Abstract
The objectives of this study were to compare ruminal total tract digestibility, bacterial communities, and eating and rumination activity between Holstein and Angus steers fed grain- or forage-based diets. Six Holstein steers (average body weight [BW] = 483 ± 23 kg) and six Angus steers (average BW = 507 ± 29 kg), previously fitted with rumen cannulae, were fed in a crossover design with a 2 × 2 factorial arrangement of four treatments: 1) Holsteins fed a grain-based diet, 2) Holsteins fed a forage-based diet, 3) Angus fed a grain-based diet, and 4) Angus fed a forage-based diet. Each period was 35 d with 26 d of diet adaptation and 9 d of sample collection. On days 1 and 2 of collection, feeding activity was recorded for 48 h. On day 3, rumen contents were sampled to measure ruminal pH at 0, 3, 6, 12, and 18 h after feeding. A portion of the strained ruminal fluid was subsampled at 0, 3, and 6 h for volatile fatty acids (VFA) analysis. Rumen contents were subsampled at 3 h for analysis of bacterial communities. From day 4 to 8, total fecal excretion, feed, and refusals samples were collected and analyzed for dry matter (DM), neutral detergent fiber (NDF), and starch. On days 8 and 9 (0 and 3 h post-feeding, respectively), total reticulorumen evacuation was conducted and contents were weighed. Data were analyzed using the MIXED procedures in SAS (v9.4 SAS Inst. Inc., Cary, NC). Repeated measures were used to analyze changes in ruminal pH and VFA over time. There were no interactions of diet × breed (P ≥ 0.07). While the main effects of diet were expected, unique to these data is the fact that bacterial diversity and richness were reduced (P < 0.01) in cattle fed grain-based diets. There was no main effect (P > 0.34) of breed on total tract DM, organic matter, and starch digestibility, but Angus cattle had greater (P = 0.01) NDF digestibility than Holsteins. The increased NDF digestibility may be associated with a numerical (P = 0.08) increased numbers of bacterial species in Angus steers compared with Holstein steers. Holstein steers also spent more time (P ≤ 0.05) ruminating than Angus steers. There was no effect (P > 0.80) of breed on reticulorumen content at feeding time; however, Holstein steers had greater (P = 0.04) reticulorumen content on a wet basis 3 h post-feeding. Although Holstein steers spent more time ruminating, Angus steers were better able to digest NDF when compared with Holsteins, regardless of basal diet, and this improvement may be related to changes in bacterial communities in the rumen or to rumination activity.
Keywords: Angus, bacterial communities, diet digestibility, Holstein, rumination activity
Introduction
According to Schaefer et al. (2017), Holsteins are the largest purebred breed in the beef supply chain in the United States. Despite the importance of Holsteins in the beef supply chain, beef breeds are more efficient in the feedlot than dairy breeds (Solis et al., 1988). Richardson and Herd (2004) stated that differences in diet digestibility account for 10% of the variation in feed efficiency, suggesting that more efficient animals digest their diets better than less efficient animals. There is strong evidence that differences in feed efficiency are associated with differences in ruminal bacterial communities and with the interaction between the rumen microbiome and the host genetics (Jewell et al., 2015; Weimer et al., 2017). However, the differences between beef and dairy breeds have not been widely studied (Schaefer et al., 2017).
Few studies have evaluated the diet digestibility differences between beef and dairy steers. Garrett (1971) reported similar dry matter (DM) digestibility between beef and dairy steers fed a grain-based diet. Tjardes et al. (2002) reported that Angus steers had greater neutral detergent fiber (NDF) digestibility (NDFD) compared with Holstein steers when fed a forage-based diet. However, information explaining the effects of breed in these experiments was limited. Moreover, there are no data evaluating the relationship among diets and cattle breeds on ruminal bacteria at the genus level and total tract digestibility in the same experiment.
Therefore, we had hypothesized that differences in diet digestibility, and ruminal bacterial communities, between breeds may be greatest when cattle are fed forage-based diets, but that these differences would be negated when cattle were fed a grain-based diet. The objectives of the study were to compare total tract diet digestibility, rumen bacterial community composition, and eating and rumination activity between Holstein and Angus steers fed grain- or forage-based diets.
Materials and Methods
All procedures involving the use of animals were approved by the Pennsylvania State University Institutional Animal Care and Use Committee (#47255) and followed the guidelines recommended in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010).
Animal and diet management
Six Holstein steers (average body weight [BW] = 483 ± 23 kg; 15 ± 1 mo of age) and six Angus steers (average BW = 507 ± 29 kg; 15 ± 1 mo of age) previously fitted with rumen cannula were fed in a crossover design with a 2 × 2 factorial arrangement of four treatments: 1) Holsteins fed a grain-based diet, 2) Holsteins fed a forage-based diet, 3) Angus fed a grain-based diet, and 4) Angus fed a forage-based diet. Diets were formulated to be isonitrogenous and met the requirements of growing British cattle breeds (NRC, 2000; Table 1). Feed was delivered twice daily (0700 and 1600 hours), with the goal of maintaining similar dry matter intake (DMI) at 2% of BW among treatments. Steers were housed in individual metabolism stalls at the Beef Nutrition Research Lab, State College, PA. Stalls (2.5 × 1.5 m) were floored with rubber mats (Ani-mat Inc., Sherbrooke, QC, Canada) and equipped with individual feed bunks and non-siphoning automatic water bowls.
Table 1.
Composition of grain-based and forage-based diets
| Item | Grain | Forage |
|---|---|---|
| Ingredients, % DM basis | ||
| Grass hay1 | 20.0 | 80.0 |
| Cracked corn | 71.0 | 9.3 |
| Soybean meal | 7.0 | 8.8 |
| Mineral and vitamin supplement2 | 2.0 | 2.0 |
| Analyzed nutrient composition | ||
| DM | 66.7 | 68.8 |
| CP | 12.1 | 11.7 |
| NDF | 17.3 | 49.0 |
| Starch | 55.8 | 9.6 |
| Calculated composition | ||
| NEm3, Mcal/kg | 1.96 | 1.27 |
| NEg3, Mcal/kg | 1.32 | 0.75 |
1Analyzed values on grass hay: 66.1% NDF, 36.8% ADF, and 7.20% CP.
2Mineral and vitamin supplement: 35.6% Urea, 1,550 g/ton Rumensin 90 (198 g of monensin/kg of DM; Elanco Animal Health, Greenfield, IN), Ca 25% (as CaSO4), NaCl 15%, Mg 1% (as MnSO4), K 3.5% (as KCl), Zn 1,000 mg/kg (as ZnSO4), Cu 180 mg/kg (as CuSO4), Se 16 mg/kg (as Na2SeO3), Vit A 286,600 IU/kg.
3Calculated based on 2000 Beef Cattle NRC tabular values for individual ingredients.
Sampling and analysis
Cattle were fed in a crossover design with two sampling periods. Each period was 35 d with 26 d for diet adaptation followed by 9 d of sample collection. On days 1 and 2 of each collection phase, feeding activity was collected by five trained personnel (one at the time) every 5 min for a 48-h period. Activity parameters recorded were eating, ruminating, and resting of each steer. Activity recorded at each given time point was assumed to persist for the 5-min period, as described by Campanili et al. (2017). At the end of the 48 h of feeding activity collection, time spent on each activity was summed within activity and divided by 2 to calculate the average in minutes of each activity performed in a 24-h period. Activities were then calculated per unit of DM and per unit of NDF intake by dividing total minutes of that activity by the average intake of DM and NDF, respectively.
On day 3 of each collection phase, ruminal pH was measured by collecting whole, mixed rumen contents via the rumen cannula at 0, 3, 6, 12, and 18 h post-morning feeding. Whole rumen content samples were strained through two layers of cheesecloth. The extracted liquid was immediately analyzed for pH using a FiveEasy FiveGo pH meter F20 with a LE438 polyoxymethylene body gel-filled electrode with Ag/AgCl reference system and 1.2 m BNC/Cinch connection (Mettler Toledo, Columbus, OH). A portion of the strained ruminal fluid was subsampled prior to pH detection at 0, 3, and 6 h post-morning feeding for volatile fatty acids (VFA) analysis. After straining, 75 mL of rumen fluid saved for VFA was mixed with 75 mL of 2 N HCl. The mixture was then placed in a refrigerator and remixed by shaking four times per day for 2 d. On day 3, samples were removed from the refrigerator, and 12 mL of diluted ruminal fluid was mixed with 3 mL of 25% m-phosphoric acid and centrifuged at 20,000 × g at 4 °C for 20 min. The supernatant was then transferred, in 1-mL aliquots, to gas chromatography vials with 0.05 mL of 2-ethyl butyrate as an internal standard. Vials were then stored at −20 °C freezer until analyzed via gas-liquid chromatography (7890A, Agilent Technologies, Santa Clara, CA) with a column 2 m long × 2. mm i.d. packed with Carbowax 20 M on 80/100 Carbopack BPA (Supelco, Inc.; Bellefonte, PA).
Samples of individual feed ingredients (100 g/d) and refusals (if present; 10% as-is) were collected daily during the sample collection period. Feces were collected in canvas bags secured by a leather harness attached to the girth and under the neck of the steers. Feces were emptied from the bags, weighed, and subsampled (10% as-is basis) twice daily over the 96-h collection phase (day 4 to 8). Feed ingredients, feed refusal, and fecal samples were composited within period of collection and dried at 55 °C for 72 h. Dry, composited samples were ground through a Wiley mill (1-mm screen, Arthur H. Thomas, Philadelphia, PA). Ground samples were analyzed for NDF (using Ankom Technology method 6; Ankom200 Fiber Analyzer, Ankom Technology, Macedon, NY), crude protein (CP; using a Costech ECS 4010 C/N/S elemental analyzer; Costech Analytical Technologies Inc., Valencia, CA), starch and soluble sugars (determined by the method of Hall, 2009), and total ash (500 °C for 12 h, using a HotPack Muffle Oven Model: 770750, HotPack Corp., Philadelphia, PA). The resulting analyses of individual feed ingredients were used to calculate the nutrient composition of the diets (Table 1).
On days 8 and 9 of the collection period, total reticulorumen evacuation was conducted manually. On day 8, before morning feed delivery (0 h), total reticulorumen evacuation was conducted, and on day 9, 3 h after morning feed delivery, a second total reticulorumen evacuation was conducted. Upon removal, the reticulorumen contents were placed in a 120-L plastic trash can, mixed, weighed, and 5% of the total wet weight was saved. The remaining ruminal contents were placed back into the rumen of the respective steer they were removed from. Ruminal content samples were stored at −20 °C until analyzed for DM (24 h at 105 °C).
One Angus steer fed the grain-based diet was excluded during the second sampling period because it became ill and had decreased DMI; therefore, that animal was removed from the analyses.
Ruminal bacterial community composition
Samples of 50 mL of whole rumen contents from the dorsal, cranial, and ventral sac of the rumen were collected and mixed on day 3 of each period through the ruminal cannula at 3 h after the morning meal and stored at −80 °C. After thawing, the samples were processed to isolate deoxyribonucleic acid (DNA) according to Weimer et al. (2017). The DNA was quantified using a Qubit (Invitrogen, Carlsbad, CA) and stored at 4 °C before preparation of the DNA library. Universal primers flanking the variable 4 region of the bacterial 16S rRNA coding region were used to perform the polymerase chain reaction, which had one reaction per sample containing a total of 50 ng of DNA (Kozich et al., 2013). Cycling conditions were as follows: an initial denaturation at 95 °C for 3 min, followed by 25 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and then a final cycle at 72 °C for 5 min. The DNA amplification was conducted by gel electrophoresis. DNA was extracted from the gel using a ZR-96 Zymoclean Gel DNA Recovery Kit (Zymo Research, Irvine, CA). Extracted DNA was quantified, equimolar pooled, combined with a 5% to 10% PhiX control DNA, and then was sequenced as paired-end reads, 250 cycles each (2 × 250), using the MiSeq 2 × 250 v2 kit for liquid samples (Illumina, San Diego, CA) with custom sequencing primers as described by Kozich et al. (2013). The program mothur v.1.41.1 was used for further processing the sequences obtained (Schloss et al., 2009). Paired-end sequences were combined to form contigs and poor quality sequences were removed. The SILVA 16S rRNA gene reference alignment database v132 was used to screen for alignment to the correct region. The GreenGenes database (DeSantis et al., 2006) was used to classify sequences with a bootstrap value cutoff of 80. Sequences classified to cyanobacteria, mitochondria, Eukarya, or Archaea were removed. Singletons (rare variant for which genetic variation is carried by a unique chromosome in a sample) were removed to streamline analysis. For the ruminal bacterial communities’ composition, the calculated sample Good’s coverage was 0.98 across all samples and the average number of sequences per sample was 16,020.
Statistical analysis
The experimental design was a crossover design with a 2 × 2 factorial arrangement of dietary treatments. Data were analyzed using the MIXED procedure of SAS (version 9.4; SAS Inst. Inc., Cary, NC). A Kenward–Roger adjustment was used. The model for eating and rumination activity, reticulorumen contents, DMI, and apparent total tract digestibility was:
in which Yijkl is the response variable; μ is the mean; pi is the random effect of period; Bj is the fixed effect of breed; Dk is the fixed effect of the diet; (BD)jk is the interaction of breed × diet; and eijkl is the experimental error. Means were separated using the LSMEANS statement with PDIFF option.
Repeated measures were used to analyze the effects of time on the response variables such as ruminal pH and VFA concentrations. The autoregressive heterogeneous, ARH(1), covariance structure was chosen based on the smallest Bayesian Information Criterion. A Kenward–Roger adjustment was used. The model was:
in which Yijklmn is the response variable; μ is the mean; pi is the random effect of period; aj(i) is the random effect of animal nested within period; Bk is the fixed effect of breed; Dl is the fixed effect of the diet; (BD)kl the fixed effect of the interaction of breed × diet; Tl is the fixed effect of time of collection; (BT)km is the fixed effect of the interaction of breed × time of collection; (DT)lm is the fixed effect of the interaction of diet × time of collection; (BDT)klm is the fixed effect of the interaction of breed × diet × time of collection; and eijklmn is the experimental error. Means were separated using the LSMEANS statement with PDIFF option.
Bacterial sequences were grouped into operational taxonomic units (OTU) at 97% sequence similarity. Good’s (1953) coverage was calculated in mother v.1.43.0 for all samples (https://mothur.org/wiki/Main_Page). The OTU counts were normalized to 10,000 sequences per sample, and the normalized counts of OTU by sample were used for further analysis. Alpha diversity (community diversity within individual animals within each period) was assessed using Chao’s estimate of species richness (Chao, 1984) and Shannon’s diversity index (Shannon, 2001). Differences in community diversity and richness between animals were assessed by overall two-way ANOVA in R v3.2.1 (R Development Core Team, 2011) following the first statistical model described above. Beta diversity (differences in community composition between samples) was assessed by using nonmetric multidimensional scaling to visualize differences between samples calculated as the Bray–Curtis metric (Bray and Curtis, 1957). Changes in total community structure (relative abundance, Bray–Curtis metric) were assessed using permutational multivariate ANOVA in R (vegan). Pairwise comparisons between each group were quantified with Permutational Multivariate ANOVA, and P-values are corrected for false discovery rate.
Individual steer was the experimental unit. Differences were declared significant at P ≤ 0.05.
Results and Discussion
There were no diet × breed interactions (P ≥ 0.07) on any of the parameters measured. Therefore, detailed discussions relative to the interaction between diet × breed will not be addressed.
Diet
The influence of grain and forage-based diets on rumen fermentation parameters are not novel information (Cipolloni et al., 1951; Prigge et al., 1984; Colucci et al., 1989; O’Mara et al., 1999). Therefore, the extent to which they are addressed here is merely to confirm that the magnitude of the responses in the current experiment align with differences previously reported in the literature.
Steers fed the grain-based diet consumed 23% more feed, on average, than steers fed the forage-based diet (Table 2). In ruminants, DMI is often regulated by the reticulorumen capacity (physical fill), chemostatic feedback (energy feedback), and feed passage rate (Allen, 1996; Fisher, 2002). Because the grain-based diet contained more energy per kilogram than the forage-based diet (Table 1), it is unlikely that the decreased DMI in steers fed the forage-based diet was caused by chemostatic feedback regulation. Therefore, the physical capacity of the reticulorumen, as well as a potential decrease in feed passage rate, was likely limiting the intake of steers fed the forage-based diet.
Table 2.
Effects of cattle breed, Holstein or Angus, and basal diet, grain or forage, on diet intake and digestibility
| Grain1 | Forage1 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Angus | Holstein | Angus | Holstein | SEM | Diet | Breed | Diet × Breed |
| Animals, n | 5 | 6 | 6 | 6 | — | — | — | — |
| Calculated DM intake, % BW2 | 2.01 | 2.13 | 1.47 | 1.68 | 0.05 | <0.01 | <0.01 | 0.27 |
| Intake, kg/d3 | ||||||||
| DM | 10.1 | 10.3 | 7.5 | 8.1 | 0.4 | <0.01 | 0.06 | 0.37 |
| OM | 9.6 | 9.8 | 7.1 | 7.7 | 0.4 | <0.01 | 0.05 | 0.35 |
| NDF | 1.7 | 1.8 | 3.5 | 3.9 | 0.2 | <0.01 | 0.08 | 0.26 |
| Starch | 5.6 | 5.8 | 0.8 | 0.8 | 0.1 | <0.01 | 0.08 | 0.25 |
| Digestibility, %3 | ||||||||
| DM | 75.6 | 74.0 | 71.7 | 69.4 | 1.3 | <0.01 | 0.13 | 0.78 |
| OM | 76.2 | 74.3 | 72.9 | 70.5 | 1.3 | 0.01 | 0.11 | 0.87 |
| NDF | 55.5 | 48.1 | 65.7 | 63.3 | 2.3 | <0.01 | <0.01 | 0.07 |
| Starch | 91.7 | 91.0 | 95.8 | 96.4 | 0.8 | <0.01 | 0.94 | 0.39 |
1Grain = Diet contained 71% cracked corn, 20% grass hay, 7% soybean meal, and 2% mineral and vitamin supplement; Forage = Diet contained 9.25% cracked corn, 80% grass hay, 8.75% soybean meal, and 2% mineral and vitamin supplement.
2% BW = kg/100 kg of BW.
3Calculated on DM basis.
While intake can drive digestibility responses in some cases, in the current experiment digestibility responses varied by nutrient. Steers fed the forage-based diet had increased NDF (P < 0.01) and starch (P < 0.01) digestibility, by 25% and 5%, respectively, compared with steers fed the grain-based diet (Table 2). But, steers fed the forage-based diet had decreased DM (P < 0.01) and organic matter (OM) (P = 0.01) digestibility, by 6% and 5%, respectively, compared with steers fed the grain-based diet steers fed the forage-based diet. In a review conducted by Dixon and Stockdale (1999), the authors stated that because microbes rapidly ferment most of the dietary carbohydrates from grains in the rumen, grain feeds are often more digestible than forages feeds. Therefore, cattle fed grain-based diets often have greater DM and OM digestibility compared with cattle fed forage-based diets (Dixon and Stockdale, 1999). However, as the fermentation of carbohydrate increases in the rumen, ruminal pH decreases, becoming detrimental to cellulolytic microbial growth. Thus, ruminants fed grain-based diets typically experience decreased NDFD compared with ruminants fed forage-based diets (Dixon and Stockdale, 1999; Nousiainen et al., 2009).
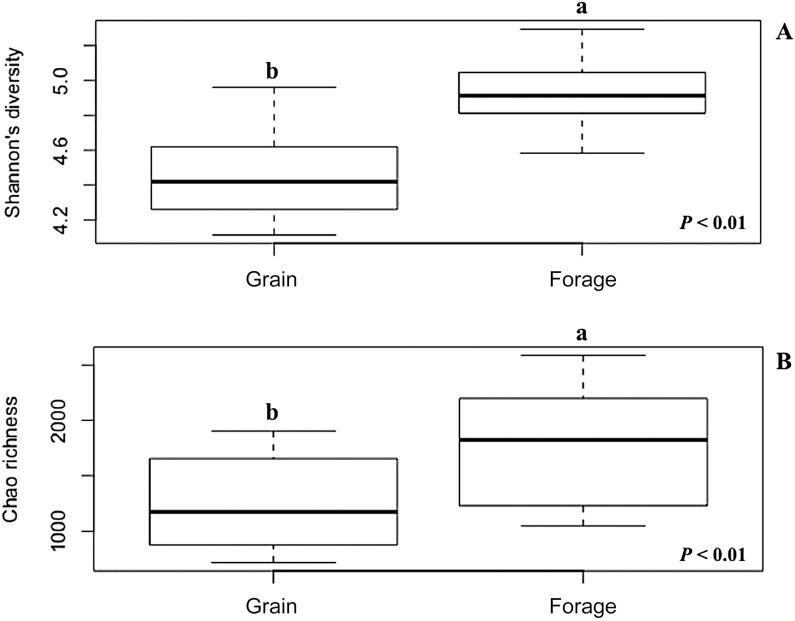
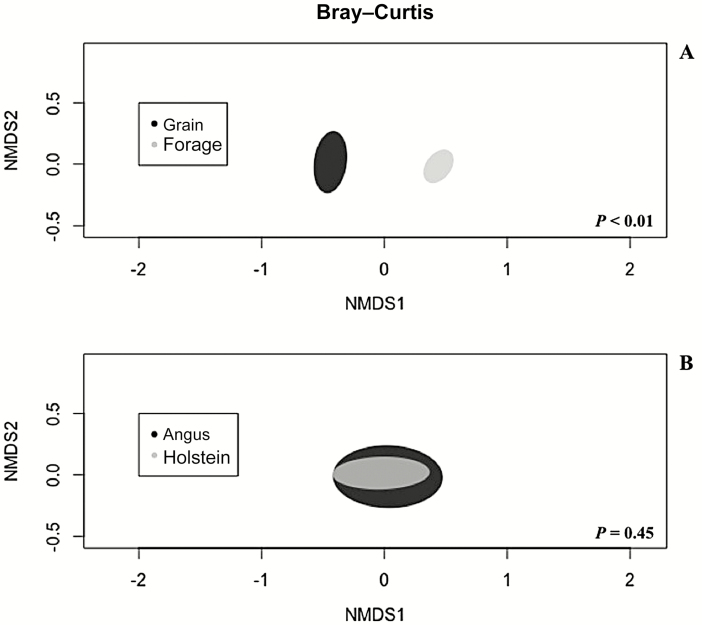
In agreement with previous literature, in the current study, bacterial diversity was reduced (P < 0.01) in cattle fed grain-based diets (Figure 1). This reduction was likely due to the reduction of substrates available to the bacteria that ferment structural carbohydrates and the subsequent lower pH (AlZahal et al., 2017). The reduction of the bacterial diversity is certainly associated with the reduction (P < 0.01) in richness (number of species), which was also observed in cattle fed the grain-based diet (Figure 1). However, cattle with different calculated residual feed intake fed the same diets, high-forage (McCann et al., 2014) or high-grain (Li and Guan, 2017), had similar ruminal bacterial communities. Therefore, decreased bacterial diversity seems to be more closely associated to the type of diet consumed than to the host feed efficiency (Carberry et al., 2012). Furthermore, the Bray–Curtis metric graphed using a nonmetric multidimensional scaling (Figure 2) shows a diet effect (P < 0.01), making possible a distinction between rumen content samples from grain- and forage-fed cattle in terms of bacterial communities composition, confirming that diet had an influence on bacterial communities composition in the rumen resulting in alterations on rumen fermentation pattern. These alterations in fermentation may have influenced the digestibility of starch as well. Steers fed the grain-based diet had decreased starch digestibility relative to steers fed forage-based diets.
Figure 1.
Effect of basal diet, grain or forage, on Shannon diversity (Panel A) and Chao’s richness (Panel B). Cattle fed grain-based diet reduced (P < 0.01) bacterial diversity and richness (number of OTU or species; SEM = 0.03), regardless of breed.
Figure 2.
Effects of basal diet, grain or forage (Panel A), and cattle breed, Holstein or Angus (Panel B), on Bray–Curtis metric graphed using a nonmetric multidimensional scaling. There was a diet effect (P<0.01), meaning that rumen content samples from cattle fed grain-based diets differed from rumen content samples from cattle fed forage-based diets in terms of bacterial communities composition, regardless of breed. However, no main effect of breed was observed (P = 0.45) on Bray–Curtis metric, meaning that it is not possible to differentiate rumen content samples collected from Angus or Holstein in terms of bacterial communities composition, regardless of diet.
Steers fed a grain-based diet consumed their diet in half of the time than steers fed a forage-based diet (131 vs. 287 min/d, respectively; Table 3). However, there was no main effect (P = 0.77) of diet on time spent eating when calculated per kilogram of NDF. When cattle are eating similar quantities of DM, forage feeds are typically consumed more slowly than concentrate feeds. However, when cattle are fed for ad libitum intake, cattle fed forage feeds often have decreased DMI compared with cattle fed concentrate feeds. Because of these differences relative to feeding strategies, differences in total time spend eating between forage- and grain-based diets are not always observed (Beauchemin, 2018).
Table 3.
Effects of cattle breed, Holstein or Angus, and basal diet, grain or forage, on eating and rumination activity1
| Grain2 | Forage2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Angus | Holstein | Angus | Holstein | SEM | Diet | Breed | Diet × Breed |
| Animals, n | 5 | 6 | 6 | 6 | — | — | — | — |
| Time spent eating | ||||||||
| min/d | 141 | 122 | 283 | 291 | 11.7 | <0.01 | 0.65 | 0.23 |
| min/kg of DM | 15 | 12 | 40 | 38 | 2.2 | <0.01 | 0.19 | 0.95 |
| min/kg of NDF3 | 84 | 68 | 81 | 75 | 8.1 | 0.77 | 0.09 | 0.27 |
| Time spent ruminating | ||||||||
| min/d | 382 | 468 | 502 | 577 | 20.8 | <0.01 | <0.01 | 0.78 |
| min/kg of DM | 40 | 48 | 70 | 76 | 5.4 | <0.01 | 0.02 | 0.64 |
| min/kg of NDF3 | 228 | 262 | 141 | 150 | 31.6 | <0.01 | 0.10 | 0.31 |
1Total rumen evacuation was conducted prior to animal feeding (0 h) and 3 h post-feeding on two consecutive days.
2Grain = diet contained 71% cracked corn, 20% grass hay, 7% soybean meal, and 2% mineral and vitamin supplement; Forage = diet contained 9.25% cracked corn, 80% grass hay, 8.75% soybean, meal and 2% mineral and vitamin supplement.
3Calculated on DM basis.
Most ruminant animals spend brief periods of time eating and spend the bulk of their time during the day ruminating (Van Soest, 1994). These observations were consistent in the current experiment. Furthermore, Beauchemin (2018) stated that there is a positive correlation between NDF intake and rumination time. In the current study, steers fed the forage-based diet consumed two times more NDF (kg/d) and spent 114 more minutes ruminating than steers fed a grain-based diet (Table 3). Jiang et al. (2017) observed that cows fed a 70% roughage diet spent more time ruminating and produced an additional 17 liters of saliva per day when compared with cows fed a 40% roughage diet. In our study, saliva production was not measured; however, steers fed the forage-based diet (80% roughage) averaged 18 more liters of liquid in their reticulorumen contents compared with steers the grain-based diet (20% roughage), regardless of breed or sampling time (Table 4), indicating a potential for greater saliva production on steers fed forage-based diet compared with steers fed grain-based diet.
Table 4.
Effects of cattle breed, Holstein or Angus, and basal diet, grain or forage, on reticulorumen content pre- and post-feeding1
| Grain2 | Forage2 | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | Angus | Holstein | Angus | Holstein | SEM | Diet | Breed | Diet × Breed |
| Animals, n | 5 | 6 | 6 | 6 | — | — | — | — |
| Reticulorumen contents, 0 h | ||||||||
| Wet, kg | 88.1 | 85.1 | 102.4 | 108.3 | 6.26 | <0.01 | 0.80 | 0.45 |
| Dry, kg3 | 12.6 | 11.6 | 13.4 | 14.4 | 1.48 | 0.08 | 0.99 | 0.35 |
| Reticulorumen contents, 3 h | ||||||||
| Wet, kg | 105.8 | 109.6 | 114.1 | 139.4 | 7.11 | 0.01 | 0.04 | 0.12 |
| Dry, kg3 | 19.3 | 19.0 | 17.0 | 20.3 | 1.54 | 0.70 | 0.31 | 0.22 |
1Total rumen evacuation was conducted prior to animal feeding (0 h) and 3 h post-feeding on two consecutive days.
2Grain = diet contained 71% cracked corn, 20% grass hay, 7% soybean meal, and 2% mineral and vitamin supplement; Forage = diet contained 9.25% cracked corn, 80% grass hay, 8.75% soybean meal, and 2% mineral and vitamin supplement.
3Calculated on DM basis.
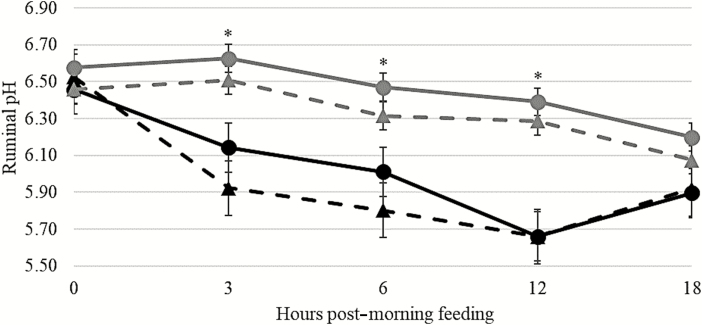
There was a diet × hour interaction (P < 0.01) on ruminal pH (Figure 3) and on ruminal acetate, propionate, and total VFA concentrations (Table 5). There was a continuous decrease in ruminal pH of steers fed the grain-based diet for 12 h post-feeding. This decrease reflects the increases in VFA concentration in the rumen of steers fed the grain-based diet. These data align with other reports that increasing intake of rapidly fermentable carbohydrates and decreasing roughage concentration in the diet decrease ruminal pH (Dixon and Stockdale, 1999) and increase ruminal concentrations of propionate and total VFA (Jiang et al., 2017). Meanwhile, steers fed forage-based diets had only minor changes in ruminal pH throughout the day; total VFA concentration in steers fed forage-based diets varied considerably less than those observed in steers fed grain-based diets. As expected, there was a greater (P < 0.01) proportion of acetate relative to propionate in steers fed the forage-based diet compared with steers fed the grain-based diet; the magnitude of this proportional difference was greatest at 0 h, before feeding.
Figure 3.
Effects of cattle breed, Holstein or Angus, and basal diet, grain or forage, on ruminal pH. Solid black line (●) = Angus steers fed a grain-based diet; solid gray line (▲) = Holstein steers fed a grain-based diet; dash black line (●) = Angus steers a fed forage-based diet; dash gray line (▲) = Holstein steers fed a forage-based diet. There was no interaction (P ≥ 0.64) of diet × breed, hour × breed, or diet × hour × breed. There was no main effect of breed (P = 0.37). There was a main effect of diet (P < 0.01) and a diet × hour interaction (P < 0.01). Steers fed grain-based diets had lesser ruminal pH than steers fed forage-based diets at 3, 6, and 12 h post-morning feeding (*). Error bars are associated with the interaction between diet × breed × hour (SEM = 0.22).
Table 5.
Effects of cattle breed, Holstein or Angus, and basal diet, grain or forage, on ruminal VFA
| Grain1 | Forage1 | P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | Time after feeding, h | Angus | Holstein | Angus | Holstein | SEM | Diet | Breed | Diet × Breed |
| Animals,n | 5 | 6 | 6 | 6 | — | — | — | — | |
| Acetate, mM | 4.1 | 0.05 | 0.25 | 0.47 | |||||
| 0 | 32.3 | 33.5 | 44.5 | 45.7 | 0.06 | ||||
| 3 | 44.6 | 44.6 | 44.5 | 46.7 | 0.04 | ||||
| 6 | 51.3 | 56.7 | 49.6 | 48.0 | 0.03 | ||||
| Propionate, mM | 2.8 | <0.01 | 0.55 | 0.20 | |||||
| 0 | 11.7 | 13.6 | 12.5 | 12.7 | 0.12 | ||||
| 3 | 19.6 | 19.9 | 14.4 | 13.8 | 0.09 | ||||
| 6 | 25.3 | 26.7 | 17.8 | 16.7 | 0.05 | ||||
| Butyrate, mM | 1.2 | <0.01 | 0.92 | 0.13 | |||||
| 0 | 7.8 | 8.1 | 5.9 | 6.7 | |||||
| 3 | 9.6 | 8.3 | 6.3 | 6.8 | |||||
| 6 | 9.8 | 9.6 | 7.6 | 7.7 | |||||
| Total VFA, mM | 12.1 | <0.01 | 0.51 | 0.62 | |||||
| 0 | 64.0 | 61.3 | 70.4 | 71.9 | 0.11 | ||||
| 3 | 73.9 | 79.1 | 72.0 | 74.3 | 0.10 | ||||
| 6 | 91.0 | 100.6 | 81.3 | 78.0 | 0.08 | ||||
| A:P3 | 0.3 | <0.01 | 0.84 | 0.35 | |||||
| 0 | 2.9 | 2.7 | 3.6 | 3.7 | |||||
| 3 | 2.4 | 2.4 | 3.1 | 3.4 | |||||
| 6 | 2.2 | 2.3 | 2.9 | 2.9 |
1Grain = diet contained 71% cracked corn, 20% grass hay, 7% soybean meal, and 2% mineral and vitamin supplement; Forage = diet contained 9.25% cracked corn, 80% grass hay, 8.75% soybean meal, and 2% mineral and vitamin supplement.
2There were no Diet × Breed × Hour interactions (P ≥ 0.12) nor Breed × Hour interactions (P ≥ 0.39); there was a Diet × Hour interaction (P ≤ 0.01) on acetate, propionate and total VFA, but not for butyrate and A:P ratio (P ≥ 0.58); there was an effect of Hour (P < 0.01) for all VFA measured.
3acetate:proprionate.
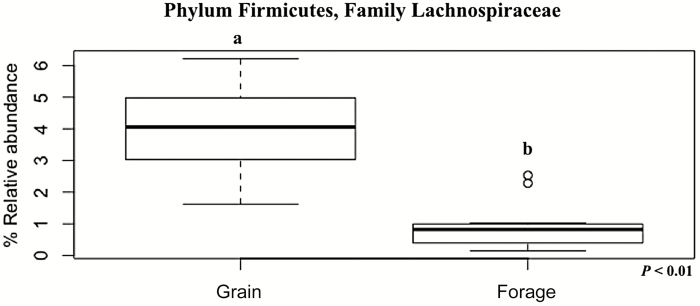
Despite the greater acetate to propionate ratio in cattle fed forage-based diets, acetate concentrations were greatest in cattle fed grain at 6 h post-feeding when compared with cattle fed forage. These data taken alone are not inherently clear because forage-fed cattle traditionally have greater ruminal acetate concentrations than grain-fed cattle (Van Soest, 1994). However, it is likely that the ruminal acetate concentration reflect the increase in the population of ruminal bacteria from phylum Firmicutes, family Lachnospiraceae (~4% of total sequences), in cattle fed grain-based diets compared with cattle fed forage-based diets (Figure 4). The family Lachnospiraceae plays a significant role in fibrolytic activities within the rumen (Biddle et al., 2013), and the abundance of unclassified Lachnospiraceae when cattle were fed grain-based diets could suggest that this group consists of metabolically diverse genera that can degrade both fiber and less complex carbohydrates (AlZahal et al., 2017), giving them a distinct advantage in the cattle fed grain-based diets.
Figure 4.
Effect of basal diet, grain or forage, on the relative abundance of OTU04, identified as bacteria from phylum Firmicutes and composed by mainly unclassified group from family Lachnospiraceae. This family was more abundant (P < 0.01) in cattle fed grain-based diet (~4% of total sequences; SEM = 0.02) compared with cattle fed forage-based diets, regardless of breed.
Breed
There was no effect of breed (P ≥ 0.11) on DM, OM, and starch digestibility (Table 2). Similar DM digestibility between beef and dairy breed steers was reported by Garrett (1971), when cattle were fed a grain-based diet, and Tjardes et al. (2002), when cattle were fed different concentrations of NDF. In the current study, Holstein steers had an 8% decrease in NDFD compared with Angus steers. These results are similar to the work of Tjardes et al. (2002) that reported a 5% decrease in NDFD when Holstein steers were compared with Angus steers. Tjardes et al. (2002) suggested that the decrease in NDFD of Holstein steers was related to a reduction in ruminal pH and the possibility of increased passage rate of the diet. Although there was no breed × diet interaction for ruminal pH in the current study, ruminal pH measured in Holstein steers remained consistently below that of their Angus counterparts (Figure 3).
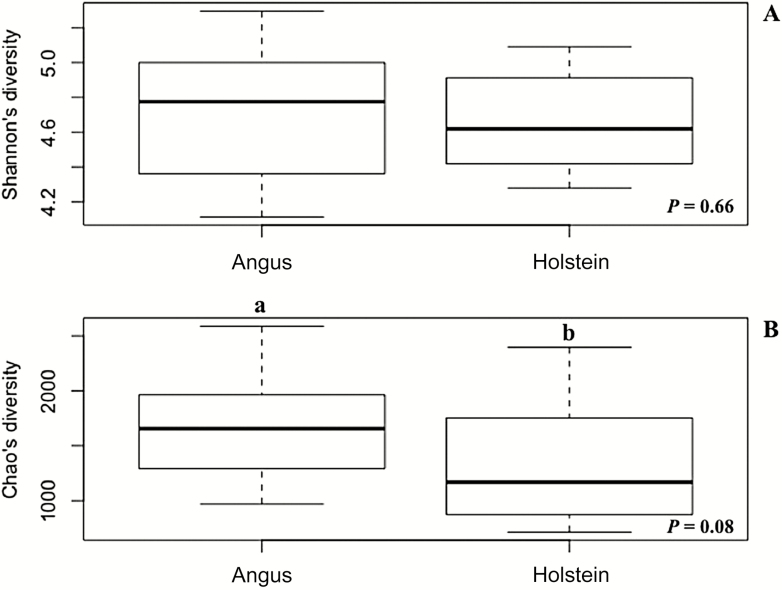
Perhaps more impactful than the shift in pH, however, was the change in intake. Despite the effort to control DMI among treatments in the current experiment, Holstein steers consumed 10% more DM, when measured on a % BW basis, than Angus steers (Table 2). The greater DMI as % of BW observed in Holstein steers compared with Angus steers occurred because even though steers had similar DMI in kg/d, Holstein steers were lighter during the collection phases than Angus steers. Allen (1996) reported that an increase in DMI could decrease fiber digestibility by an increase in the passage rate of the diet. The potential that the increased intake of the Holstein steers may have promoted an increased passage rate exists; however, the passage rate was not determined in the current experiment and would need to be further investigated. Fibrolytic ruminal bacteria do have a longer lag time when compared with amylolytic bacteria (Zhang et al., 2017). Thus, another factor that may have played a role in changes in NDFD is the ruminal bacterial community composition between Angus and Holstein steers (Figure 5). Even though Angus steers had numerically greater bacterial richness (number of bacterial species) when compared with Holstein steers, it was only a trend (P = 0.08). Additional research may be necessary to corroborate these results. Breed did not affect bacterial diversity (Figure 5), and Bray–Curtis metric (Figure 2), which means that it is not possible to differentiate rumen content samples collected from Angus or Holstein in terms of bacterial community composition.
Figure 5.
Effects of cattle breed, Angus or Holstein, on Shannon diversity (Panel A) and Chao’s richness (Panel B). Even though Holstein steers had a numerical decrease in richness (number of OTU or species; SEM = 0.03) when compared with Angus steers, richness only tended to be different (P = 0.08) between the two breeds.
Despite the greater DMI observed in Holstein steers compared with Angus steers, steers from both breeds spent similar (P ≥ 0.09) amount of time eating (Table 3). However, Holstein steers spent 80 additional min/d ruminating on average when compared with Angus steers. The greater total amount of time ruminating observed in Holstein steers compared with Angus steers may have increased the digestion rate of the diet by decreasing physical size of particles, thereby increasing the passage rate as well. Tjardes et al. (2002) also suggested that the potential increase in passage rate could be the primary reason for the decrease in NDFD on diets fed to Holstein compared with Angus steers; however, Beauchemin (2018) stated the quantitative aspects of rumination time and fiber digestibility are not well documented and require further investigation.
Although the effects of rumination time on digestibility are not clear, rumination time may influence saliva production (Jiang et al., 2017; Beauchemin, 2018). In the current study, Holstein steers that ruminated more had 14 extra liters of liquid in the reticulorumen at 3 h post-feeding compared with Angus steers. The greater (P = 0.04) reticulorumen content weight on a wet basis of Holstein steers 3 h post-feeding may be associated with the increased saliva production caused by increased rumination. However, saliva production was not directly measured in the current study. Therefore, it is also possible that increased water intake of Holstein steers compared with Angus caused the difference in the wet weight of reticulorumen contents. Peters (2014) and Zinn et al. (2016) have previously suggested that water intake may be greater in Holstein steers when compared with beef breeds. However, as neither saliva production nor water intake were measured in the current experiment, further research needs to be conducted to evaluate the effects of breed on saliva production and water intake.
Even though Holstein steers spent more time ruminating, and potentially produced a greater amount of saliva than Angus steers, ruminal pH (Figure 3) and VFA concentrations (Table 5) were similar (P ≥ 0.22) between Holstein and Angus steers.
Although Holstein steers spent more time ruminating, Angus and Holstein steers digested DM and starch similarly, regardless of diet. However, Angus steers had a greater NDF digestibility than Holstein steers, which may be associated with the trend for increased rumen bacterial richness in Angus steers. Additional research validating changes in bacterial richness and investigating the potential effects of passage rate, which was not part of this study, are needed to further elucidate these differences between Angus and Holstein steers.
Acknowledgments
We would like to acknowledge the training and expertise of Dr. Garret Suen’s laboratory at the University of Wisconsin. In addition, funding support for the project was provided by the Pennsylvania State University.
Glossary
Abbreviations
- BW
body weight
- CP
crude protein
- DM
dry matter
- DMI
dry matter intake
- DNA
deoxyribonucleic acid
- NDF
neutral detergent fiber
- NDFD
neutral detergent fiber digestibility
- NEg
net energy for gain
- NEm
net energy for maintenance
- NRC
nutrient requirements of cattle
- OM
organic matter
- OUT
operational taxonomic unit
- rRNA
ribosomal ribonucleic acid
- VFA
volatile fatty acids
Conflict of interest statement
The authors have no real or perceived conflicts of interest to declare.
Literature Cited
- Allen M. S. 1996. Physical constraints on voluntary intake of forages by ruminants. J. Anim. Sci. 74:3063–3075. doi: 10.2527/1996.74123063x [DOI] [PubMed] [Google Scholar]
- AlZahal O., Li F., Guan L. L., Walker N. D., and McBride B. W.. . 2017. Factors influencing ruminal bacterial community diversity and composition and microbial fibrolytic enzyme abundance in lactating dairy cows with a focus on the role of active dry yeast. J. Dairy Sci. 100:4377–4393. doi: 10.3168/jds.2016-11473 [DOI] [PubMed] [Google Scholar]
- Beauchemin K. A. 2018. Invited review: current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 101:4762–4784. doi: 10.3168/jds.2017-13706 [DOI] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., and Leschine. S.. 2013. Understanding the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity (Basel). 5:627–640. doi: 10.3390/d5030627 [DOI] [Google Scholar]
- Bray R. J., and Curtis J. T.. . 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 27:325–349. doi. 10.2307/1942268 [DOI] [Google Scholar]
- Campanili P. R. B., Sarturi J. O., Ballou M. A., Trojan S. J., Sugg J. D., Ovinge L. A., Alrumaih A. U., Pellarin L. A., and Hoffman A. A.. . 2017. Effects of silage type and inclusion level on ruminal characteristics and feeding behavior of steers fed finishing diets. J. Anim. Sci. 95:4623–4637. doi: 10.2527/jas2017.1510 [DOI] [PubMed] [Google Scholar]
- Carberry C. A., Kenny D. A., Han S., McCabe M. S., and Waters S. M.. . 2012. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 78:4949–4958. doi: 10.1128/AEM.07759-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. 1984. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11:265–270. [Google Scholar]
- Cipolloni M. A., Schneider B. H., Lucas H. L., and Pavlech H. M.. . 1951. Significance of the differences in digestibility of feeds by cattle and sheep. J. Anim. Sci. 10:337–343. doi: 10.2527/jas1951.102337x [DOI] [PubMed] [Google Scholar]
- Colucci P. E., Macleod G. K., Grovum W. L., Cahill L. W., and McMillan I.. . 1989. Comparative digestion in sheep and cattle fed different forage to concentrate ratios at high and low intakes. J. Dairy Sci. 72:1774–1785. doi: 10.3168/jds.S0022-0302(89)79294-8 [DOI] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., Huber T., Dalevi D., Hu P., and Andersen G. L.. . 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. M., and Stockdale C. R.. . 1999. Associative effects between forages and grains: consequences for feed utilization. Aust. J. Agric. Res. 50:757–773. doi: 10.1071/AR98165 [DOI] [Google Scholar]
- Federation of Animal Science Societies. 2010. Guide for the care and use of agricultural animals in agricultural research and teaching, 3rd ed. Champaign (IL): Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.
- Fisher D. S. 2002. A review of a few key factors regulating voluntary feed intake in ruminants. Crop Sci. 42:1651–1655. doi: 10.2135/cropsci2002.1651 [DOI] [Google Scholar]
- Garrett W. N. 1971. Energetic efficiency of beef and dairy steers. J. Anim. Sci. 32:451–456. doi: 10.2527/jas1971.323451x [DOI] [Google Scholar]
- Good I. J. 1953. The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264. doi: 10.2307/2333344 [DOI] [Google Scholar]
- Hall M. B. 2009. Determination of starch, including maltooligosaccharides, in animal feeds: comparison of methods and a method recommended for AOAC collaborative study. J. AOAC Int. 92:42–49. doi: 10.1093/jaoac/92.1.42 [DOI] [PubMed] [Google Scholar]
- Jewell K. A., McCormick C. A., Odt C. L., Weimer P. J., and Suen G.. . 2015. Ruminal bacterial community composition in dairy cows is dynamic over the course of multiple lactations and correlates with feed efficiency. Appl. Environ. Microbiol. 81:4697–4710. doi: 10.1128/AEM.00720-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F. G., Lin X. Y., Yan Z. G., Hu Z. Y., Liu G. M., Sun Y. D., Liu X. W., and Wang Z. H.. . 2017. Effect of dietary roughage level on chewing activity, ruminal pH, and saliva secretion in lactating Holstein cows. J. Dairy Sci. 100:2660–2671. doi: 10.3168/jds.2016-11559 [DOI] [PubMed] [Google Scholar]
- Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., and Schloss P. D.. . 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., and Guan L. L.. . 2017. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol. 83(9):e00061–17. doi. 10.1128/AEM.00061-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. C., Wiley L. M., Forbes T. D., Rouquette F. M. Jr, and Tedeschi L. O.. . 2014. Relationship between the rumen microbiome and residual feed intake-efficiency of Brahman bulls stocked on bermudagrass pastures. PLoS One. 9:e91864. doi: 10.1371/journal.pone.0091864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen J., Rinne M., and Huhtanen P.. . 2009. A meta-analysis of feed digestion in dairy cows. 1. The effects of forage and concentrate factors on total diet digestibility. J. Dairy Sci. 92:5019–5030. doi: 10.3168/jds.2008-1833 [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- O’Mara F. P., Coyle J. E., Drennan M. J., Young P., and Caffrey P. J.. . 1999. A comparison of digestibility of some concentrate feed ingredients in cattle and sheep. Anim. Feed Sci. Technol. 81:167–174. doi. 10.1016/S0377-8401(99)00082-6 [DOI] [Google Scholar]
- Peters T. M. 2014. Dairy beef management considerations: from conception to consumption. The Plains Nutrition Council, Amarillo, TX; p. 66–94. http://amarillo.tamu. edu/files/2010/10/2014-Proceedings-final.pdf [Accessed January 21, 2020]. [Google Scholar]
- Prigge E. C., Baker M. J., and Varga G. A.. . 1984. Comparative digestion, rumen fermentation and kinetics of forage diets by steers and wethers. J. Anim. Sci. 59:237–245. doi: 10.2527/jas1984.591237x [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2011. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. Available from http://www.R-project.org
- Richardson E. C., and Herd R. M.. . 2004. Biological basis for variation in residual feed intake in beef cattle. 2. Synthesis of results following divergent selection. Aust. J. Exp. Agr. 44:431–440. doi: 10.1071/EA02221 [DOI] [Google Scholar]
- Schaefer D. M., Chester-Jones H., and Boetel. B.. 2017. Beef production from the dairy herd. In: Beede, D. K., editor, Large dairy herd management. Vol. 1969 Champaign (IL): American Dairy Science Association; p. 143–164. [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J., . et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C. E. 2001. A mathematical theory of communication. Mob.Comput. Commun. Rev. 5:3–55. doi: 10.1145/584091.584093 [DOI] [Google Scholar]
- Solis J. C., Byers F. M., Schelling G. T., Long C. R., and Greene L. W.. . 1988. Maintenance requirements and energetic efficiency of cows of different breed types. J. Anim. Sci. 66:764–773. doi: 10.2527/jas1988.663764x [DOI] [PubMed] [Google Scholar]
- Tjardes K. E., Buskirk D. D., Allen M. S., Tempelman R. J., Bourquin L. D., and Rust S. R.. . 2002. Neutral detergent fiber concentration in corn silage influences dry matter intake, diet digestibility, and performance of Angus and Holstein steers. J. Anim. Sci. 80:841–846. doi: 10.2527/2002.803841x [DOI] [PubMed] [Google Scholar]
- Van Soest P. J. 1994. Function of the ruminant forestomach. In: Nutritional ecology of the ruminant. 2nd ed. Ithaca (NY): Cornell University Press; p. 230–252. [Google Scholar]
- Weimer P. J., Cox M. S., Vieira de Paula T., Lin M., Hall M. B., and Suen G.. . 2017. Transient changes in milk production efficiency and bacterial community composition resulting from near-total exchange of ruminal contents between high- and low-efficiency Holstein cows. J. Dairy Sci. 100:7165–7182. doi: 10.3168/jds.2017-12746 [DOI] [PubMed] [Google Scholar]
- Zhang L., Chung J., Jiang Q., Sun R., Zhang J., Zhongb Y., and Ren. N.. 2017. Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values. RSC Adv. 7:40303–40310. doi: 10.1039/c7ra06588d [DOI] [Google Scholar]
- Zinn S. A., Ivey S. L., Lalman D. L., Long N. M., and Zinn R. A.. . 2016. Beef Cattle Nutrition Symposium: feeding Holstein steers. J. Anim. Sci. 94:3135–3136. doi: 10.2527/jas.2016-0412 [DOI] [PubMed] [Google Scholar]