Abstract
Cardiovascular diseases, such as atherosclerosis, are the leading cause of death worldwide. Although mice are currently the most commonly used model for atherosclerosis, zebrafish are emerging as an alternative, especially for inflammatory and lipid metabolism studies. Here, we review the history of in vivo atherosclerosis models and highlight the potential for future studies on inflammatory responses in lipid deposits in zebrafish, based on known immune reactions in humans and mice, in anticipation of new zebrafish models with more advanced atherosclerotic plaques.
Keywords: atherosclerosis, animal models, mouse, zebrafish, immune response, human disease, APOE, LDLR
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of death worldwide (1). Research on atherosclerosis is of great clinical importance, because it increases the risk of CVDs.
Atherosclerosis is a chronic inflammatory disease that leads to myocardial infarction and cerebrovascular events. In addition to independent risk factors, such as blood pressure, hypertriglyceridemia, diet, and smoking, genetic disorders can have major pro-atherosclerotic effects. Atherosclerotic plaques develop when low density lipoproteins (LDL) accumulate in the sub-endothelial space of the arterial wall, where they are oxidized, leading to inflammatory responses (Figure 1). Then, dendritic cells, T-cells, and monocyte-derived macrophages are recruited to the affected area. Macrophages absorb oxidized LDL (oxLDL), which can accumulate and convert macrophages into foam cells, which in turn stimulate the proliferation and migration of vascular smooth muscle cells (VSMCs) into the developing plaque. As the plaque grows, its stability may change. Decreasing stability potentially leads to plaque rupture, which triggers a thrombosis cascade. The consequences of rupture include occluded arteries, ischemic events, myocardial infarction, stroke, and sudden death. Several animal models have been developed to further understanding of the pathomechanisms underlying CVD, especially atherosclerosis. The ideal disease model should develop various stages of atherosclerotic plaques and have human-like lipid metabolism and immune responses. In this review, we discuss rabbit, rat, mouse, and zebrafish, four of the most commonly used animal models for atherosclerosis. Primarily, we compared mice and zebrafish as models for human patients.
Figure 1.
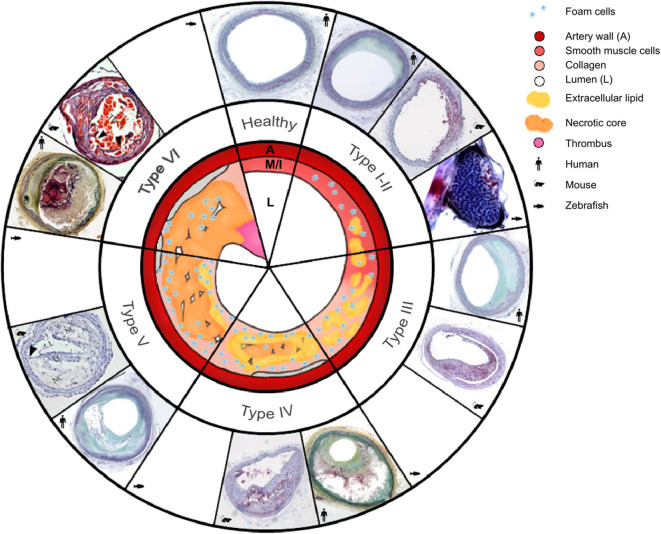
Morphology of the six types of atherosclerosis in human, mouse, and zebrafish: a schematic overview with a comparison of micrographs. Healthy arteries have an adventitia surrounding the media (M), a very thin intima (I) layer, and a large lumen (L). As atherosclerosis initiates and progresses, different types are classified as follows: Type I, intima thickening; type II, fatty streaks, with or without macrophages; type III, intermediate lesion; type IV, advanced atheroma; type V, fibroatheroma; type VI, complicated plaques with surface defects, leading to plaque rupture. The type VI mouse lesion image shows an unstable plaque in the right common carotid artery in the tandem stenosis mouse model, and it is used to represent future mouse plaque rupture models. Micrographs representing human atherosclerosis, are from Yahagi et al. (2); micrographs of ApoE−/− mouse type I–IV atherosclerosis in the brachiocephalic artery and zebrafish belong to the Institute for Cardiogenetics. A set of micrographs of mouse types V and VI were taken from Chen et al. (3) Permission was granted for the use of all micropgraphs.
Atherosclerosis in Human, Mouse, and Zebrafish
Pathogenesis of Atherosclerosis in Humans
Atherosclerosis pathogenesis comprises a complex interaction of hemodynamics, lipid metabolism, immune response and vascular injury response. Human atherosclerosis was classified into six fluent developmental stages, Type I-VI [Figure 1; (4)]. Lesions usually develop in regions of the arterial tree with local flow field disturbances leading to low shear stress, that induces endothelial cell (EC) activation and thereby the development of type I atherosclerosis (intimal thickening) (5, 6). Additionally, flow field changes contribute to accumulation of excess LDL in the sub-endothelial space of the arterial wall (LDL concentration polarization), where LDL is oxidized to oxLDL (5, 7). This reaction induces a T-helper type 1 (TH1)-driven inflammatory response, which stimulates expression of adhesion proteins, such as vascular cell adhesion molecule (VCAM)-1, E-selectin, P-selectin, and chemokines, including CCL2, CCL5, and CX3CL1 (8). One of the key players in plaque development is interferon-γ of TH1, which elevates chemokine secretion and upregulates adhesion molecule levels. Subsequently, dendritic cells, T-cells, and monocytes are recruited to the affected area in the arterial wall. Endothelial cells of normal and atherosclerotic arteries, as well as monocyte-derived macrophages, express various pattern recognition receptors (PRRs), including toll-like receptor (TLR) 1, TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 and inflammasomes [e.g., nucleotide oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing protein-3 (NLRP3)] (8, 9). The latter are damage-associated molecular pattern (DAMP)-activated intracellular innate immune signaling complexes, that activate pro-inflammatory transcription factors such as NF-κB and p53 (6, 10). Dendritic cells in healthy arterial walls silence T-cells, whereas in plaques, activation of danger signals, such as DAMPs [e.g., oxLDL, necrotic cell debris, cholesterol crystals; (11, 12)], promotes dendritic cell tolerance to T-cell antigens. Concurrently, monocytes differentiate into macrophages that absorb oxLDL through their scavenger receptors (SRs), such as CD36, MARCO, SRA-1 and -2, and SR-B1. SRs mediate the import of oxLDL via fluid phase uptake, CD36-dependent endocytosis, or micropinocytosis (13). Atherogenesis can be mediated by cholesterol crystals that directly and indirectly prime and activate macrophages via neutrophil extracellular traps (NETs) and NLRP3s (6, 14). When oxLDL accumulates as cytosolic droplets, it converts macrophages into foam cells (15). Newest in vitro findings, that are still under discussion, indicate that oxLDL activates NLRP3 inflammasomes and restricts autophagy, thereby reducing the inflammatory response. One explanation is, that low level oxLDL stimulation dampens the inflammatory response, whereas hyperlipidemia ultimately leads to chronic inflammation (12, 16). The transition from type I to type II atherosclerosis, the fatty streak, is very fluid; accordingly, we chose to merge these types together in Figure 1.
In type III atherosclerosis (intermediate lesions), extracellular lipid droplets are scattered throughout the intima. Foam cells present antigens [e.g., heat-shock protein 60 (Hsp60), interleukin-6 (IL-6) and IL-1ß] to immune cells, such as monocytes and T-cells, thereby stimulating the proliferation of VSMCs in the developing plaque (17). Eventually, extracellular lipids concentrate into a growing lipid core (type IV). Concurrently, apoptotic foam cell membranes stimulate endothelial cells to recruit additional monocytes, creating an inflammatory positive-feedback loop that leads to the formation of a necrotic core (type V) (6, 14). Additionally, migrating VSMCs contribute to the development of a fibrous cap. Lesion growth eventually restricts blood circulation and thereby increases blood pressure, which in turn can lead to hypertension and thrombus formation.
In type VI atherosclerosis, the complicated plaque, lesions grow further until the artery is sealed and blood flow is prevented, resulting in myocardial infarction. Low shear stress is not only an induction, but also a progression factor of atherogenesis, that reduces collagen fibers, increases the necrotic core and causes thinning of the fibrous cap. Taken together, this makes the fibrous cap more susceptible to tensile stress and can lead to rupture, which triggers a thrombosis cascade that occludes the artery and causes ischemic events, myocardial infarction, unstable angina, stroke, acute coronary syndrome, and sudden death (18).
Overview of Animal Models Over the Last 100 Years
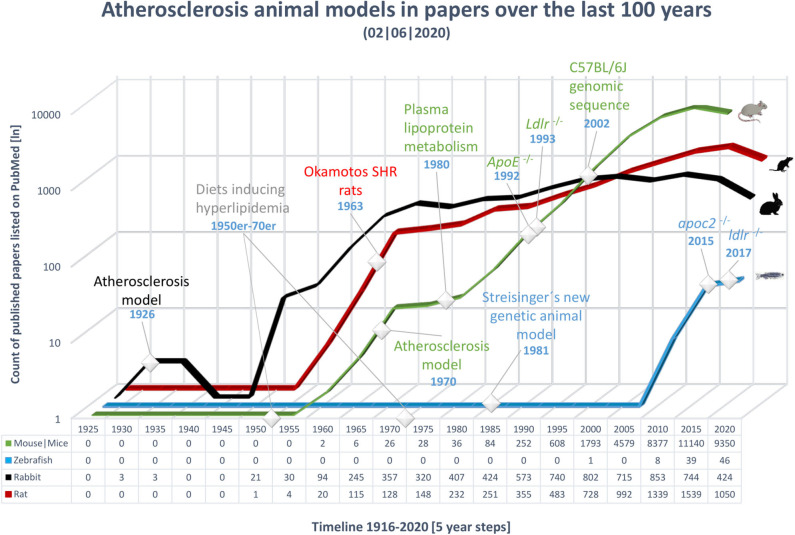
Over the last 100 years, many processes involved in the pathogenesis of atherosclerosis have been revealed; however, many aspects of this disease still require clarification. In 1908, Ignatowski discovered the potential of rabbits as an atherosclerosis model by describing the thickening of the intima accompanied by the formation of large cells in the aorta of rabbits fed an animal protein-enriched diet (19–21). In 1926, Clarkson and Newburgh were the first to publish on atherosclerosis using rabbits. They evaluated the effect of different diets varying in cholesterol and protein concentration and discovered that high-cholesterol diet (HCD) as well as high protein diet led to atherosclerosis and hypercholesterolemia (22). Further research on diet-induced modifications of arteries was performed from 1926 to 1935. After World War II ended in 1945, new animal models for CVD emerged; first the rat, later the mouse, and in the last 20 years, the zebrafish (Figure 2).
Figure 2.
Overview of publications over the last 100 years on the topic of atherosclerosis in various animal models. The x-axis shows time, from 1921 to 2018, in 5 year bins; the last time point includes only 3 years. The most important events in the history of atherosclerosis research have been marked. The y-axis shows the number of publications in PubMed, on a logarithmic scale; an exact count is shown below the timeline for each animal model. Results were gathered using the MeSH term atherosclerosis in combination with the model to include a wide range of publications. Black, rabbit; red, rat; green, mouse; blue, zebrafish.
From the 1950s to 1970s, various diets capable of inducing hyperlipidemia were developed and tested in rats and rabbits (21). Two examples of prominent diets used to experimentally induce atherosclerosis include the Paigen diet (PD) (15% fat, 1.25% cholesterol, and 0.5% cholic acid) and the Western-type diet (WTD) (21% fat by weight, 0.15% cholesterol, and no cholic acid) (21). Hence, investigations of diet-inducible atherosclerosis have made critical contributions to the understanding of the pathogenesis of this condition.
In the 1970s and 1980s, intensive investigations of atherosclerosis began in mice. The characterization of plasma lipoprotein metabolism in the 1980s, in combination with the emergence of transgenic technologies in the 1990s, led to the development of the transgenic knockout mouse lines: ApoE−/− in 1992 (23) and Ldlr−/− in 1993 (24). Further, the complete DNA sequence of the diet-sensitive mouse strain, C57BL/6, was published in 2002 (25).
In 1981, Streisinger et al. were the first to show that forward genetics can be performed in zebrafish (26). The first zebrafish study to mention atherosclerosis, in the context of loss of the C-terminal end of the mammalian lipoprotein lipase protein, was published in 1996 (27). Starting in 2007, the number of studies published has increased, including research that led to the first genetic model of hyperlipidemia, the apoc2−/− zebrafish mutant (28). Recently, Liu et al. reported the first Ldlr−/− zebrafish mutant, a genetic model of hypercholesterolemia (29). Lipid metabolism and lipid trafficking genes are conserved in zebrafish, making this mutant an important tool for atherosclerosis studies (30).
We observed that the focus on animal models for CVDs has shifted over the last 100 years: from rabbit and rat, to mouse, and, possibly soon, to zebrafish. As mice and zebrafish need less space than rabbits and rats, their husbandry is cheaper. Furthermore, they are easier to genetically manipulate. Therefore, in the following sections we critically evaluate the mouse and zebrafish as the most prominent animal models for atherosclerosis.
Mouse
Due to its low associated costs, easy maintenance, and breeding, extensive tools for genetic manipulation, and the ability to control its diet to rapidly induce atherosclerosis in various mutants, the mouse (Mus musculus) is a very useful model. Physiological similarities based on its phylogenetic relationship to humans, in addition to other factors mentioned below, make the mouse an important model for scientific experiments and the development of therapies (31). Therefore, we described the advantage and disadvantage of using genetically modified models in research and drug development.
Wild-type (WT) rodents are mostly resistant to atherosclerosis when fed a HCD (15); however, the development of inbred strains, such as C57BL/6J, C57L/J, and DBA/2J (32), has enabled atherosclerosis research in mice. Forward and reverse genetics play crucial roles in the development of atherosclerosis models. Knockouts, knockins, knockdowns, and transgenic modifications can be generated by various methods, including the CRISPR/Cas9 system, the Cre-loxP-system, Vivo-Morpholinos (MOs), lentiviral infection, and ethylnitrosourea (ENU) mutagenesis (33, 34). Genetic manipulation yielded the two most commonly used mouse models of atherosclerosis, namely Ldlr−/− and ApoE−/− (Table 1).
Table 1.
Phenotypes and traits of mouse and zebrafish atherosclerosis models.
| Model | Induction (age) | Lesion size | Lesion type | Plaque stability | Incidence | Long(L) /Short(S) term | Advantages | Limitations |
|---|---|---|---|---|---|---|---|---|
| MOUSE | ||||||||
| ApoE−/− (35–39) | CD (8–10 w)(3–4 m) | n.i. 3,157 ± 437 μm2 | n.i.Type II | Stable | Plaque 100% | S (4–5 w),L (3–4 m) | - Higher total cholesterol levels than Ldlr−/−
- Severe hypercholesterolemia - Diet hyper-responsive |
- Only homozygous mice display the phenotype - ApoE−/− impacts immune response - No xanthomatosis - Hypercholesterolemia is much more severe than the human phenotype - Occurring sudden deaths are unpredictable and differ greatly |
| WTD (8–10 w) | 9,200 ± 4,700 μm2 | Type V | Stable | Plaque 100% | S (4–5 w) | - Severe hypercholesterolemia - Lesions are consistent, pronounced, and more widespread |
||
| WTD (8–48 w) | 1,19,300 ± 17,800 μm2 1,75,400 ± 9,800 μm2 | Type VI | Unstable | Plaque 55.4% 78.8% | L (16 w)L (40 w) | - Provides a model for intervention studies for unstable plaques | ||
| ApoE−/−Fbn1C1039G+/− (40–44) | WTD (6–26 w) ♀ only | AAo 2,21,000 ± 35,000 μm2 DAo 17–49 × 103 ± 15–18 × 103 μm2 BCA 1,93,000 ± 29,000 μm2 | Type V | Stable | Coronary plaques & MI (<75%) | S (10 w) | - Disturbed cerebral blood flow (<73% of cases) - Brain hypoxia in 64% of cases, indicating stroke - Mimics pathology of aged vessels - Spontaneous plaque rupture - Intraplaque neovascularization and hemorrhage |
- Long-term WTD <70% sudden death in mice (increasing with longer WTD), whereas controls survived - Head tilt, disorientation, and motor disturbances (66% of cases) - Phenotypes vary strongly from study to study |
| CD (6–26 w) | AAo 7,62,000 ± 51,000 μm2 BCA 2,25,000 ± 10,000 μm2 | Type V | Stable | Plaque rupture, rarely | L (20 w) | |||
| WTD (6–26 w) | AAo 8,14,000 ± 60,000 μm2 BCA 4,56,000 ± 22,000 μm2 | Type VI | Unstable | Plaque rupture AAo 70% BCA 50% | ||||
| ApoE*3-Leiden (45–48) | WTD (10–34 w) ♀ only | 15,000 μm2 | Type IV | Stable | n.i. | L (24 w) | - Diet inducible hyperlipidemia | - Reported poor breeding in homozygotes - Great variation of plaque progression (no lesions-severe lesions) - No spontaneous plaque rupture |
| WTD (8–28 w) | Ø 5 × 104 μm2 | Type 0-V | Stable | Type 0, 30%;Type I–III, 65%;Type IV–V, 5% | L (19 w) | |||
| CD (8–24 w) ♀ vs. ♂ | ♀ Ø 1 × 104 μm2 ♂ Ø 1,000 μm2 | n.i. | Stable | n.i. | S-L (4–28 w) | |||
| WTD (8–24 w) ♀ vs. ♂ | ♀ Ø 12 × 104 μm2 ♂ Ø 500 μm2 | n.i. | Stable | n.i. | ||||
| ApoE*3-Leiden.CETP (46, 47) | WTD (8–28 w) | Ø 28 × 104 μm2 | Type I-V | Stable | Type I–III 30%; Type IV–V 70% | L (19 w) | - Lipoprotein cholesterol distribution is more human-like - VLDL cholesterol levels are very susceptible to dietary cholesterol levels - CETP is a pro-atherogenic factor in ApoE*3-Leiden mice - Diet inducible hyperlipidemia |
- Decreased SR-BI-mediated cholesterol efflux - Gender-dependent phenotype - No spontaneous plaque rupture |
| CD (8–24 w) ♀ vs. ♂ | ♀ Ø 1 × 105 μm2 ♂ Ø 7,500 μm2 | n.i. | Stable | n.i. | S/L (4–28 w) | |||
| WTD (8–24 w) ♀ vs. ♂ | ♀ Ø 18 × 104 μm2 ♂ Ø 3,000 μm2 | n.i. | Stable | n.i. | ||||
| Tandem stenosis in ApoE−/− (3) | HFD (6–12 w); 150 μm, 450 μm TS (12–23 w) | n.i. | Type V | Stable | Plaque 100%; 14% | L (8–17 w) | - Mimics type VI atherosclerosis - Models intraplaque hemorrhage (IH) |
- Surgery must be performed on each mouse |
| n.i. | Type VI | Unstable | IH (50.6%, 0%) Rupture (32%, 0%) | |||||
| AVV-PCSK9DY (49) | HFD (0.75% chol.) (60–144 day) | C57BL/6J 350 ± 30 μm2 ApoE−/− 710 ± 150 μm2 | n.i. | Stable | n.i. | L (8 w) | - Stable PCSK9DY mRNA expression - Persistent IDL/LDL hyperlipidemia - No long-term breeding needed to generate the model - Can be performed in different genetic backgrounds, but C57BL/6J seems to be most susceptible to PCSK9DY - Synergistic effect of ApoE−/− and PCSK9DY |
- Lesion development requires a longer time than in ApoE−/− - No spontaneous plaque rupture - Gender-dependent phenotype |
| 112 ± 27 μm2; n.i. | n.i. | Stable | n.i. | L (12 w) L (<1 year) | ||||
| Injected to Tie2-rtTA/TRE-Gpr124 (50) | WTD; (n.i.) | 7 × 103 μm2 | Type III | Stable | n.i. | L (16 w) | - Increased serumlevels of TC and LDL-C | |
| Ldlr−/− (24, 36) | CD | n.i. | Type I–II | Stable | n.i. | S (2 w), L (<1 year) | - Elevated plasma cholesterol - Diet hyper-responsive - Elevated apoB-100, apoB-48, and apoE levels |
- No Xanthomatosis - No spontaneous plaque rupture |
| PD | n.i. | Type V | Stable | n.i. | S (2 w), L (6–8 m) | - Xanthomatosis - Greatly increased apoB-100, apoB-48 and apoE levels |
||
| ApoE−/−Ldlr−/− (51) | CD (16–28 w) | Ø 11.7 × 105 μm2 | Type IV | Stable | n.i. | L (10 w) | - LCHP could cause inflammation-driven unstable plaque rupture | - No spontaneous plaque rupture with WTD |
| WTD (16–28 w) | Ø 15.2 × 105 μm2 | Type V | Stable | n.i. | ||||
| LCHP (16–28 w) | Ø 20.2 × 105 μm2 | Type V | (Stable) | n.i. | ||||
| Zebrafish | ||||||||
| WT (28, 52–54) | HCD, adults; larvae (30 dpf) | 100–500 μm (length) | Type II | Stable | Plaque 75% | S (5–14 day); L (<10 w) | - Hypercholesterolemia - Accelerated lipid oxidation - 2-week HCD leads to up to 70x increase of oxCE - Phenotype in adults and larvae |
- Only mimics beginning stages of atherosclerosis |
| apoc2−/− (28, 55) | ND; larvae (3–14 dpf) | n.i. | Type II | Stable | n.i. | S (11 day) | - Hypercholesterolemia - LCAT deficiency limits HDL cholesterol efflux capacity - Free cholesterol (FC)/CE ratio is increased as in LPL-def. patients |
- Impacts ISV and SIV growth in early developmental stages, which is corrected 14 dpf - Only mimics beginning stages of atherosclerosis |
| ldlr−/− (29) | No feeding (<5 dpf) | n.a. | n.a. | n.a. | n.a. | S (5 day) | - Hypercholesterolemia - Increased susceptibility to HCD |
- Only mimics beginning stages of atherosclerosis |
| ND, Larvae (4.5–9 dpf) | n.i. | Type II | Stable | n.i. | S (5 day) | - Moderate, sign. increase of vascular lipid deposits | ||
| HCD, Larvae (4.5–9 dpf) | n.i. | Type II | Stable | n.i. | S (5 day) | - Dramatic increase of vascular lipid deposits | ||
AAo, ascending aorta; DAo, descending aorta; BCA, brachiocephalic arteries; ISV, intersegmental vessel; SIV, subintestinal vessel; CD, Chow diet; HCD, high cholesterol diet; LCHP, low-carbohydrate, high protein; ND, Normal diet; PD, Paigen diet; WTD, Western-type diet; n.a., not applicable; n.i., no information.
The major apolipoprotein in chylomicrons, Apolipoprotein E (ApoE), can serve as a ligand for the LDL receptor (LDLR). Synthesis of ApoE occurs in the brain, liver, and other tissues in both humans and mice, although humans have high-density lipoprotein (HDL) subsets and ApoE isoforms that mice lack (56, 57). In addition to its effects on lipoprotein metabolism, ApoE has roles in macrophage biology, immune function, and adipose tissue biology (56, 58). Mutations in mouse ApoE result in human-like phenotypes, such as hyperlipoproteinemia type III, xanthomatosis, or dysbetalipoproteinemia (35). At the age of 3 months on a normal diet, ApoE-deficient mice show signs of type II fatty streaks (Figure 1). Type III diffusely intimal foam cell deposits in the aortic sinus can be observed at 5 months. Finally, at 8 months, the atherosclerotic plaques in ApoE−/− mice seal the artery almost completely. The relatively early development of lesions and the anthropomorphous lipid metabolism are major advantages of the mouse as an animal model (35). Yet, lesion rupture is usually only observed under severe stress (30).
Along with LDL, ApoE is a structural component of all lipoproteins and a critical ligand for hepatic clearance of plasma lipoproteins, and it is mediated by LDLR (59). Hepatic clearance may explain why mice are naturally resistant to atherosclerosis (36). Deficiency of LDLR, more common in humans than non-functional ApoE, leads to reduced uptake and clearance of lipoproteins, resulting in the predominance of LDL as a cholesterol carrier and to familial hypercholesterolemia, which in turn increases the risk of CVDs (59). The human-like lipid metabolism of Ldlr−/− mice enables the study of macrophages loaded with cholesterol creating xanthomas in the skin and subcutaneous tissue, as well as atherosclerotic lesions in arteries (36). In 2017 Emini Veseli et al. confirmed the plaque rupture in 20-week-old ApoE−/−Fbn1C1039G+/− and Il1r1−/−ApoE−/− mice, that mimicked human type VI atherosclerosis (17). Fragmentation of elastic fibers in the vessel wall, increases arterial stiffness and thereby leads to plaque rupture in 50% of the brachiocephalic arteries (40, 41). Inactivation of IL-1 signaling in atherosclerotic plaques decreased plaque collagen levels in Il1r1−/−ApoE−/− mice, subsequently destabilizing the lesion (60); however, 50% of the mice die suddenly after receiving a HCD for 16–23 weeks (40). Lack of natural type VI atherosclerosis (i.e., lesion rupture; Figure 1), remained a detractor of the mouse model for many years. This changed not only with development of the ApoE−/−Fbn1C1039G+/− and Il1r1−/−ApoE−/− but also the tandem stenosis (TS) mouse model (3), which can mimic various stages of human atherosclerosis, including type VI lesion rupture (Figure 1). Chen et al. demonstrated that treatment with atorvastatin increased collagen content, and they concluded that plaque stability increased, although they observed no difference in total plaque area. Earlier studies showed that statins, combined with ezetimibe, could reduce lesion size by 17%, and had anti-inflammatory effects in mice, although there was no effect on serum oxLDL (61).
As an alternative approach Roche-Molina et al. (49) and Bjørklund et al. (62), established the AAV-PCSK9DY model with adeno-associated virus (AAV) vectors serotype 8 resp. 9 carrying the human gain-of-function mutation Asp374Tyr in protein convertase subtilisin/kexin type 9 (PCSK9DY), that developed atherosclerosis and hyperlipidemia in the timespan of 84 day [Table 1; (49)]. Injection of PCSK9DY into 30 day-old mice resulted in stable PCSK9DY mRNA expression and persistent intermediate density lipoprotein (IDL)/LDL hyperlipidemia up to 1 year (49). Bjørklund et al. reported a lesion progression up to type V atherosclerosis (62). Since development of this approach it has been widely used, e.g., for highly efficient PCSK9-targeted genome editing via zinc-finger nuclease (ZFN) mRNAs, that were delivered to the liver by lipid nanoparticles (63); and to demonstrate the gender-dependent role of the EC-mineralocorticoid receptor (MR) for atherosclerosis and vascular inflammation, revealing the potential of EC-MR inhibitor therapy in male patients (64). Transexpression of human disease-causing genes in mice is a refined concept, that reduces time and number of animals required for the generation of mutants (65) and could provide a new effective and sustainable approach to study long-term atherosclerosis and hyperlipidemia (49) as well as to perform high-content experiments, in which it would reduce time and costs (62).
A particular disadvantage of genetically modified mouse models is that transport of cholesterol is mediated by HDL, rather than LDL (56), as lipid metabolism plays a crucial role in the development of lesions in the vascular wall. Recent publications report the use of very low density lipoprotein (VLDL) as the major plasma cholesterol carrier in ApoE−/− mutants, as well as having atheroprotective properties (59). Another point to consider is passenger gene effects, which can arise due to various types of incestuous pairings (66). Passenger gene effects do not influence the fitness of an individual but can lead to subtle changes in the genetic background. This situation arises when a donor strain is bred to a recipient strain that carries the mutation of interest. The F1 generation is then bred again with the recipient strain, and repetition of this process for ten generations leads to a statistically 99.8% pure strain; however, 0.2% contamination from the donor strain remains. Considering crossover during meiosis, a small contamination can lead to additional variation in features such as cellular composition, calcification, and lesion size in the newly-developed strain (67, 68).
Very few animal model studies lead to new therapies and drugs, as mice and humans differ not only in size, maturation, and metabolism per gram of tissue (and in nutrient requirements), but also in telomere length and microbiome (31). For example, mice with human inflammation-associated diseases, such as lupus, psoriasis, and rheumatoid arthritis, develop more atherosclerotic lesions than patients with these conditions. Thus, promotion of vascular inflammation by atherosclerosis is more likely to result from general inflammation than from shared risk genes (69, 70). Among 25,000 compounds investigated in labs, only 25 will be tested in humans, of which five will come to market (71), corresponding to a success rate of 0.02% for individual compounds. One rare example of success is the development of PCSK9 monoclonal antibodies (mAbs) (alirocumab and evolocumab) in mice, which contributes to proven therapy for high LDL cholesterol (LDLc) levels and atherosclerosis in humans (56). While these mAbs are approved vaccines, their short in vivo half-time makes them expensive and thereby frequent administration is required (72). In recent years, two further PCSK9-based drugs were investigated in a clinical trial (ClinicalTrials.gov NCT02508896), the phase I of which was already completed in August 2017, although no results have yet been published. In ApoE*3-Leiden.CETP mice immunization using this peptide-based vaccine elicits antibodies against PCSK9, that reduced lesion size, especially decreased necrotic core-formation and macrophage inflammation and lowered plasma lipid levels through humoral response (72, 73). This approach promises to be more cost effective and might offer a long-term LDLc management (72).
Zebrafish
The advantages of mice, such as easy care and breeding, and the ability to control the diet to induce atherosclerotic lesions, are shared by zebrafish (Danio rerio), making them another promising candidate animal model to study atherosclerosis.
The translucency of zebrafish larvae until about 30 days post-fertilization (dpf) permits observation of vascular development in vivo in real time (74, 75), which is augmented by the existence of transgenic lines such as fli1:eGFP and lyz:DsRed2 (52, 76). The zebrafish is especially fecund, with females laying 200–300 eggs/day (77); however, excessive inbreeding leads to infertility (78), impeding the development of purer inbred lines. Although the vascular system of the zebrafish is different from that of mammals, its development and anatomy are similar (79).
During embryonic and first larval stages, zebrafish must rely on their innate immune system, since the adaptive immune system is not yet mature. Both adaptive and innate immune systems are highly conserved between zebrafish and mammals. The dominant leucocyte in zebrafish is the neutrophil, which responds to H2O2 gradients in injured tissues, while eosinophils provide an important host defense against parasites. Furthermore, macrophages are the key phagocytic cells that regulate cytokine-mediated immunity (80). In addition, zebrafish are likely to have dendritic and other antigen-presenting cells (81).
Zebrafish are ideal models for large-scale screens; the early larval stages are only 2–3 mm long, they develop rapidly, and their results are representative for mammals (82). Various genetic tools, such as MOs, targeting-induced local lesions in genes (TILLING), RNA caging, transgenic reporter lines expressing fluorescent marker proteins (i.e., the UAS/GAL4-system), ENU mutagenesis, the CRISPR/Cas9 system, and transcription activator-like effector nuclease (TALEN), facilitate evaluation of gene functions in zebrafish (83, 84). Like mammals, zebrafish process lipid throughout the intestine and hepatobiliary system (79, 83). Thus, they are often used to study vascular lipid accumulation and lipoprotein oxidation. In contrast to mammals, zebrafish cannot synthesize their own vitamin C and must obtain it from their diet; consequently, vitamin C levels can be manipulated to trigger oxidative stress [Table 2; (79)].
Table 2.
Comparison of animal models for atherosclerosis.
| Mouse | Zebrafish | Rat (57, 85–87) | Rabbit (88–92) | ||
|---|---|---|---|---|---|
| Larvae | Adult | ||||
| ApoE−/− | ✓ | X | ✓ | ✓ | |
| Ldlr−/− | ✓ | ✓ | ✓ | ✓ | |
| Apoc2−/− | ✓ | ✓ | X | X | |
| lcat | ✓ | ✓ | ✓ | ✓ | |
| cetp | X | ✓ | X | ✓ | |
| Maturation | 2 months | 3 months | 2–3 months | 5–6 months | |
| Progeny | 5–8 | 200–300 | Ø10 | 4–12 | |
| Transparency | X | ✓ | X | X | X |
| Housing | Group (6–10) | Swarm (4–10/L) | Group (2–4) | Group (2–8) | |
| Dominating the lipoprotein profile | LDL | HDL | LDL | VLDL and LDL | |
| Cholesterol transport | HDL | HDL | HDL | HDL | |
| Vitamin C synthesis | ✓ | X | ✓ | ✓ | |
| Favored source of energy | Carbohydrates | Lipid | Carbohydrates | Carbohydrates | |
| Collection of blood samples | Non-lethal | None from individuals; lethal | Mostly lethal | Non-lethal | Non-lethal |
| Intima thickening | ✓ | X | ✓ | ✓ | ✓ |
| Lesions | Aorta and carotids | Caudal vein | Dorsal aorta | Aorta | Aorta and carotids |
| Lesion rupture | Rarely | X | X | Rarely | |
| Highest atherosclerotic classification | Type V to type VI rupture | Type II fatty streak | Type III | Type V to type VI rupture | |
| Oxygenated cholesteryl esters | In atherosclerotic lesions | In body liquids | In atherosclerotic lesions | In atherosclerotic lesions | |
In recent years zebrafish larvae, younger than 5 dpf, which are not considered animals until they start independent feeding according to the Directive 2010/63/EU of the European Parliament, have become a valuable animal model of drug discovery (93). Effects of administered compounds on vital developmental processes, such as vasculogenesis and organogenesis can be assessed in the whole organism during embryogenesis in a cost-effective and time-saving manner (93, 94). Today two approaches in terms of drug discovery are being pursued: Molecular target-based screens and high-throughput phenotypical screens. While performing target-based screens is fast and efficient, the risk that the compounds are eliminated downstream in the pipeline of preclinical and clinical trials is significantly higher than with phenotypic screens, as efficacy is lacking (95–97). Automated imaging systems and script-based evaluation tools improve the efficiency of phenotypic screens and thereby create a platform with enhanced data quality output, disease relevance, and reduced risk of elimination (95, 98).
Zebrafish animal models also have limitations. Collecting blood samples from juveniles from 40 to 45 dpf is possible, but lethal (52), and the larvae are so small that homogenates of several individuals are required for analysis. Another disadvantage of zebrafish is that poikilothermic vertebrates favor lipids as a source of energy, whereas carbohydrates are favored by homoeothermic mammals (52). Furthermore, mammals and fish differ not only in terms of favored energy source but also in their lipoprotein profile and LDL makeup. In zebrafish, their lipoprotein profile is dominated by HDL and their LDL contains more triglycerides and fewer cholesteryl esters (CEs) relative to human LDL (52). This is because zebrafish HDL functions as the cholesterol transporter, while in mammals it transports excess cholesterol from peripheral cells back to the liver, where it is recycled and excreted (99). Feeding a HCD to WT zebrafish causes their lipoprotein profiles to more closely resemble those of humans (74) and results in hypercholesterolemia, vascular lipid accumulation, and myeloid cell recruitment, among other symptoms (100). In both zebrafish and human, cholesteryl ester transfer proteins (CETPs) are involved in the transfer of CE from HDL to other lipoproteins (30, 101), which may explain why a HCD results in hypercholesterolemia in WT zebrafish.
All major classes of apolipoproteins (ApoA, ApoB, ApoC, and ApoE) are expressed in zebrafish and are highly similar to human apolipoproteins (79). As mentioned above, hypertriglyceridemia is an independent CVD risk factor caused by single mutations in the genes encoding lipoprotein lipase (LPL) or in those encoding LPL cofactors, such as APOC2 (28, 55). Liu et al. demonstrated that apoc2−/− zebrafish exhibit properties of human patients, including dyslipidemia, specifically hypertriglyceridemia as early as 14 dpf, which resulted in type II atherosclerosis. To examine whether vascular lipid deposits accumulated in macrophages, they crossed apoc2−/− with Tg (mpeg1-eGFP), that expresses eGFP in macrophages and emerged them in BODIPY to fluorescently stain neutral circulating lipoproteins and intracellular lipid droplets in vivo. They were able to show a co-localization of vascular lipid deposits with macrophages in apoc2−/− mpeg1-eGFP 14 dpf as well as IK17 co-localization in selected areas of the larval fatty streaks, which is observed in early atherosclerotic lesions in human and mice (28, 100). In concordance other studies demonstrated a significant increase of LDL concentrations at vascular bifurcations (LDL concentration polarization) in vivo in larvae 52 hpf after fluorescent DiI-LDL-injection 48 hpf (5) and after feeding larvae fluorescence-labeled HCD short term (10 day) and long term (<10 weeks) (53).
Recently, Liu et al. reported the first ldlr−/− zebrafish mutant, created using the CRISPR/Cas9 system, thus introducing a new model for atherosclerosis and hypercholesterolemia, that resembles the corresponding model in mice (24, 36). This mutant exhibits elevated susceptibility to HCDs and vascular lipid accumulation at 9 dpf, after 5 days of feeding a HCD (29).
Overall, studies conducted on zebrafish larvae may only be able to complement mammalian studies (102). Nevertheless, eight compounds discovered in zebrafish drug screens are being tested in clinical trials (93) and a promising approach showed that persistent expression of the human monoclonal antibody IK17 prevents vascular lipid accumulation and reduces existing lipid deposits in zebrafish (100). It is suspected that IK17 accumulates in vascular lesions and neutralizes oxidation-specific epitopes (74). Further, reduction in oxLDL, mediated by IK17, is predicted to decrease foam cell formation.
Atherogenic Immune Responses in Mice and Zebrafish
Very few studies have characterized the whole immune response involved in atherosclerosis in zebrafish, hence our comparisons of them with mice and humans are also based on non-CVD focused studies (Table 3).
Table 3.
Immune response factors in human atherosclerotic lesions.
| Human | Mouse | Zebrafish | Human | Mouse | Zebrafish | ||
|---|---|---|---|---|---|---|---|
| B-cells | ✓ | ✓ | ✓ | TLR7 | ✓ | ✓ | ✓ |
| T-cells | ✓ | ✓ | ✓ | TLR9 | ✓ | ✓ | ✓ |
| Granulocytes | ✓ | ✓ | ✓ | MyD88 | ✓ | ✓ | ✓ |
| Dendritic cells | ✓ | ✓ | ✓ | MARCO | ✓ | ✓ | ✓ |
| Macrophages | ✓ | ✓ | ✓ | CD36 | ✓ | ✓ | ✓ |
| E-selectin | ✓ | ✓ | ✓ | SRA-1 | ✓ | ✓ | ✓ |
| P-selectin | ✓ | ✓ | ✓ | SRA-2 | ✓ | ✓ | n. i. |
| VCAM-1 | ✓ | ✓ | Vcam1a/b | SR-B1 | ✓ | ✓ | ✓ |
| CCL2 | ✓ | ✓ | ✓ | MCP-1 | ✓ | ✓ | n. i. |
| CCL5 | ✓ | ✓ | ✓ | IL-1α/β | ✓ | ✓ | ✓ |
| CXCL10 | ✓ | ✓ | n. i. | IL-6 | ✓ | ✓ | ✓ |
| CX3CL1 | ✓ | ✓ | n. i. | IL-8 | ✓ | ✓ | ✓ |
| TLR1 | ✓ | ✓ | ✓ | IL-12 | ✓ | ✓ | Il-12a/b |
| TLR2 | ✓ | ✓ | ✓ | IL-17 | ✓ | ✓ | Il-17a/f2 |
| TLR3 | ✓ | ✓ | (Tlr22) | TGF-β | ✓ | ✓ | ✓ |
| TLR4 | ✓ | ✓ | Tlr4b.a/b | IFN-γ | ✓ | ✓ | Ifn-γ1-2 |
| TLR5 | ✓ | ✓ | Tlr5a/b |
In the early stages of atherosclerosis, TH1-induced adhesion molecules, such as P-selectin and E-selectin, are highly conserved from zebrafish to mammals and have important roles in the inflammatory response (109). Mammalian chemokines, such as CXCL11, have homologs in zebrafish (CXC-64), although initial functional analyses revealed differences in their release from monocytes following stimulation by bacteria (103). The TLR family is conserved from insects to mammals; however, their signaling pathways can differ. Li et al. analyzed the PRRs and their corresponding homologs in mammals and zebrafish, and found that some TLRs have two counterparts in zebrafish, due to gene duplication events during evolution (104). They identified the TLR22 of zebrafish as a homolog of mammalian TLR3. Although mice lack TLR10, they have three additional TLRs: TLR11, TLR12, and TLR13 (110). TLR4 is required for efficient lipid uptake by macrophages in humans, mice, and zebrafish (52). Myeloid differentiation primary response 88 (MyD88) is a key pro-atherosclerotic adaptor protein in signaling cascades mediated by IL-1R and various TLRs (105).
The phylogenetically-conserved autoantigens, Hsp60 and LDL, stand out as important promoters of atherosclerosis. Hsp60 functions as a chaperone at the cell surface, folding freshly-synthesized proteins, and protecting them under stress conditions such as heat shock. In some cases, Hsp60 can trigger adaptive and innate immune responses in vivo (111). Studies of Hsps in mice lead to preclinical trials of DiaPep277, a vaccine, that had the potential to change Hsp-mediated immune regulation and was intended to treat type-I diabetes (112), but was terminated due to scientific misconduct (113).
In mice, immunization against oxLDL attenuates atherosclerosis. Phospholipids are ubiquitous molecules that form integral parts of cell membranes and lipoproteins such as LDL. SRs of macrophages, such as CD36, TLR2, and TLR4, take up oxidized phospholipids (oxPLs) and oxLDL, thereby promoting inflammation because they are recognized by the innate immune system. Knockout of SRs that recognize oxPLs leads to abatement of atherosclerosis in both models. oxPLs are found in all types of atherosclerotic lesions, indicating their contribution to atherogenesis (114). Accessory proteins found in humans are also present in zebrafish, indicating similarities in the activation and folding of zebrafish TLRs. The SR accessory protein, CD36, fine-tunes TLR assembly, especially in response to TLR2/6 ligands (106), and drives atherosclerosis through the aforementioned interaction with oxLDL (107, 115). It was shown, that while highly conserved, alternative splicing may not occur and differences in C-terminal amino acids of Cd36 may alter the function of the protein to being a co-receptor in contrast to mammalian CD36 (107).
Discussion
In this review, we compared the most important aspects of mouse and zebrafish models for human atherosclerosis. In the last decade, models mimicking human atherogenesis, different diets, and tools for forward and reverse genetics were also developed, accelerating, and increasing the efficiency of research progress.
Currently, mice are the most commonly used atherosclerosis model because mutant strains are susceptible to dietary interventions, and plaques progress to type V lesions in the aorta and carotids (35). Nevertheless, the development of late-stage lesions takes months, even when mice are fed a HCD and rupture is usually only observed under severe stress (30). Since mouse immune responses and pathways are similar to those of humans, they are a good model for studying atherosclerosis-related inflammatory responses, whereas the HDL-based lipid metabolism and the lack of CETP in mice are clear disadvantages, that are only abolished in ApoE*3-Leiden.CETP (Table 1). Increased use of the relatively new PCSK9DY mouse model negates the need to generate inbred strains. One recent example is the use of PCSK9DY-injections into mice expressing GPR124 under control of the Tie-2 promoter. Gong et al. found that GPR124, a receptor known to increase angiogenesis in the brain, influences the pathogenesis of atherosclerosis by activating nitrosative stress and NLRP3 inflammasome signaling (50). Nevertheless, while PCSK9DY itself is suitable to create atherosclerosis models user errors and gender-dependent susceptibility to PCSK9DY could lead to great variations of study outcomes between laboratories (64, 65).
Zebrafish have recently emerged as models for atherosclerosis and are also susceptible to diet, and they have the added advantage of their amenability to high-throughput screenings. The adaptive and innate immune systems are highly conserved between zebrafish and mammals. Use of transgenic zebrafish lines that can track macrophages and neutrophils in vivo has recently provided new insights into the early development of atherosclerosis. Chronological in vivo imaging over a 10-day period revealed, that neutrophils rather than macrophages may accumulate in the sub-endothelial space before LDL concentration polarization (53). These results illustrate the importance of appropriate in vivo models for studying the pathophysiology of complex diseases such as atherosclerosis.
Similarities with humans in lipid metabolism and expression of CETP, along with the development of the ldlr−/− and apoc2−/− mutant, demonstrate other advantages of this new model. Furthermore, the data from the apoc2−/− mutant reported by Liu et al. suggests, that zebrafish suitably and even more favorably than mice, model human dyslipidemia, a major risk factor for atherosclerosis (28, 116, 117). While mutant mice can exhibit atheroprotective properties, which can bias experimental results, passenger gene effects may not affect atherogenic zebrafish models because WT animals are already affected by HCD, negating the for inbred strains.
With advancing technology, zebrafish could also be treated with a TS approach to test whether the phenotype of atherosclerotic plaques will also be enhanced in this model. Haemodynamic forces in the arterial wall of zebrafish can be measured using transgenic lines, BODIPY, imaging systems and analysis tools such as ZebraLab (5, 28). The recent creation of the ldlr−/− zebrafish mutant will enhance the importance of zebrafish as an animal model for atherosclerosis. ldlr−/− mutants on a regular diet develop moderate hypercholesterolemia, whereas even short-term consumption of a HCD results in hypercholesterolemia and aggravated accumulation of lipids in the vascular wall. Therefore, ldlr−/− as well as apoc2−/− zebrafish could enable high-content screens for novel therapeutics and drug repositioning for atherosclerosis. Research on zebrafish atherosclerosis is heavily leaning on the lipid metabolism as the lesions are very small, due to the models size and disease progression limitations. On one hand, this could be a drawback for the model depending on the hypothesis, but on the other hand its easy genetic manipulation and opacity offers an opportunity to study early atherosclerosis pathomechanisms using e.g., transgenic lines, BODIPY, or injections with fluorescent dyes, into the bloodstream of larvae 48 dpf.
Considering the very low costs associated with a model that can be used at just 9 dpf, even laboratories with low budgets could drive research on atherosclerosis forward using zebrafish. The development of ApoE−/− zebrafish would create a new tool for atherosclerosis research that could be compared with mouse models. To date, there is no mouse model with consistent plaque instability, while the ApoE−/− mouse mutant appears to be key to the development of a transgenic model that completely mirrors human atherosclerosis. Feeding ApoE−/− mice a HCD is sufficient for them to exhibit complex and multi-layered lesions, but loss of ApoE makes this model much more complex, as it's an integral part of the immune response and lipid metabolism (118). Nevertheless, the mortality rate of this mouse line varies greatly for the same diet and length of the experiment (Table 1). While Van der Donckt et al. reported no dead animals, which is consistent with our own experience, Stöhr et al. published a 20% mortality and Johnson et al. a mortality rate of 70% after 10 months on WTD (37, 38, 40). Other reports of WTD for shorter periods show the same variability in mortality rates (42, 43). It is unclear whether this variation is a consequence of the ApoE KO or husbandry differences.
Studies of the atheroprotective characteristics of oxLDL antibodies, which were demonstrated in mice and rabbits, could also be performed in zebrafish, followed by immunization studies (53, 119). Furthermore, functional analysis of chemokines in growing plaques and regulation of their promoters in zebrafish could further drive atherosclerosis research.
The use of new imaging systems and tools, such as magnetic resonance imaging (MRI), Ultrasound biomicroscopy (UBM) and improved contrast enhancers for computerized tomography (CT) will provide more reliable information about plaque size and position in vivo. Currently gold nanoparticles (AuNP) are most commonly used as contrast agent for CTs, as they can attenuate X-rays, are rarely toxic for humans and can target specific tissues when combined with fluorescent dyes and surface molecules (120). Several toxicity studies in zebrafish embryos revealed size- and chemical-dependent biocompatibility and toxicity (121), resulting in small, under-pigmented eyes, neurological defects, and abnormal behavior activity (122–124). Studies in mice showed, that the use of AuNP, such as AuroVist™ can visualize macrophage infiltration in vascular bifurcations in mice in vivo. However, scans have to be performed pre- and post-contrast injection, which increases radiation time for each animal and thus may increase the mortality rate if an animal has to be examined several times within a study. Additionally animals must be fixed in place to reduce repositioning effects, that impact readout of the scans (125, 126). Taken together, a contrast agent for CT is needed that reduces the burden on animals to enable a time-lapse recording of plaque progression in vivo with reduced influence on the survival rate and pathogenesis for a reasonable price. Another imaging technique that has been used for decades in humans and mice is MRI. It enables differentiation of plaque components based on their biophysical and biochemical composition without radiation in vivo (127). In recent years new techniques have been developed, that not only increased the resolution, but enable MRI of adult zebrafish as well (128). Repeated MRIs with and without use of non-toxic, gadolinium-based contrast agents, were performed without side-effects in mice and zebrafish (128–131). Altogether, MRI is a well-established imaging technique, that provides in depth information for several animal models as well as for humans, but interpretation of the data still needs a lot of experience (127) and the images are static and cannot display hemodynamics. Another imaging technique, that allows to calculate blood flow based on Doppler blood velocity and vessel diameter as well as detection of atherosclerotic plaques is the multifrequency UBM combined with Doppler ultrasound (132). Readout quality from UBMs is frequently increasing, enabling reliable diagnosis of small animals with higher heart rates and smaller vessel sizes (133–136). Nevertheless, interpretation of the acquired data requires even more experience. To date, the best imaging techniques with the highest resolution, such as microCT and light-sheet microscopy, are still lethal or only applicable to one model (137–139). While in vivo imaging of mice can be achieved by adapting human imaging technologies and vice versa, imaging of zebrafish larvae can be achieved with simple microscopes. Automated microscopes enable high-throughput imaging of the small fish with high quality data output. Therefore, zebrafish are more commonly used for drug discovery. However, they also have limitations, that do not apply to murine models. Drug screening in zebrafish only succeeds if the compounds to be tested are predominantly water-soluble or are injected into the fish. Additionally, in-water dosing could yield unpredictable exposures (93). Furthermore, protocols to conduct high-content screens in zebrafish are very diverse, therefore results in different laboratories may vary. Taken together, zebrafish are a good model for primary drug screens, provided that all limitations have been considered. Moreover, primary screens in zebrafish reduce the number of mice needed for secondary drug screening.
Overall, mice will continue to be pioneers to study atherosclerosis pathogenesis until the majority of the mechanisms underlying atherosclerosis in zebrafish have been elucidated and mutant animals have been established. Zebrafish have clear advantages in studying the influence of lipid metabolism on atherosclerosis. Therefore, the model should be chosen according to the hypothesis and a combination of mouse and zebrafish experiments should be considered for in depth studies. Additionally, it is important to find an in vivo imaging method that meets the challenges of different models and at the same time creates a platform that delivers consistent, reliable, and above all comparable results, regardless of the model chosen.
Author Contributions
ZA and VV contributed conception and design of the review. VV collected data, organized the database, and wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Anna Schmidt and Karim Tarhbalouti for providing their micrographs and Loreto Muñoz Venegaz (https://orcid.org/0000-0003-2308-4158) for proofreading.
Footnotes
Funding. This review was supported by the DZHK (German Centre for Cardiovascular Research). This work was supported by the Institute for Cardiogenetics and the University of Osnabrück.
References
- 1.World Health Organization Cardiovascular Diseases (CVDs). World Health Organization (2017). Available online at: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed November 27, 2017).
- 2.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. (2016) 13:79–98. 10.1038/nrcardio.2015.164 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y-C, Bui AV, Diesch J, Manasseh R, Hausding C, Rivera J, et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res. (2013) 113:252–65. 10.1161/CIRCRESAHA.113.301562 [DOI] [PubMed] [Google Scholar]
- 4.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. (1995) 15:1512–31. 10.1161/01.ATV.15.9.1512 [DOI] [PubMed] [Google Scholar]
- 5.Xie X, Tan J, Wei D, Lei D, Yin T, Huang J, et al. In vitro and in vivo investigations on the effects of low-density lipoprotein concentration polarization and haemodynamics on atherosclerotic localization in rabbit and zebrafish. J R Soc Interface. (2013) 10:20121053. 10.1098/rsif.2012.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf MP, Hunziker P. Atherosclerosis: insights into vascular pathobiology and outlook to novel treatments. J Cardiovasc Transl Res. (2020). 10.1007/s12265-020-09961-y. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. (2019) 2019:1–32. 10.1155/2019/8563845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. (2011) 12:204–12. 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 9.O Krogmann A, Lüsebrink E, Lahrmann C, Flender A, Nickenig G, Zimmer S. Toll-like receptor 7 stimulation promotes the development of atherosclerosis in apolipoprotein E-deficient mice. Int Heart J. (2020) 61:364–72. 10.1536/ihj.19-365 [DOI] [PubMed] [Google Scholar]
- 10.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. (2012) 249:158–75. 10.1111/j.1600-065X.2012.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-α. Circulation. (2006) 114:2482–9. 10.1161/CIRCULATIONAHA.106.642801 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Zhu X, Yin R, Liu T, Yang S, Zhou L, et al. K63 ubiquitin chains target NLRP3 inflammasome for autophagic degradation in ox-LDL-stimulated THP-1 macrophages. Aging. (2020) 12:1747–59. 10.18632/aging.102710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller YI, Choi S-H, Fang L, Harkewicz R. Toll-like receptor-4 and lipoprotein accumulation in macrophages. Trends Cardiovasc Med. (2009) 19:227–32. 10.1016/j.tcm.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. (2019) 39:1724–38. 10.1161/ATVBAHA.119.312463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 16.Miller YI, Shyy JYJ. Context-dependent role of oxidized lipids and lipoproteins in inflammation. Trends Endocrinol Metab. (2017) 28:143–52. 10.1016/j.tem.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, et al. Animal models of atherosclerosis. Eur J Pharmacol. (2017) 816:3–13. 10.1016/j.ejphar.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 18.Moore KJ, Tabas I. The cellular biology of macrophages in atherosclerosis. Cell. (2011) 145:341–55. 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignatowski AC. Influence of animal food on the organism of rabbits. Izv Imp Voyenno-Med Akad Peter. (1908) 16:154–73. [Google Scholar]
- 20.Leong X-F, Ng C-Y, Jaarin K. Animal models in cardiovascular research: hypertension and atherosclerosis. BioMed Res Int. (2015) 2015:528757. 10.1155/2015/528757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jawien J, Nastałek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. (2004) 55:503–17. [PubMed] [Google Scholar]
- 22.Clarkson S, Newburgh LH. The relation between atherosclerosis and ingested cholesterol in the rabbit. J Exp Med. (1926) 43:595–612. 10.1084/jem.43.5.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. (1992) 89:4471–5. 10.1073/pnas.89.10.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. (1993) 92:883–93. 10.1172/JCI116663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouse Genome Sequencing Consortium. Waterston RH, Lindblad-Toh K, Birney E. Initial sequencing and comparative analysis of the mouse genome. Nature. (2002) 420:520–62. 10.1038/nature01262 [DOI] [PubMed] [Google Scholar]
- 26.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebrafish (Brachydanio rerio). Nature. (1981) 291:293–6. 10.1038/291293a0 [DOI] [PubMed] [Google Scholar]
- 27.Arnault F, Etienne J, Noé L, Raisonnier A, Brault D, Harney JW, et al. Human lipoprotein lipase last exon is not translated, in contrast to lower vertebrates. J Mol Evol. (1996) 43:109–15. 10.1007/BF02337355 [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Gates KP, Fang L, Amar MJ, Schneider DA, Geng H, et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis Model Mech. (2015) 8:989–98. 10.1242/dmm.019836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Kim YS, Kim J, Pattison J, Kamaid A, Miller YI. Modeling hypercholesterolemia and vascular lipid accumulation in LDL receptor mutant zebrafish. J Lipid Res. (2017) 59:391–9. 10.1194/jlr.D081521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlegel A. Zebrafish models for dyslipidemia and atherosclerosis research. Front Endocrinol. (2016) 7:159. 10.3389/fendo.2016.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlman RL. Mouse models of human disease: an evolutionary perspective. Evol Med Public Health. (2016) 2016:170–6. 10.1093/emph/eow014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. (1985) 57:65–73. 10.1016/0021-9150(85)90138-8 [DOI] [PubMed] [Google Scholar]
- 33.Svenson KL, Ahituv N, Durgin RS, Savage H, Magnani PA, Foreman O, et al. A new mouse mutant for the LDL receptor identified using ENU mutagenesis. J Lipid Res. (2008) 49:2452–62. 10.1194/jlr.M800303-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miano JM, Zhu QM, Lowenstein CJ. A crispr path to engineering new genetic mouse models for cardiovascular research. Arterioscler Thromb Vasc Biol. (2016) 36:1058–75. 10.1161/ATVBAHA.116.304790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. (1992) 258:468–71. 10.1126/science.1411543 [DOI] [PubMed] [Google Scholar]
- 36.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. (1994) 93:1885–93. 10.1172/JCI117179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stöhr R, Kappel BA, Carnevale D, Cavalera M, Mavilio M, Arisi I, et al. TIMP3 interplays with apelin to regulate cardiovascular metabolism in hypercholesterolemic mice. Mol Metab. (2015) 4:741–52. 10.1016/j.molmet.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson J, Carson K, Williams H, Karanam S, Newby A, Angelini G, et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. (2005) 111:1422–30. 10.1161/01.CIR.0000158435.98035.8D [DOI] [PubMed] [Google Scholar]
- 39.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. (1992) 71:343–53. 10.1016/0092-8674(92)90362-G [DOI] [PubMed] [Google Scholar]
- 40.Van der Donckt C, Van Herck JL, Schrijvers DM, Vanhoutte G, Verhoye M, Blockx I, et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J. (2015) 36:1049–58. 10.1093/eurheartj/ehu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Herck JL, De Meyer GRY, Martinet W, Van Hove CE, Foubert K, Theunis MH, et al. Impaired fibrillin-1 function promotes features of plaque instability in apolipoprotein E–deficient mice. Circulation. (2009) 120:2478–87. 10.1161/CIRCULATIONAHA.109.872663 [DOI] [PubMed] [Google Scholar]
- 42.Roth L, Van Dam D, Van der Donckt C, Schrijvers DM, Lemmens K, Van Brussel I, et al. Impaired gait pattern as a sensitive tool to assess hypoxic brain damage in a novel mouse model of atherosclerotic plaque rupture. Physiol Behav. (2015) 139:397–402. 10.1016/j.physbeh.2014.11.047 [DOI] [PubMed] [Google Scholar]
- 43.Roth L, Van der Donckt C, Emini Veseli B, Van Dam D, De Deyn PP, Martinet W, et al. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability and reduces myocardial infarction in mice. Vascul Pharmacol. (2019) 118–119:106561. 10.1016/j.vph.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 44.Van der Veken B, De Meyer GRY, Martinet W. Axitinib attenuates intraplaque angiogenesis, haemorrhages and plaque destabilization in mice. Vascul Pharmacol. (2018) 100:34–40. 10.1016/j.vph.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 45.Wong MC, van Diepen JA, Hu L, Guigas B, de Boer HC, van Puijvelde GH, et al. Hepatocyte-specific IKKβ expression aggravates atherosclerosis development in APOE*3-Leiden mice. Atherosclerosis. (2012) 220:362–8. 10.1016/j.atherosclerosis.2011.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerterp M, van der Hoogt CC, de Haan W, Offerman EH, Dallinga-Thie GM, Jukema JW, et al. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden Mice. Arterioscler Thromb Vasc Biol. (2006) 26:2552–9. 10.1161/01.ATV.0000243925.65265.3c [DOI] [PubMed] [Google Scholar]
- 47.Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, et al. Genetic architecture of atherosclerosis in mice: a systems genetics analysis of common inbred strains. PLOS Genet. (2015) 11:e1005711. 10.1371/journal.pgen.1005711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Maagdenberg AM, Hofker MH, Krimpenfort PJ, de Bruijn I, van Vlijmen B, van der Boom H, et al. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J Biol Chem. (1993) 268:10540–5. [PubMed] [Google Scholar]
- 49.Roche-Molina M, Sanz-Rosa D, Cruz FM, García-Prieto J, López S, Abia R, et al. Induction of sustained hypercholesterolemia by single adeno-associated virus–mediated gene transfer of mutant hPCSK9. Arterioscler Thromb Vasc Biol. (2015) 35:50–9. 10.1161/ATVBAHA.114.303617 [DOI] [PubMed] [Google Scholar]
- 50.Gong D-M, Zhang Y-L, Chen D-Y, Hong L-J, Han F, Liu Q-B, et al. Endothelial GPR124 exaggerates the pathogenesis of atherosclerosis by activating inflammation. Cell Physiol Biochem. (2018) 45:547–57. 10.1159/000487032 [DOI] [PubMed] [Google Scholar]
- 51.Kostogrys RB, Franczyk-Zarów M, Maślak E, Gajda M, Mateuszuk Ł, Jackson CL, et al. Low carbohydrate, high protein diet promotes atherosclerosis in apolipoprotein E/low-density lipoprotein receptor double knockout mice (apoE/LDLR–/–). Atherosclerosis. (2012) 223:327–31. 10.1016/j.atherosclerosis.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 52.Stoletov K, Fang L, Choi S-H, Hartvigsen K, Hansen LF, Hall C, et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res. (2009) 104:952–60. 10.1161/CIRCRESAHA.108.189803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo H, Li Q-Q, Wu N, Shen Y-G, Liao W-T, Yang Y, et al. Chronological in vivo imaging reveals endothelial inflammation prior to neutrophils accumulation and lipid deposition in HCD-fed zebrafish. Atherosclerosis. (2019) 290:125–35. 10.1016/j.atherosclerosis.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 54.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi S-H, Almazan F, et al. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J Biol Chem. (2010) 285:32343–51. 10.1074/jbc.M110.137257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Gaudet D, Miller YI. Deficient cholesterol esterification in plasma of apoc2 knockout zebrafish and familial chylomicronemia patients. PLoS ONE. (2017) 12:e0169939. 10.1371/journal.pone.0169939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daugherty A, Tall AR, Daemen MJAP, Falk E, Fisher EA, García-Cardeña G, et al. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Circ Res. (2017) 121:e53–79. 10.1161/RES.0000000000000169 [DOI] [PubMed] [Google Scholar]
- 57.Gao M, Xin G, Qiu X, Wang Y, Liu G. Establishment of a rat model with diet-induced coronary atherosclerosis. J Biomed Res. (2017) 31:47–55. 10.7555/JBR.31.20160020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martínez-Martínez AB, Torres-Perez E, Devanney N, Del Moral R, Johnson LA, Arbones-Mainar JM. Beyond the CNS: the many peripheral roles of APOE. Neurobiol Dis. (2020) 138:104809. 10.1016/j.nbd.2020.104809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee YT, Lin HY, Chan YWF, Li KHC, To OTL, Yan BP, et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. (2017) 16:12. 10.1186/s12944-016-0402-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. (2012) 122:70–9. 10.1172/JCI43713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tie C, Gao K, Zhang N, Zhang S, Shen J, Xie X, et al. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS ONE. (2015) 10:e0142430. 10.1371/journal.pone.0142430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjørklund MM, Hollensen AK, Hagensen MK, Dagnæs-Hansen F, Christoffersen C, Mikkelsen JG, et al. Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res. (2014) 114:1684–9. 10.1161/CIRCRESAHA.114.302937 [DOI] [PubMed] [Google Scholar]
- 63.Conway A, Mendel M, Kim K, McGovern K, Boyko A, Zhang L, et al. Non-viral delivery of zinc finger nuclease mRNA enables highly efficient in vivo genome editing of multiple therapeutic gene targets. Mol Ther J Am Soc Gene Ther. (2019) 27:866–77. 10.1016/j.ymthe.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moss ME, Lu Q, Iyer SL, Engelbertsen D, Marzolla V, Caprio M, et al. Endothelial mineralocorticoid receptors contribute to vascular inflammation in atherosclerosis in a sex-specific manner. Arterioscler Thromb Vasc Biol. (2019) 39:1588–601. 10.1161/ATVBAHA.119.312954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarrett KE, Lee C, De Giorgi M, Hurley A, Gillard BK, Doerfler AM, et al. Somatic editing of Ldlr with adeno-associated viral-CRISPR is an efficient tool for atherosclerosis research. Arterioscler Thromb Vasc Biol. (2018) 38:1997–2006. 10.1161/ATVBAHA.118.311221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, et al. Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metab. (2017) 25:248–61. 10.1016/j.cmet.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackert-Bicknell CL, Rosen CJ. Passenger gene mutations: unwanted guests in genetically modified mice. J Bone Miner Res. (2016) 31:270–3. 10.1002/jbmr.2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allayee H. Using mice to dissect genetic factors in atherosclerosis. Arterioscler Thromb Vasc Biol. (2003) 23:1501–9. 10.1161/01.ATV.0000090886.40027.DC [DOI] [PubMed] [Google Scholar]
- 69.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. (2008) 121(10 Suppl. 1):S9–14. 10.1016/j.amjmed.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. (2014) 34:2658–68. 10.1161/ATVBAHA.114.304108 [DOI] [PubMed] [Google Scholar]
- 71.Torjesen I. Drug development: the journey of a medicine from lab to shelf. The Pharmaceutical Journal. (2015). Available online at: http://www.pharmaceutical-journal.com/publications/tomorrows-pharmacist/drug-development-the-journey-of-a-medicine-from-lab-to-shelf/20068196.article (accessed November 13, 2017).
- 72.Landlinger C, Pouwer MG, Juno C, van der Hoorn JWA, Pieterman EJ, Jukema JW, et al. The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice. Eur Heart J. (2017) 38:2499–507. 10.1093/eurheartj/ehx260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy P, Ali AJ, Kobiyama K, Ghosheh Y, Ley K. Opportunities for an atherosclerosis vaccine: from mice to humans. Vaccine. (2020) 38:4495–506. 10.1016/j.vaccine.2019.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang L, Miller YI. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic Biol Med. (2012) 53:1411–20. 10.1016/j.freeradbiomed.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkinson RN, van Eeden FJM. The zebrafish as a model of vascular development and disease. Prog Mol Biol Transl Sci. (2014) 124:93–122. 10.1016/B978-0-12-386930-2.00005-7 [DOI] [PubMed] [Google Scholar]
- 76.Asnani A, Peterson RT. The zebrafish as a tool to identify novel therapies for human cardiovascular disease. Dis Model Mech. (2014) 7:763–7. 10.1242/dmm.016170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. (2012) 122:2337–43. 10.1172/JCI60434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. (2004) 20:367–79. 10.1016/S1074-7613(04)00084-6 [DOI] [PubMed] [Google Scholar]
- 79.Fang L, Liu C, Miller YI. Zebrafish models of dyslipidemia: relevance to atherosclerosis and angiogenesis. Transl Res J Lab Clin Med. (2014) 163:99–108. 10.1016/j.trsl.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Renshaw SA, Trede NS. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech. (2012) 5:38–47. 10.1242/dmm.007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lugo-Villarino G, Balla KM, Stachura DL, Bañuelos K, Werneck MBF, Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci USA. (2010) 107:15850–5. 10.1073/pnas.1000494107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson RT, Macrae CA. Systematic approaches to toxicology in the zebrafish. Annu Rev Pharmacol Toxicol. (2012) 52:433–53. 10.1146/annurev-pharmtox-010611-134751 [DOI] [PubMed] [Google Scholar]
- 83.Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. (2001) 2:956–66. 10.1038/35103567 [DOI] [PubMed] [Google Scholar]
- 84.Hwang WY, Peterson RT, Yeh JRJ. Methods for targeted mutagenesis in zebrafish using TALENs. Methods San Diego Calif. (2014) 69:76–84. 10.1016/j.ymeth.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang HY, Quan C, Hu C, Xie B, Du Y, Chen L, et al. A lipidomics study reveals hepatic lipid signatures associating with deficiency of the LDL receptor in a rat model. Biol Open. (2016) 5:979–86. 10.1242/bio.019802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ou L, Li X, Chen B, Ge Z, Zhang J, Zhang Y, et al. Recombinant human cytoglobin prevents atherosclerosis by regulating lipid metabolism and oxidative stress. J Cardiovasc Pharmacol Ther. (2018) 23:162–73. 10.1177/1074248417724870 [DOI] [PubMed] [Google Scholar]
- 87.Kaliste E, Mering S. The welfare of laboratory rats. In: Kaliste E, editor. The Welfare of Laboratory Animals. Dordrecht: Springer; (2007) p. 153–80. 10.1007/978-1-4020-2271-5_8 [DOI] [Google Scholar]
- 88.Lu R, Yuan T, Wang Y, Zhang T, Yuan Y, Wu D, et al. Spontaneous severe hypercholesterolemia and atherosclerosis lesions in rabbits with deficiency of low-density lipoprotein receptor (LDLR) on exon 7. EBioMedicine. (2018) 36:29–38. 10.1016/j.ebiom.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lidfors L, Edström T, Lindberg L. The welfare of laboratory rabbits. In: Kaliste E, editor. The Welfare of Laboratory Animals. Dordrecht: Springer; (2007) p. 211–43. 10.1007/978-1-4020-2271-5_10 [DOI] [Google Scholar]
- 90.Shiomi M, Ishida T, Kobayashi T, Nitta N, Sonoda A, Yamada S, et al. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction–prone strain of the Watanabe Heritable Hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. (2013) 33:2518–23. 10.1161/ATVBAHA.113.301303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chalmers AD, Bursill CA, Myerscough MR. Nonlinear dynamics of early atherosclerotic plaque formation may determine the efficacy of high density lipoproteins (HDL) in plaque regression. PLoS ONE. (2017) 12:e0187674. 10.1371/journal.pone.0187674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niimi M, Yang D, Kitajima S, Ning B, Wang C, Li S, et al. ApoE knockout rabbits: a novel model for the study of human hyperlipidemia. Atherosclerosis. (2016) 245:187–93. 10.1016/j.atherosclerosis.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 93.Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, et al. Use of zebrafish in drug discovery toxicology. Chem Res Toxicol. (2020) 33:95–118. 10.1021/acs.chemrestox.9b00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams CH, Hong CC. Zebrafish small molecule screens: taking the phenotypic plunge. Comput Struct Biotechnol J. (2016) 14:350–6. 10.1016/j.csbj.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aulner N, Danckaert A, Ihm J, Shum D, Shorte SL. Next-generation phenotypic screening in early drug discovery for infectious diseases. Trends Parasitol. (2019) 35:559–70. 10.1016/j.pt.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 96.Vincent F, Loria P, Pregel M, Stanton R, Kitching L, Nocka K, et al. Developing predictive assays: the phenotypic screening “rule of 3.” Sci Transl Med. (2015) 7:293ps15. 10.1126/scitranslmed.aab1201 [DOI] [PubMed] [Google Scholar]
- 97.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med. (2010) 7:e1000245. 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moffat JG. Turning the light on in the phenotypic drug discovery black box. Cell Chem Biol. (2017) 24:545–7. 10.1016/j.chembiol.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 99.Fang L, Miller YI. Targeted cholesterol efflux. Cell Cycle Georget Tex. (2013) 12:3345–6. 10.4161/cc.26401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang L, Green SR, Baek JS, Lee S-H, Ellett F, Deer E, et al. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J Clin Invest. (2011) 121:4861–9. 10.1172/JCI57755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.CETP Gene - GeneCards (2017). Available online at: http://www.genecards.org/cgi-bin/carddisp.pl?gene=CETP (accessed December 16, 2017).
- 102.Brown DR, Samsa LA, Qian L, Liu J. Advances in the study of heart development and disease using zebrafish. J Cardiovasc Dev Dis. (2016) 3:13. 10.3390/jcdd3020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen L-C, Wu J-L, Shiau C-Y, Chen J-Y. Organization and promoter analysis of the zebrafish (Danio rerio) chemokine gene (CXC-64) promoter. Fish Physiol Biochem. (2010) 36:511–21. 10.1007/s10695-009-9321-y [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Li Y, Cao X, Jin X, Jin T. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cell Mol Immunol. (2017) 14:80–9. 10.1038/cmi.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Vaart M, van Soest JJ, Spaink HP, Meijer AH. Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis Model Mech. (2013) 6:841–54. 10.1242/dmm.010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanwal Z, Wiegertjes GF, Veneman WJ, Meijer AH, Spaink HP. Comparative studies of Toll-like receptor signalling using zebrafish. Dev Comp Immunol. (2014) 46:35–52. 10.1016/j.dci.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 107.Fink IR, Benard EL, Hermsen T, Meijer AH, Forlenza M, Wiegertjes GF. Molecular and functional characterization of the scavenger receptor CD36 in zebrafish and common carp. Mol Immunol. (2015) 63:381–93. 10.1016/j.molimm.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 108.Svensson S, Abrahamsson A, Rodriguez GV, Olsson A-K, Jensen L, Cao Y, et al. CCL2 and CCL5 are novel therapeutic targets for estrogen-dependent breast cancer. Clin Cancer Res. (2015) 21:3794–805. 10.1158/1078-0432.CCR-15-0204 [DOI] [PubMed] [Google Scholar]
- 109.Sun G, Pan J, Liu K, Wang X, Wang S. Molecular cloning and expression analysis of P-selectin from zebrafish (Danio rerio). Int J Mol Sci. (2010) 11:4618–30. 10.3390/ijms11114618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pietretti D, Spaink HP, Falco A, Forlenza M, Wiegertjes GF. Accessory molecules for Toll-like receptors in teleost fish. Identification of TLR4 interactor with leucine-rich repeats (TRIL). Mol Immunol. (2013) 56:745–56. 10.1016/j.molimm.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 111.Tsan M-F, Gao B. Heat shock proteins and immune system. J Leukoc Biol. (2009) 85:905–10. 10.1189/jlb.0109005 [DOI] [PubMed] [Google Scholar]
- 112.Wick C. Tolerization against atherosclerosis using heat shock protein 60. Cell Stress Chaperones. (2016) 21:201–11. 10.1007/s12192-015-0659-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hyperion Therapeutics Terminates DiaPep277(R) Program | FierceBiotech Available online at: https://www.fiercebiotech.com/biotech/hyperion-therapeutics-terminates-diapep277-r-program (accessed April 6, 2020).
- 114.Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. (2005) 353:9–11. 10.1056/NEJMp058118 [DOI] [PubMed] [Google Scholar]
- 115.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. (2014) 46:e99. 10.1038/emm.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yin W, Carballo-Jane E, McLaren DG, Mendoza VH, Gagen K, Geoghagen NS, et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res. (2012) 53:51–65. 10.1194/jlr.M019927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andreadou I, Schulz R, Badimon L, Adameová A, Kleinbongard P, Lecour S, et al. Hyperlipidaemia and cardioprotection: animal models for translational studies. Br J Pharmacol. (2020). 10.1111/bph.14931. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y, Wen S, Jiang M, Zhu Y, Ding L, Shi H, et al. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE –/– mice fed a high-fat diet. Atherosclerosis. (2017) 262:78–86. 10.1016/j.atherosclerosis.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 119.Nilsson J, Hansson GK, Shah PK. Immunomodulation of atherosclerosis: implications for vaccine development. Arterioscler Thromb Vasc Biol. (2005) 25:18–28. 10.1161/01.ATV.0000149142.42590.a2 [DOI] [PubMed] [Google Scholar]
- 120.Mahan MM, Doiron AL. Gold nanoparticles as X-ray, CT, and multimodal imaging contrast agents: formulation, targeting, and methodology. J Nanomater. (2018) 2018:1–15. 10.1155/2018/5837276 [DOI] [Google Scholar]
- 121.Browning LM, Huang T, Xu X-HN. Real-time in vivo imaging of size-dependent transport and toxicity of gold nanoparticles in zebrafish embryos using single nanoparticle plasmonic spectroscopy. Interface Focus. (2013) 3:20120098. 10.1098/rsfs.2012.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim K-T, Zaikova T, Hutchison JE, Tanguay RL. Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci. (2013) 133:275–88. 10.1093/toxsci/kft081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chakraborty C, Sharma AR, Sharma G, Lee S-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J Nanobiotechnol. (2016) 14:65. 10.1186/s12951-016-0217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cordeiro M, Carvalho L, Silva J, Saúde L, Fernandes A, Baptista P. Gold nanobeacons for tracking gene silencing in zebrafish. Nanomaterials. (2017) 7:10. 10.3390/nano7010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Wilde D, Trachet B, Van der Donckt C, Vandeghinste B, Descamps B, Vanhove C, et al. Vulnerable plaque detection and quantification with gold particle–enhanced computed tomography in atherosclerotic mouse models. Mol Imaging. (2015) 14:9–19. 10.2310/7290.2015.00009 [DOI] [PubMed] [Google Scholar]
- 126.Schürmann C, Gremse F, Jo H, Kiessling F, Brandes RP. Micro-CT technique is well suited for documentation of remodeling processes in murine carotid arteries. PLoS ONE. (2015) 10:e0130374. 10.1371/journal.pone.0130374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corti R, Fuster V. Imaging of atherosclerosis: magnetic resonance imaging. Eur Heart J. (2011) 32:1709–19. 10.1093/eurheartj/ehr068 [DOI] [PubMed] [Google Scholar]
- 128.Koth J, Maguire ML, McClymont D, Diffley L, Thornton VL, Beech J, et al. High-resolution magnetic resonance imaging of the regenerating adult zebrafish heart. Sci Rep. (2017) 7:2917. 10.1038/s41598-017-03050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parant M, Sohm B, Flayac J, Perrat E, Chuburu F, Cadiou C, et al. Impact of gadolinium-based contrast agents on the growth of fish cells lines. Ecotoxicol Environ Saf. (2019) 182:109385. 10.1016/j.ecoenv.2019.109385 [DOI] [PubMed] [Google Scholar]
- 130.Hockings PD, Roberts T, Galloway GJ, Reid DG, Harris DA, Vidgeon-Hart M, et al. Repeated three-dimensional magnetic resonance imaging of atherosclerosis development in innominate arteries of low-density lipoprotein receptor-knockout mice. Circulation. (2002) 106:1716–21. 10.1161/01.CIR.0000030188.50326.8D [DOI] [PubMed] [Google Scholar]
- 131.Jung C, Christiansen S, Kaul MG, Koziolek E, Reimer R, Heeren J, et al. Quantitative and qualitative estimation of atherosclerotic plaque burden in vivo at 7T MRI using Gadospin F in comparison to en face preparation evaluated in ApoE KO mice. PLoS ONE. (2017) 12:e0180407. 10.1371/journal.pone.0180407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou YQ, Foster FS, Qu DW, Zhang M, Harasiewicz KA, Adamson SL. Applications for multifrequency ultrasound biomicroscopy in mice from implantation to adulthood. Physiol Genomics. (2002) 10:113–26. 10.1152/physiolgenomics.00119.2001 [DOI] [PubMed] [Google Scholar]
- 133.Li R-J, Yang Y, Wang Y-H, Xie J-J, Song L, Wang Z, et al. Micro-ultrasonographic imaging of atherosclerotic progression and correlation with inflammatory markers in apolipoprotein-E knockout mice. Tex Heart Inst J. (2011) 38:364–70. 10.7555/JBR.31.20160020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou X, Sun L, Yu Y, Qiu W, Lien C-L, Shung KK, et al. Ultrasound bio-microscopic image segmentation for evaluation of zebrafish cardiac function. IEEE Trans Ultrason Ferroelectr Freq Control. (2013) 60:718–26. 10.1109/TUFFC.2013.2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benslimane FM, Alser M, Zakaria ZZ, Sharma A, Abdelrahman HA, Yalcin HC. Adaptation of a mice Doppler echocardiography platform to measure cardiac flow velocities for embryonic chicken and adult zebrafish. Front Bioeng Biotechnol. (2019) 7:96. 10.3389/fbioe.2019.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo Y, Liu X-C, Wang Y-J, Li Q, Yang Q, Weng X-G, et al. Effects of Shenlian extract on experimental atherosclerosis in ApoE-deficient mice based on ultrasound biomicroscopy. BMC Complement Altern Med. (2016) 16:469. 10.1186/s12906-016-1449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Becher T, Riascos-Bernal DF, Kramer DJ, Almonte VM, Chi J, Tong T, et al. Three-dimensional imaging provides detailed atherosclerotic plaque morphology and reveals angiogenesis after carotid artery ligation. Circ Res. (2020) 126:619–32. 10.1161/CIRCRESAHA.119.315804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salman HE, Yalcin HC. Advanced blood flow assessment in zebrafish via experimental digital particle image velocimetry and computational fluid dynamics modeling. Micron. (2020) 130:102801. 10.1016/j.micron.2019.102801 [DOI] [PubMed] [Google Scholar]
- 139.Ding Y, Vanselow DJ, Yakovlev MA, Katz SR, Lin AY, Clark DP, et al. Computational 3D histological phenotyping of whole zebrafish by X-ray histotomography. Elife. (2019) 8:e44898 10.7554/eLife.44898 [DOI] [PMC free article] [PubMed] [Google Scholar]