Abstract
A receptive endometrium is a prerequisite for successful embryo implantation, and about one-third of repeated embryo implantation failure attribute to defective endometrial receptivity. Integrin-linked kinase (ILK), a 59kDa serine/threonine-protein kinase, plays a vital role in multiple cellular processes, including cell proliferation, apoptosis, and invasion. However, its role in endometrial receptivity is still unclear. In the current study, we demonstrated that ILK level was significantly downregulated in the serum of patients with unexplained infertility compared with healthy non-pregnancy. Functionally, ILK knockdown inhibited endometrial epithelial cells (EECs) proliferation and invasion, whereas ILK overexpression promoted endometrial EECs proliferation and invasion. ILK inhibition also repressed the adhesion rate of embryonic cells to EECs. In vivo studies further demonstrated that ILK inhibition suppressed endometrium receptivity formation and embryo implantation potential. Mechanistically, the downregulation of ILK inactivated Wnt/β-catenin signaling and thus resulted in the downregulation of MMP-3 and MMP-9 expression. Importantly, activation of Wnt/β-catenin signaling, partially recovered ILK inhibition-caused endometrium receptivity defects, and embryo implantation failure. Considered all the current data, it verified that the low expression of ILK exacerbates endometrial receptivity formation by inactivating Wnt/β-catenin signaling and decreasing the MMP-3/9 expression and indicated that ILK may be applied as an indicator of endometrial receptivity, and as a diagnostic and therapeutic target for infertility.
Keywords: ILK, endometrial receptivity, Wnt/β-catenin signaling, MMPs
Introduction
The receptive endometrium is a prerequisite for successful embryo implantation [1]. Currently, approximately 30% of embryo implantation failure correlated with low uterine receptivity [2,3]. Lots of genes and associated signaling pathways have been identified to involve in the regulation of endometrial activity. Our previous studies showed that 317 proteins are differentially expressed between the receptive and proliferative phase uterine [4]. For instance, CKB (creatine kinase B-type) overexpression in the receptive phase is correlated with endometrium receptivity [4]. Despite recent advances in understanding uterine receptivity, the underlying mechanisms of poor endometrial receptivity in abortion and infertility remain poorly understood.
Integrin-linked kinase (ILK) encodes a 59k serine/threonine-protein kinase, plays a critical role in various normal cellular processes [5], including cellular proliferation [6], migration [7], apoptosis [8] and production of pro-inflammatory cytokines [9]. Gil D et al. reported the suppression of epithelial-mesenchymal transition (EMT) via ILK overexpression [10]. Fassler R et al. found that intracellular signal transduction pathway, for example, ILK and Wnt/β-catenin signaling are critical in controlling of the EMT process [11]. Yan et al. indicated that bacilin can up-regulate FUT4 through Wnt/β-catenin signal pathway to promote embryo adhesion and implantation [12]. There are several studies reported that ILK is essential for embryonic development [13-16], indicating that ILK may be involved in endometrial receptivity.
In this study, we examined the contents of ILK in the serum of healthy control and unexplained infertility. Next, we analyzed the protein expression, which belonged to Wnt/β-catenin signaling pathway as well as matrix metallopeptidase family. We assessed the association of ILK/Wnt axis with receptivity formation and embryo implantation potential of the uterus. The results showed that ILK level was significantly decreased in the serum of patients with unexplained infertility compared with healthy non-pregnancy. ILK inhibition inactivated the Wnt/β-catenin signaling and resulted in the downregulation of MMP-3 and MMP-9 expression. The potential of uterine receptivity formation and embryo implantation was decreased by repression of ILK. Generally speaking, ILK improved endometrial receptivity formation by activating Wnt/β-catenin signaling and enhancing the MMP-3 and MMP-9 expression. This study suggests that ILK might become a biomarker for endometrial receptivity and diagnostic and therapeutic target for infertility.
Materials and methods
Serum samples
This study was conducted in accordance with the declaration of Helsinki. The Institutional Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, approved all samples collections (serum from the healthy control, unexplained infertility; There were ten samples in each group). Women aged 26-30 years old who attended the Centre for Assisted Reproductive Technology (ART) were recruited in the study. Patients were excluded if they presented intrauterine or ovarian abnormalities (e.g., endometriosis, adenomyosis, leiomyomas) or received steroid hormone therapy in the last 3 months.
The healthy non-pregnancy control group was formed by fertile women who had at least one child (malefactor was the previous problem of fertility in the couple). The unexplained infertility group was defined as those who regularly tried to have pregnancy for 12 months or more and failed to achieve a pregnancy without any apparent barriers to conception by clinical investigations (e.g., pathological tubal obstruction or malefactors). All the women underwent ultrasonography every other day from day 9 of the menstrual cycle. When dominant follicle diameter was >15 mm, ultrasonography was performed daily, and serum LH and E2 were detected daily using a chemoluminescence technique (Beckman) until follicular rupture. Serum samples were collected on the 7th day post-ovulation. The ILK and progesterone (P) levels were detected. Ovulation was confirmed by P levels of >8 ng/ml 7 d post-ovulation.
Cell culture
Four human endometrial cancer cell lines, HEC-1-A, RL95-2, Ishikawa and KLE, and one human placental chorionic carcinoma cell line JAR cells were all purchased from American Type Culture Collection (ATCC; Manassas, VA). Cells were maintained with DMEM/F12 containing 10% foetal bovine serum (FBS), 1% penicillin/streptomycin (Sangon Biotech, China). They were cultivated at 37°C in a humidified atmosphere with 5% CO2.
ILK overexpression
The plasmids pcDNA-ILK were designed and purchased from GenePharma (Shanghai, China) for ILK overexpression. RL95-2 cells (3×105/per plate), which were used to overexpress ILK, were plated into 6-well plates to cultivate for 24 h. Then, transfection was applied by 2 μg/mL of pcDNA-ILK, empty pcDNA, and Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA) in line as instruction’s process and incubated at 37°C for 48 h. After transfecting 48 h, the cells were maintained into corresponding medium 21 days.
Construction of lentiviral vectors expressing ILK-specific shRNA
The ILK-specific target sequence was designed and purchased from GenePharma. Double-stranded DNA, contained the interference sequences, was synthesized to generate the lentiviral vectors as the manufacturer’s instructions and then infected HEC-1-A cells. Before infection, the medium was substituted with a proper titer of virus supernatant and incubated at 37°C for 12 h. Western blot was used to analyze the silencing efficiency of shRNA three days after transfection.
RNA isolation and real-time PCR
Total RNA was obtained from four serum samples and cells with a Trizol reagent (TaKaRa, Tokyo, Japan) following the manufacturer’s instructions, and then, reverse transcription was performed with PrimeScriptTM RT Reagent reagent. SYBR Premix Ex Taq II Kit (TaKaRa) was applied for Real-time PCR detection and run by ABI Prism 7500 Detection system. After the reaction, the PCR amplification curve and the melting curve were confirmed for PCR analysis. The expression levels of ILK and MMPs (MMP-3 and MMP-9) were analyzed with GAPDH as an internal parameter. All samples were tested three times, and the relevant primer sequences were respectively: ILK, Forward, 5’-AAGGTACTCGAGCTATGGACGACATTTTCACTC-3’ and reversed, 5’-ATCCAAGAATTCTCTACTTGTCCTGCATCTTCT-3’; MMP-3, forward, 5’-TTCCTGGCATCCCGAAGTGG-3’ and reversed, 5’-ACAGCCTGGAGAATGTGAGTGG-3’; MMP-9, forward, 5’-GATGCGTGGAGAGTCGAAAT-3’ and reversed, 5’-CACCAAACTGGATGACGATG-3’.
Western blot
The protein extracts from serum samples or cells were collected with RIPA lysis buffer (Solarbio, Beijing, China), and the harvested protein concentration was assayed by a BCA Assay Kit (Pierce, Rockford, IL, USA). The samples were loaded onto SDS-polyacrylamide gel electrophoresis (PAGE, 12%) for separating protein and transferred to polyvinylidene fluoride membranes (PVDF; Roche, Basel, Switzerland). Primary antibodies: ILK (1:1000; ab52480, Abcam, Cambridge, MA, USA), MMP-3 (1:1000; ab53015, Abcam), MMP-9 (1 µg/ml; ab73734, Abcam), p-GSK3β (1:1000; ab75745, Abcam) and β-catenin (1:5000; ab32572, Abcam) were applied to incubate membranes overnight at 4°C after 5% non-fat skim milk blocking 2 h. After washing with 1× TBST for 3 times, and then, the membranes were incubated with corresponding secondary antibody Goat Anti-Rabbit IgG (HRP) (1:10000; ab205718, Abcam) for 40 min. The protein bands were identified with the ECL detection system.
Immunofluorescent assay
The endometrial cancer cells were fixed with 4% paraformaldehyde about 20 min and then washed with PBS. The cells were incubated with anti-ILK antibody (1:200; ab236455, Abcam) overnight at 4°C, and then, the sections were incubated with the corresponding second antibody (1:10000) for 1 h after PBS washing 3 times. The nuclei were stained with DAPI (Sigma-Aldrich) for 20 min. A fluorescence microscope was used to observe and record the images.
Cell proliferation assay
The proliferation ability of RL95-2, RL95-2-pcDNA3-ILK, HEC-1-A, and HEC-1-A-shILK cells were detected using a Cell Counting Kit-8 (CCK-8, TaKaRa). The cells (2×103) were plated into 96-well plates, and CCK-8 solution (10 μl) was added into per plate after the cells were cultured for a series of gradient time (1, 2, 3, 4 and 5 day). Then, the culture plate was incubated in the incubator for 2 h at 37°C, and then the absorbance (450 nm) was measured with an enzyme label instrument.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde in the 4°C environments for 24 h. After that, all tissues were dehydrated in the embedding box and embedded using liquid paraffin as instruction’s procedure (Sigma-Aldrich). The sections (4 μm) were prepared through a slicer and baked on the slide warmers for 30 min. The sections were placed in a wet box, in which a small amount of distilled water was added, plus 3% hydrogen peroxide, and incubated at room temperature for 10 min. Washing with PBS and distilled water 3 times each, 3 min each time, wet box was added goat serum occlusive fluid to incubate sections for 10 min. Immunohistochemistry was carried out by primary antibodies and corresponding secondary antibody according to instruction procedures. 100 μl DAB solution (Sigma-Aldrich) was added to each section, restained with hematoxylin for 1-2 min.
Scanning electron microscope (SEM)
Cells and tissues were fixed in glutaraldehyde fixative (2.5%), and then osmium tetroxide (2%). The samples, which were washed with PBS 2 times, were dehydrated with a series of gradient ethanol (30%, 50%, 70%, 80%, 90%) twice at each concentration for 15 min. Samples were dried by acetonitrile drying method after dehydration, vacuum drying followed. The surfaces of cells were coated and then observed by scanning electron microscopy (Hitachi, Tokyo).
Cell adhesion assay
RL95-2 and HEC-1-A cells were cultured in a 96-well plate to form a fused monolayer. JAR cells, which were stained with Cell Tracker Green for 1 h, were collected and counted after digestion with 0.25% trypsin. JAR cells were gently seeded into the monolayer of RL95-2 and HEC-1-A cells and adhered for 1 h. PBS was performed to wash out un-adhered JAR cells. Unstained JAR cells were used as blank controls. Adhesion rate = (number of attached JAR cells/number of JAR cells entering the mouth) * 100%. Fluorescence microscope (Olympus) was used to capture and display representative images.
Transwell assay
The 24-well transwell chambers (Thermo Fisher Scientific, Waltham, MA, USA) coated with eight μM pore-size Matrigel (Coring, Corning, NY, USA) were used to assess the invasiveness of RL95-2, RL95-2-pcDNA3-ILK, HEC-1-A, HEC-1-A-shILK and HEC-1-A treated with SKL2001. The transfected RL95-2 and HEC-1-A cells (1×104 cells) were resuspended in 150 μl serum-free DMEM/F12 medium and seeded onto the top of the invasion chambers. The lower chambers were filled up with 600 μl DMEM/F12 containing 10% FBS as a chemoattractant. After 24 h incubation at 37°C, noninvasive cells inside the upper chamber were scraped off with cotton swabs, and invading cells on the lower membrane surface were fixed in 4% paraformaldehyde for 15 min and then stained with 0.1% crystal violet for another 15 min. Cells were photographed and counted in ten fields at 100× magnification using a microscope (Nikon, Tokyo, Japan).
Animal experiments
Kunming mice (KM; 6-8 weeks; n=5/each group) were purchased from SeBiona BioTech (Guangzhou, China). All animal experiments were approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The mice were raised under standard conditions with adequate food and water. To get a pregnant mouse, a female mouse was left alone with a male mouse. Pregnancy is the first day of a vaginal blockage. On the second day of pregnancy, the females were anesthetized by injecting pentobarbital sodium, and ten μl solution, including 1 μg Lv-ILK, was injected into the right uterus horn and the left with normal saline. RNA and protein samples from mouse endometrium were collected on day 7. The number of embryos implanted was analyzed statistically.
Statistical analysis
All data were presented as mean ± SD. The difference between the two groups was assayed using the Student’s t-test (Figures 1B, 1D, 2D-J, 3B and 3D) or one-way analysis of variance (ANOVA) followed by the Scheffé test (Figures 3F-H, 4). SPSS17.0 (SPSS, Inc., USA) was applied to analyze statistical analysis. P<0.05 was indicated to be statistically significant.
Figure 1.
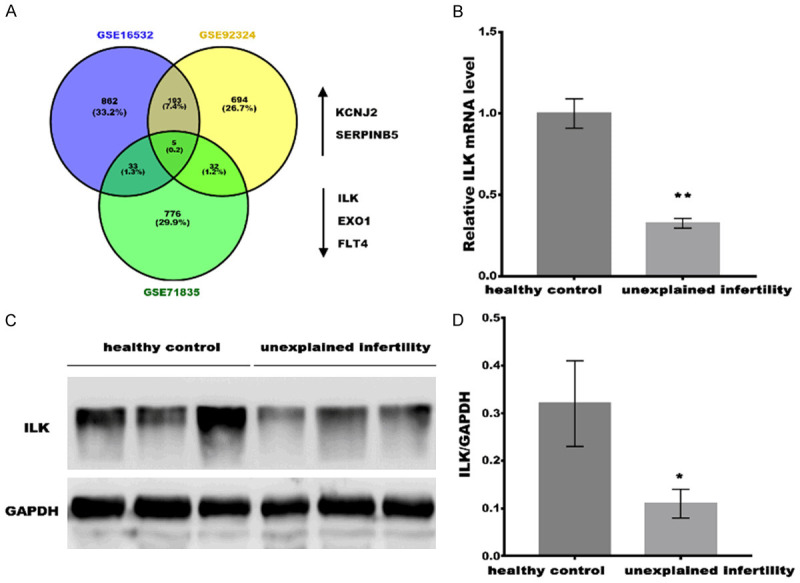
ILK was downregulated in unexplained infertility patients. A. Three GEO public datasets (GSE16532, GSE92324, and GSE71835) were acquired to carry out bioinformatics analysis, and five commonly dysregulated genes (KCNJ2, SERPINB3, ILK, EXO1 and FLT4) were identified. B. qPCR analysis of ILK mRNA expression in serum from healthy control (Non-pregnancy, n=10), unexplained infertility (n=10). All samples were tested 3 times, and data were presented as mean ± SD. C. Western blot analysis of ILK protein expression in Non-pregnancy (n=3) and unexplained infertility (n=3). D. Quantitative analysis of ILK protein expression in Non-pregnancy (n=3) and unexplained infertility (n=3). GAPDH was used as loading control in western blot analysis. *P<0.05, **P<0.01.
Figure 2.
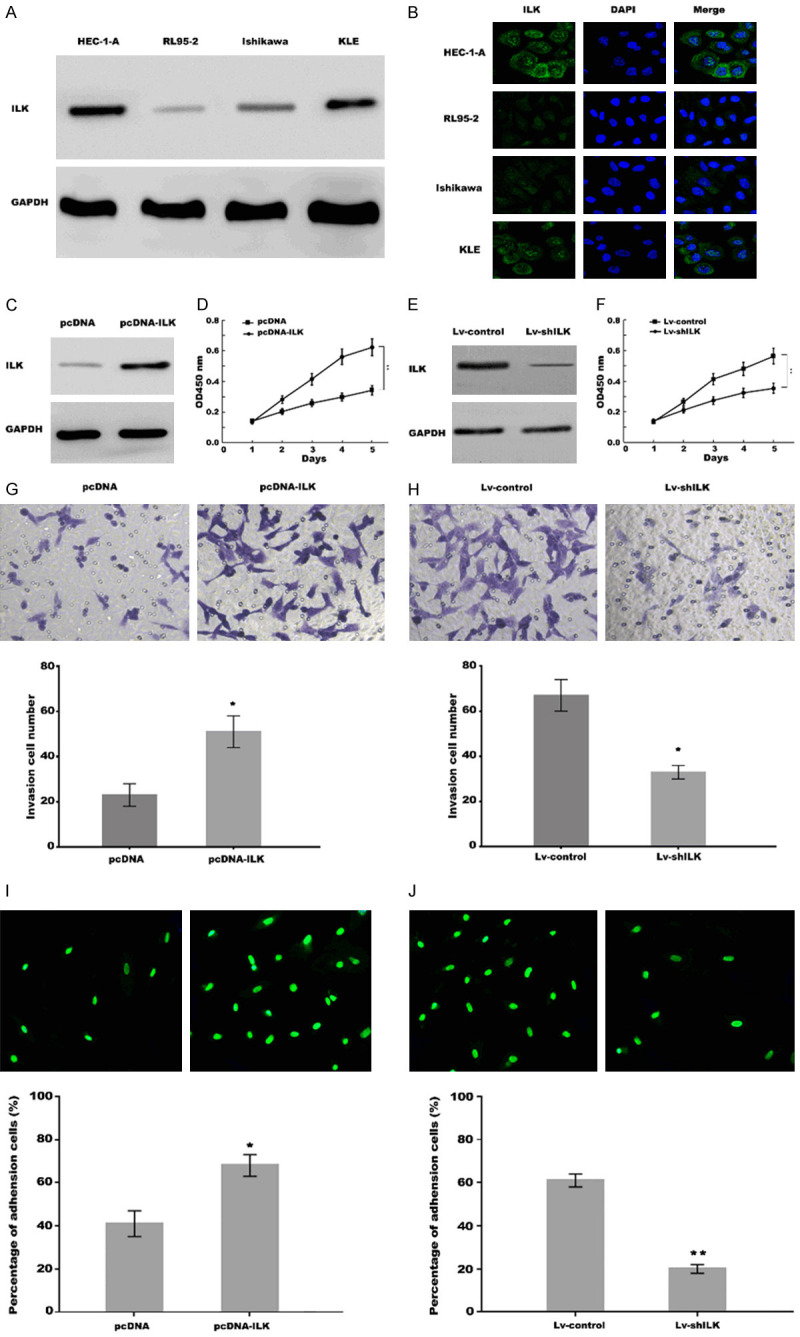
ILK promoted proliferation and invasion, and improved receptive ability of uterine epithelial cells in vitro. (A) Western blot analysis of ILK expression in uterine epithelial cell lines HEC1-A, RL95-2, Ishikawa and KLE. GAPDH was used as loading control. (B) Immunofluorescence analysis for cell location of ILK in uterine epithelial cell lines HEC1-A, RL95-2, Ishikawa and KLE. (C) Western blot analysis of ILK expression in RL95-2 cells treated with pcDNA control or pcDNA-ILK. (D) RL95-2 cell proliferation was assessed using CCK-8 assay after ILK overexpression. (E) Western blot analysis of ILK expression in HEC-1-A cells treated with shRNA control or shILK group. (F) HEC-1-A cells proliferation was assessed using CCK-8 assay after ILK knockdown. (G, H) Transwell invasion assay to analyze RL95-2 cells invasion after ILK overexpression (G) and HEC-1-A cells invasion after ILK knockdown (H). (I, J) Cell adhesion assay showed that adhesion rate of JAR cells to RL95-2 cells was significantly enhanced after ILK overexpression, while the adhesion rate of JAR cells to HEC-1-A was obviously reduced after ILK knockdown compared with control cells. *P<0.05, **P<0.01.
Figure 3.
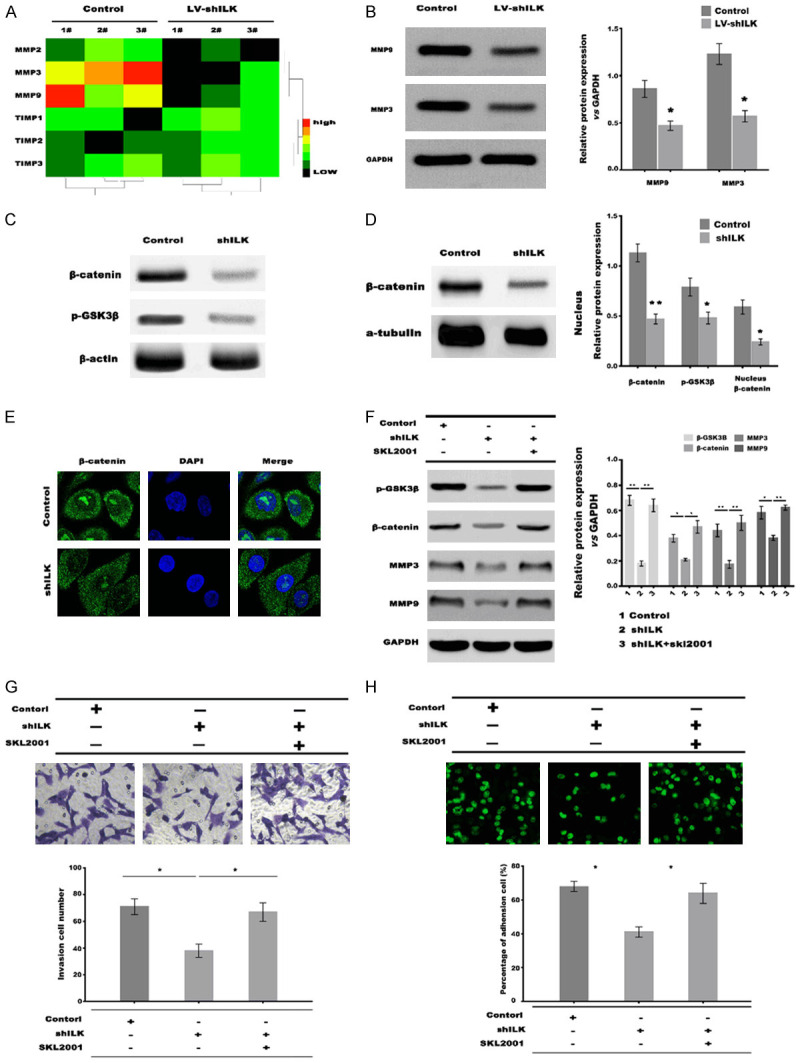
ILK knockdown decreased the expression of MMP3 and MMP9 by inactivating Wnt/β-catenin signaling. A. qPCR analysis of MMP2, MMP3, MMP9, TIMP1, TIMP2, and TIMP3 mRNA expression in HEC-1-A cells after ILK knockdown. B. Western blot analysis of MMP3 and MMP9 protein expression in HEC-1-A cells after ILK knockdown. C. Western blot analysis of total β-catenin and p-GSK3β in HEC-1-A cells after ILK knockdown. D. Western blot analysis of nuclear β-catenin in HEC-1-A cells after ILK knockdown, α-tubulin was used as loading control. E. Immunostaining analysis of β-catenin in HEC-1-A cells after ILK knockdown. F. Western blot analysis of p-GSK3β, β-catenin, MMP-3 and MMP-9 in HEC-1-A cells after ILK knockdown in the presence or absence of SKL2001. G. Transwell invasion assay of HEC-1-A cells invasion after ILK knockdown in the presence or absence of SKL2001. H. Cells adhesion analysis of JAR cells to HEC-1-A cells after ILK knockdown in the presence or absence of SKL2001. *P<0.05, **P<0.01.
Figure 4.

ILK improved the uterine receptivity and embryo implantation in vivo. Mouse uterus was injected with Lv-control or Lv-shILK in the presence of absence of SKL2001 2 day before pregnancy. A. Number of implanted embryos in the uterus on day 7 pregnancy. B. Western blot and quantitative analysis of ILK, β-catenin, MMP9, and MMP3 protein expression in the uterine endometrium of pregnancy mouse. C. Immunohistochemistry analysis MMP3 and MMP9 in the uterine endometrium of pregnancy mouse. D. Representative SEM images in the uterine endometrium at Lv-control or Lv-shILK group with or without SKL2001 treatment on day 7 of pregnancy. *P<0.05, **P<0.01.
Results
ILK was downregulated in unexplained infertility patients
To identify the molecules that may be associated with the acquisition of endometrium receptivity phenotype, three GEO public datasets (GSE16532, GSE92324, and GSE71835) were acquired to carry out bioinformatics analysis. As shown in Figure 1A, five common molecules KCNJ2, SERPINB3, ILK, EXO1, and FLT4 were identified. Our previous studies verified the up-regulated expression of ILK in the proliferative phage compared within the endometrium’s receptive phase [4]. In the study, we attempted to investigate the association of ILK with endometrium receptivity. Real-time PCR and Western blot analysis were applied to determine the ILK level in the serum of the healthy control (non-pregnancy) and unexplained infertility women. Figure 1B-D showed that the ILK mRNA and protein levels were decreased in unexplained infertility patients compared to healthy women. These data suggest the potential role of ILK in the formation of uterine receptivity.
ILK inhibition repressed EECs proliferation and invasion and decreased the adhesion rate of embryonic cells to EECs
We then described the functional characteristics of ILK, with emphasis on its effects on the EECs proliferation and invasion, and adhesion rate of embryonic cells to EECs. The expression of ILK in four kinds of EECs lines HEC1-A, RL95-2, Ishikawa, and KLE was detected using western blot analysis. As shown in Figure 2A, ILK is highly expressed in HEC1-A and KLE cell lines compared to RL95-2 and Ishikawa cell lines. Next, the cell location of ILK was assessed in uterine epithelial cells by immunofluorescence. Figure 2B showed that strong intense cytoplasmic staining for ILK was detected in HEC1-A and KLE cell lines, whereas weak cytoplasmic staining in RL95-2 and Ishikawa cell lines.
CCK-8 assay was applied to assess the effect of ILK on EECs proliferation. ILK was overexpressed in RL95-2 cells by transfection with pcDNA3-ILK (Figure 2C). Figure 2D showed that ILK overexpression significantly promoted RL95-2 cell proliferation compared with control cells. In contrast, the ILK expression was repressed in HEC-1-A through treatment with ILK-shRNA (Figure 2E). The results from CCK-8 assay showed that ILK inhibition significantly repressed HEC-1-A cell proliferation compared with control (Figure 2F). Invasion assay was applied to analysis the effect of ILK on EECs invasion. Figure 2G and 2H showed that ILK overexpression significantly promoted RL95-2 cell invasion, whereas ILK knockdown repressed HEC-1-A cell invasion compared with control. We further investigated the role of ILK in adhesion of embryo onto endometrium using in vitro embryo adhesion model. Figure 2I and 2J showed that the adhesion rate of JAR cells (embryonic cells) to RL95-2 cells was significantly enhanced after ILK overexpression, while the adhesion rate of JAR cells to HEC-1-A was obviously reduced after ILK knockdown compared with control cells. These results demonstrated that ILK promoted uterine epithelial cell proliferation and invasion, and improved its receptive ability in vitro.
ILK inhibition repressed matrix metalloproteases expression by inactivating Wnt/β-catenin signaling
The degradation of extracellular matrix proteins, achieved by increasing the matrix metalloproteases (MMPs), plays a vital role in embryo implantation [17,18]. Previous studies have demonstrated that MMPs are crucial factors in trophoblast invasion during implantation [17,19]. To test the potential regulating effects of ILK on MMPs expression, the expression of MMPs (MMP-2, MMP-3, and MMP-9) and tissue inhibitors of MMPs (TIMP1, TIMP2, and TIMP3) was assessed in RL95-2 cells after the ILK knockdown. Figure 3A and 3B showed that ILK inhibition significantly reduced the mRNA and protein levels of MMP-3 and MMP-9. MMP-3 and MMP-9 are known targets of Wnt/β-catenin signaling [20,21]. Previous studies demonstrated that ILK positively regulates Wnt/β-catenin signaling [22]. Therefore, we then investigated whether ILK inhibition reduced MMP-3 and MMP-9 expression by inactivating Wnt/β-catenin signaling. Phosphorylation of GSK3β (p-GSK3β), total and nuclear β-catenin protein levels were remarkably decreased in the ILK-knockdown group compared to the control group (Figure 3C-E). Importantly, additional SKL2001 (a specific activator of Wnt/β-catenin signaling) treatment rescued ILK inhibition-induced Wnt/β-catenin signaling inactivation as indicated by increased p-GSK3β and β-catenin, and thus increased the expression of MMP-3 and MMP-9 (Figure 3F).
Invasion assay and in vitro embryo adhesion model was applied to analyze the effect of Wnt/β-catenin signaling on ILK-mediated EECs function. Figure 3G showed that ILK knockdown significantly inhibited RL95-2 cell invasion compared with control cells, while additional SKL2001 treatment rescued the invasion of RL95-2. Figure 3H further showed that the adhesion rate of JAR cells to RL95-2 was obviously reduced after ILK knockdown compared with control cells, while additional SKL2001 treatment recovered the adhesion rate of JAR cells to RL95-2. These results demonstrated that the Wnt/β-catenin signaling mediated the function of ILK on EECs in vitro.
ILK improved uterine receptivity and embryo implantation by regulating Wnt/β-catenin signaling in vivo
We further explored if ILK improved uterine receptivity formation and subsequent embryo implantation in vivo by regulating Wnt/β-catenin signaling. The effects of ILK on uterine receptivity and embryo implantation were observed in a mouse model. Lv-shILK was introduced into the mouse uterus on day 2 of pregnancy, and the uterine receptivity was assessed on day 7. Figure 4A showed that ILK inhibition obviously decreased embryo implantation rate in mice, whereas the decrease was partially reversed after additional treatment with SKL2001.
To verify that ILK regulated MMP-3 and MMP-9 expression by activating Wnt/β-catenin signaling in vivo, we assessed Wnt/β-catenin signaling activation and MMP-3 and MMP-9 expression in the pregnant uterus after ILK inhibition in the presence or absence of SKL2001. The results from western blot and immunohistochemistry analysis showed that ILK inhibition inactivated Wnt/β-catenin signaling and repressed MMP-3 and MMP-9 expression, whereas the decreased MMP-3 and MMP-9 level was partially reversed after additional SKL2001 treatment within the pregnant uterus (Figure 4B and 4C).
To clarify the fact that ILK inhibition impaired uterine receptivity by inactivating Wnt/β-catenin signaling, the structure changes of uterine endometrium were observed by SEM. The results showed that the microvilli of uterus in the ILK knockdown group were remarkably less than those in the control group, indicating the critical role of ILK on uterine receptivity formation. Importantly, additional SKL2001 treatment rescued the microvilli of uterus (Figure 4D). These results demonstrated that ILK improves uterine receptivity formation by activating Wnt/β-catenin signaling and up-regulating MMP-3/9 expression.
Discussion
In the current study, the function of ILK on regulating endometrial receptivity was verified, and the underlying mechanism was uncovered. The present data verified that: (I) The expression of ILK was downregulated in unexplained infertility patients, (II) ILK inhibition repressed EECs proliferation and invasion, and decreased the adhesion rate of embryonic cells to EECs, (III) ILK inhibition repressed MMP-3 and MMP-9 expression by inactivating Wnt/β-catenin signaling, (IV) ILK improved uterine receptivity and embryo implantation by regulating Wnt/β-catenin signaling in vivo. These data indicated that ILK/Wnt/MMPs axis may be applied as an indicator of endometrial receptivity, and as a diagnostic and therapeutic target for infertility.
The previous study has shown that ILK was associated with the human endometrial stromal cells (ESCs) [23]. Chen et al. demonstrated that the migrated and invasive abilities of ESCs was enhanced through facilitating EMT by ILK overexpression [24]. ILK also regulates the morphologic transformation of ESCs during endometrial decidualization [25]. However, the effect of ILK on regulating EECs biological behavior remains unclear. Given the importance of stromal-epithelial communication in human endometrium, here we investigated the regulatory role of ILK in EECs. The current data showed that ILK inhibition suppressed EECs proliferation and invasion, whereas ILK overexpression promoted EECs proliferation and invasion compared with control. Notably, the adhesion rate of embryonic cells to EECs was also increased after ILK overexpression. In contrast, ILK inhibition suppressed the adhesion rate of embryonic cells to EECs, indicating that ILK promoted EECs proliferation and invasion, and improved its receptive ability in vitro.
Extracellular matrix (ECM) degradation is a fundamental process to initialize the blastocyst invasion [26]. Matrix metalloproteinases (MMPs) are the main enzymes for ECM degradation and play a critical role in the embryo implantation and placentation process. Hiden et al. reported that the MMP-14 expression is downregulated in fetal growth restriction and thus results in the decrease of trophoblast-derived cell migration, proliferation, and trophoblast fusion [27]. Up-regulated MMP-14 has been identified in extravillous trophoblast cells and is helpful for trophoblast invasion and pregnancy outcomes [17]. MMP-2 and MMP-9 also play an important role in facilitating the degradation of ECM and cytotrophoblast invasion [28].
In the study, to test the association of ILK with MMPs expression, the expression of MMPs (MMP-2, MMP-3, and MMP-9) and tissue inhibitors of MMPs (TIMP1, TIMP2, and TIMP3) was assessed in EECs after the ILK knockdown. The current data showed that ILK inhibition repressed MMP-3 and MMP-9 expression. Given that MMP-3 and MMP-9 are known targets of Wnt/β-catenin signaling, we thus investigated whether ILK inhibition reduced MMP-3 and MMP-9 expression by inactivating Wnt/β-catenin signaling. As expected, ILK knockdown resulted in the inactivation of Wnt/β-catenin signaling and the repression of MMP-3 and MMP-9 expression, whereas forced activation of Wnt/β-catenin signaling, partially recovered the expression of MMP-3 and MMP-9 in the presence of ILK inhibition. Finally, we verified the mediated role of Wnt/β-catenin signaling in ILK-regulating uterine receptivity formation. ILK knockdown inhibited EECs invasion and the adhesion rate of embryonic cells to EECs, while forced activation of Wnt/β-catenin signaling recovered EECs invasion and the adhesion rate of embryonic cells to EECs in the presence of ILK inhibition. More critical, forced activation of Wnt/β-catenin signaling partially reversed ILK inhibition-induced decrease of the microvilli of uterus and embryo implantation rate. In conclusion, the current results demonstrated that ILK improves uterine receptivity formation by regulating Wnt/MMPs axis.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81671517 and no. 81873857) and Scientific Research Foundation of Shanghai Municipal Commission of Health and Planning (grant no. 201840060).
Disclosure of conflict of interest
None.
References
- 1.Mahajan N. Endometrial receptivity array: clinical application. J Hum Reprod Sci. 2015;8:121–129. doi: 10.4103/0974-1208.165153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissonnette L, Drissennek L, Antoine Y, Tiers L, Hirtz C, Lehmann S, Perrochia H, Bissonnette F, Kadoch IJ, Haouzi D, Hamamah S. Human S100A10 plays a crucial role in the acquisition of the endometrial receptivity phenotype. Cell Adh Migr. 2016;10:282–298. doi: 10.1080/19336918.2015.1128623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharkey AM, Smith SK. The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol. 2003;17:289–307. doi: 10.1016/s1521-6934(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Zhang A, Yu F, Gao J, Liu Y, Yu C, Zhou H, Xu C. Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J Proteome Res. 2015;14:1831–1842. doi: 10.1021/acs.jproteome.5b00038. [DOI] [PubMed] [Google Scholar]
- 5.Zheng CC, Hu HF, Hong P, Zhang QH, Xu WW, He QY, Li B. Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am J Cancer Res. 2019;9:186–197. [PMC free article] [PubMed] [Google Scholar]
- 6.Ho B, Bendeck MP. Integrin linked kinase (ILK) expression and function in vascular smooth muscle cells. Cell Adh Migr. 2009;3:174–176. doi: 10.4161/cam.3.2.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirley LA, McCarty S, Yang MC, Saji M, Zhang X, Phay J, Ringel MD, Chen CS. Integrin-linked kinase affects signaling pathways and migration in thyroid cancer cells and is a potential therapeutic target. Surgery. 2016;159:163–170. doi: 10.1016/j.surg.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raman A, Reif GA, Dai Y, Khanna A, Li X, Astleford L, Parnell SC, Calvet JP, Wallace DP. Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J Am Soc Nephrol. 2017;28:2708–2719. doi: 10.1681/ASN.2016111235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alasseiri M, Ahmed AU, Williams BRG. Mechanisms and consequences of constitutive activation of integrin-linked kinase in acute myeloid leukemia. Cytokine Growth Factor Rev. 2018;43:1–7. doi: 10.1016/j.cytogfr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Gil D, Ciolczyk-Wierzbicka D, Dulinska-Litewka J, Zwawa K, McCubrey JA, Laidler P. The mechanism of contribution of integrin linked kinase (ILK) to epithelial-mesenchymal transition (EMT) Adv Enzyme Regul. 2011;51:195–207. doi: 10.1016/j.advenzreg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Volckaert T, Yuan T, Yuan J, Boateng E, Hopkins S, Zhang JS, Thannickal VJ, Fassler R, De Langhe SP. Hippo signaling promotes lung epithelial lineage commitment by curbing Fgf10 and beta-catenin signaling. Development. 2019;146:dev166454. doi: 10.1242/dev.166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YM, Zhang YY, Bulbul A, Shan X, Wang XQ, Yan Q. Baicalin promotes embryo adhesion and implantation by up-regulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett. 2015;589:1225–1233. doi: 10.1016/j.febslet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Qin J, Wu C. ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol. 2012;24:607–613. doi: 10.1016/j.ceb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejaeger M, Bohm AM, Dirckx N, Devriese J, Nefyodova E, Cardoen R, St-Arnaud R, Tournoy J, Luyten FP, Maes C. Integrin-linked kinase regulates bone formation by controlling cytoskeletal organization and modulating BMP and wnt signaling in osteoprogenitors. J Bone Miner Res. 2017;32:2087–2102. doi: 10.1002/jbmr.3190. [DOI] [PubMed] [Google Scholar]
- 15.Moik D, Bottcher A, Makhina T, Grashoff C, Bulus N, Zent R, Fassler R. Mutations in the paxillin-binding site of integrin-linked kinase (ILK) destabilize the pseudokinase domain and cause embryonic lethality in mice. J Biol Chem. 2013;288:18863–18871. doi: 10.1074/jbc.M113.470476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 17.Latifi Z, Fattahi A, Ranjbaran A, Nejabati HR, Imakawa K. Potential roles of metalloproteinases of endometrium-derived exosomes in embryo-maternal crosstalk during implantation. J Cell Physiol. 2018;233:4530–4545. doi: 10.1002/jcp.26259. [DOI] [PubMed] [Google Scholar]
- 18.Benkhalifa M, Zayani Y, Bach V, Copin H, Feki M, Benkhalifa M, Allal-Elasmi M. Does the dysregulation of matrix metalloproteinases contribute to recurrent implantation failure? Expert Rev Proteomics. 2018;15:311–323. doi: 10.1080/14789450.2018.1464915. [DOI] [PubMed] [Google Scholar]
- 19.Wu LZ, Liu XL, Xie QZ. Osteopontin facilitates invasion in human trophoblastic cells via promoting matrix metalloproteinase-9 in vitro. Int J Clin Exp Pathol. 2015;8:14121–14130. [PMC free article] [PubMed] [Google Scholar]
- 20.Royer PJ, Henrio K, Pain M, Loy J, Roux A, Tissot A, Lacoste P, Pison C, Brouard S, Magnan A COLT consortium. TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/beta-catenin signaling. Respir Res. 2017;18:208. doi: 10.1186/s12931-017-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Zhang Z, Pan S, Shang S, Li C. Interaction between the Wnt/beta-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res. 2018;53:842–852. doi: 10.1111/jre.12574. [DOI] [PubMed] [Google Scholar]
- 22.Yoganathan TN, Costello P, Chen X, Jabali M, Yan J, Leung D, Zhang Z, Yee A, Dedhar S, Sanghera J. Integrin-linked kinase (ILK): a “hot” therapeutic target. Biochem Pharmacol. 2000;60:1115–1119. doi: 10.1016/s0006-2952(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Q, Xu Y, Lu J, Zhao J, Wei X, Liu P. Emodin inhibits migration and invasion of human endometrial stromal cells by facilitating the mesenchymal-epithelial transition through targeting ILK. Reprod Sci. 2016;23:1526–1535. doi: 10.1177/1933719116645192. [DOI] [PubMed] [Google Scholar]
- 24.Zheng QM, Chen XY, Bao QF, Yu J, Chen LH. ILK enhances migration and invasion abilities of human endometrial stromal cells by facilitating the epithelial-mesenchymal transition. Gynecol Endocrinol. 2018;34:1091–1096. doi: 10.1080/09513590.2018.1498477. [DOI] [PubMed] [Google Scholar]
- 25.Yen CF, Kim SH, Liao SK, Atabekoglu C, Uckac S, Arici A, Arlier S, Lee CL, Wang HS, Kayisli UA. Increased expression of integrin-linked kinase during decidualization regulates the morphological transformation of endometrial stromal cells. Fertil Steril. 2017;107:803–812. doi: 10.1016/j.fertnstert.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Maia-Filho VO, Rocha AM, Ferreira FP, Bonetti TC, Serafini P, Motta EL. Matrix metalloproteinases 2 and 9 and e-cadherin expression in the endometrium during the implantation window of infertile women before in vitro fertilization treatment. Reprod Sci. 2015;22:416–422. doi: 10.1177/1933719114529373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiden U, Ghaffari-Tabrizi N, Gauster M, Tam-Amersdorfer C, Cetin I, Dieber-Rotheneder M, Lang U, Desoye G. Membrane-type matrix metalloproteinase 1 regulates trophoblast functions and is reduced in fetal growth restriction. Am J Pathol. 2013;182:1563–1571. doi: 10.1016/j.ajpath.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. doi: 10.1242/dev.122.6.1723. [DOI] [PubMed] [Google Scholar]


