Abstract
Brain responses to external stimuli such as light are preserved under general anesthesia. In nocturnal animals, acute light exposure can induce sleep, and acute dark can increase wakefulness. This study aims to investigate the effect of acute continuous nocturnal light exposure (ACNLE) on burst-suppression patterns under sevoflurane anesthesia using electroencephalogram (EEG) monitoring in mice. We set the initial sevoflurane dose to 2.0% and increased it by 0.5% every 20 min until it reached 4.0%. Burst-suppression ratio (BSR), EEG power and quantitative burst analysis were used to assess the effects of ACNLE on burst suppression patterns under sevoflurane anesthesia. Blood serum corticosterone measurement and c-Fos immunofluorescent staining of the suprachiasmatic nucleus (SCN) and ventrolateral preoptic nucleus (VLPO) were used to demonstrate the biological consequence induced by ACNLE. Compared to darkness, ACNLE caused significant changes in EEG power and decrease of BSR at 2.5%, 3.0% and 3.5% sevoflurane. ACNLE was also associated with an increase in burst duration and burst frequency as well as a decrease in burst maximum peak-to-peak amplitude and burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands. ACNLE increased the concentration of serum corticosterone and the expression of c-Fos in the SCN, while not changed c-Fos expression in the VLPO. These results demonstrated that ACNLE influences the BSR under sevoflurane anesthesia, possibly by activating light-sensitive nonvisual pathways including SCN and increasing of peripheral serum corticosterone levels.
Keywords: Light exposure, nocturnal, burst suppression electroencephalogram, suprachiasmatic nucleus, ventrolateral preoptic nucleus, sevoflurane
Introduction
Burst-suppression activity, first described by Swank and Watson in 1949 [1], is defined as an electroencephalography (EEG) pattern comprising alternative periods of high-amplitude slow waves (the burst) and flat EEG (suppression) [2], and it is often observed in coma [3], hypothermia [4], epilepsy [5], and deep general anesthesia [6,7]. The duration of suppression is prolonged as anesthesia deepens. Eventually, a completely isoelectric (flatline) EEG can be attained. Increasing levels of anesthesia are thought to produce a progressive loss of brain responsiveness to external stimuli. However, current evidence supports the idea that general anesthesia is not a uniform state of the brain. Ongoing activity of brain differs as the depth of anesthesia changes, and cerebral response properties are modulated depending on the anesthetic dosage [8]. General anesthetics, which are expected to silence brain activity, often spare brain responses to sensory stimuli [9-15]. Under the deep levels of anesthesia that induce burst suppression, brain excitability is dramatically increased [16], such that external stimuli, including somatosensory, auditory and visual stimuli, have been shown to elicit bursts of whole-brain activity. Previous studies demonstrated that light pulses can induce transient EEG bursts during general anesthesia-induced burst suppression in both humans [9,12] and rodents [13,14]. In addition, light-induced gamma oscillations are preserved or enhanced in anesthesia induced by volatile anesthetics [14,17,18]. At present, the biological consequences and neural pathway of this light-induced brain activity during anesthesia-induced burst suppression are largely unknown.
Beyond its widely appreciated role in vision, light exerts a wide range of powerful biological effects on nonvisual responses, including the regulation of circadian rhythms, sleep and wake cycles, pupil constriction, heart rate, hormone release, learning and memory, through distinct light-sensitive brain pathways [19,20]. In addition, light-induced gamma oscillations result in the preservation of neuronal and synaptic density across multiple brain regions in neurodegeneration mouse models and are associated with improved cognitive function [21]. Aside from acute changes in EEG activities, it would be interesting to know the local and organism-level biophysical consequences following light exposure during anesthesia-induced burst suppression. Acute nocturnal light exposure in rodents has been shown to result in rapid sleep induction through intrinsically photosensitive retinal ganglion cells (ipRGCs) [22]. A recent study demonstrated that ipRGCs project to nonvisual systems, including the suprachiasmatic nucleus (SCN) and ventrolateral preoptic area (VLPO), which are related to arousal-promoting responses and sleep-promoting responses to light in mice, respectively [23]. The SCN and VLPO are differentially involved in the action of general anesthetics [24,25]. Whether acute light exposure impacts brain activity and to what extent the process and consequence of light-induced nonvisual response is preserved during sevoflurane-induced burst suppression remain unknown.
In the present study, EEG was used to monitor burst suppression under sevoflurane anesthesia. The burst-suppression ratio (BSR) and EEG power were calculated to assess the effects of acute light exposure at night on burst suppression under sevoflurane anesthesia in C57BL/6L mice kept under a light-dark cycle. SCN and VLPO c-Fos immunofluorescent staining and blood serum corticosterone measurement were used to demonstrate the neural pathway involved in and biological consequences associated with continuous acute nighttime light exposure (ACNLE) induced brain activities during sevoflurane-induced burst suppression. We aimed to test the hypothesis that ACNLE has an impact on burst suppression patterns under sevoflurane anesthesia. Differentially preserved light-sensitive nonvisual pathways may be involved in ACNLE induced changes of brain activity and biological consequences.
Materials and methods
Animals
All procedures were approved by the Experimental Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology and carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Male C57BL/6J mice aged 10-12 weeks old weighing 22-26 g were used in this study. All animals were provided with at least 7 days of rest before experiments and were kept on a standard light/dark cycle (light on: 07:00-19:00) at constant ambient temperature (21 ± 1°C) and humidity (50 ± 5%). All experiments were performed between 20:00 and 24:00.
Electroencephalogram electrode placement and recording
Mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). The hair was removed from the scalp with an electric razor. Then, the animals were placed in a stereotaxic apparatus (RWD Inc., Shenzhen, China). A heating pad (RWD Inc., Shenzhen, China) was used to maintain a stable body temperature. With additional subcutaneous bupivacaine for analgesia, extradural EEG electrodes were implanted surgically. Two stainless steel screws were fixed in the right frontal cortex (0.4 mm lateral and 1.75 mm anterior to bregma) and left cerebellum for EEG recording. All leads were connected to a miniature plug. Then, the screws, cables and miniature plug were all permanently fixed to the skull with Super-Bond C&B and dental acrylic [26,27]. After surgery, the mice were wrapped in a heating pad (RWD Inc., Shenzhen, China) until complete recovery from anesthesia. All animals that underwent EEG electrode placement surgery were kept on a standard light/dark cycle (light on: 07:00-19:00) for at least 7 days before anesthesia and EEG recording.
Mice were connected to a cable attached to a rotative connector to allow free movement within the cylindrical chambers. EEG was performed with a sampling frequency of 500 Hz using a Model 1700 differential alternating-current amplifier (A-M system, Carlsborg, WA, USA) and a PCIe 6323 data acquisition board (National Instruments, Austin, TX). EEG signals were continuously recorded with Spikehound software [28]. Recordings began approximately 20 min prior to the start of sevoflurane administration and ended after animals regained movement.
Anesthesia protocol
Four days before the test, we placed all mice into individual anesthesia chambers adapted for at least 2 hr. As shown in Figure 1, baseline recordings were taken for 20 min in the waking state before sevoflurane was administered. Then, anesthesia was induced with 2.0% sevoflurane (RWD Inc., Shenzhen, China) in oxygen with a fresh gas flow rate of 500 ml/min. The sevoflurane concentration was increased in steps of 0.5% every 20 min until a final concentration of 4.0% was reached. This maximal dose was maintained for an additional 20 min. During the first 10 min at each dose, the animals were allowed to equilibrate to the anesthetic concentration in the chamber. For the entire recording period, the mice within the cylindrical chambers were placed on a far infrared warming pad (RightTemp®, Kent Scientific, USA) that had been warmed to 37°C. After the onset of loss of righting reflex, a rectal temperature probe (RET-3, Kent Scientific, USA) was placed to monitor the rectal temperature of the mice. The pulse oxygen saturation (SpO2) of the mice was continuously monitored by a pulse oximeter (MouseSTAT® Jr., Kent Scientific, USA). We recorded the rectal temperature and SpO2 of each mouse during the maintenance of anesthesia as of 5 min, 10 min, and 20 min into each state for analysis. Gas was continuously sampled from the downstream portion of the chamber, and sevoflurane concentrations in the chamber were monitored using an anesthetic agent analyzer (G60, PHILIPS, Shenzhen, China).
Figure 1.
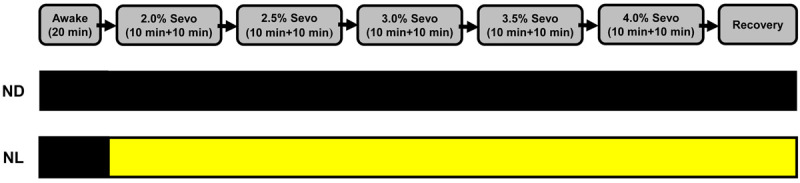
Flowchart of the anesthesia protocol; lighting conditions during sevoflurane administration. Mice were recorded for 20 min in the waking state before sevoflurane was administered. Then, anesthesia was induced with 2.0% sevoflurane in oxygen with a fresh gas flow rate of 500 mL/min. The sevoflurane concentration was increased by 0.5% every 20 min until a final concentration of 4.0% was attained. For first 10 min at each dose, the animals were allowed to equilibrate to the anesthetic concentration in the chamber. The second line: black box indicates dark condition; the third line: black box indicates dark condition; yellow box indicates light condition. Sevo = sevoflurane. ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Mice were randomly divided into 2 groups (Figure 1). Before anesthesia, mice in both groups were kept in the dark. In the night dark group (ND), sevoflurane was administered in the dark. In the night light group (NL), sevoflurane was administered along with continuous acute exposure to light (LED, 5 W, 2700-3500 K, 50 Hz).
EEG burst-suppression identification and BSR calculation
Artifact-free EEG data under each sevoflurane concentration were selected for analysis after the 10 min equilibration of the animal to the chamber concentration. Large artifacts in the electroencephalogram were observed only during gross motor movement; we searched for a recorded period free from such events. The raw EEG data were preliminarily bandpass filtered (10-80 Hz) and band-block filtered (48-52 Hz) for removal of line noise. Each EEG recording (n = 10 in each group) was detrended and smoothed by convolution with a Gaussian function. The BSR is calculated by segmenting the EEG into bursts and suppressions using a voltage- and duration-based threshold. Suppression is commonly defined as an interval in which the amplitude of the time-differentiated EEG signal remains within a -15 to 15 μV/s window for at least 200 ms; suppression events were considered part of the same suppression when the interevent interval was ≤ 50 ms. EEG epochs between suppression periods were considered bursts. For the BSR algorithm, suppressions are given a value of 1 and bursts are given a value of 0 to create a binary time series [29]. This binary time series is then smoothed with a windowing function to calculate the BSR over time [10,29]. Quantitative burst analysis was performed on identified burst episodes during burst suppression to illustrate the effect of acute nocturnal light exposure on burst frequency, burst duration, and burst maximum peak-to-peak amplitude (rectified signal) according to previously reported methods [10]. Burst frequency (Hz) was the number of burst appearances over a period of time. Duration (s) was the absolute length of the individual burst. The maximum peak-to-peak amplitude (mV) was the absolute difference between the maximum and minimum amplitude values within each individual burst [10]. The raw EEG signals were exported into MATLAB R2019a (MathWorks, Natick, MA) and analyzed using appropriate code.
Power spectral analysis
We computed spectrograms using multitaper methods from the Chronux toolbox (version 2.1.2, http://chronux.org/) in MATLAB R2019a (MathWorks, Natick, MA) [30]. An EEG spectrogram of spontaneous brain activity was computed every 4 s (50% overlapping) with a 5-taper FFT. The average power spectral density (PSD) over the whole frequency band (0.5-80 Hz) and for single frequency bands of the raw EEG signal was computed as follows: 300 s of spontaneous brain activity was computed every 4 s (50% overlapping), bandpass filtered (0.5-80 Hz) and band-block filtered (48-52 Hz) for removal of line noise, followed by a 5-taper fast Fourier transform (FFT). The averaged power values were calculated for the delta (0.5-4 Hz), theta (4-8 Hz), alpha (8-12 Hz), spindle (12-15 Hz), beta (15-25 Hz) and gamma (25-80 Hz) frequency bands for each studied sample [31,32]. For the analysis of power of identified bursts during the burst suppression patterns, a wavelet spectrogram was generated via continuous wavelet transform (Morlet kernel) for each frequency (0.5 Hz bin size) and normalized to be coherent with PSD as described above [33]. The wavelet decomposition was chosen over more traditional fast Fourier transform-based methods because it offers variable time and frequency resolutions and enables reliable detection of rapid transient changes in signal amplitude at higher frequencies [34].
Blood collection and corticosterone analysis
Mice in groups ND and NL were administered 2.5% sevoflurane. After anesthesia was maintained for 40 min, the mice were decapitated, and approximately 1 ml of blood was collected into a serum separator tube. After clot formation, the samples were centrifuged at 3000×g for 10 min at 4°C to separate out the serum. The serum was stored at -20°C until corticosterone analysis. All blood sampling was conducted within the same 3-h period in an effort to minimize the effect of circadian rhythms on corticosterone release.
Serum corticosterone concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) kit (ab108821; Abcam, Cambridge, MA, United States). According to the manufacturer’s protocol, 25 μl of sample and standard solutions and 25 μl biotinylated corticosterone protein were added to the precoated antibody plate provided with the kit and incubated for 2 h at room temperature. The plate was manually washed five times with 200 μL of 1× wash buffer. Next, 50 µl of 1× streptavidin-peroxidase conjugate was added to each well and incubated for 30 min at room temperature. Then, the microplate was washed as described above. A 50 µl volume of chromogen substrate was added to each well and incubated for 25 min at room temperature. The reaction was stopped by adding 50 μl of stop solution to each well. The optical density (O.D.) of corticosterone was read at a wavelength of 450 nm using a microplate reader (Model 680; Bio-Rad, California, USA). The concentration of serum corticosterone was calculated according to the standard curves.
Immunofluorescent staining and cell counting
Mice in groups ND and NL were administered 2.5% sevoflurane. After anesthesia was maintained for 40 min, the mice were perfused intracardially with saline followed by 4% ice-cold paraformaldehyde (PFA) in 0.1 M phosphate buffer saline (PBS). After perfusion, the brains were harvested, postfixed in 4% PFA for 4 h, and dehydrated in 30% sucrose in PBS at 4°C until they sank. The brains were coronally sectioned into 20-μm slices on a cryostat (CM1900; Leica, Germany), mounted on polylysine-coated slides, and stored at -80°C until use.
For c-Fos staining, the brain sections were permeabilized with 0.3% Triton X-100 for 15 min and blocked with 10% donkey serum for 1 h at room temperature, incubated overnight at 4°C with the rabbit anti-c-Fos antibody (1:500; Cat. No. 226 003; Synaptic Systems, Germany). Then, the sections were incubated with Alexa Fluor 488-labeled donkey anti-rabbit secondary antibody (1:300; A-21206; Invitrogen, Carlsbad, CA, United States) for 2 h at room temperature and washed with PBS. After being covered with DAPI (AR1177; Boster, Wuhan, China) for 5 min at room temperature, sections were rinsed, mounted, and cover-slipped with 50% glycerol. Images were captured using a fluorescence microscope (DM2500, Leica, Germany). The c-Fos-positive cells were counted on alternate sections in the SCN nucleus from -0.22 to -0.82 mm relative to bregma. VLPO c-Fos-positive cell counts were performed on sections spanning from +0.26 mm to -0.10 mm relative to bregma using a standardized 400×250 μm box positioned 300 μm lateral to the midline.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, San Diego, CA) was used for statistical analysis. In the repeated-measures designs (body temperature and SpO2, BSR, burst analysis and EEG PSD), principal effects were tested with one-way or two-way repeated-measures ANOVA (rANOVA). The Greenhouse-Geisser correction was applied when sphericity could not be assumed. Group comparisons in repeated-measures design were tested with two-way rANOVA, and Sidak’s multiple comparison test or Fisher’s least significant difference (LSD) test was applied for multiple hypothesis testing. The two-sample t-test was chosen for non-repeated measures designs (serum corticosterone, SCN and VLPO c-Fos), and the Welch correction for unequal variance was applied. All data are presented as the mean ± standard error of the mean. P < 0.05 was considered statistically significant.
Results
Initial assessment of depth of sevoflurane anesthesia
To evaluate the changes of depth of anesthesia, we recorded cortical activity by EEG monitoring. The sevoflurane concentrations used in this study ranged from 2.0 to 4.0%. Figures 2B-D, 3B-D present representative EEG power spectrograms, raw EEG traces and BSR responses for groups ND and NL. In the waking state, spontaneous cortical activity showed the characteristic low amplitude and high frequency EEG with no obvious burst-suppression activity (Figures 2E, 3E). When the sevoflurane concentration was increased, a clear burst-suppression pattern was induced (Figures 2F, 2G, 3F, 3G), reflecting strong brain inactivation, which is characteristic of deep general anesthesia.
Figure 2.
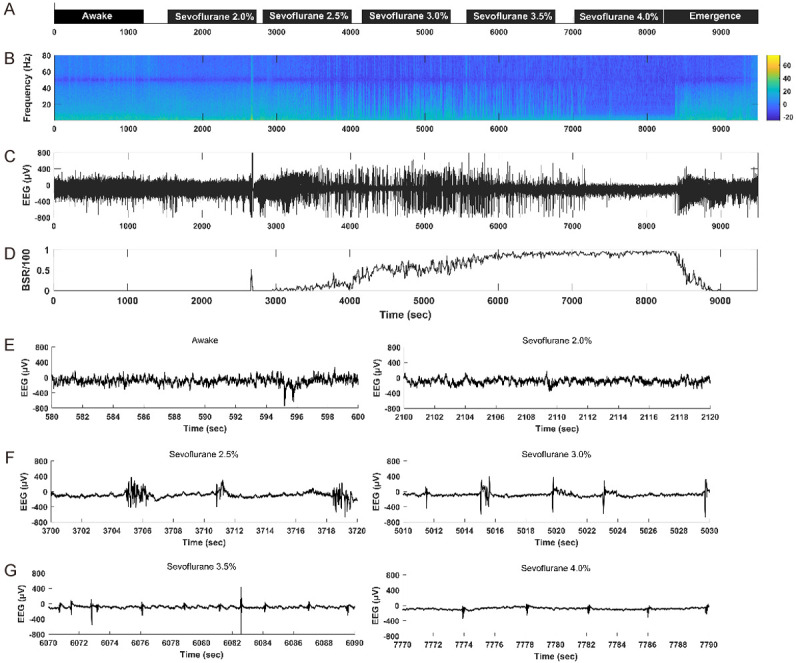
Representative electroencephalogram (EEG) power spectrogram, burst-suppression ratio (BSR) graph and raw EEG traces in an ND mouse. (A), the concentration and duration of sevoflurane anesthesia; black boxes indicate dark conditions. (B), EEG power spectrogram. Color bar, scale in decibels (dB); warm colors indicate frequency components with high power; cool colors indicate frequency components with low power. The EEG energy is decreased with the increase of the sevoflurane concentration. The deeper the anesthesia, the lower the EEG energy. (C), raw EEG trace under sevoflurane anesthesia. (D), BSR response under sevoflurane anesthesia. The BSR is increased with the increase of the sevoflurane concentration. (E-G), representative raw EEG traces (20 s) in the waking state (E left) and under 2.0% sevoflurane (E right), 2.5% sevoflurane (F left), 3.0% sevoflurane (F right), 3.5% sevoflurane (G left), 4.0% sevoflurane (G right).
Figure 3.
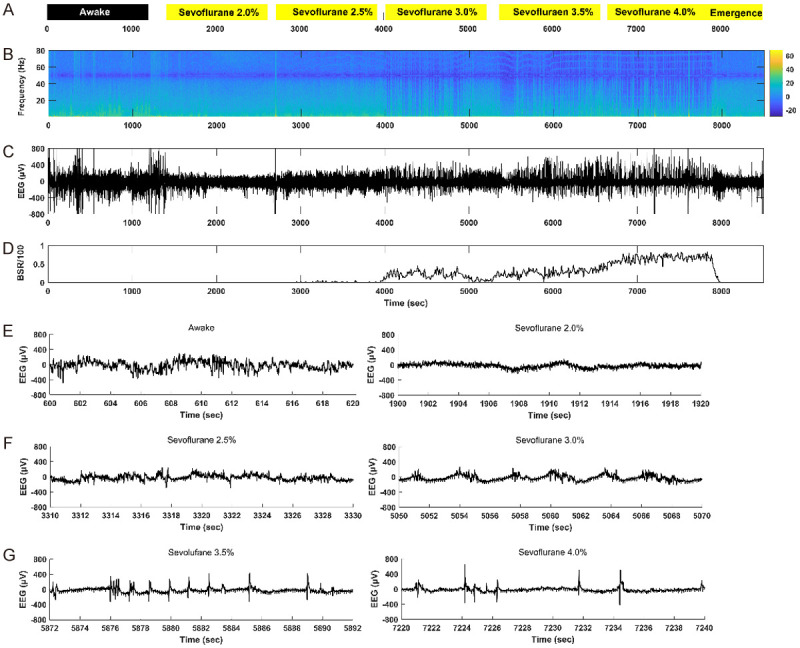
Representative electroencephalogram (EEG) power spectrogram, burst-suppression ratio (BSR) and raw EEG traces in an NL mouse. (A), the concentration and duration of sevoflurane anesthesia; black box indicates dark condition, yellow boxes indicate light conditions. (B), EEG power spectrogram. Color bar, scale in decibels (dB); warm colors indicate frequency components with high power; cool colors indicate frequency components with low power. The EEG energy is decreased with the increase of the sevoflurane concentration. The deeper the anesthesia, the lower the EEG energy. (C), raw EEG trace under sevoflurane anesthesia. (D), BSR response under sevoflurane anesthesia. The BSR is increased with the increase of the sevoflurane concentration. (E-G), representative raw EEG traces (20 s) in the waking state (E left) and under 2.0% sevoflurane (E right), 2.5% sevoflurane (F left), 3.0% sevoflurane (F right), 3.5% sevoflurane (G left), 4.0% sevoflurane (G right).
Effect of ACNLE on BSR and EEG power under sevoflurane anesthesia
Burst suppression ratio (BSR) was zero in the waking state, and a large increase in BSR was found in groups ND and NL when the sevoflurane concentration was increased (Figure 4A). Compared to group ND, acute light exposure in group NL caused a significant decrease in BSR at 2.5%, 3.0% and 3.5% sevoflurane (Figure 4A). With the lowest (2.0%) and highest (4.0%) concentrations of sevoflurane, there were no significant differences in BSR between groups NL and ND (Figure 4A). The mean EEG power over the whole frequency band (0.5-80 Hz) was increased in group NL compared to group ND at 2.5%, 3.0% and 3.5% sevoflurane (Figure 4B). The administration of sevoflurane produced a significant reduction in EEG power in almost all individual frequency bands, with the exception of the spindle band in group ND (Figure 4C and 4E). However, administration of sevoflurane produced a significant reduction in EEG power only in the delta, theta and gamma bands in the group NL (Figure 4D and 4F).
Figure 4.
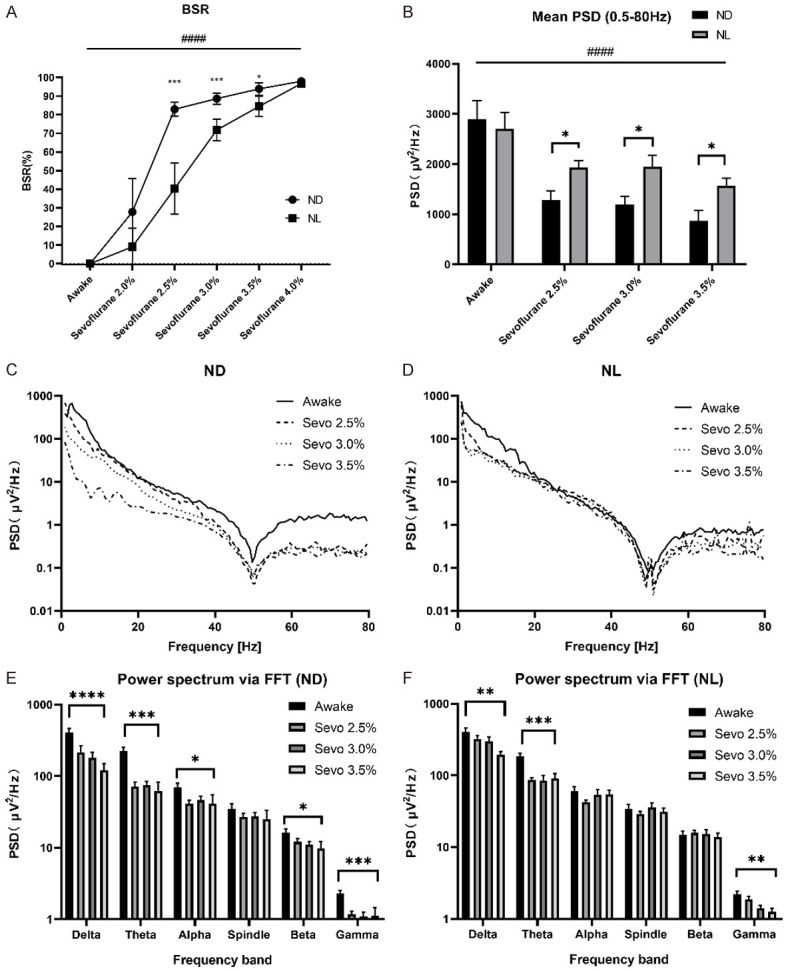
Effect of acute continuous nocturnal light exposure (ACNLE) on electroencephalogram (EEG) burst-suppression ratio (BSR) and EEG power spectral density (PSD) under sevoflurane anesthesia. (A), BSR for sevoflurane at each state. BSR increased as the sevoflurane concentration increased in groups ND and NL (####P < 0.0001, main effect of sevoflurane concentration by two-way rANOVA). Compared to group ND, acute light exposure in group NL caused a significant decrease in BSR at 2.5%, 3.0% and 3.5% sevoflurane. With the lowest (2.0%) and highest (4.0%) concentrations of sevoflurane, there were no significant differences in BSR between groups NL and ND (*P < 0.05, ***P < 0.001 compared with ND, n = 10 in each group, two-way rANOVA followed by Sidak’s multiple comparison test). (B), mean EEG PSD over the whole frequency band (0.5-80 Hz) from quantitative EEG spectral analysis in the waking state and under 2.5%, 3.0% and 3.5% sevoflurane anesthesia for groups NL and ND. The mean EEG power decreased as the sevoflurane concentration increased in groups ND and NL (####P < 0.0001, main effect of sevoflurane concentration by two-way rANOVA). The mean EEG power over the whole frequency band (0.5-80 Hz) was increased in group NL compared to group ND at 2.5%, 3.0% and 3.5% sevoflurane (*P < 0.05 compared with ND, n = 10 in each group, two-way rANOVA followed by Fisher’s LSD test). (C and D), for normalization, the squared magnitude of the fast Fourier transform (FFT) was divided by the number of samples (N) and by the sampling frequency (Fs). Average periodograms from four representative EEG recordings (4 s sweeps, 5-taper FFT, total trace length 300 s) obtained as above for ND (C) and NL (D). (E and F); mean EEG power from quantitative spectral analysis for ND (E) and NL (F) in single frequency bands under sevoflurane in each state. Group ND produced a significant reduction in EEG power in almost all individual frequency bands, with the exception of the spindle band. However, Group NL produced a significant reduction in EEG power only in the delta, theta and gamma bands (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 10 in each group, main effect of sevoflurane concentration by one-way rANOVA). Sevo = sevoflurane. ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Effect of ACNLE on features of spontaneous bursts under sevoflurane anesthesia
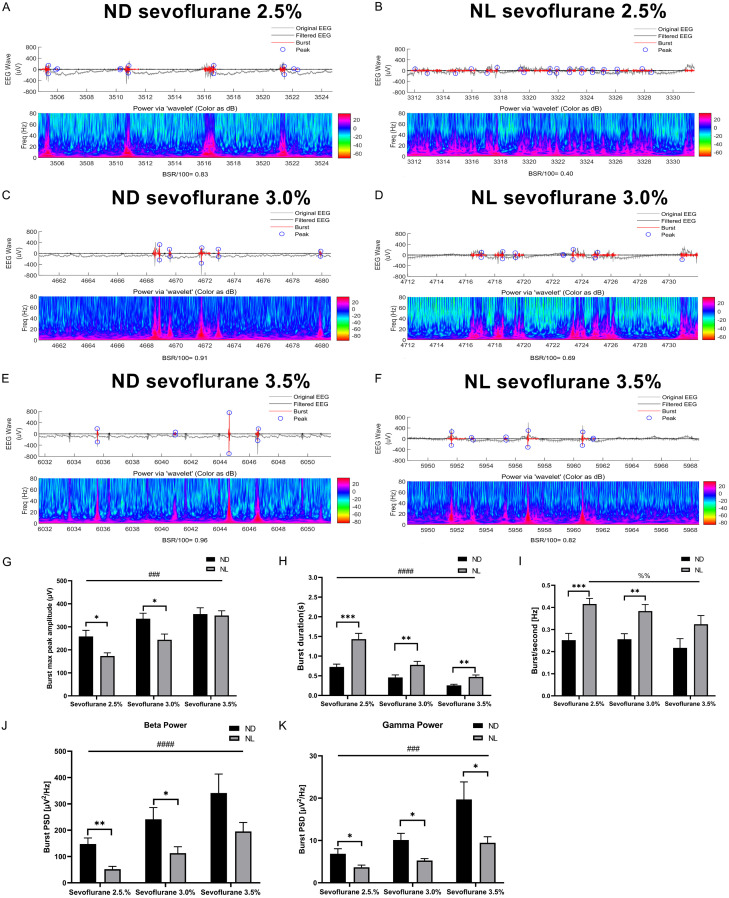
The burst duration and burst frequency decreased with increasing sevoflurane concentration in both groups. The peak-to-peak amplitude and burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands were increased with increasing sevoflurane concentration in both groups.
Compared to group ND, the analysis of individual bursts showed that burst duration and burst frequency were significantly increased in group NL (Figure 5H, 5I). The burst maximum peak-to-peak amplitude was decreased in group NL compared to group ND (Figure 5G). Compared to group ND, burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands was decreased in group NL (Figure 5J, 5K).
Figure 5.
Effect of acute continuous nocturnal light exposure (ACNLE) on spontaneous bursts under sevoflurane anesthesia. (A-F), representative EEG traces (up) and burst power (down) at increasing concentrations of sevoflurane for groups ND and NL. When the anesthetic concentration is increased, a clear burst-suppression pattern is induced. In the EEG trace, both the original EEG (gray line) and the filtered EEG (black line) are presented. Black on the filtered EEG trace indicates suppression, and red indicates a burst. Blue circles indicate the maximum and minimum amplitude values within each individual burst. (G-K), quantitative burst analysis obtained on identified burst episodes during burst suppression to illustrate the effect of acute nighttime light exposure on burst maximum peak-to-peak amplitude (G); burst duration (H); burst frequency (I); and burst power in beta (J) and gamma (K) bands. The burst duration and burst frequency decreased with increasing sevoflurane concentration in both groups. The peak-to-peak amplitude and burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands were increased with increasing sevoflurane concentration in both groups. Compared to group ND, the analysis of individual bursts showed that burst duration and burst frequency were significantly increased in group NL. The burst maximum peak-to-peak amplitude and burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands were decreased in group NL (##P < 0.01, ###P < 0.001, ####P < 0.0001, main effect of sevoflurane concentration by two-way rANOVA; %%P < 0.01 main effect of sevoflurane concentration by one-way rANOVA; *P < 0.05, **P < 0.01, ***P < 0.001 compared with ND, n = 10 in each group, two-way rANOVA followed by Fisher’s LSD test). Sevo = sevoflurane. ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Effect of ACNLE on c-Fos expression in the SCN and VLPO under sevoflurane anesthesia
To determine whether the SCN and VLPO were involved in changes induced by ACNLE during sevoflurane anesthesia, we examined immunofluorescent expression of c-Fos, a marker of antecedent neuronal activity. Figures 6 and 7 show that ACNLE under sevoflurane anesthesia elicited a significant increase in c-Fos expression in the SCN but had no effect on the number of c-Fos-positive cells in the VLPO, suggesting that ACNLE during sevoflurane anesthesia could activate only the SCN.
Figure 6.
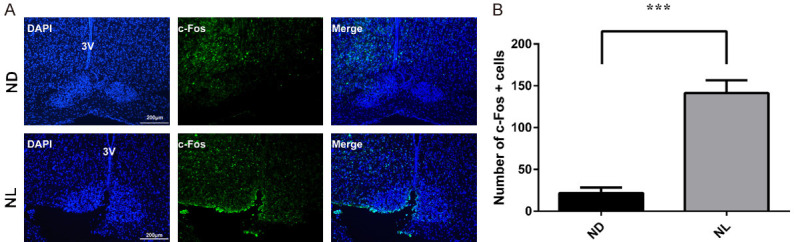
Effect of acute continuous nocturnal light exposure (ACNLE) on c-Fos expression in the suprachiasmatic nucleus (SCN) under sevoflurane anesthesia. A. c-Fos immunostaining and DAPI nuclear staining in the SCN. 3V, the third ventricle. Bar = 200 μm. B. quantitative analysis of the numbers c-Fos-positive cells in groups ND and NL. Compared to group ND, acute light exposure in group NL caused a higher c-Fos expression in the SCN (***P < 0.001 compared with ND, n = 4 in group ND, n = 6 in NL group, unpaired t-test). ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Figure 7.
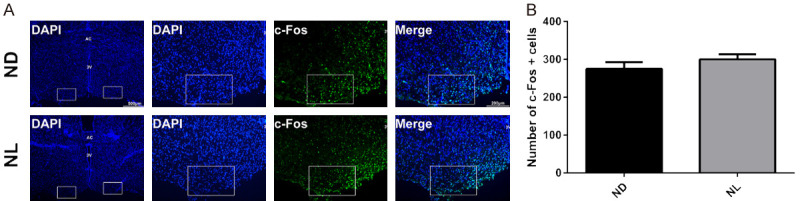
Effect of acute continuous nocturnal light exposure (ACNLE) on c-Fos expression in the ventrolateral preoptic nucleus (VLPO) under sevoflurane anesthesia. (A), c-Fos immunostaining and DAPI nuclear staining in the VLPO. 3V, the third ventricle. AC, the anterior commissure. Bar = 500 μm. Bar = 200 μm. The white boxes in (A) are 250 μm tall by 400 μm wide and correspond to the location used for counting. (B), quantitative analysis of the merged number of c-Fos and cells in the ND and NL groups. There was no difference in c-Fos expression depending on whether sevoflurane was administered in light (NL) or darkness (ND). (n = 7 in group ND, n = 8 in group NL, unpaired t-test). ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Effect of ACNLE on serum corticosterone levels under sevoflurane anesthesia
We measured the serum corticosterone levels in groups ND and NL at 2.5% sevoflurane, and we found that the serum corticosterone concentration of group NL was higher than that of group ND (Figure 8).
Figure 8.
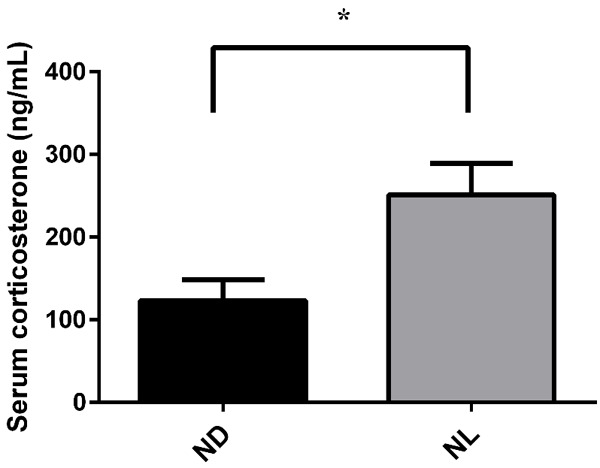
Effect of acute continuous nocturnal light exposure (ACNLE) on serum corticosterone levels under sevoflurane anesthesia. Compared to group ND, acute light exposure in group NL caused a higher serum corticosterone level (*P < 0.05 compared with ND, n = 6 in group ND, n = 6 in NL group, unpaired t-test). ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Body temperature and pulse oxygen saturation under sevoflurane anesthesia
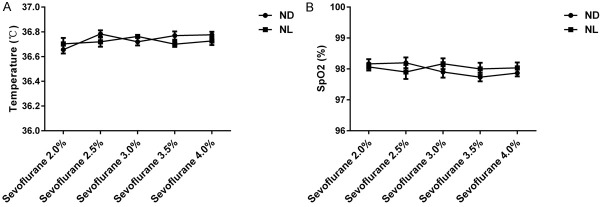
To verify that the differences in BSR and EEG power were not caused by hypothermia or hypoxia, we recorded rectal temperature and SpO2 of each mouse as of 5 min, 10 min, and 20 min into each state. The mean of three rectal temperature measurements was considered the final temperature; likewise, three SpO2 measurements were averaged to calculate the final SpO2. A two-way ANOVA showed no significant difference in rectal temperature (Figure 9A) or SpO2 (Figure 9B) between groups ND and NL under sevoflurane anesthesia.
Figure 9.
Rectal temperature and pulse oxygen saturation (SpO2) in groups ND and NL showed no statistically significant differences under sevoflurane anesthesia. A. There was no significant difference in rectal temperature between groups ND and NL under sevoflurane anesthesia (n = 10 in each group, two-way repeated-measures ANOVA). B. SpO2 did not vary between groups ND and NL under sevoflurane anesthesia. (n = 10 in each group, two-way repeated-measures ANOVA). ND, nighttime administration of sevoflurane (20:00-24:00) in the dark; NL, nighttime administration of sevoflurane (20:00-24:00) with exposure to light.
Discussion
This study demonstrated that ACNLE influences burst-suppression patterns under sevoflurane anesthesia. Our findings can be briefly described as follows: (1) ACNLE under sevoflurane anesthesia decreased the depth of anesthesia and was associated with reduced BSR, increased mean EEG power over the whole frequency range and less suppression of EEG power in single frequency bands compared to darkness at night. (2) ACNLE under sevoflurane anesthesia increased burst duration and burst frequency while decreasing burst maximum peak-to-peak amplitude and burst power in the beta (15-25 Hz) and gamma (25-80 Hz) bands. (3) ACNLE under sevoflurane anesthesia increased the expression of SCN c-Fos and the concentration of serum corticosterone. (4) ACNLE had no effect on VLPO c-Fos expression.
Burst suppression is an EEG pattern characterized by periods of high-voltage electrical activity alternating with periods of inactivity in the brain. Burst suppression was formally defined by the International Federation of Societies for Electroencephalography and Clinical Neurophysiology (IF-SECN) as a “pattern characterized by theta and/or delta waves, at times intermixed with faster waves, and intervening periods of relative quiescence” [35]. This EEG pattern has been considered to be associated with profound brain inactivation and unconsciousness and can be observed in patients with inactivated brain states, such as coma [3], hypothermia [4], and deep general anesthesia [6].
Increasing levels of anesthesia are thought to produce a progressive loss of brain responsiveness to external stimuli. In anesthetized subjects, sensory stimuli fail to gain access to the cerebral cortex because of a block or disruption of thalamocortical information transfer [36,37]. However, under the deep levels of anesthesia that induce burst suppression, brain excitability is still preserved or even dramatically increased [16]; external auditory [38], mechanical [39] and visual stimuli [9,12-14] have been shown to elicit bursts of whole-brain activity. In a human study, Hartikainen et al. demonstrated that the central nervous system reacts strongly to photic stimulation under deep anesthesia [12]. In humans under isoflurane anesthesia at 1.5 minimum alveolar concentration (MAC), 100% of photic stimuli, 98% of somatosensory stimuli and 94% of auditory stimuli given during EEG suppression evoked burst activity [9]. The effect of light on brain activity in nocturnal animals is distinct from its effect in humans, but this light-induced burst excitatory activity is preserved in nocturnal animals under anesthesia. In a rat model, burst activation of the cerebral cortex can be induced by flash stimuli under isoflurane anesthesia [13]. Robust gamma oscillations in the visual cortex have been observed in rats in response to flashes at all useful anesthetic levels with various agents [14]. Visual and auditory stimulation were able to evoke burst activity in the visual cortex and subiculum under progressively increasing levels of isoflurane anesthesia in mice [40]. However, this hypersensitivity seems to occur only in a specific range of anesthesia depth. Kroeger and Amzica demonstrated that external stimuli fail to induce bursting at isoflurane levels less than 2% or greater than 3.5% (the latter of which produces an isoelectric EEG state) [16]. Previous studies have focused mainly on the acute effects of external stimuli on anesthesia-induced burst suppression. Using an acute light exposure model in mice, we have provided the first demonstration of the effect of ACNLE on brain reactivity during sevoflurane-induced burst suppression. Consistent with a previous study, our study demonstrated that ACNLE decreased BSR at 2.5%, 3.0% and 3.5% sevoflurane but not at 2.0% or 4.0% sevoflurane. We also noticed that along with decreased BSR, the mean EEG power over the whole frequency band increased in group NL. In addition, group NL showed smaller changes of EEG power in single frequency bands than group ND at 2.5% to 3.5% sevoflurane. These data implied an increase in brain activity and a decrease in anesthesia depth following ACNLE in group NL. In line with the decrease in BSR at 2.5% to 3.5% sevoflurane in group NL, the burst duration and burst frequency increased in group NL compared to group ND. Consistent with a previous report [10], the burst amplitude and burst power in the beta and gamma frequency bands increased as the sevoflurane concentration increased from 2.5% to 3.5%. In contrast to previous studies [14,17], ACNLE decreased the burst amplitude and burst power in the beta and gamma frequency bands in our study. These differences imply that brain activity induced by ACNLE and bursts introduced by pulsed flashes may have completely different mechanisms.
In humans, light promotes activity during the day by entraining the master circadian clock located in the hypothalamic SCN [41,42]. In contrast, acute light exposure in nocturnal rodents has been shown to result in rapid sleep induction through intrinsically photosensitive retinal ganglion cells (ipRGCs) [22]. A recent study demonstrated that mouse ipRGCs projecting to the SCN and VLPO are related to arousal-promoting and sleep-promoting responses to light, respectively [23]. The spatial aspects of visual response properties in the lateral geniculate nucleus, lateral posterior nucleus and primary visual cortex have been found to remain unchanged between anesthetized and awake mice [11]. The normal metabolic oscillation of the SCN is also reported to be resistant to general anesthesia [43]. We demonstrated that the reactivity of the SCN to light was preserved during sevoflurane-induced burst suppression, which may be responsible for the arousal-promoting responses of acute light exposure at night [23]. Acute light exposure can activate VLPO neurons responsible for the sleep-promoting responses to light in mice [23]. Our data demonstrated no significant difference in c-Fos-positive neuron counts in the VLPO between groups ND and NL. General anesthetics can activate VLPO neurons responsible for natural sleep promoting [25]. Because the VLPO neurons have already been activated by exposure to sevoflurane, the acute light pulse would not be able to further increase the activity of these cells. This may explain why only the wakefulness-promoting effect (decreased depth of anesthesia) and not the sleep-promoting effect (increased depth of anesthesia) of acute light exposure could be observed in our mice during sevoflurane anesthesia-induced burst suppression.
It has been reported that the response of brain activity to light varies over the course of the day [44-47]. The light responsiveness of light-activated SCN neurons showed a significant circadian rhythm, with high responsiveness at night and low responsiveness during the day [47]. Light presented during the subjective night increased corticosterone levels in Sprague Dawley rats, while light presented during the subjective day did not [48]. The SCN-sympathetic nervous system pathway has been reported to be involved in this time-dependent increase in plasma and brain corticosterone levels following acute light exposure in C57BL/6J mice [49]. A preserved sympathetic reflex can be observed during spontaneous bursts under anesthesia [50,51] and bursts evoked by visual stimulation [12]. The increase in corticosterone release in group NL provides further evidence of the preserved reactivity of the SCN-sympathetic pathway to acute nighttime light exposure during sevoflurane-induced burst suppression. Corticosterone in a dose that approximates stress-induced plasma concentrations increased mesencephalic extracellular concentrations of dopamine, and this increase was augmented in the dark phase [52]. Acute intramuscular injection of corticosterone caused a dose-dependent increase in seizure response to a flashing light stimulus in Papio papio [53]. Treatment of epileptic animals with exogenous corticosterone can induce a persistent increase in interictal epileptiform activity [54]. Based on the above evidence, we inferred that acute light-induced corticosterone release might partly account for the decrease in BSR in group NL by increasing the excitability of the brain during sevoflurane-induced burst suppression.
Worldwide, general anesthesia is administered to an estimated 234 million surgical patients per year [55]. General anesthesia is often followed by sleep disorders, mood alteration and cognitive dysfunction, especially in aged patients and critically ill patients [56]. A burst-suppression EEG can be deliberately induced by anesthetic agents for therapeutic use [57,58]. Unintentionally induced burst-suppression EEG under deeper anesthesia has been reported to be correlated with unfavorable outcomes, including postoperative delirium [59-64]. EEG-based depth-of-anesthesia monitors have been recommended as an option in patients likely to suffer the adverse effects of excessively deep anesthesia [65]. Studies using intraoperatively processed quantitative EEG monitoring suggest that postoperative delirium can be decreased by maintaining the patient at a lighter level of anesthesia [66]. However, recent clinical trials were unable to demonstrate the benefit of EEG-based monitoring during surgery in high-risk patients [67,68]. These controversial results cast doubt on the reliability of the current EEG-based index as a clinical indicator of anesthesia depth. A thorough understanding of static and stimulus-induced EEG activity will help us understand the state of brain function under anesthesia and develop new indicators of anesthetic depth. This change in burst suppression in response to ACNLE may help to explain the confounding effects of clinical EEG-based depth-of-anesthesia monitoring.
In this study, EEG recording was performed during the first half of the night; thus, our findings cannot precisely reflect the whole day and night. The flickering of our light source may also play a role in the phenomenon we have observed. We did not consider the effect of the flicker frequency or wavelength of light, which are crucial factors affecting experiments [21]. Furthermore, we focused only on male mice in this study, which, to some extent, may make our results unrepresentative of the species in general. Finally, the nonvisual effects of light have several same signs in nocturnal animals as in diurnal humans, although this is not always the case. The clinical relevance of this phenomenon remains unclear because mice are nocturnal animals. Hence, further studies with surgical patients in the routine perioperative period are warranted.
In conclusion, this study demonstrated that, in addition to light-induced changes in brain activities, local and organism-level biological consequences such as changes in c-Fos expression in the SCN and levels of corticosterone in plasma can also happen following ACNLE under sevoflurane induced burst suppression patterns. The function of light-sensitive brain pathways is differentially preserved under sevoflurane induced burst suppression. These may help us understand the nature of general anesthesia state and provide further references for anesthesia monitoring.
Acknowledgements
Supported by the National Natural Science Foundation of China (81571357 and 81873793 to WM).
Disclosure of conflict of interest
None.
References
- 1.Swank RL, Watson CW. Effects of barbiturates and ether on spontaneous electrical activity of dog brain. J Neurophysiol. 1949;12:137–160. doi: 10.1152/jn.1949.12.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–3100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Westover MB, Ching S, Kumaraswamy VM, Akeju SO, Pierce E, Cash SS, Kilbride R, Brown EN, Purdon PL. The human burst suppression electroencephalogram of deep hypothermia. Clin Neurophysiol. 2015;126:1901–1914. doi: 10.1016/j.clinph.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toda Y, Kobayashi K, Hayashi Y, Inoue T, Oka M, Endo F, Yoshinaga H, Ohtsuka Y. High-frequency EEG activity in epileptic encephalopathy with suppression-burst. Brain Dev. 2015;37:230–236. doi: 10.1016/j.braindev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niu B, Fang Y, Miao JM, Yu Y, Cao F, Chen HX, Zhang ZG, Mei W, Tian YK. Minimal alveolar concentration of sevoflurane for induction of isoelectric electroencephalogram in middle-aged adults. Br J Anaesth. 2014;112:72–78. doi: 10.1093/bja/aet280. [DOI] [PubMed] [Google Scholar]
- 8.Gross WL, Lauer KK, Liu X, Roberts CJ, Liu S, Gollapudy S, Binder JR, Li SJ, Hudetz AG. Propofol sedation alters perceptual and cognitive functions in healthy volunteers as revealed by functional magnetic resonance imaging. Anesthesiology. 2019;131:254–265. doi: 10.1097/ALN.0000000000002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartikainen KM, Rorarius M, Perakyla JJ, Laippala PJ, Jantti V. Cortical reactivity during isoflurane burst-suppression anesthesia. Anesth Analg. 1995;81:1223–1228. doi: 10.1097/00000539-199512000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Arena A, Lamanna J, Gemma M, Ripamonti M, Ravasio G, Zimarino V, De Vitis A, Beretta L, Malgaroli A. Linear transformation of the encoding mechanism for light intensity underlies the paradoxical enhancement of cortical visual responses by sevoflurane. J Physiol. 2017;595:321–339. doi: 10.1113/JP272215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand S, Iyer R, Mizuseki K, de Vries S, Mihalas S, Reid RC. A comparison of visual response properties in the lateral geniculate nucleus and primary visual cortex of awake and anesthetized mice. J Neurosci. 2016;36:12144–12156. doi: 10.1523/JNEUROSCI.1741-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartikainen K, Rorarius M, Makela K, Perakyla J, Varila E, Jantti V. Visually evoked bursts during isoflurane anaesthesia. Br J Anaesth. 1995;74:681–685. doi: 10.1093/bja/74.6.681. [DOI] [PubMed] [Google Scholar]
- 13.Hudetz AG, Imas OA. Burst activation of the cerebral cortex by flash stimuli during isoflurane anesthesia in rats. Anesthesiology. 2007;107:983–991. doi: 10.1097/01.anes.0000291471.80659.55. [DOI] [PubMed] [Google Scholar]
- 14.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–947. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Jeczmien-Lazur JS, Orlowska-Feuer P, Smyk MK, Lewandowski MH. Modulation of spontaneous and light-induced activity in the rat dorsal lateral geniculate nucleus by general brain state alterations under urethane anesthesia. Neuroscience. 2019;413:279–293. doi: 10.1016/j.neuroscience.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci. 2007;27:10597–10607. doi: 10.1523/JNEUROSCI.3440-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imas OA, Ropella KM, Wood JD, Hudetz AG. Halothane augments event-related gamma oscillations in rat visual cortex. Neuroscience. 2004;123:269–278. doi: 10.1016/j.neuroscience.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal A, Brennan C, Shortal B, Contreras D, Kelz MB, Proekt A. Coherence of visual-evoked gamma oscillations is disrupted by propofol but preserved under equipotent doses of isoflurane. Front Syst Neurosci. 2019;13:19. doi: 10.3389/fnsys.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM, Hattar S. Light affects mood and learning through distinct retina-brain pathways. Cell. 2018;175:71–84. e18. doi: 10.1016/j.cell.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adaikkan C, Middleton SJ, Marco A, Pao PC, Mathys H, Kim DN, Gao F, Young JZ, Suk HJ, Boyden ES, McHugh TJ, Tsai LH. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron. 2019;102:929–943. e928. doi: 10.1016/j.neuron.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupi D, Oster H, Thompson S, Foster RG. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008;11:1068–1073. doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 23.Pilorz V, Tam SK, Hughes S, Pothecary CA, Jagannath A, Hankins MW, Bannerman DM, Lightman SL, Vyazovskiy VV, Nolan PM, Foster RG, Peirson SN. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14:e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vacas S, Kurien P, Maze M. Sleep and anesthesia-common mechanisms of action. Sleep Med Clin. 2013;8:1–9. doi: 10.1016/j.jsmc.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore JT, Chen J, Han B, Meng QC, Veasey SC, Beck SG, Kelz MB. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol. 2012;22:2008–2016. doi: 10.1016/j.cub.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor S, Szabo L, Varga J, Cuesta M, Morton AJ. Progressive sleep and electroencephalogram changes in mice carrying the Huntington’s disease mutation. Brain. 2013;136:2147–2158. doi: 10.1093/brain/awt128. [DOI] [PubMed] [Google Scholar]
- 28.Lott GK 3rd, Johnson BR, Bonow RH, Land BR, Hoy RR. g-PRIME: a free, windows based data acquisition and event analysis software package for physiology in classrooms and research labs. J Undergrad Neurosci Educ. 2009;8:A50–54. [PMC free article] [PubMed] [Google Scholar]
- 29.Vijn PC, Sneyd JR. I.v. anaesthesia and EEG burst suppression in rats: bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–421. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 30.Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng. 2014;61:1555–1564. doi: 10.1109/TBME.2014.2311996. [DOI] [PubMed] [Google Scholar]
- 31.Hambrecht-Wiedbusch VS, Li D, Mashour GA. Paradoxical emergence: administration of subanesthetic ketamine during isoflurane anesthesia induces burst suppression but accelerates recovery. Anesthesiology. 2017;126:482–494. doi: 10.1097/ALN.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai SK, Ma J, Leung LS. Medial septal cholinergic neurons modulate isoflurane anesthesia. Anesthesiology. 2014;120:392–402. doi: 10.1097/ALN.0b013e3182a7cab6. [DOI] [PubMed] [Google Scholar]
- 33.Liebe S, Hoerzer GM, Logothetis NK, Rainer G. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci. 2012;15:456–462. S451–452. doi: 10.1038/nn.3038. [DOI] [PubMed] [Google Scholar]
- 34.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandon Westover M, Shafi MM, Ching S, Chemali JJ, Purdon PL, Cash SS, Brown EN. Real-time segmentation of burst suppression patterns in critical care EEG monitoring. J Neurosci Methods. 2013;219:131–141. doi: 10.1016/j.jneumeth.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ries CR, Puil E. Isoflurane prevents transitions to tonic and burst firing modes in thalamic neurons. Neurosci Lett. 1993;159:91–94. doi: 10.1016/0304-3940(93)90806-v. [DOI] [PubMed] [Google Scholar]
- 37.Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829:77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 38.Joas TA, Stevens WC, Eger EI 2nd. Electroencephalographic seizure activity in dogs during anaesthesia. Br J Anaesth. 1971;43:739–745. doi: 10.1093/bja/43.8.739. [DOI] [PubMed] [Google Scholar]
- 39.Yli-Hankala A, Jantti V, Pyykko I, Lindgren L. Vibration stimulus induced EEG bursts in isoflurane anaesthesia. Electroencephalogr Clin Neurophysiol. 1993;87:215–220. doi: 10.1016/0013-4694(93)90021-m. [DOI] [PubMed] [Google Scholar]
- 40.Land R, Engler G, Kral A, Engel AK. Auditory evoked bursts in mouse visual cortex during isoflurane anesthesia. PLoS One. 2012;7:e49855. doi: 10.1371/journal.pone.0049855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sollars PJ, Pickard GE. The neurobiology of circadian rhythms. Psychiatr Clin North Am. 2015;38:645–665. doi: 10.1016/j.psc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy JF, Wright KP Jr. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz WJ. In vivo metabolic activity of hamster suprachiasmatic nuclei: use of anesthesia. Am J Physiol. 1987;252:R419–422. doi: 10.1152/ajpregu.1987.252.2.R419. [DOI] [PubMed] [Google Scholar]
- 44.Askaripoor T, Motamedzadeh M, Golmohammadi R, Farhadian M, Babamiri M, Samavati M. Non-image forming effects of light on brainwaves, autonomic nervous activity, fatigue, and performance. J Circadian Rhythms. 2018;16:9. doi: 10.5334/jcr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iskra-Golec I, Golonka K, Wyczesany M, Smith L, Siemiginowska P, Watroba J. Daytime effect of monochromatic blue light on EEG activity depends on duration and timing of exposure in Young Men. Adv Cogn Psychol. 2017;13:241–247. doi: 10.5709/acp-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohawk JA, Pargament JM, Lee TM. Circadian dependence of corticosterone release to light exposure in the rat. Physiol Behav. 2007;92:800–806. doi: 10.1016/j.physbeh.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Yli-Hankala A, Jantti V. EEG burst-suppression pattern correlates with the instantaneous heart rate under isoflurane anaesthesia. Acta Anaesthesiol Scand. 1990;34:665–668. doi: 10.1111/j.1399-6576.1990.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 51.Yli-Hankala A, Heikkila H, Varri A, Jantti V. Correlation between EEG and heart rate variation in deep enflurane anaesthesia. Acta Anaesthesiol Scand. 1990;34:138–143. doi: 10.1111/j.1399-6576.1990.tb03058.x. [DOI] [PubMed] [Google Scholar]
- 52.Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehlers CL, Killam EK. The influence of cortisone on EEG and seizure activity in the baboon Papio papio. Electroencephalogr Clin Neurophysiol. 1979;47:404–410. doi: 10.1016/0013-4694(79)90156-1. [DOI] [PubMed] [Google Scholar]
- 54.Castro OW, Santos VR, Pun RY, McKlveen JM, Batie M, Holland KD, Gardner M, Garcia-Cairasco N, Herman JP, Danzer SC. Impact of corticosterone treatment on spontaneous seizure frequency and epileptiform activity in mice with chronic epilepsy. PLoS One. 2012;7:e46044. doi: 10.1371/journal.pone.0046044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 56.Wu CL, Berenholtz SM, Pronovost PJ, Fleisher LA. Systematic review and analysis of postdischarge symptoms after outpatient surgery. Anesthesiology. 2002;96:994–1003. doi: 10.1097/00000542-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 57.Doyle PW, Matta BF. Burst suppression or isoelectric encephalogram for cerebral protection: evidence from metabolic suppression studies. Br J Anaesth. 1999;83:580–584. doi: 10.1093/bja/83.4.580. [DOI] [PubMed] [Google Scholar]
- 58.Reinsfelt B, Westerlind A, Houltz E, Ederberg S, Elam M, Ricksten SE. The effects of isoflurane-induced electroencephalographic burst suppression on cerebral blood flow velocity and cerebral oxygen extraction during cardiopulmonary bypass. Anesth Analg. 2003;97:1246–1250. doi: 10.1213/01.ANE.0000086732.97924.BE. [DOI] [PubMed] [Google Scholar]
- 59.Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, Escallier KE, Ben Abdallah A, Lin N, Avidan MS. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–242. doi: 10.1213/ANE.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fritz BA, Maybrier HR, Avidan MS. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth. 2018;121:241–248. doi: 10.1016/j.bja.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willingham M, Ben Abdallah A, Gradwohl S, Helsten D, Lin N, Villafranca A, Jacobsohn E, Avidan M, Kaiser H. Association between intraoperative electroencephalographic suppression and postoperative mortalitydouble dagger. Br J Anaesth. 2014;113:1001–1008. doi: 10.1093/bja/aeu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willingham MD, Karren E, Shanks AM, O’Connor MF, Jacobsohn E, Kheterpal S, Avidan MS. Concurrence of intraoperative hypotension, low minimum alveolar concentration, and low bispectral index is associated with postoperative death. Anesthesiology. 2015;123:775–785. doi: 10.1097/ALN.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorrilla-Vaca A, Healy RJ, Wu CL, Grant MC. Relation between bispectral index measurements of anesthetic depth and postoperative mortality: a meta-analysis of observational studies. Can J Anaesth. 2017;64:597–607. doi: 10.1007/s12630-017-0872-6. [DOI] [PubMed] [Google Scholar]
- 65.Checketts MR, Alladi R, Ferguson K, Gemmell L, Handy JM, Klein AA, Love NJ, Misra U, Morris C, Nathanson MH, Rodney GE, Verma R, Pandit JJ Association of Anaesthetists of Great Britain and Ireland. Recommendations for standards of monitoring during anaesthesia and recovery 2015: association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2016;71:85–93. doi: 10.1111/anae.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 67.Sieber F, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JP, Jaberi M, Hasenboehler EA, Wang NY. Depth of sedation as an interventional target to reduce postoperative delirium: mortality and functional outcomes of the strategy to reduce the incidence of postoperative delirium in elderly patients randomised clinical trial. Br J Anaesth. 2019;122:480–489. doi: 10.1016/j.bja.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, Kronzer A, McKinnon SL, Park D, Torres BA, Graetz TJ, Emmert DA, Palanca BJ, Goswami S, Jordan K, Lin N, Fritz BA, Stevens TW, Jacobsohn E, Schmitt EM, Inouye SK, Stark S, Lenze EJ, Avidan MS ENGAGES Research Group. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. 2019;321:473–483. doi: 10.1001/jama.2018.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]