant differences were observed between the M0 and the PBS group. Data are presented as means ± SD. ***P < 0.001.
Discussion
Liver grafts from BN rats are spontaneously accepted when transplanted into Lewis rats; this is referred to as tolerance combination, in which the recipient has extended survival time and mild inflammatory infiltration. However, in the reverse direction, BN rats exhibit severe rejection of grafts from Lewis rats, due to intensive lymphocytic infiltration; this is referred to as rejection combination [32]. This rejection/tolerance phenotype of a particular strain is due to genetically determined levels of immune responses against alloantigens from different donors; however, the mechanisms behind spontaneous tolerance induction in specific rat combinations remain to be further elucidated [25].
Recently, the protective role of hepatic macrophages in LT has been demonstrated in several studies. For example, Kupffer cells induce T-cell apoptosis in spontaneous acceptance allografts in rats via the Fas/FasL pathway [33]. KC also promote the timely and effective removal of apoptotic cells, which subsequently suppress immune responses by up-regulating anti-inflammatory factors and down-regulating pro-inflammatory factors [34,35]. More specifically, IL-10, which can be highly expressed by the anti-inflammatory M2 subtype, exhibits anti-inflammatory properties, including down-regulating the expression of MHC-II and co-stimulatory molecules, raising the antigenic threshold required for T cell activation, transforming naïve lymphocytes into regulatory T-cells, and suppressing the proliferation of various inflammatory cells, such as CD4+ T-cells, CD8+ T-cells, NKT cells, and dendritic cells [13,36]. Though M2 macrophages are further divided into several subsets, which of these dominate the anti-rejection function in LT remains unknown. CD163, the M2c surface marker, was largely increased in tolerant allografts, especially in the portal inflammatory infiltration area, with the simultaneous elevation of serum IL-10 and TGF-β1 in the tolerance group. We therefore focused on the role of M2c macrophages in AR.
Although adoptive transfer of KC isolated from tolerance allografts induces tolerance, due to their higher proportion of M2 macrophages, the precise roles of different macrophage subsets have not been thoroughly evaluated [15]. To further demonstrate the protective role of M2c in AR, ex vivo cultured M2c macrophages were infused into recipients. Our results showed a significantly prolonged survival rate and improvement of liver functions in M2c treatment groups, hinting that M2c macrophages can alleviate AR in rat liver transplantation.
M2c are believed to inhibit the proliferation of CD4+ T-cells but induce regulatory T-cells [20]. However, the numbers of CD4+ T-cells and Tregs in this study remained constant after M2c infusion (data not shown). Interestingly, CD8+ T-cells, which are considered to be predominant in acute cellular rejection, were decreased in the M2c treatment groups [37]. Furthermore, an increased number of apoptotic cells was observed in infiltrating cells around the portal area, indicating that the therapeutic effects of M2c macrophages included preventing CD8+ T-cell infiltration and induction of inflammatory cell apoptosis [38].
Several studies have revealed that M2c macrophages alleviate inflammatory infiltration by expressing high level of IL-10 [19,36,39]. The elevation of IL-10 in the M2c groups was observed, which was consistent with previous studies. Series of proteins act as the targets of IL-10, including MHC-II and MGAT5. MGAT5 is a glycosyl-transferase that enhance branched N-glycans on T-cell receptor, which raise T-cell activation thresholds by limiting the capacity of TCR to interact with MHC peptide-loading complex [40]. MGAT5 deficient mice showed spontaneous kidney autoimmune disease and increased susceptibility to experimental autoimmune encephalomyelitis [30,41]. Besides, IL-10 can directly restrict CD8+ T cell activation by upregulating MGAT5 in CD8+ T cells thus promoted establishment of chronic viral infections [31]. In this study, adoptive transfer of M2c macrophages up-regulated the expression of MGAT5 within acute rejected liver graft, hinting that the M2c macrophages may inhibit the activation of CD8+ T cell through IL-10/MGAT5/TCR pathway.
Moreover, MHC-II plays pivotal roles in mediating the priming of naïve T cells to develop strong responses leading to graft rejection [42,43]. Previous research indicated that IL-10 can stimulate the expression of E3 ubiquitin ligase in activated macrophages, thereby down-regulating the MHC-II and inhibiting the antigen presentation ability of macrophages [44]. Besides, marked reduction of MHC-II molecules could alleviate immune responses against the allograft [29]. In this study, M2c macrophages infusion significantly reduced the expression of MHC-II, indicating that M2c macrophages might exhibit their protective functions through IL-10-mediated down-regulation of MHC-II molecules.
Interestingly, M2c macrophages from both the hosts and donors alleviated AR in our results. We speculate that the underlying mechanism of this phenomenon may be that M2c macrophages exhibit anti-inflammatory effects through IL-10 secretion rather than by inducing donor-specific tolerance. Overall, our approach is the first to examine the use of bone marrow-derived M2c macrophages in alleviating AR following rat LT. Polarization of macrophages towards the M2c phenotype may serve as a novel and effective therapeutic approach for AR after transplantation.
Acknowledgements
This work was supported by National Key Research and Development Program (2017YFC1103703), National Basic Research Program (2015CB554100), National Natural Science Foundation (81870446, 81671838, 81670593, 81970566, 81900571). Provincial Natural Science Foundation of Shaanxi (2016JM8026, 2020JQ-451).
Disclosure of conflict of interest
None.
References
- 1.Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869–1884. doi: 10.1002/hep.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton M, Levitsky J, Aqel B, O’Grady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, Little EC, Berenguer M, Shaked A, Trotter J, Roberts J, Rodriguez-Davalos M, Rela M, Pomfret E, Heyrend C, Gallegos-Orozco J, Saliba F. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 3.Kalil AC, Sandkovsky U, Florescu DF. Severe infections in critically ill solid organ transplant recipients. Clin Microbiol Infect. 2018;24:1257–1263. doi: 10.1016/j.cmi.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Zheng L, He W, Qiu M, Gao L, Liu J, Huang A. Cotransfection with IL-10 and TGF-beta1 into immature dendritic cells enhances immune tolerance in a rat liver transplantation model. Am J Physiol Gastrointest Liver Physiol. 2014;306:G575–581. doi: 10.1152/ajpgi.00283.2013. [DOI] [PubMed] [Google Scholar]
- 5.Saei A, Hadjati J. Tolerogenic dendritic cells: key regulators of peripheral tolerance in health and disease. Int Arch Allergy Immunol. 2013;161:293–303. doi: 10.1159/000350328. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y, Wu Y, Xin K, Wang JJ, Xu H, Ildstad ST, Leventhal J, Yang GY, Zhang Z, Levitsky J. Delayed donor bone marrow infusion induces liver transplant tolerance. Transplantation. 2017;101:1056–1066. doi: 10.1097/TP.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125–1136. doi: 10.1111/ajt.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu LY, Wang XH, Zhang F, Li XC, Yao AH, Yu Y, Lv L, Li GQ. Adoptive transfusion of ex vivo donor alloantigen-stimulated CD4(+)CD25(+) regulatory T cells ameliorates rejection of DA-to-Lewis rat liver transplantation. Surgery. 2007;142:67–73. doi: 10.1016/j.surg.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Qi H, Chen G, Huang Y, Si Z, Li J. Foxp3-modified bone marrow mesenchymal stem cells promotes liver allograft tolerance through the generation of regulatory T cells in rats. J Transl Med. 2015;13:274. doi: 10.1186/s12967-015-0638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu J, Yue W, Song Y, Zhang Y, Qi X, Wang Z, Liu B, Shen H, Hu X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin Exp Immunol. 2014;176:473–484. doi: 10.1111/cei.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu B, Song HL, Yang Y, Yin ML, Zhang BY, Cao Y, Dong C, Shen ZY. Improvement of liver transplantation outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem cells in rats. Stem Cells Int. 2016;2016:9235073. doi: 10.1155/2016/9235073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage phenotype in liver injury and repair. Scand J Immunol. 2017;85:166–174. doi: 10.1111/sji.12468. [DOI] [PubMed] [Google Scholar]
- 13.Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222–229. doi: 10.1016/j.molimm.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9:489–497. doi: 10.1053/jlts.2003.50091. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Pan G, Tang C, Li Z, Zheng D, Wei X, Wu Z. IL-34 inhibits acute rejection of rat liver transplantation by inducing kupffer cell M2 polarization. Transplantation. 2018;102:e265–e274. doi: 10.1097/TP.0000000000002194. [DOI] [PubMed] [Google Scholar]
- 17.Pan G, Zhao Z, Tang C, Ding L, Li Z, Zheng D, Zong L. Soluble fibrinogen-like protein 2 ameliorates acute rejection of liver transplantation in rat via inducing Kupffer cells M2 polarization. Cancer Med. 2018;7:3168–3177. doi: 10.1002/cam4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 19.Tang L, Zhang H, Wang C, Li H, Zhang Q, Bai J. M2A and M2C macrophage subsets ameliorate inflammation and fibroproliferation in acute lung injury through interleukin 10 pathway. Shock. 2017;48:119–129. doi: 10.1097/SHK.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 20.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, Zheng G, Tan TK, Ince J, Alexander SI, Harris DC. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilliard BA, Zizzo G, Ulas M, Linan MK, Schreiter J, Cohen PL. Increased expression of Mer tyrosine kinase in circulating dendritic cells and monocytes of lupus patients: correlations with plasma interferon activity and steroid therapy. Arthritis Res Ther. 2014;16:R76. doi: 10.1186/ar4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas J, Salazar J, Martinez MS, Palmar J, Bautista J, Chavez-Castillo M, Gomez A, Bermudez V. Macrophage heterogeneity and plasticity: impact of macrophage biomarkers on atherosclerosis. Scientifica (Cairo) 2015;2015:851252. doi: 10.1155/2015/851252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 24.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 25.Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55:369–389. [PMC free article] [PubMed] [Google Scholar]
- 26.Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int J Mol Sci. 2018;19:2208–2238. doi: 10.3390/ijms19082208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 28.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 29.Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (IL-10)-mediated immunosuppression: march-I induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem. 2015;290:27158–27167. doi: 10.1074/jbc.M115.682708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan R, Gao G, Pawling J, Dennis JW, Demetriou M, Li B. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J Immunol. 2004;173:7200–7208. doi: 10.4049/jimmunol.173.12.7200. [DOI] [PubMed] [Google Scholar]
- 31.Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, Butler NS, Bruneau J, Shoukry NH, Krawczyk CM, Richer MJ. Interleukin-10 directly Inhibits CD8(+) T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity. 2018;48:299–312. e5. doi: 10.1016/j.immuni.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuchimoto S. Orthotopic liver transplantation in the rat. Hokkaido Igaku Zasshi. 1986;61:776–799. [PubMed] [Google Scholar]
- 33.Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J, Peng Y, Yan L, Gong J. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008;14:823–836. doi: 10.1002/lt.21450. [DOI] [PubMed] [Google Scholar]
- 34.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravishankar B, McGaha TL. O death where is thy sting? Immunologic tolerance to apoptotic self. Cell Mol Life Sci. 2013;70:3571–3589. doi: 10.1007/s00018-013-1261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Curr Opin Organ Transplant. 2015;20:446–453. doi: 10.1097/MOT.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokota S, Yoshida O, Ono Y, Geller DA, Thomson AW. Liver transplantation in the mouse: insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl. 2016;22:536–546. doi: 10.1002/lt.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 39.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 41.Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 42.Caballero A, Fernandez N, Lavado R, Bravo MJ, Miranda JM, Alonso A. Tolerogenic response: allorecognition pathways. Transpl Immunol. 2006;17:3–6. doi: 10.1016/j.trim.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Liu G, He X, Lu N, Qiu Y, Wang H. Attenuation of allograft rejection by intragraft inhibition of class II transcativator in high responder rat liver transplantation. Microsurgery. 2015;35:52–59. doi: 10.1002/micr.22265. [DOI] [PubMed] [Google Scholar]
- 44.Thibodeau J, Bourgeois-Daigneault MC, Huppe G, Tremblay J, Aumont A, Houde M, Bartee E, Brunet A, Gauvreau ME, de Gassart A, Gatti E, Baril M, Cloutier M, Bontron S, Fruh K, Lamarre D, Steimle V. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–1230. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]