Abstract
Background: Epilepsy (EP) is a very dangerous neurological disease. MiR-181b was reported to play a regulatory role during the progression of EP. However, the mechanism by which miR-181b regulates the process of EP remains unclear. Methods: Hippocampal neurons were extracted from rats, which were treated with magnesium-free to mimic EP in vitro. CCK-8 assay was performed to test the cell viability. Gene and protein expressions in hippocampal neurons were detected by qRT-PCR, immunofluorescence and western blot, respectively. In addition, TUNEL staining was performed to test the cell apoptosis. Finally, dual luciferase report assay was used to verify the relation between miR-181b, ZNF883 and RASSF1A. Results: Magnesium-free significantly inhibited the proliferation of hippocampal neurons, which was reversed by miR-181b mimics. In consistent, magnesium-free induced apoptosis of cells was notably inhibited by miR-181b mimics. In addition, miR-181b suppressed the progression of EP via directly targeting RASSF1A and activating PI3K/Akt signaling. Finally, upregulation of miR-181b notably suppressed the progression of EP via regulation of ZNF883. Conclusion: MiR-181b suppressed the progression of epilepsy via regulation of RASSF1A and lncRNA ZNF883. Thus, miR-181b might serve as a new target for treatment of EP.
Keywords: Epilepsy, miR-181b, lncRNA ZNF883, RASSF1A
Introduction
Epilepsy (EP) is a dangerous and drug-refractory neurological disorder, which was characterized by recurrent spontaneous seizures [1]. Nearly 30% of patients with EP do not respond adequately to anti-epileptic drug (AED) treatment and need long-time medical care [2,3]. Surgical resection of the epileptic focus is an invasive approach that is not the best choice for most of patients [4]. In addition, EP has many pathological conditions including inflammation, gliosis and synaptic remodeling [5-7]. An imbalance between excitatory and sequelae of brain surgery that results in hypersynchronous discharge of neurons in a focal area of the brain or all over the entire brain is the most commonly accepted mechanism [7]. Current AEDs primarily target voltage-gated ion channels to reduce neuronal excitability directly or synaptic transmission [8]. However, AEDs lack efficacy in most of the patients with EP because of patient’s maladaptation to the drug [9]. Therefore, a systematic understanding of the mechanisms regulate this imbalance is important for us to control seizure susceptibility and develop new therapeutic methods.
It has been reported that magnesium-free could promote the progression of EP by transcriptional activation of the relevant genes [10,11]. In addition, previous studies have indicated that magnesium-free plays a regulatory role in the recurrence of EP [12,13]. MicroRNAs (miRNAs) are endogenic noncoding small RNAs, 21~24 nucleotides in length, which function in the post-transcriptional regulation [14]. Upregulation or downregulation of miRNA has been related with the progression of multiple diseases [14]. It has been previously reported that miR-137 suppressed seizure activity and neuronal excitability during the occurrence of EP [1]. Moreover, Geng JF et al indicated that miR-495 downregulation could inhibit the progression of EP [15]. Meanwhile, it has been previously been reported that miR-181b could inhibit the progression of EP [16]. However, the molecular mechanism by which miR-181b mediates the progression of EP remains unclear. Thus, we will investigate the biological function of miR-181b during the progression of EP and its underlying mechanism.
Material and methods
Animal and cell culture
Hippocampal neurons were isolated from the brain tissues of rats according to the previous reference [17]. Rats were purchased from the Chinese Academy of Science (Shanghai, China) and bred in the animal facility of the Third Xiangya Hospital of Central South University. All mice used were housed in a specific pathogen-free (SPF) conditions with a 12/12 h light/dark cycle. All animal care and experimental protocols were approved by the Use Committee of the Third Xiangya Hospital of Central South University. National Institutes of Health guide for the care and use of laboratory animals was strictly followed by us. The efficiency of hippocampal neurons extraction was observed by morphology and immunofluorescence.
Hippocampal neurons were maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS), 1% penicillin (Thermo Fisher Scientific) and 1% streptomycin (Thermo Fisher Scientific) in a humidified incubator with 5% CO2 at 37°C. To establish in vitro EP model, hippocampal neurons were treated with magnesium-free for 48 h.
Immunofluorescence staining
Hippocampal neurons were seeded in 24-well plates overnight. Then, cells were treated with nothing (control) or magnesium free for 48 h. Next, cells were blocked with 10% goat serum for 30 min at room temperature and then incubated with anti-NSE antibody (Abcam; 1:1000) or anti-MAP2 antibody (Abcam; 1:1000) at 4°C overnight. Then cells were incubated with goat anti-rabbit IgG (Abcam; 1:5000) at 37°C for 1 h and the nuclei were stained with DAPI (Beyotime, Shanghai, China) for 5 min. Finally, cells were observed under a fluorescence microscope (Olympus CX23, Tokyo, Japan).
Cell transfection
Hippocampal neurons were transfected with miR-181b mimics or NC according to the previous reference [18]. MiR-181b mimics and negative control RNAs were purchased from GenePharma (Shanghai, China). MiR-181b mimics or negative control RNAs was transfected into the hippocampal neurons according to the manufactures’ protocol.
MTT assay
Hippocampal neurons were seeded in 96-well plates (5×103 per well) overnight. Then, cells were treated with magnesium-free, miR-181b mimics or magnesium-free + miR-181b mimics for 48 h, respectively. After that, 100 μl MTT were added to each well and further incubated for 2 h at 37°C. Then, cell supernatants were removed and 150 μl DMSO was added to each well. Finally, the absorbance of hippocampal neurons was measured at 490 nm using a microplate reader (Thermo Fisher Scientific).
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from hippocampal neurons using TRIzol reagent (TaKaRa, Tokyo, Japan) according to the manufacturer’s protocol. cDNA was synthesized using the reverse transcription kit (TaKaRa, Ver.3.0) according to the manufacturer’s protocol. Real-Time qPCRs were performed in triplicate under the following protocol: 2 minutes at 94°C, followed by 35 cycles (30 s at 94°C and 45 s at 55°C). The primers were obtained from GenePharma (Shanghai, China). miR-181b forward, 5’-GGCGAGGACTTTAATCTTGGTG-3’ and reverse, 5’-AGACACCACTTTGCCATCCACT-3’; ZNF883: forward, 5’-GTCCTTTACCCGGAACAGTAATC-3’ and reverse, 5’-TAAGGGATGACCTATGACTAAAG-3’; SNHG12: forward, 5’-AGTTTCCTGGCAGTGTGTGATAC-3’ and reverse, 5’-TGTTGTTTCTACCTAAAATGACCG-3’; XIST: forward, 5’-GCTTCCTCGTTGCTTACATCG-3’ and reverse, 5’-ACCATTGATAACTGCCATTGT-3’; LINC00667: forward, 5’-GATAGTTCAGGCCCAGAGAC-3’ and reverse, 5’-TTTTTTCCTGGTAGTGGCCAG-3’; MALAT1: 5’-GACTTCTGTAAAGGACTGGGGC-3’ and reverse 5’-ACAGCTAAGATAGCAGCACAAC-3’; GAPDH: forward, 5’-CATCATCCCTGCCTCTACTGG-3’ and reverse, 5’-GTGGGTGTCGCTGTTGAAGTC-3’; U6: forward, 5’-CTCGCTTCGGCAGCACAT-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’. The relative fold changes were calculated using the 2-ΔΔCt method by the formula: 2-(sample ΔCt - control ΔCt), where ΔCt is the difference between the amplification fluorescent thresholds of the gene of interest and the internal reference gene (U6 or GAPDH) used for normalization.
Western-blot detection
Total protein was isolated from cell lysates by using RIPA buffer (Thermo Fisher Scientific), and quantified by BCA protein assay kit (Beyotime, Shanghai, China). Proteins were separated with 10% SDS-PAGE and transferred to PVDF (Bio-Rad) membranes. After blocking, the membranes were incubated with primary antibodies at 4°C overnight. After washing, the membranes were incubated with secondary anti-rabbit antibody (Abcam; 1:5000) at room temperature for 1 h. Membranes were scanned by using an Odyssey Imaging System and analyzed with Odyssey v2.0 software (LICOR Biosciences, Lincoln, NE, USA). Then, the primary antibodies used in this study as follows: anti-Bax (Abcam; 1:1000), anti-Bcl-2 (Abcam; 1:1000), anti-Cleaved caspase3 (Abcam; 1:1000), anti-Bcl-2 (Abcam; 1:1000), anti-cleaved caspase3 (Abcam; 1:1000), anti-RASSF1A (Abcam; 1:1000), anti-MOAP1 (Abcam; 1:1000), anti-Akt (Abcam; 1:1000), anti-ERK (Abcam; 1:1000) and anti-GAPDH (Abcam; 1:1000). GAPDH was used as an internal control.
TUNEL staining
Cell apoptosis was also determined by the TUNEL assay. Briefly, paraffin sections were washed, permeabilized, and then incubated with 50 μl TUNEL reaction mixture in a wet box for 60 min at 37°C in the dark. For signal conversion, slides were incubated with 50 μl of peroxidase (POD) for 30 min at 37°C, rinsed with PBS, and then incubated with 50 μl diaminobenzidine (DAB) substrate solution for 10 min at 25°C. Finally, the expression of apoptotic cells was observed under an optical microscope.
Fluorescence in situ hybridization (FISH) detection
To explore the relation between ZNF883 and miR-181b, colocalization of miR-181b and ZNF883 on cytoplasm was investigated by FISH detection according to the previous reference [19].
Dual luciferase reporter assay
The partial squence of ZNF883 and 3’-UTR of RASSF1A containing the putative binding sites of miR-181b were synthetized and obtained from Sangon Biotech (Shanghai, China), then were cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vectors (Promega, Madison, WI, USA) to construct wild-type reporter vectors ZNF883 (WT) and RASSF1A (WT), respectively. The mutant ZNF883 sequences and 3’-UTR of RASSF1A sequences containing the putative binding sites of miR-181b were performed by Q5 Site-Directed Mutagenesis Kit (New England Biolabs, Ipswich, MA, USA) and then cloned into pmirGLO vectors respectively, to construct mutant-type reporter vectors ZNF883 (MUT) and RASSF1A (MUT). The ZNF883 (WT) or ZNF883 (MUT) were transfected into neurons cells together with control, vector-control (NC) or miR-181b mimics using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Similarly, the RASSF1A (WT) or RASSF1A (MUT) was transfected into neurons cells together with control, vector-control (NC) or miR-181b mimics. The relative luciferase activity was analyzed by the Dual-Glo Luciferase Assay System (Promega).
Statistical analysis
Measurement data were expressed as the mean ± standard deviation (SD). The comparisons among multiple groups were made with one-way analysis of variance (ANOVA) followed by Tukey’s test. P < 0.05 was considered significant.
Results
Hippocampal neurons were successfully isolated from brain tissues of rats
The morphology of hippocampal neurons isolated from brain tissues of rats was firstly observing under microscope. As showed in Figure 1A, cells exhibited slender. This morphology was consistent to the characteristic of hippocampal neurons [20]. In addition, the result of immunofluorescence staining demonstrated that the expressions of Neuron-Specific Enolase (NSE) and MAP2 were notably high (Figure 1B). Since NSE and MAP2 are key biomarkers in hippocampal neurons [21], these data suggested that hippocampal neurons were successfully isolated from brain tissues of rats.
Figure 1.
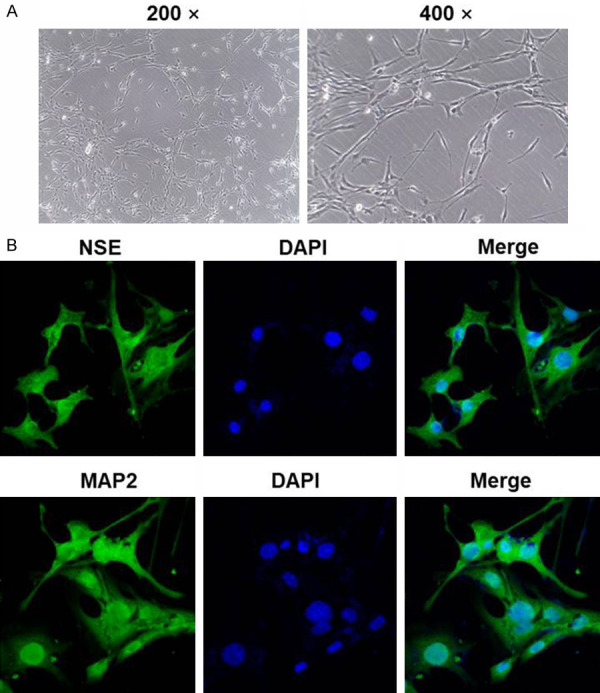
Hippocampal neurons were successfully isolated form brain tissues of rats. Hippocampal neurons were isolated from brain tissues of mice. Then, (A) the efficacy of isolation was observed by a light microscope at 200× or 400× magnification. (B) Efficiency of isolation was detected using immunofluorescence staining. The green fluorescence indicated NSE or MAP2 and the blue fluorescence indicated nuclear staining (DAPI).
Magnesium-free induced growth inhibition of hippocampal neurons was reversed by miR-181b mimics
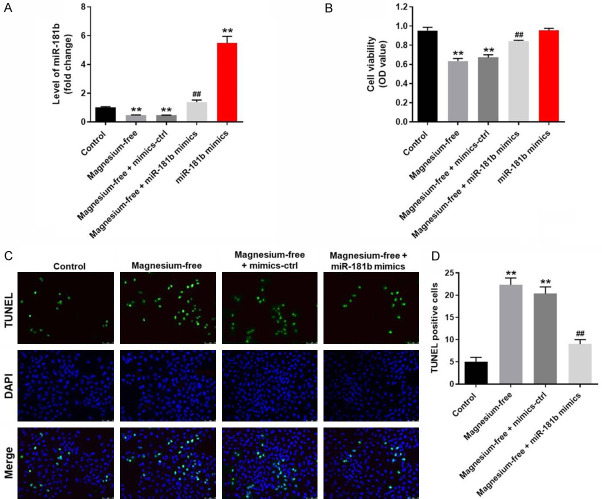
To explore the expression of miR-181b during the progression of EP, q-PCR was used. As revealed in Figure 2A, the expression of miR-181b in hippocampal neurons was significantly downregulated by magnesium free, which was completely reversed by miR-181b mimics. Moreover, miR-181b was notably activated in hippocampal neurons after miR-181b mimics transfection. Next, MTT assay was performed to evaluate the cell viability. The data indicated that the proliferation of hippocampal neurons was obviously inhibited by magnesium free (Figure 2B). However, magnesium-free induced growth inhibition of hippocampal neurons was notably reversed by miR-181b mimics. On the other hand, miR-181b mimics alone had limited effect on proliferation of hippocampal neurons (Figure 2B). In addition, as revealed in TUNEL staining upregulation of miR-181b significantly inhibited magnesium-free induced apoptosis of hippocampal neurons (Figure 2C and 2D).
Figure 2.
Overexpression of miR-181b significantly promoted the growth of magnesium-free-treated hippocampal neurons. Hippocampal neurons were treated with nothing, magnesium-free, miR-181b mimics, magnesium-free + mimics-control or magnesium-free + miR-181b mimics for 48 h. Then, (A) the expression of miR-181b in hippocampal neurons was detected by q-PCR. (B) The viability (OD value) of hippocampal neurons was tested using MTT assay. (C) Hippocampal neurons were treated with magnesium-free, magnesium-free + mimics-control or magnesium-free + miR-181b mimics for 48 h. Apoptosis in hippocampal neurons was detected by TUNEL staining. (D) TUNEL positive cell rate in each group was calculated. **P < 0.01 compared to control. ##P < 0.01 compared to magnesium-free.
Next, to detect the effect of miR-181b mimics on apoptosis-related proteins in hippocampal neurons, western blot was used. As demonstrated in Figure 3A-D, the expressions of pro-apoptotic proteins (Bax and Cleaved caspase3) in hippocampal neurons were significantly upregulated by magnesium-free, which were partially rescued in the presence of miR-181b mimics. In contrast, magnesium free notably inhibited the expression of Bcl-2 in hippocampal neurons. As expected, magnesium-free induced Bcl-2 downregulation in cells was partially revered by miR-181b mimics. Taken together, overexpression of miR-181b significantly reversed magnesium-free induced growth inhibition of hippocampal neurons via inhibiting apoptosis.
Figure 3.
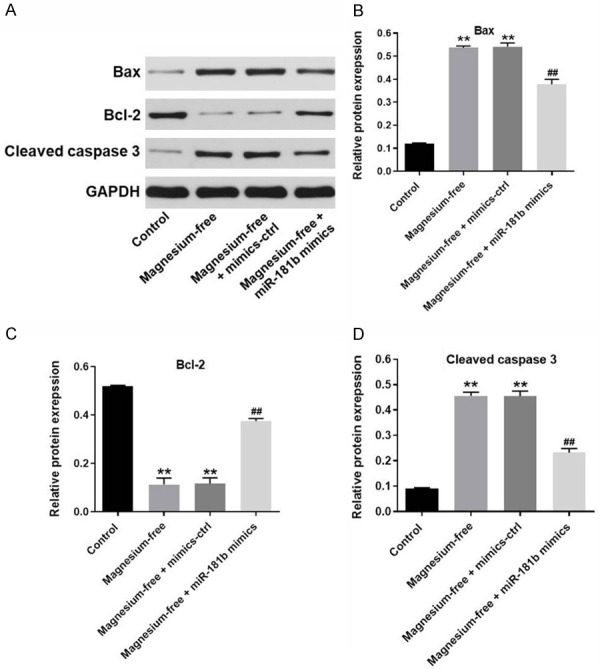
MiR-181b mimics greatly mediated the apoptosis-related proteins in magnesium-free-treated hippocampal neurons. A. The expression of Bax, Bcl-2 and cleaved caspase3 in hippocampal neurons was detected by western-blot. B. The relative expressions of Bax was quantified via normalizing to GAPDH. C. The relative expressions of Bcl-2 was quantified via normalizing to GAPDH. D. The relative expressions of cleaved caspase3 were quantified via normalizing to β-actin. **P < 0.01 compared to control. ##P < 0.01 compared to magnesium-free.
MiR-181b directly targeted RASSF1A
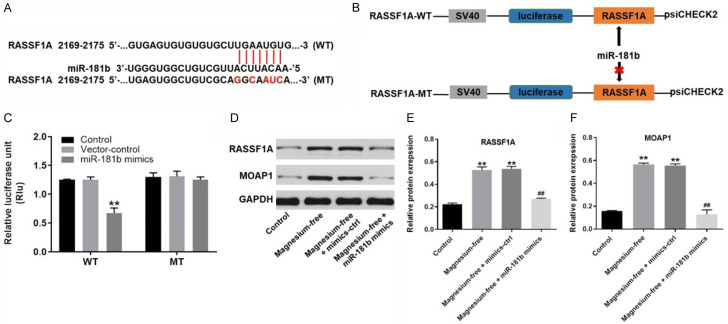
To explore the downstream target of miR-181b, Targetscan (http://www.targetscan.org/vert_71/), miRDB (http://www.mirdb.org/) and dual luciferase report assay were used. As demonstrated in Figure 4A-C, RASSF1A was found to a direct target of miR-181b. In addition, western blot was used to confirm the outcome. The results demonstrated that the protein levels of RASSF1A and MOAP1 in hippocampal neurons were obviously upregulated by magnesium-free, which were significantly reversed by miR-181b mimics (Figure 4D-F). Taken together, miR-181b mimics inhibited the progression of EP in vitro via directly targeting RASSF1A.
Figure 4.
MiR-181b directly targeted RASSF1A. A, B. Gene structure of RASSF1A at the position of 2169-2175 indicated the predicted target site of miR-181b in its 3’UTR. C. The luciferase activity was measured in hippocampal neurons following co-transfecting with WT/MT RASSF1A 3’-UTR plasmid and miR-181b with the dual luciferase reporter assay. D. The protein expression of RASSF1A and MOAP1 in hippocampal neurons were detected by western-blot. E. The relative expression of RASSF1A was quantified via normalizing to GAPDH. F. The relative expression of MOAP1 was quantified via normalizing to GAPDH. **P < 0.01 compared to control. ##P < 0.01 compared to magnesium-free.
MiR-181b suppressed the progression of EP in vitro through activation of PI3K/Akt signaling
To further investigate the mechanism by which miR-181b mediated the progression of EP in vitro, western blot was performed. As demonstrated in Figure 5A-C, the expression of p-Akt and p-ERK in hippocampal neurons were significantly downregulated by magnesium-free. However, the inhibitory effect of magnesium-free on these two proteins was significantly suppressed in by miR-181b mimics (Figure 5A-C). There data suggested miR-181b suppressed the progression of EP in vitro might through activation of PI3K/Akt signaling.
Figure 5.
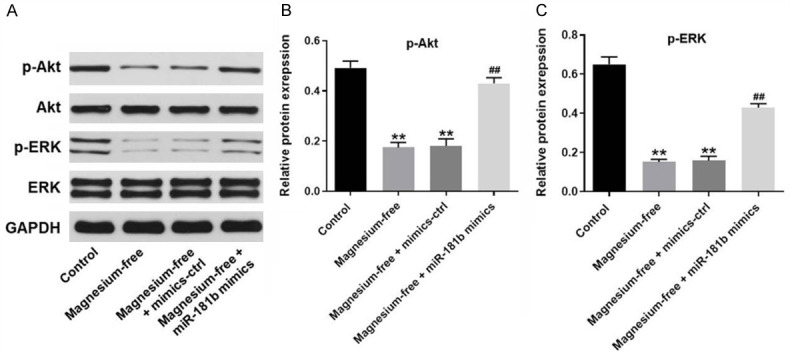
MiR-181b suppressed the progression of EP in vitro through activation of PI3K/Akt signaling. A. The protein expressions of Akt, p-Akt, ERK and p-ERK in hippocampal neurons were detected by western-blot. B. The relative expression of p-Akt was quantified via normalizing to GAPDH. C. The relative expression of p-ERK was quantified via normalizing to GAPDH. **P < 0.01 compared to control. ##P < 0.01 compared to magnesium-free.
MiR-181b inhibited the development of EP via downregulation of lncRNA ZNF883
Since previous reports indicated the differentially expressed lncRNAs in EP [22,23], q-PCR was performed to detect the expressions of some lncRNAs in hippocampal neurons. As revealed in Figure 6A, the expressions of ZNF883, SNHG12, XIST, LINC00667 and MALAT1 in hippocampal neurons were significantly upregulated by magnesium free, compared with control. Moreover, the expression of ZNF883 exhibited most significant changes. Thus, it was selected of use in following experiments. Then, ENCORI, dual luciferase report assay and q-PCR were performed to detect the relation with miR-181b and ZNF883. The results suggested that lncRNA ZNF883 was downregulated by miR-181b mimics (Figure 6B-D). In addition, the result of FISH demonstrated that ZNF883 and miR-181b partly shared the same location in cells (Figure 6E). Altogether, miR-181b inhibited the development of EP might via downregulation of lncRNA ZNF883.
Figure 6.
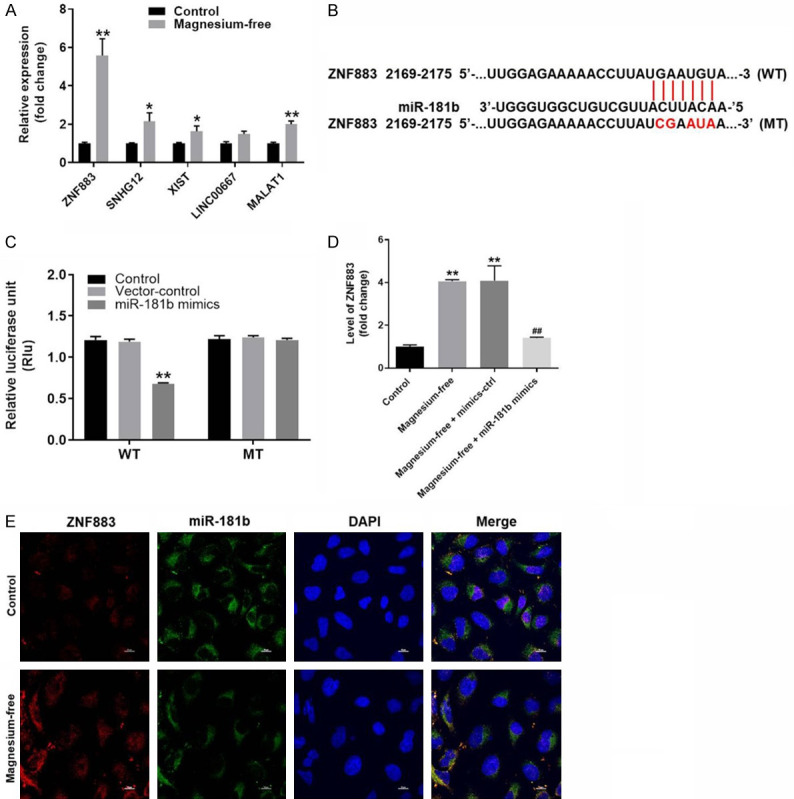
MiR-181b inhibited the development of EP via downregulation of lncRNA ZNF883. A. The gene expression of ZNF883, SNHG12, XIST, LINC00667 and MALAT1 in hippocampal neurons were detected by q-PCR. B. Gene structure of ZNF883 at the position of 2169-2175 indicated the predicted target site of miR-181b in its 3’UTR. C. The luciferase activity was measured in hippocampal neurons following co-transfecting with WT/MT ZNF883 3’-UTR plasmid and miR-181b with the dual luciferase reporter assay. D. The expression of ZNF883 in hippocampal neurons was investigated by q-PCR. E. The co-location of miR-181b and ZNF883 was detected by FISH detection. **P < 0.01 compared to control. ##P < 0.01 compared to magnesium-free.
Discussion
MiRNAs have been confirmed to play important roles during the development of multiple diseases, including EP [24-26]. In this research, we found that upregulation of miR-181b could significantly promote the growth of hippocampal neurons in the presence of magnesium-free. Otherwise, previous studies have reported that miR-181b could inhibit the cell proliferation, invasion and migration in multiple malignant tumors [27-29]. This discrepancy may due to different type of diseases. In addition, our findings further confirmed the biological function of miR-181b, indicating that miR-181b could be a key regulator during the progression of EP.
Ras association domain family member 1 (RASSF1A) has been identified to be present in the cancerous samples [30]. It is an important transcription factor that regulates the growth, differentiation and apoptosis of cancer cells [31]. Additionally, overexpression of RASSF1A has been studied in multiple malignant tumors and has been found to regulate various biological functions, including cell survival and metastasis [32,33]. In this research, we have indicated that RASSF1A was a direct target of miR-181b. It has been previously confirmed that miR-181b overexpression induced the apoptosis of gastric carcinoma cells by directly targeting RASSF1A [34]. However, we have found that miR-181b mimics promoted the growth of hippocampal neurons. This discrepancy might due to the different cell type. Based on these findings, it can be confirmed that RASSF1A might be a promoter in cell growth.
Previous studies have indicated that lncRNAs played a critical role during the progression of various diseases, including EP [35-37]. In the present research, we confirmed that the expression of lncRNA ZNF883 was significantly upregulated in hippocampal neurons. Wang X et al found that the expression of lncRNA ZNF883 was notably downregulated in ovarian cancer cells [38]. This study supplemented the biological role of ZNF883, suggesting that ZNF883 could be a biomarker in EP. Besides, in this research, we also found that miR-181b mimics inhibited the progression of EP through mediation of ZNF883. This finding was similar to the previous study [39], indicating that miR-181b might act as a suppressor in occurrence of EP.
PI3K/Akt signaling was involved in growth of many types of cells [40,41]. A previous studies has reported that PI3K/Akt signaling played a key role in cancer progression, drug resistance, and treatment [42]. Recent studies found that PI3K/Akt signaling lead to reduced apoptosis and increased proliferation of various cells [43-45]. This finding was similar to our current research. Moreover, our present study demonstrated that miR-181b downregulation activated PI3K/Akt signaling pathway in vitro, indicating that miR-181b may act as a PI3K/Akt signaling inhibitor. Moreover, it has been previously reported that MALAT1 could relieve the symptom of EP via PI3K/Akt [46]. This research further verified the function of PI3K/Akt, suggesting that PI3K/Akt could be a suppressor during the progression of EP. In addition, Ji M et al found that RASSF1A had inhibitory effect on PI3K/Akt signaling [47]. Our research was consistent to this result, confirming that miR-181b could suppress the progression of EP via mediation of RASSF1A/PI3K/Akt axis. Since it has been reported that JAK/STAT signaling could play a key role during the development of EP [48], we will further investigate the effect of miR-181b on JAK/STAT signaling.
In conclusion, miR-181b suppresses the progression of epilepsy by inhibition of lncRNA ZNF883, which may serve as a new target for treatment of EP.
Acknowledgements
This research was supported by Youth Science Foundation of the National Natural Science Foundation of China (81901329) and Youth Fund of Natural Science Foundation of Hunan Province in 2017 (2017JJ3466).
Disclosure of conflict of interest
None.
References
- 1.Wang W, Guo Y, He L, Chen C, Luo J, Ma Y, Li J, Yang Y, Yang Q, Du C, Zhang Y, Li Z, Xu X, Tian X, Wang X. Overexpression of miRNA-137 in the brain suppresses seizure activity and neuronal excitability: a new potential therapeutic strategy for epilepsy. Neuropharmacology. 2018;138:170–181. doi: 10.1016/j.neuropharm.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Cho YJ, Kim H, Kim WJ, Chung S, Kim YH, Cho I, Lee BI, Heo K. Trafficking patterns of NMDA and GABAA receptors in a Mg(2+)-free cultured hippocampal neuron model of status epilepticus. Epilepsy Res. 2017;136:143–148. doi: 10.1016/j.eplepsyres.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73. doi: 10.1016/j.seizure.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Xiang L, Ren Y, Li X, Zhao W, Song Y. MicroRNA-204 suppresses epileptiform discharges through regulating TrkB-ERK1/2-CREB signaling in cultured hippocampal neurons. Brain Res. 2016;1639:99–107. doi: 10.1016/j.brainres.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Wong SB, Cheng SJ, Hung WC, Lee WT, Min MY. Rosiglitazone suppresses in vitro seizures in hippocampal slice by inhibiting presynaptic glutamate release in a model of temporal lobe epilepsy. PLoS One. 2015;10:e0144806. doi: 10.1371/journal.pone.0144806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall AM, Vilasi A, Garcia-Perez I, Lapsley M, Alston CL, Pitceathly RD, McFarland R, Schaefer AM, Turnbull DM, Beaumont NJ, Hsuan JJ, Cutillas PR, Lindon JC, Holmes E, Unwin RJ, Taylor RW, Gorman GS, Rahman S, Hanna MG. The urinary proteome and metabonome differ from normal in adults with mitochondrial disease. Kidney Int. 2015;87:610–622. doi: 10.1038/ki.2014.297. [DOI] [PubMed] [Google Scholar]
- 7.Serafini R, Dettloff S, Loeb JA. Neocortical slices from adult chronic epileptic rats exhibit discharges of higher voltages and broader spread. Neuroscience. 2016;322:509–524. doi: 10.1016/j.neuroscience.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Xiang L, Ren Y, Cai H, Zhao W, Song Y. MicroRNA-132 aggravates epileptiform discharges via suppression of BDNF/TrkB signaling in cultured hippocampal neurons. Brain Res. 2015;1622:484–495. doi: 10.1016/j.brainres.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Thieffry S, Klein P, Baulac M, Plumb J, Pelgrims B, Steeves S, Borghs S. Understanding the challenge of comparative effectiveness research in focal epilepsy: a review of network meta-analyses and real-world evidence on antiepileptic drugs. Epilepsia. 2020;61:595–609. doi: 10.1111/epi.16476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Huang Z, Luo Y, Zheng F, Hu Y, Liu H, Zhu S, He M, Xu D, Li Y, Yang M, Yang Y, Wei X, Gao X, Wang W, Ma J, Ma Y, Wang X, Wang Q. Inhibition of Nwd1 activity attenuates neuronal hyperexcitability and GluN2B phosphorylation in the hippocampus. EBioMedicine. 2019;47:470–483. doi: 10.1016/j.ebiom.2019.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, Russo E, Whalley BJ, Di Marzo V, Stephens GJ. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 12.Gong XW, Li JB, Lu QC, Liang PJ, Zhang PM. Effective connectivity of hippocampal neural network and its alteration in Mg2+-free epilepsy model. PLoS One. 2014;9:e92961. doi: 10.1371/journal.pone.0092961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frye RE, Rossignol D, Casanova MF, Brown GL, Martin V, Edelson S, Coben R, Lewine J, Slattery JC, Lau C, Hardy P, Fatemi SH, Folsom TD, Macfabe D, Adams JB. A review of traditional and novel treatments for seizures in autism spectrum disorder: findings from a systematic review and expert panel. Front Public Health. 2013;1:31. doi: 10.3389/fpubh.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y, Ren J. Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci. 2019;76:441–451. doi: 10.1007/s00018-018-2940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng JF, Liu X, Zhao HB, Fan WF, Geng JJ, Liu XZ. LncRNA UCA1 inhibits epilepsy and seizure-induced brain injury by regulating miR-495/Nrf2-ARE signal pathway. Int J Biochem Cell Biol. 2018;99:133–139. doi: 10.1016/j.biocel.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Song LF, Chen XY, Ma YL, Suo JF, Shi JH, Chen GH. MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neurosci Ther. 2019;25:112–122. doi: 10.1111/cns.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Miao W, Teng J, Zhang L. Ginsenoside Rb1 protects the brain from damage induced by epileptic seizure via Nrf2/ARE signaling. Cell Physiol Biochem. 2018;45:212–225. doi: 10.1159/000486768. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Huang H, Zhou X, Liu X, Ou S, Xu T, Li R, Ma L, Chen Y. MicroRNA-132 interact with p250GAP/Cdc42 pathway in the hippocampal neuronal culture model of acquired epilepsy and associated with epileptogenesis process. Neural Plast. 2016;2016:5108489. doi: 10.1155/2016/5108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balazs M, Koroknai V, Szasz I, Ecsedi S. Detection of CCND1 locus amplification by fluorescence in situ hybridization. Methods Mol Biol. 2018;1726:85–100. doi: 10.1007/978-1-4939-7565-5_9. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson H, Lowhagen Henden P, Rentzos A, Pujol-Calderon F, Karlsson JE, Hoglund K, Blennow K, Zetterberg H, Rosengren L, Zelano J. Acute symptomatic seizures and epilepsy after mechanical thrombectomy. Epilepsy Behav. 2020;104:106520. doi: 10.1016/j.yebeh.2019.106520. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Xu Y, Dai H, Tan S, Mao X, Chen Z. Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-kappaB pathway. Cell Biosci. 2020;10:42. doi: 10.1186/s13578-020-00387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo ZH, Walid AA, Xie Y, Long H, Xiao W, Xu L, Fu Y, Feng L, Xiao B. Construction and analysis of a dysregulated lncRNA-associated ceRNA network in a rat model of temporal lobe epilepsy. Seizure. 2019;69:105–114. doi: 10.1016/j.seizure.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Vieira AS, Dogini DB, Lopes-Cendes I. Role of non-coding RNAs in non-aging-related neurological disorders. Braz J Med Biol Res. 2018;51:e7566. doi: 10.1590/1414-431X20187566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henshall DC, Hamer HM, Pasterkamp RJ, Goldstein DB, Kjems J, Prehn JHM, Schorge S, Lamottke K, Rosenow F. MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol. 2016;15:1368–1376. doi: 10.1016/S1474-4422(16)30246-0. [DOI] [PubMed] [Google Scholar]
- 25.Lovisari F, Simonato M. Gene networks and microRNAs: promises and challenges for treating epilepsies and their comorbidities. Epilepsy Behav. 2019:106488. doi: 10.1016/j.yebeh.2019.106488. [DOI] [PubMed] [Google Scholar]
- 26.Antonio LGL, Freitas-Lima P, Pereira-da-Silva G, Assirati JA Jr, Matias CM, Cirino MLA, Tirapelli LF, Velasco TR, Sakamoto AC, Carlotti CG Jr, Tirapelli DPDC. Expression of microRNAs miR-145, miR-181c, miR-199a and miR-1183 in the blood and hippocampus of patients with mesial temporal lobe epilepsy. J Mol Neurosci. 2019;69:580–587. doi: 10.1007/s12031-019-01386-w. [DOI] [PubMed] [Google Scholar]
- 27.Han Z, Zhan R, Chen S, Deng J, Shi J, Wang W. miR-181b/Oncostatin m axis inhibits prostate cancer bone metastasis via modulating osteoclast differentiation. J Cell Biochem. 2020;121:1664–1674. doi: 10.1002/jcb.29401. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Huang J, Xu T, Ye Z, Jin F, Li N, Lv B. MicroRNA-181b blocks gensenoside Rg3-mediated tumor suppression of gallbladder carcinoma by promoting autophagy flux via CREBRF/CREB3 pathway. Am J Transl Res. 2019;11:5776–5787. [PMC free article] [PubMed] [Google Scholar]
- 29.Yun J, Han SB, Kim HJ, Go SI, Lee WS, Bae WK, Cho SH, Song EK, Lee OJ, Kim HK, Yang Y, Kwon J, Chae HB, Lee KH, Han HS. Exosomal miR-181b-5p downregulation in ascites serves as a potential diagnostic biomarker for gastric cancer-associated malignant ascites. J Gastric Cancer. 2019;19:301–314. doi: 10.5230/jgc.2019.19.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.S SK, Swamy SN, Premalatha CS, Pallavi VR, Gawari R. Aberrant promoter hypermethylation of RASSF1a and BRCA1 in circulating cell-free tumor DNA serves as a biomarker of ovarian carcinoma. Asian Pac J Cancer Prev. 2019;20:3001–3005. doi: 10.31557/APJCP.2019.20.10.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois F, Bergot E, Levallet G. Cancer and RASSF1A/RASSF1C, the two faces of janus. Trends Cancer. 2019;5:662–665. doi: 10.1016/j.trecan.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Tokarz P, Pawlowska E, Bialkowska-Warzecha J, Blasiak J. The significance of DNA methylation profile in metastasis-related genes for the progression of colorectal cancer. Cell Mol Biol (Noisy-le-grand) 2017;63:79–87. doi: 10.14715/cmb/2017.63.2.12. [DOI] [PubMed] [Google Scholar]
- 33.Liao A, Tan G, Chen L, Zhou W, Hu H. RASSF1A inhibits gastric cancer cell proliferation by miR-711-mediated downregulation of CDK4 expression. Oncotarget. 2016;7:5842–5851. doi: 10.18632/oncotarget.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao LD, Zheng WW, Wang GX, Kang XC, Qin L, Ji JJ, Hao S. Epigenetic silencing of miR-181b contributes to tumorigenicity in colorectal cancer by targeting RASSF1A. Int J Oncol. 2016;48:1977–1984. doi: 10.3892/ijo.2016.3414. [DOI] [PubMed] [Google Scholar]
- 35.Li BG, Wu WJ, Zheng HC, Yang HF, Zuo YX, Cui XP. Long noncoding RNA GAS5 silencing inhibits the expression of KCNQ3 by sponging miR-135a-5p to prevent the progression of epilepsy. Kaohsiung J Med Sci. 2019;35:527–534. doi: 10.1002/kjm2.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henshall DC. Epigenetics and noncoding RNA: recent developments and future therapeutic opportunities. Eur J Paediatr Neurol. 2020;24:30–34. doi: 10.1016/j.ejpn.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhao T, Ding Y, Li M, Zhou C, Lin W. Silencing lncRNA PVT1 inhibits activation of astrocytes and increases BDNF expression in hippocampus tissues of rats with epilepsy by downregulating the Wnt signaling pathway. J Cell Physiol. 2019 doi: 10.1002/jcp.28264. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Han L, Zhou L, Wang L, Zhang LM. Prediction of candidate RNA signatures for recurrent ovarian cancer prognosis by the construction of an integrated competing endogenous RNA network. Oncol Rep. 2018;40:2659–2673. doi: 10.3892/or.2018.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecera M, Sana J, Butova R, Reguli S, Hermanova M, Kren L, Lipina R, Smrcka M, Slaby O. Dysregulation of long non-coding RNAs in glioblastoma multiforme and their study through use of modern molecular-genetic approaches. Klin Onkol. 2018;31(Suppl 1):168–170. [PubMed] [Google Scholar]
- 40.Li Z, Liu J, Que L, Tang X. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer. 2019;10:5770–5784. doi: 10.7150/jca.29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang N, Dong Q, Zhou XN. LMO4 promotes the invasion and proliferation of gastric cancer by activating PI3K-Akt-mTOR signaling. Am J Transl Res. 2019;11:6534–6543. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, Zhu Z, Li L, Ding J. Resibufogenin inhibits ovarian clear cell carcinoma (OCCC) growth in vivo, and migration of OCCC cells in vitro, by down-regulating the PI3K/AKT and actin cytoskeleton signaling pathways. Am J Transl Res. 2019;11:6290–6303. [PMC free article] [PubMed] [Google Scholar]
- 43.Elsherbiny NM, Abdel-Mottaleb Y, Elkazaz AY, Atef H, Lashine RM, Youssef AM, Ezzat W, El-Ghaiesh SH, Elshaer RE, El-Shafey M, Zaitone SA. Carbamazepine alleviates retinal and optic nerve neural degeneration in diabetic mice via nerve growth factor-induced PI3K/Akt/mTOR activation. Front Neurosci. 2019;13:1089. doi: 10.3389/fnins.2019.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu H, Ma L, Feng F. PICK1 attenuates high glucose-induced pancreatic beta-cell death through the PI3K/Akt pathway and is negatively regulated by miR-139-5p. Biochem Biophys Res Commun. 2020;522:14–20. doi: 10.1016/j.bbrc.2019.11.051. [DOI] [PubMed] [Google Scholar]
- 45.Peng X, Zhou J, Li B, Zhang T, Zuo Y, Gu X. Notch1 and PI3K/Akt signaling blockers DAPT and LY294002 coordinately inhibit metastasis of gastric cancer through mutual enhancement. Cancer Chemother Pharmacol. 2020;85:309–320. doi: 10.1007/s00280-019-03990-4. [DOI] [PubMed] [Google Scholar]
- 46.Wu Q, Yi X. Down-regulation of long noncoding RNA MALAT1 protects hippocampal neurons against excessive autophagy and apoptosis via the PI3K/Akt signaling pathway in rats with epilepsy. J Mol Neurosci. 2018;65:234–245. doi: 10.1007/s12031-018-1093-3. [DOI] [PubMed] [Google Scholar]
- 47.Ji M, Guan H, Gao C, Shi B, Hou P. Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC) BMC Cancer. 2011;11:147. doi: 10.1186/1471-2407-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, Meng WJ, Hu W, Zhang JG, Li L, Meng FG. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J Neuroinflammation. 2018;15:103. doi: 10.1186/s12974-018-1139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]