Abstract
Thyroid-associated ophthalmopathy (TAO) remains a vexing autoimmune component of Graves’ disease that can diminish the quality of life as a consequence of its impact on visual function, physical appearance and emotional well-being. Because of its relative rarity and variable presentation, the development of highly effective and well-tolerated medical therapies for TAO has been slow relative to other autoimmune diseases. Contributing to the barriers of greater insight into TAO has been the historical absence of high-fidelity preclinical animal models. Despite these challenges, several agents, most developed for other diseases, have found their way into consideration for use in active TAO through repurposing from their original purposes. Among these, teprotumumab is a fully human monoclonal antibody that acts as a β- arrestin agonist of the insulin-like growth factor I receptor. It has shown remarkable effectiveness in moderate to severe, active TAO in two completed multicenter, double masked, and placebo controlled clinical trials. The drug exhibits a favorable safety profile. Should teprotumumab become approved by the U.S. F.D.A, it may rapidly become the first line therapy for this disfiguring and potentially blinding condition.
Keywords: autoimmune, insulin-like growth factor-I receptor, Graves’ disease, biological agents, monoclonal antibodies, teprotumumab
1.0. Introduction
Thyroid-associated ophthalmopathy (TAO, aka Graves’ ophthalmopathy/orbitopathy or thyroid eye disease) is a disfiguring, potentially blinding autoimmune component most frequently associated with Graves’ disease (GD) but also occurring in patients with Hashimoto’s thyroiditis (Figure 1) (1–3). In both instances, it is presumed to represent a consequence of similar if not identical autoimmune processes occurring within the thyroid gland. Lack of a more complete understanding of TAO has been delayed by the wide variations in clinical presentation seen in afflicted patients. In addition, many aspects of the disease (i.e. eyelid swelling, redness, dry eye symptoms) are shared with other ocular disease processes, some of which occur with far greater frequency. The long-standing absence of high-fidelity preclinical models of TAO in which to study disease pathophysiology and test candidate therapeutics has also proven to represent an important barrier; however, recent developments in generating small animal models represent significant improvements (4). Uncertainty surrounding many aspects of disease development has in turn severely constricted development of specific, targeted medical therapies which have proven safe and effective in adequately-powered, prospective, and placebo-controlled clinical trials. To date, no medical therapy has achieved registration by the U.S. Food and Drug Administration. But the insights gathered in recent years concerning disease development has led to recent identification of plausible therapeutic targets for TAO. In this brief, focused review, I have attempted to provide an overview of the current understanding of the mechanistic underpinnings of TAO, how it is diagnosed, and to identify both current therapeutic options and those emerging treatments in various stages of development for this vexing disease.
Figure 1. Moderate to severe thyroid-associated ophthalmopathy characterized by bilateral proptosis, periorbital edema, scleral injection, and lid retraction.
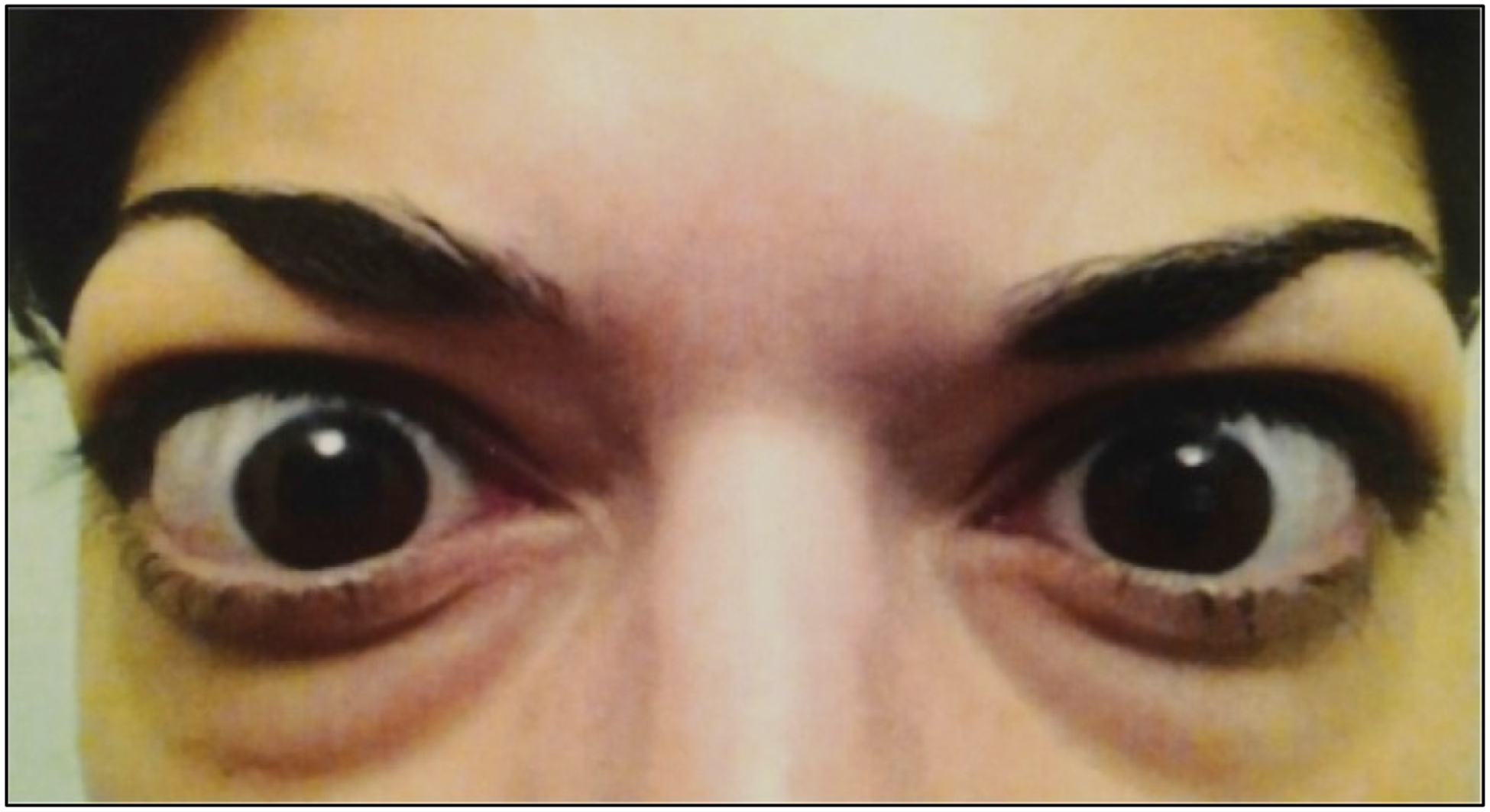
From N. Engl. J. Med, Smith T.J. and Hegedus L., Graves’ Disease, 375; 1552–1565. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission.
1.1. Pathogenic mechanisms
The basis for thyroid-centric GD and TAO is thought to be shared by the two anatomically dispersed components of the disease and revolves around the thyrotropin receptor (TSHR) (Figure 2) (5). TSHR becomes targeted by pathogenic antibodies that uniquely in GD activate the receptor and result in dysthyroidemia (6). Like other autoantigens, immunoreactivity to TSHR is initiated as the consequence of defects of either central or peripheral tolerance (or both). More complete understanding the loss of immune tolerance to TSHR in GD has been the focused topic of inquiry for many years and substantial advances have been reported recently (7). It appears, based on studies conducted in hTSHR/NOD.H2h4 transgenic mice, that the availability of TSHR A-subunit protein is important to the generation of pathogenic anti-TSHR antibodies in GD (8). But the generation of anti-TSHR antibodies represents only one aspect of the autoimmunity against TSHR. Further, while these receptor-targeting IgGs can be identified in a majority of patients with active TAO (9), they cannot be detected in a small fraction of those individuals affected by GD, some of whom exhibit severe ocular disease (10,11). This raises the important question of whether another autoantigen might also play a role in the development of TAO. Antigen-specific autoreactive T cells are also generated and their roles in disease development include their endorsement of B cells and the production of IgG1 antibodies. While these TSIs engage TSHR, activate signaling, and increase hormone production and thyroid gland volume expansion (2), their consequences within orbital connective tissues and extraocular muscles remain uncertain. Further, the roles of T cells infiltrating the TAO orbit remain to be fully understood but they likely participate in the tissue activation and remodeling characteristic of the disease. Access to orbital fibroblasts from patients with TAO has allowed investigation into the effects of TSH and TSI on these cells in vitro (12–14). But while these studies in several laboratories have yielded much useful information, they are inherently limited, as are all such experimental models with isolated cells and especially those maintained in monolayer. Three-dimensional organoid culture models of orbital fibroblasts might represent an improvement but their importance has yet to be established (15). TSHR displayed on these fibroblasts and the closely related cells coming from the bone marrow, known as fibrocytes, can initiate the induction of multiple cytokines, including IL-1 receptor antagonist, IL-6, IL-8, and IL-12 (14,16–18). But the absence of high-fidelity mouse models has deprived investigators of the experimental systems that have propelled major advances in other diseases, especially those with an uncertain molecular basis.
Figure 2. Theoretical model of thyroid-associated ophthalmopathy (TAO) pathogenesis.
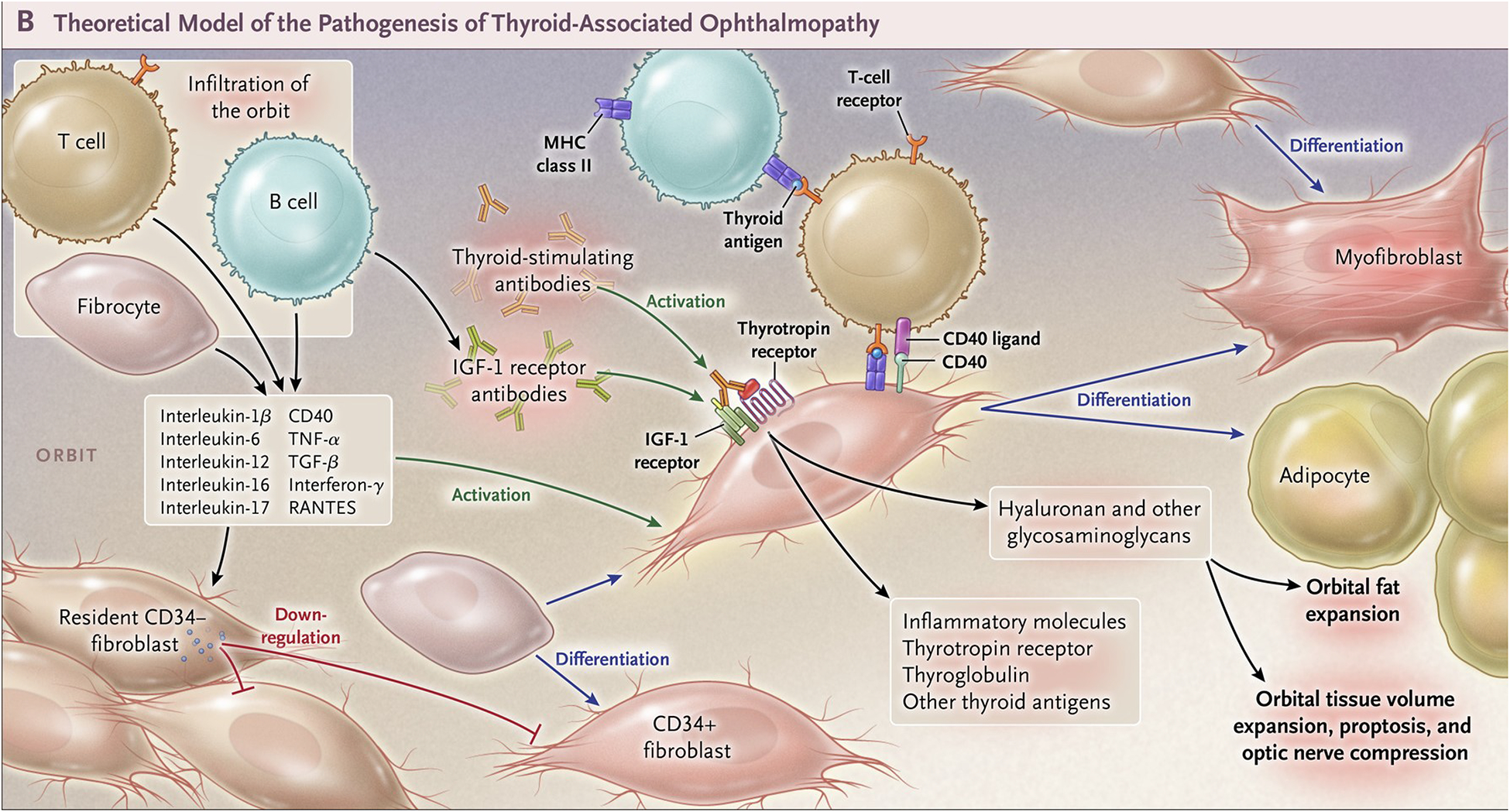
CD34+ fibrocytes, monocyte-derived progenitor cells coming from the bone marrow, circulate in Graves’ disease at higher abundance than in healthy control individuals. Fibrocytes express the thyrotropin receptor (TSHR), thyroglobulin, thyroperoxidase and sodium-iodide symporter. They constitutively express Class II major histocompatibility complex (MHC) and present antigens to T cells. They can differentiate into CD34+ fibroblasts, myofibroblasts, and adipocytes. Many of the genes expressed by fibrocytes are detected at considerably lower levels in CD34+ fibroblasts. When activated, CD34+ fibroblasts generate several pro-inflammatory or anti-inflammatory cytokines, including interleukins 1β, 6, 8, 10, 12, 16, tumor necrosis factor α, and regulated on activation, normal T expressed and secreted (RANTES), CXCL-12 and CD40-CD154. Orbital fibroblasts display insulin-like growth factor-I receptor (IGF-IR) on their cell surfaces. Orbital fibroblasts express three mammalian hyaluronan synthase isoenzymes and UDP glucose dehydrogenase and synthesize hyaluronan, the glycosaminoglycan associated with expanding orbital tissue in TAO. From N. Engl. J. Med, Smith T.J. and Hegedus L., Graves’ Disease, 375; 1552–1565. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission.
1.2. Orbital fibroblasts as major effector cells in TAO
Heterogeneity of fibroblasts derived from many human tissues has been described by several laboratory groups studying both normal tissue function and the abnormalities associated with disease (19,20). This is certainly the case with regard to orbital connective tissues and the fibroblasts that can be outgrown from them(21–24). The orbital fibroblast population can be divided into discrete subsets; among those are cells surface-displaying CD90 (Thy-1) and in which this determinant cannot be detected (24). This mixed population can be found in fibroblast cultures from patients with TAO and those from healthy donors. We have reported that Thy-1+ fibroblasts can differentiate into myofibroblasts when treated with TGF-β and thus activating the Smad pathway while Thy-1− cells develop into mature adipocytes when exposed to PPARγ agonists (23,24). Using another set of markers, Douglas et al identified two discrete cellular subsets in the TAO orbit, based on their exhibiting a phenotype including CD34+CXCR4+Collagen I+ display (12). These cells uniformly express relatively high levels of TSHR and respond to both TSH and TSIs with the production of several cytokines, including IL-6 and IL-8 (12,14). Another subset comprises cells with the CD34−CXCR4−Col I− phenotype, fibroblasts with very different characteristics from those the CD34+CXCR4+Collagen I+ array of markers. Each subset exhibits distinct patterns of proliferation and capacity to undergo differentiation. Further, each expresses profiles of cytokines following activation with TSH and inflammatory mediators such as IL-1β. In contrast to the TAO orbit, fibroblasts from tissues in healthy orbits have a uniformly CD34−CXCR4−Col I− phenotype (12). They display a more uniform morphology under a variety of culture conditions (25).
The CD34+CXCR4+Col I+ fibroblasts inhabiting the TAO orbit most likely represent fibrocytes, cells that originate in the bone marrow, and have infiltrated from the circulation (26). These cells derive from a monocyte lineage and appear to play important roles in tissue remodeling and scar formation (27). They share many of the phenotypic and functional attributes of CD34+ orbital fibroblasts, supporting their presumptive identity as the precursors of those fibroblasts. Fibrocytes have been directly implicated in the development of fibrosis, a concept aligning well with their capacity to differentiate into myofibroblasts (23,24,28,29). They promiscuously express the autoimmune regulator protein (AIRE), a non-canonical transcription factor central to the editing of autoreactive T cells in the thymus (30,31). AIRE in these fibrocytes supports the expression of several thyroid autoantigens, including TSHR, thyroglobulin (Tg), sodium iodide symporter, and thyroperoxidase (32,33). Substantial levels of collagen are synthesized by fibrocytes as well as other components of the extracellular matrix, which is well-suited to their functions concerning wound healing. They efficiently present antigens to T cells by constitutively expressing MHC class II at high levels in addition to several costimulatory molecules (34,35). Fibrocytes prime T cells while reciprocally depending on those cells for their capacity to undergo differentiation (36).
Like fibrocytes cultured from the peripheral blood, orbital fibroblasts can also participate in immune regulation by virtue of their expressing MHC II when treated with interferon γ (37). The levels of MHC II induction are considerably higher in fibroblasts from patients with TAO than in those from healthy control subjects. This induction can be markedly attenuated by physiologically relevant levels of glucocorticoids (38). Orbital fibroblasts also express a myriad of cytokines, especially when activated through the TSHR/IGF-IR signaling complex (3). IGF-I and IgGs from patients with GD (GD-IgG) can induce physiologically meaningful T cell chemoattractants such as IL-16 and regulated on activation, normal T cell expressed and secreted (RANTES, CCL5) (39,40). Activation of TSHR displayed on the surface of these cells results in expression of IL-1β, IL-1 receptor antagonist, IL-4, IL-6, IL-8, IL-12, and IL-13 (2,3,12,14,16). Thus, orbital fibroblasts possess the capacity to actively participate in the recruitment of professional immune cells to the TAO orbit and therefore represent potentially attractive therapeutic targets at multiple stages of disease development.
A critical component of the tissue remodeling occurring in TAO involves the dysregulated accumulation of hyaluronan (HA) in fatty connective tissues (41). This accumulation can be attributed to the increased biosynthetic activity of orbital fibroblasts (42). Human fibroblasts, including those in the orbit, fail to exhibit substantial HA- degrading activity (42,43). Thus, HA synthesis is likely responsible for the increased abundance of glycosaminoglycan in TAO. This absence in HA degradation by fibroblasts does not exclude the uncertain potential for exhibiting hyaluronidase activity by other cells within the TAO orbit, including the inflammatory cells recruited during the disease process. HA synthesis can be downregulated in fibroblasts by glucocorticoids (44) whereas thyroid hormones, which inhibit synthesis in human dermal fibroblasts, fail to alter HA production in orbital fibroblasts (42). Orbital fibroblasts express all three known mammalian HA synthase isoenzymes (45). Of these three, hyaluronan synthase 2 (HAS2) is the most abundant and is therefore presumed to be responsible for most of the HA synthetic activity found in orbital fibroblasts. Orbital fibroblasts also express an inducible UDP glucose dehydrogenase gene (46) through the activation of its promoter (47). Accumulating HA in the confines of the bony orbit can be consequential in that the glycosaminoglycan, when fully hydrated, can directly increase tissue volume. This expansion can be substantial, leading in some cases to orbital congestion and to the expulsion of the globe, resulting in proptosis. Further, accumulation of HA between the intact muscle cells comprising the extraocular musculature can underlie development of diplopia and can result in compressive optic neuropathy..
1.3. Long-standing autoantigen candidates implicated in TAO
Among the early contributions to the early literature surrounding development of TAO are the innovative studies conducted by the Kriss group at Stanford (48). He and his colleagues proposed the potential importance of Tg, a molecule they identified in the TAO orbit and which they proffered had its origins in the thyroid gland. Their theoretical model appears to represent the initial effort to integrate the processes involved in GD occurring within the thyroid and the ocular manifestations of the disease. Their studies remained descriptive and other investigators failed to follow their line of investigation for several decades. Much more recent studies have suggested that Tg can bind to orbital fibroblasts and Marino and colleagues reported detecting the molecule in the connective tissues of several patients with TAO (49,50). Detection of Tg in the TAO orbit can now be plausibly reconciled with the recognition that it is expressed by fibrocytes cultivated from the circulation (32,33) while much lower levels can be identified in their derivative CD34+ orbital fibroblasts. But the issue of whether Tg plays any role in the pathogenesis of TAO remains an open and as yet unresolved question.
A shifting focus to the potential involvement of TSHR as an autoantigen shared by the thyroid and orbit was provoked by the successful molecular cloning of the receptor (5). Feliciello and colleagues reported soon thereafter detecting TSHR mRNA in orbital tissues from both healthy donors and those with TAO (51). Its expression in orbital fibroblasts was subsequently detected (52), suggesting that these cells might be responsive to TSH. Indeed, IL-6 among other cytokines has been shown to be highly inducible by TSH and GD-IgG at the gene transcriptional level (14). Further, both TSH and GD-IgG were found to induce HA synthesis in orbital fibroblasts from patients with TAO (53).
Anti-TSHR antibodies generated in patients with GD and TAO are presumed to play significant roles in orbital disease development, although the evidence for that involvement is far more circumstantial than their established roles in thyroid over-activity. Supporting this widely held view are the correlations thus far identified between the clinical behavior of TAO and the levels of these antibodies (54). On the other hand, infrequent patients, some with severe, active TAO, present with undetectable TSIs and TRabs (10,11). The latter occurrences suggest the possible involvement of another autoantigen, besides TSHR and its cognate autoantibodies in the development of TAO. Identification of other antigenic determinants could broaden the potential targets for interrupting the immune responses occurring in TAO. That prospect led us to explore the potential for involvement of insulin-like growth factor I (IGF-IR) in the disease.
1.4. Implicating IGF-IR in the pathogenesis of TAO
As stated above, anti-TSHR antibodies cannot be detected in a small proportion of patients with TAO, sometimes with severe, active forms of the disease (10,11). Their absence suggested to us that another autoantigen besides TSHR might be involved in the development of TAO. An early study had disclosed autoantibodies in patients with the disease which could displace IGF-I binding to the surface of orbital fibroblast derived from patients with GD (55). A decade later, the relevant binding site was identified as IGF-IR and subsequent studies showed that GD-IgG could mimic recombinant IGF-I in inducing cytokine expression in orbital fibroblasts from patients with TAO whereas fibroblasts from healthy individuals failed to respond (39,40). These actions of GD-IgG were mediated through the PI3K/FRAP/mTOR/p70s6k pathway and were sensitive to inhibition with rapamycin (39). They could be attenuated with either an inhibitory monoclonal anti-IGF-IR monoclonal antibody (1H7) or by transfecting responding fibroblasts with a dominant negative IGF-IR (40). In contrast, recombinant human TSH failed to elicit the response in either TAO fibroblasts or those from healthy donors. Subsequently, Tsui et al demonstrated that TSHR and IGF-IR co-localize on the plasma membranes of orbital fibroblasts, thyroid epithelial cells and orbital fat in situ (56). The receptor proteins were found to co-precipitate, being pulled out of solution with monoclonal antibodies to either receptor. In addition, the IGF-IR inhibitory antibody, 1H7, was found to attenuate the downstream signaling initiated at either receptor. Specifically, 1H7 was found to attenuate the phosphorylation of Erk 1/2 provoked by IGF-I (a positive control), rhTSH and GD-IgG. Thus it appeared that TSHR signaling was dependent, at least in part, on the activity of IGF-IR (56). The aggregate findings implicating IGF-IR in TAO, either directly or indirectly, were generated, almost entirely in vitro and provided the initial rationale for pursuing development of drugs similar to teprotumumab as a therapy for the disease. We have consistently acknowledged the gaps in current understanding of the disease and how disrupting IGF-IR might be eliciting responses.
In studies similar to those conducted by our laboratory group, others subsequently began to examine the potential for IGF-IR to play a meaningful role in TAO. Among these studies, Gershengorn and colleagues have also begun to characterize the relationship between TSHR and IGF-IR pathways. They have strongly promoted the point of view that activating anti-IGF-IR antibodies are not generated in GD and TAO, especially those that in some manner initiate signaling through direct interactions with that receptor protein (57). Their view is consistent with anti-IGF-IR not playing any role in the pathogenesis of TAO. While their concept regarding antibodies directly targeting IGF-IR and directly initiating signaling might ultimately be proven correct, the negative findings from studies conducted in their laboratory as well as in others relied on assays and experimental conditions that may have precluded the detection of either canonical or non-canonical IGF-IR signaling. This same group has recently published the putative role of β arrestin in mediating cross-talk between TSHR and IGF-IR (58). Those studies and the proximate role of β arrestin are very interesting and may lead to more granular understanding of the complex interactions between IGF-IR and TSHR.
2.0. Clinical presentation of TAO
Among the currently debated issues concerning GD is whether TAO represents an integral manifestation of the disease or if the two are distinct but frequently occurring as comorbidities. In my view, and based on our current level of understanding, GD and TAO share common etiologic factors but given their often dissociated clinical courses and divergent severities, may be fairly considered clinically distinct. Difficulties in diagnosing early TAO stem from its variable presentation and sometimes subtle symptoms and signs. These often resemble other causes of periocular inflammation (1,59). Conditions such as seasonal allergies and viral conjunctivitis can bear remarkable resemblance to mild, active TAO. If the initial diagnosis of GD is made in the absence of TAO, specific clinical features have been suggested as predictive of future ocular disease development (60). Among these are tobacco smoke exposure, prolonged hyperthyroidism, extremely high level T3 toxicosis and relatively high levels of TSH-binding inhibitory immunoglobulins.
The initial presentation of TAO can vary widely. Many patients have already been diagnosed with hyperthyroidism or will soon be found to harbor abnormalities of thyroid function (2). Others may develop thyroid dysfunction decades later or may never manifest clinically detectable glandular abnormalities. The ocular manifestations can be very subtle, limited to one or more of the following: dry eye, eyelid retraction and swelling, mild stare, foreign body sensation, and excessive tearing. Many patients exhibit inflammatory signs of the upper face. These can resolve, sometimes without treatment or worsen and progress to clinically significant proptosis, diplopia, optic neuropathy, and/or deterioration of the ocular surface, the consequence of exposure keratopathy. Most authors agree that the early recognition of TAO and the initiation of therapies, both local and systemic, can optimize the clinical outcome. In any event, the early recognition of TAO can represent a diagnostic challenge.
2.1. Currently available therapeutic options for TAO
Managing TAO, regardless of its level of activity or severity, represents substantial challenges given the imperfections in assessing the disease and treating it. A major impediment is the absence of medical therapies that have been proven effective and safe in large, multicenter, placebo-controlled trials and that have been approved by the U.S. FDA or analogous regulatory agencies in other countries (61). Among experts who manage patients are those who forcefully argue that glucocorticoids, administered either by IV pulse or orally, are effective treatments, the benefits of which might be enhanced with concomitant orbital radiotherapy (62,63). Many of these advocates refer to steroids as the “gold standard”. Evidence for continuing to use steroids as “first-line” therapy continues to be weak. Most studies purporting their value have been uncontrolled and/or were statistically underpowered. But many of even the most ardent of these “steroid stalwarts” are those now conceding that better and safer drugs are greatly needed (63). By far, the most frequently employed systemic therapy in moderate to severe TAO remain glucocorticoids. Their administration often consists of 12 weekly methylprednisolone infusions where 500 mg is administered for the initial 6 doses and 250 mg for the remainder (4.5 g total) (64). Among the most informative studies examining the benefits of steroids was that performed by Bartalena et al who examined three different total dosages of IV methylprednisolone (2.25 g, 4.98 g or 7.47 g) (65). These were administered as 12 weekly infusions. Effects of the steroid on the clinical activity scores (CAS) were assessed at week 12 where transient benefit was identified to be greatest in those patients receiving the highest dosage. A clinically inconsequential reduction of 0.6 mm reduction in proptosis compared with baseline was detected in that treatment group. A drawback to high-dose steroid infusions relates to its potential hepatic toxicity, which is well-recognized in the literature but can be under-stressed in practice (66,67). Appropriate precautions must be taken since an appreciable fraction of patients manifesting severe liver dysfunction from steroids have histories of viral or autoimmune hepatitis or other forms of liver disease (68). Baseline studies are recommended by many authorities. Patients can experience at least temporary relief from steroid dosing; however, symptomatic benefit is frequently transient and many individuals fail to respond. Of considerable importance, no convincing evidence has thus far been generated that these agents alter the course of TAO, lessen its ultimate severity as assessed by proptosis and strabismus, or reduce the need for rehabilitative ophthalmic surgery. Many authorities in the field believe that meaningful responses to glucocorticoids are limited to reduction in inflammation, pain and edema, as reflected in an improving CAS.
Besides steroids, biologics such as those targeting cytokines and B cells have been introduced into the autoimmune disease space with appreciable success (69). Rituximab, a monoclonal antibody targeting B cells displaying CD20, has demonstrated effectiveness in rheumatoid arthritis and systemic lupus erythematosus (70). Several authors had proposed consideration of rituximab in the therapy of both GD and TAO (71). Several case studies appeared but none was particularly convincing because of small, inadequate patient numbers and the lack of appropriate controls (72–74). Finally, results from two trials of rituximab in patients with moderate to severe TAO were published simultaneously several years ago. These were performed in separate, single institutions. The studies have left the question of whether anti-CD20 therapy is effective in TAO. One study compared rituximab to methylprednisolone and found that the former was more effective in reducing CAS (75). The other trial was placebo controlled and failed to disclose any differences between rituximab and placebo in reducing disease activity (76). In neither study was there a meaningful improvement in proptosis among any of the treatment arms. A recently reported trial compared the combination of methylprednisolone and mycophenolate to treatment with methypredniolone as a single agent in 164 patients with moderate to severe, active TAO and failed to demonstrate convincing benefit of adding mycophenolate to the steroid only regimen (77). No differences in response at 12 weeks or relapse at 24 and 36 weeks were seen in the two treatment groups; however post-hoc analysis suggested that mycophenolate plus methylprednisolone improved the response at 24 weeks compared to the steroid alone. The IL-6 receptor has been successfully targeted in autoimmune diseases, most recently in neuromyelitis optica spectrum disorder, with the fully humanized monoclonal antibody, satralizumab (78). Examination of the anti-IL-6 receptor monoclonal antibody, tocilizumab, in a cohort of steroid-resistant patients demonstrated superiority compared to placebo in reducing CAS, measured as change from baseline at 16 weeks (the primary outcome) (79,80). The study included 32 patients with moderate to severe TAO. Infusions were administered at weeks 0, 4, 8 and 12. Among those receiving active drug, 93.3% responded compared to 58.8% of controls (p=0.04). Among the secondary outcomes, patients treated with tocilizumab experienced a greater reduction in proptosis (1.5 mm reduction versus 0.0 mm in controls, p=0.01) at 16 weeks. This difference was not detected at follow-up, suggesting that any effect on proptosis might be transient. The enhanced reduction in CAS coupled with the modest, transient improvement in proptosis compared to placebo suggests that tocilizimab may represent the most encouraging agent among the aforementioned candidate steroid alternatives for the treatment of active TAO. But based on the results thus far reported, none of these candidate agents appears to effectively reduce the severity of TAO or to offer substantial improvement over steroids.
2.2. Potential therapeutic targeting of TSHR in TAO
Another strategy for attenuating upstream events thought to be involved in TAO concerns the interruption of TSHR signaling activities. Both small molecule and monoclonal antibody approaches have been attempted to target the receptor. Recent interest in the broad category of G-protein coupled receptors has resulted from a better understanding of their protean involvement in both physiological and pathological processes (81). This certainly applies to the TSHR. With regard to TSHR agonists, org41841, a partial agonist for both the leutinizing hormone receptor and TSHR was utilized to demonstrate that small molecules could effectively target the TSHR transmembrane domain (82). Whether it or any derivative molecule might prove useful in preparing patients with thyroid cancer for surveillance and treatment is uncertain given the compound’s potential for off-target actions. A series of multiple TSHR agonists exhibiting activity in vitro and in mice, suggest that they or similar agents might be developed into drugs (83). TSHR inhibitory small molecules similarly have exhibited activity, both in cell culture and in vivo, providing evidence that they might prove effective therapies in GD (84,85). Among these are molecules that behave as inverse agonists (86). Several monoclonal antibodies directed at TSHR have also demonstrated potential as drugs, including both agonists such as M22 (87) and antagonists (88–91). Perhaps even more exciting than molecules interfering with TSHR signaling are those strategies designed to restore immune tolerance to that receptor protein. Among these is the approach described by Jansson et al (92) who utilized HLA-DR3 transgenic mice and administered two TSHR immunodominant region peptides. These animals exhibited suppressed T cell and antibody responses to the antigen. A phase I study was recently reported where a mixture of two soluble, synthetic TSHR peptides, designated ATX-GD-59, were used to treat 10 adult patients with otherwise untreated hyperthyroidism resulting from GD (93). The therapy appeared to be well-tolerated although issues of their efficacy will require later stage clinical testing before they can be resolved.
2.3. Results from two clinical trials suggest that anti-IGF-IR monoclonal antibodies may shift treatment paradigm for active severe TAO
Based on the experimental results amassed over two decades concerning a potential role for IGF-IR in TAO (39,40,55,56,94,95), an initial phase 2 clinical trial was sponsored by River Vision examining an inhibitory monoclonal antibody originally developed by Roche/Genmab for the treatment of solid cancers (96). Teprotumumab is a fully human, IgG 1 that acts as a β-arrestin-biased IGF-IR agonist. Clinical studies in several tumor types including Ewing Sarcoma showed some benefit of teprotumumab in subgroups of patients but the findings were generally viewed as disappointing (97). In addition, appreciable side effects were observed with IGF-IR-inhibitors in cancer cohorts which were frequently administered in combination with other chemotherapeutics. Some adverse events appeared to be specific for particular agents (98). As a consequence of the perceived barriers toward progress in further developing these drugs for treatment of cancer, teprotumumab became available for repurposing to TAO.
A randomized trial of teprotumumab was organized and an experimental protocol developed between 2010 and 2013. It was designed to assess safety and efficacy of the drug in adult patients with moderate to severe, active TAO. (Figure 3) The trial involved 15 performance sites in North America and western Europe (99). Patients were enrolled between 2 July, 2013 and 23 September, 2015. A total of 112 patients underwent screening which required one to three visits during which inclusion and exclusion criteria were assessed. Inclusion criteria included recent onset of TAO (developed within 9 months of earliest ocular signs and symptoms), CAS > 4 points on a 7-point scale, no history of previous immunotherapy with rituximab, cumulative systemic glucocorticoid steroid for TAO ≤ 1 gram with a 6-week washout period, and no history of ophthalmic surgery for TAO. Eighty-eight patients were randomized to one of the treatment arms. Forty-five patients received placebo and 42 were assigned to receive active drug. Thus, 87 patients comprised the intention-to-treat (ITT) cohort. Clinical assessments were conducted at baseline and every three weeks during the next 24-week treatment phase. Efficacy was determined at week 6, 12, 18 and 24. Primary and secondary endpoint responses were determined at week 24. An aggregate primary response included reduction in CAS ≥ 2 points and reduction in proptosis ≥ 2 mm, both assessed in the study (more severely affected) eye. This response had to occur in the absence of a similar worsening in the fellow (contralateral) eye. Secondary responses included reduction from baseline in CAS ≥ 2 points and reduction in proptosis ≥ 2 mm measured as independent variables, improvement in strabismus and quality of life score using a validated survey questionnaire (GO-Qol) (100). Patients were stratified with regard to smoking status.
Figure 3. Phase 2 trial design, patient disposition and responder analyses.
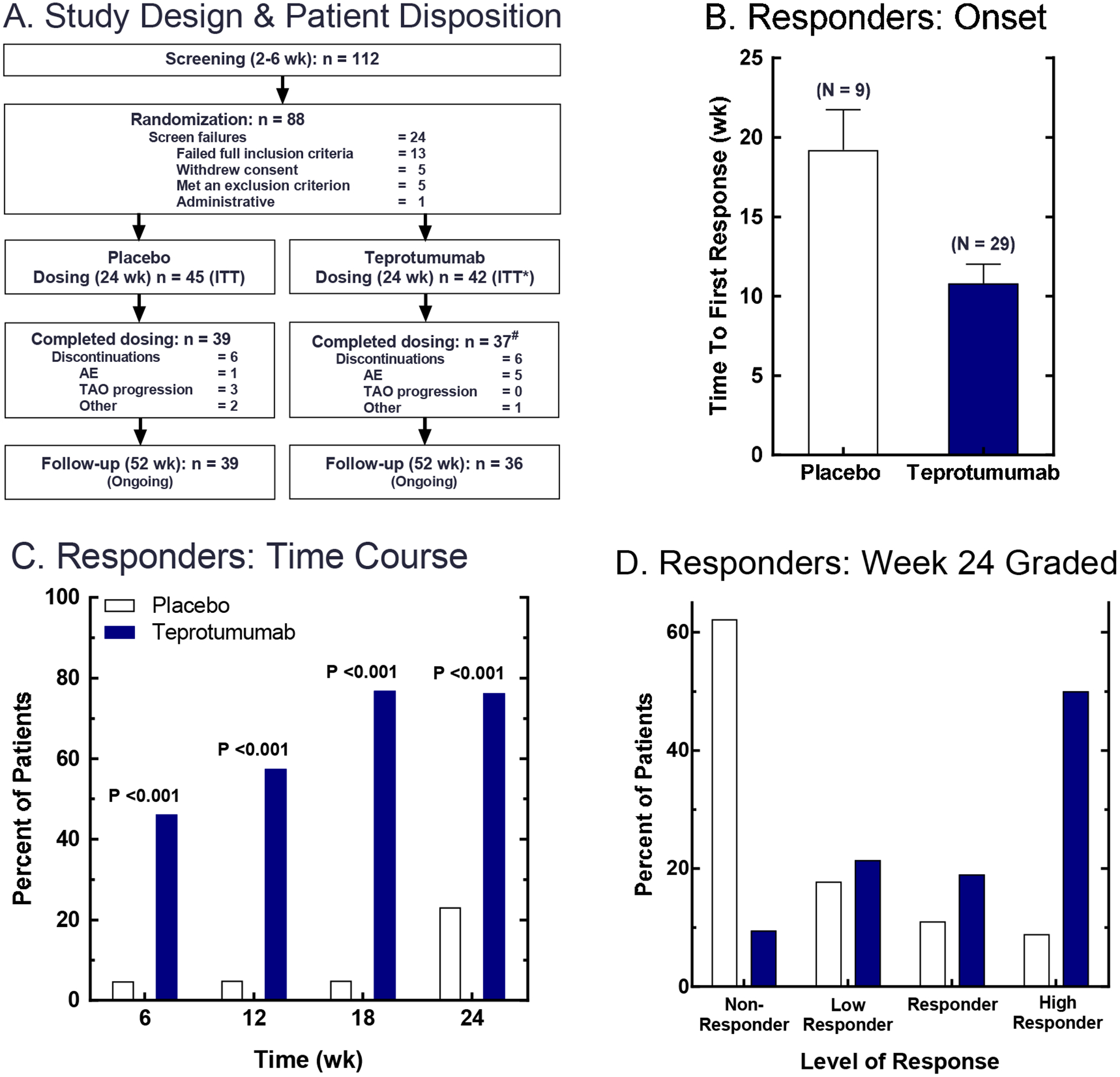
(Panel A) Patients who met primary inclusion criteria for disease onset ≤ 9 months and clinical activity score ≥ 4 were entered into the screening phase of the study. At the baseline visit, patients meeting all inclusion and exclusion criteria were randomized to receive teprotumumab or placebo. Teprotumumab was administered as 8 IV infusions, given at 3 week intervals over 24 weeks. Placebo was IV saline. (Panels B-D) Primary endpoint was a logistic regression on responder status at Week 24. Responders were defined as patients who had reduction in proptosis of ≥2 mm AND reduction in clinical activity score of ≥2 in the study eye. (Panel B) The time to first response (i.e. for meeting responder criteria); data are expressed as mean ± SE. (Panel C) Time course for patients meeting responder criteria. (Panel D) Grading of Week 24 responders; p <0.001 obtained from a logistic regression model. From N. Engl. J. Med, Smith T.J., Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, et al, Teprotumumab for Thyroid-Associated Ophthalmopathy, 376:1748–61. Copyright © (2017) Massachusetts Medical Society. Reprinted with permission.
Thirty-seven patients (88%) in the Tepro arm completed the intervention phase while 39 (87%) did so in the placebo group. Baseline proptosis, CAS and GO-QoL were identical in the two treatment arms. With regard to the primary outcome, 9/45 (20%) of those receiving placebo versus 29/42 (69%) of those in the Teprotumumab cohort met the aggregate primary response at week 24 (p<0.001). The time to first response was shorter and the fraction of patients achieving response was greater in the teprotumumab group at weeks 6, 12 and 18 (p<0.001 at every time point). Thus, the effects of Teprotumumab appear to be very rapid. When graded, the primary response levels were greater in the Teprotumumab arm than those in placebo cohort; more patients receiving Teprotumumab had proptosis reductions of ≥3 mm and reductions of CAS ≥ 3 points. With regard to the secondary endpoints, reduction in CAS and proptosis, measured as continuous variables from baseline, was substantially greater in the teprotumumab group (p<0.001). While 17/42 patients receiving teprotumumab experienced a reduction in proptosis ≥ 4 mm at week 24, 0/45 patients receiving placebo exhibited this level of proptosis reduction. CAS reduction was also greater and more rapid in the teprotumumab-receiving group and the differences from placebo were significantly different at every time point. Teprotummumab appears to be effective in mitigating other aspects of the disease. Secondary endpoint responses such as improvement in diplopia and the visual functioning subscale of GO-QoL were also highly significant in the teprotumumab-treated patients compared to placebo-receiving controls (p=0.009 and p<0.001 at week 24, respectively). Thus, results from this initial trial strongly suggest that teprotumumab may meaningfully alter the clinical course of TAO and therefore may become a useful strategy for managing active disease. Initial assessment of the durability of the clinical benefit suggests that a sizable patient group so treated and experiencing a response may have sustained benefit (101–103). The safety profile exhibited by teprotumumab in the phase 2 trial is promising. Several patients in the active treatment arm experienced auditory changes; however, these were transient. Other subjects with diabetes mellitus diagnosed prior to study participation required upward adjustment of their diabetes medications. Several patients in the teprotumumab group experienced grade 2 or 3 hyperglycemia. Importantly, the need for these increased dosages disappeared following the end of the treatment phase of the study. Other side effects observed included muscle cramps, 1 patient developed suspected Hashimoto’s encephalopathy and weight loss was observed in 3 patients receiving teprotumumab.
A phase 3 trial has been completed very recently and the topline results have been made public. This study protocol was similar to that of the phase 2 trial in that 8 infusions of teprotumumab or placebo were administered over a 24 week intervention phase but differed from the initial trial in that the primary endpoint was simplified to a proptosis reduction of ≥ 2 mm in the study eye as assessed at week 24. Secondary endpoints included overall response of proptosis reduction ≥ 2 mm and at least a 2-point reduction in CAS, and improved diplopia and GO-QoL scores. All primary and secondary endpoints were met at week 24 of the treatment phase. Of those receiving Tepro, 82.9% met the primary response compared to 9.5% of patients receiving placebo (p<0.001). The change in proptosis with teprotumumab was a mean of −3.32 mm at Week. 78% of the teprotumumab group were overall responders (CAS reduction ≥ 2 points and proptosis reduction ≥ 2 mm). Significantly more patients in the teprotumumab group achieved secondary responses. At least one diplopia grade improvement occurred in 50.0% in the teprotumumab group at week 6 compared to 3.6% of patients receiving placebo. Go-Qol scores improved with teprotumumab at 6 weeks compared to placebo. A study extension is currently being conducted where all non-responders, regardless of whether they were in the active drug or placebo cohort, are qualified for open label treatment with teprotumumab (8 infusions over 24 weeks). With regard to safety, no additional adverse effects were identified in the phase 3 study.
3.0. Conclusions
Despite the currently incomplete understanding of TAO, recent insights into its pathogenesis have led to the development of several novel medical therapeutic candidates for active, moderate to severe disease. Among them are biological agents that target specific molecular pathways suspected of playing roles in TAO. Based on the results of two double masked, multicenter, placebo controlled therapeutic trials, teprotumumab, an anti-IGF-IR monoclonal antibody that behaves as a β arrestin biased agonist has emerged as a promising treatment for moderate to severe, active TAO with unprecedented effectiveness that may change the therapeutic paradigm for active TAO. While the drug has not yet achieved registration by the U.S. FDA at the time of this writing, it remains possible that some patients may achieve benefit substantial and durable enough as to lessen the need for many remedial surgeries. It safety profile remains very promising, thus teprotumumab represents a potential first line therapy that may supplant steroids for this disease.
Practice points
TAO represents a vexing, disfiguring, and potentially blinding autoimmune disease
Current limitation to medical therapeutic options for active TAO
Glucocorticoids are currently the mainstay of medical therapy but are often ineffective and associated with side effects
Newer, more specific drug candidates are on the horizon based on newly identified, plausible molecular targets, including IL-6 receptor, B cells, TSHR and IGF-IR
Teprotumumab, an IGF-IR inhibitory antibody has shown remarkable effectiveness and promising safety profile in two multicenter, double-masked, placebo-controlled trials
Research agenda
Bring trials of TSHR-targeting agents to late-phase
Fully develop TSHR re-tolerization programs to full development
Expand clinical trial program of anti-IL-6 agents to adequate design and size
Post-approval Stage 4 trials to determine optimal dosing, expand indications
Continue basic and translation investigation of anti-IGF-IR therapeutic agents to more fully elucidate mechanism and cellular targets
Figure 4. Secondary efficacy endpoints in the phase 2 trial; time course of changes in proptosis, CAS and GO-QoL as continuous variables, and responders achieving CAS 0 or 1 and grade changes in subjective diplopia.
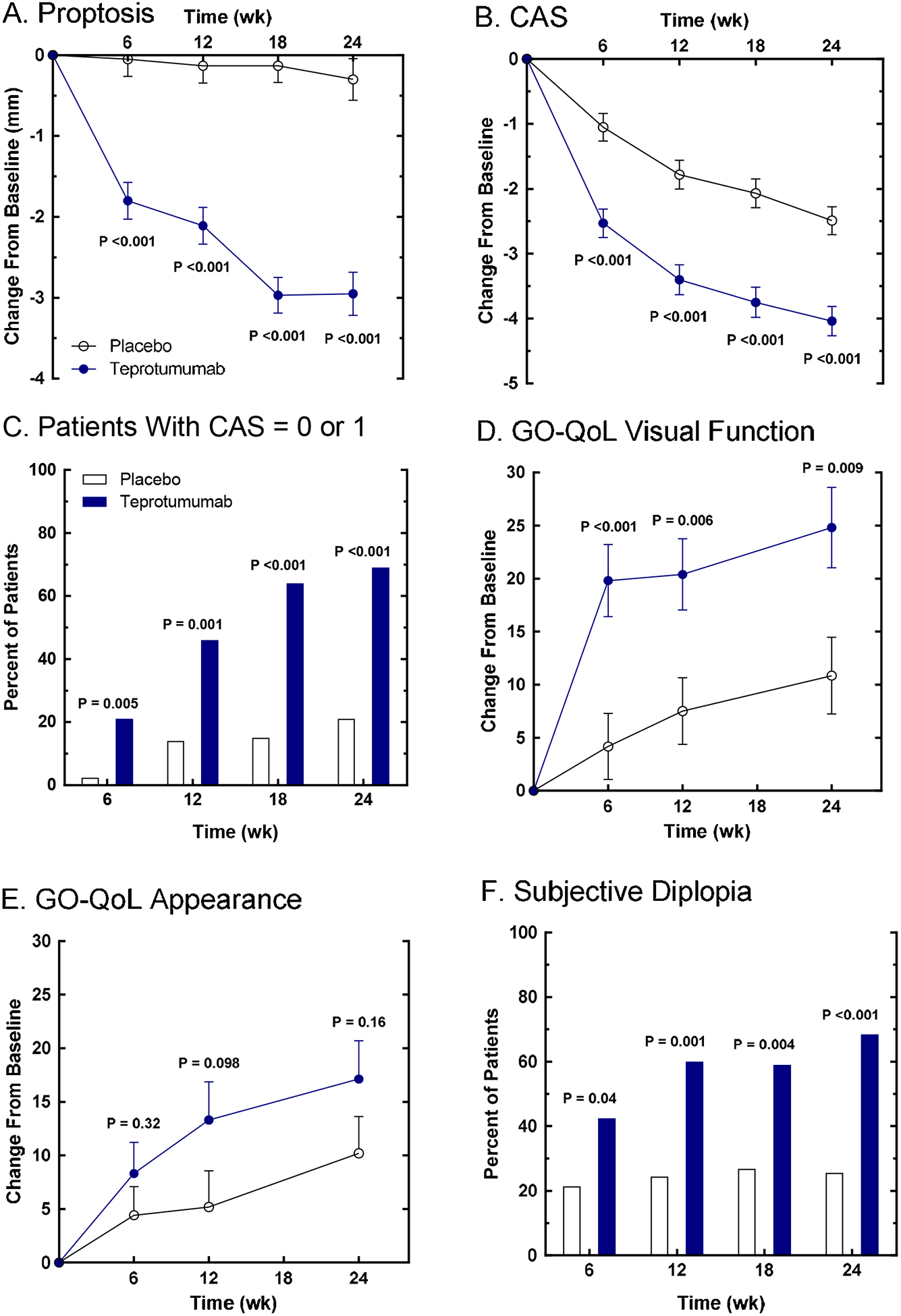
(Panel A) Proptosis change from baseline. (Panel B) CAS (7-component scale) change from baseline. (Panel C) Post-hoc analysis; time-course of percentage of patients achieving CAS of 0 or 1. (Panel D) GO-QoL Visual Function change from baseline. (Panel E) GO-QoL Appearance change from baseline. (Panel F) Subjective diplopia responders. Quality of life was evaluated using the Graves’ ophthalmopathy quality of life scale (GO-QoL), comprising two subscales each with a range of 0–100, where a change of 8 points is considered clinically relevant. Subjective diplopia was assessed where patients are categorized into four grades, and a change ≥1 grade is considered clinically relevant. From N. Engl. J. Med, Smith T.J., Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, et al, Teprotumumab for Thyroid-Associated Ophthalmopathy, 376:1748–61. Copyright © (2017) Massachusetts Medical Society. Reprinted with permission.
Acknowledgements
The author is grateful for the expert assistance of Leslie Bordine in preparing the manuscript. This work was supported in part by National Institutes of Health grants EY008976, EY11708, DK063121, 5UM1AI110557, Center for Vision core grant EY007002 from the National Eye Institute, an unrestricted grant from Research to Prevent Blindness, and by the Bell Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Bahn RS (2010) Graves’ ophthalmopathy. The New England journal of medicine 362, 726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *2.Smith TJ, and Hegedus L (2016) Graves’ Disease. The New England journal of medicine 375, 1552–1565 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, and Smith TJ (2014) Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 55, 1735–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moshkelgosha S, So PW, Deasy N, Diaz-Cano S, and Banga JP (2013) Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology 154, 3008–3015 [DOI] [PubMed] [Google Scholar]
- 5.Parmentier M, Libert F, Maenhaut C, Lefort A, Gerard C, Perret J, Van Sande J, Dumont JE, and Vassart G (1989) Molecular cloning of the thyrotropin receptor. Science 246, 1620–1622 [DOI] [PubMed] [Google Scholar]
- 6.Michalek K, Morshed SA, Latif R, and Davies TF (2009) TSH receptor autoantibodies. Autoimmun Rev 9, 113–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Marin-Sanchez A, Alvarez-Sierra D, Gonzalez O, Lucas-Martin A, Selles-Sanchez A, Rudilla F, Enrich E, Colobran R, and Pujol-Borrell R (2019) Regulation of TSHR Expression in the Thyroid and Thymus May Contribute to TSHR Tolerance Failure in Graves’ Disease Patients via Two Distinct Mechanisms. Front Immunol 10, 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapoport B, and McLachlan SM (2018) Reflections on Thyroid Autoimmunity: A Personal Overview from the Past into the Future. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 50, 840–852 [DOI] [PubMed] [Google Scholar]
- 9.Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, and Kahaly GJ (2011) Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology 118, 2279–2285 [DOI] [PubMed] [Google Scholar]
- 10.Wall JR, Lahooti H, El Kochairi I, Lytton SD, and Champion B (2014) Thyroid-stimulating immunoglobulins as measured in a reporter bioassay are not detected in patients with Hashimoto’s thyroiditis and ophthalmopathy or isolated upper eyelid retraction. Clinical ophthalmology (Auckland, N.Z.) 8, 2071–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabasum A, Khan I, Taylor P, Das G, and Okosieme OE (2016) Thyroid antibody-negative euthyroid Graves’ ophthalmopathy. Endocrinology, diabetes & metabolism case reports 2016, 160008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, and Smith TJ (2010) Increased generation of fibrocytes in thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism 95, 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Schiefer R, Coenen MJ, and Bahn RS (2010) A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid 20, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raychaudhuri N, Fernando R, and Smith TJ (2013) Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One 8, e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hikage F, Atkins S, Kahana A, Smith TJ, and Chun TH (2019) HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 160, 20–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, and Smith TJ (2014) Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. The Journal of clinical endocrinology and metabolism 99, E625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, and Douglas RS (2014) Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. The Journal of clinical endocrinology and metabolism 99, E1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, Mester T, Gupta S, Sun F, Smith TJ, and Douglas RS (2016) Thyrotropin and CD40L Stimulate Interleukin-12 Expression in Fibrocytes: Implications for Pathogenesis of Thyroid-Associated Ophthalmopathy. Thyroid 26, 1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, and Phipps RP (1994) Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 72, 283–292 [DOI] [PubMed] [Google Scholar]
- 20.Lynch MD, and Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Invest 128, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, and Phipps RP (1995) Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. The Journal of clinical endocrinology and metabolism 80, 2620–2625 [DOI] [PubMed] [Google Scholar]
- 22.Koumas L, Smith TJ, and Phipps RP (2002) Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol 32, 477–485 [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ (2002) Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid 12, 197–203 [DOI] [PubMed] [Google Scholar]
- 24.Koumas L, Smith TJ, Feldon S, Blumberg N, and Phipps RP (2003) Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol 163, 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith TJ, Wang HS, Hogg MG, Henrikson RC, Keese CR, and Giaever I (1994) Prostaglandin E2 elicits a morphological change in cultured orbital fibroblasts from patients with Graves ophthalmopathy. Proc Natl Acad Sci U S A 91, 5094–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucala R, Spiegel LA, Chesney J, Hogan M, and Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1, 71–81 [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda N, Masuya M, Tawara I, Tsuboi J, Yoneda M, Nishikawa K, Kageyama Y, Hachiya K, Ohishi K, Miwa H, Yamada R, Hamada Y, Tanaka K, Kato T, Takei Y, and Katayama N (2019) Infiltrating CCR2(+) monocytes and their progenies, fibrocytes, contribute to colon fibrosis by inhibiting collagen degradation through the production of TIMP-1. Sci Rep 9, 8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack M (2018) Inflammation and fibrosis. Matrix Biol 68-69, 106–121 [DOI] [PubMed] [Google Scholar]
- 29.Hempel F, Roderfeld M, Savai R, Sydykov A, Irungbam K, Schermuly R, Voswinckel R, Kohler K, Churin Y, Kiss L, Bier J, Pons-Kuhnemann J, and Roeb E (2019) Depletion of Bone Marrow-Derived Fibrocytes Attenuates TAA-Induced Liver Fibrosis in Mice. Cells 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar PG, Laloraya M, Wang CY, Ruan QG, Davoodi-Semiromi A, Kao KJ, and She JX (2001) The autoimmune regulator (AIRE) is a DNA-binding protein. J Biol Chem 276, 41357–41364 [DOI] [PubMed] [Google Scholar]
- 31.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, and Mathis D (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 [DOI] [PubMed] [Google Scholar]
- 32.Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, and Smith TJ (2012) Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A 109, 7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, and Smith TJ (2014) Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. The Journal of clinical endocrinology and metabolism 99, E1236–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesney J, Bacher M, Bender A, and Bucala R (1997) The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 94, 6307–6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grab DJ, Lanners H, Martin LN, Chesney J, Cai C, Adkisson HD, and Bucala R (1999) Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol Med 5, 46–54 [PMC free article] [PubMed] [Google Scholar]
- 36.Abe R, Donnelly SC, Peng T, Bucala R, and Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. Journal of immunology (Baltimore, Md. : 1950) 166, 7556–7562 [DOI] [PubMed] [Google Scholar]
- 37.Heufelder AE, Smith TJ, Gorman CA, and Bahn RS (1991) Increased induction of HLA-DR by interferon-gamma in cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial dermopathy. The Journal of clinical endocrinology and metabolism 73, 307–313 [DOI] [PubMed] [Google Scholar]
- 38.Heufelder AE, Bahn RS, and Smith TJ (1992) Regulation by glucocorticoids of interferon gamma-induced HLA-DR antigen expression in cultured human orbital fibroblasts. Clinical endocrinology 37, 59–63 [DOI] [PubMed] [Google Scholar]
- 39.Pritchard J, Horst N, Cruikshank W, and Smith TJ (2002) Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. Journal of immunology (Baltimore, Md. : 1950) 168, 942–950 [DOI] [PubMed] [Google Scholar]
- *40.Pritchard J, Han R, Horst N, Cruikshank WW, and Smith TJ (2003) Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. Journal of immunology (Baltimore, Md. : 1950) 170, 6348–6354 [DOI] [PubMed] [Google Scholar]
- 41.Smith TJ, Bahn RS, and Gorman CA (1989) Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev 10, 366–391 [DOI] [PubMed] [Google Scholar]
- 42.Smith TJ, Bahn RS, and Gorman CA (1989) Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. The Journal of clinical endocrinology and metabolism 69, 1019–1023 [DOI] [PubMed] [Google Scholar]
- 43.Arbogast B, Hopwood JJ, and Dorfman A (1975) Absence of hyaluronidase in cultured human skin fibroblasts. Biochem Biophys Res Commun 67, 376–382 [DOI] [PubMed] [Google Scholar]
- 44.Smith TJ (1984) Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J Clin Invest 74, 2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaback LA, and Smith TJ (1999) Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1beta in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. The Journal of clinical endocrinology and metabolism 84, 4079–4084 [DOI] [PubMed] [Google Scholar]
- 46.Spicer AP, Kaback LA, Smith TJ, and Seldin MF (1998) Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem 273, 25117–25124 [DOI] [PubMed] [Google Scholar]
- 47.Tsui S, Fernando R, Chen B, and Smith TJ (2011) Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem 286, 24487–24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriss JP (1970) Radioisotopic thyroidolymphography in patients with Graves’ disease. The Journal of clinical endocrinology and metabolism 31, 315–323 [DOI] [PubMed] [Google Scholar]
- 49.Lisi S, Marino M, Pinchera A, Mazzi B, Di Cosmo C, Sellari-Franceschini S, and Chiovato L (2002) Thyroglobulin in orbital tissues from patients with thyroid-associated ophthalmopathy: predominant localization in fibroadipose tissue. Thyroid 12, 351–360 [DOI] [PubMed] [Google Scholar]
- 50.Marino M, Lisi S, Pinchera A, Marcocci C, Menconi F, Morabito E, Macchia M, Sellari-Franceschini S, McCluskey RT, and Chiovato L (2003) Glycosaminoglycans provide a binding site for thyroglobulin in orbital tissues of patients with thyroid-associated ophthalmopathy. Thyroid 13, 851–859 [DOI] [PubMed] [Google Scholar]
- 51.Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, and Fenzi G (1993) Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet (London, England) 342, 337–338 [DOI] [PubMed] [Google Scholar]
- 52.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, and Bahn RS (1993) Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid 3, 297–300 [DOI] [PubMed] [Google Scholar]
- 53.Smith TJ (2004) Novel aspects of orbital fibroblast pathology. Journal of endocrinological investigation 27, 246–253 [DOI] [PubMed] [Google Scholar]
- 54.Woo YJ, Jang SY, Lim TH, and Yoon JS (2015) Clinical Association of Thyroid Stimulating Hormone Receptor Antibody Levels with Disease Severity in the Chronic Inactive Stage of Graves’ Orbitopathy. Korean J Ophthalmol 29, 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weightman DR, Perros P, Sherif IH, and Kendall-Taylor P (1993) Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity 16, 251–257 [DOI] [PubMed] [Google Scholar]
- *56.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, and Smith TJ (2008) Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. Journal of immunology (Baltimore, Md. : 1950) 181, 4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcus-Samuels B, Krieger CC, Boutin A, Kahaly GJ, Neumann S, and Gershengorn MC (2018) Evidence That Graves’ Ophthalmopathy Immunoglobulins Do Not Directly Activate IGF-1 Receptors. Thyroid 28, 650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Krieger CC, Boutin A, Jang D, Morgan SJ, Banga JP, Kahaly GJ, Klubo-Gwiezdzinska J, Neumann S, and Gershengorn MC (2019) Arrestin-beta-1 Physically Scaffolds TSH and IGF1 Receptors to Enable Crosstalk. Endocrinology 160, 1468–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazim M, Goldberg RA, and Smith TJ (2002) Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol 120, 380–386 [DOI] [PubMed] [Google Scholar]
- 60.Wiersinga W, Zarkovic M, Bartalena L, Donati S, Perros P, Okosieme O, Morris D, Fichter N, Lareida J, von Arx G, Daumerie C, Burlacu MC, Kahaly G, Pitz S, Beleslin B, Ciric J, Ayvaz G, Konuk O, Toruner FB, Salvi M, Covelli D, Curro N, Hegedus L, and Brix T (2018) Predictive score for the development or progression of Graves’ orbitopathy in patients with newly diagnosed Graves’ hyperthyroidism. European journal of endocrinology 178, 635–643 [DOI] [PubMed] [Google Scholar]
- *61.Wiersinga WM (2017) Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol 5, 134–142 [DOI] [PubMed] [Google Scholar]
- 62.Nicosia L, Reverberi C, Agolli L, Marinelli L, De Sanctis V, Minniti G, Valeriani M, and Osti MF (2019) Orbital Radiotherapy Plus Concomitant Steroids in Moderate-to-Severe Graves’ Ophthalmopathy: Good Results After Long-Term Follow-Up. International journal of endocrinology and metabolism 17, e84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith TJ, and Bartalena L (2019) Will biological agents supplant systemic glucocorticoids as the first-line treatment for thyroid-associated ophthalmopathy? European journal of endocrinology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kahaly GJ, Pitz S, Hommel G, and Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. The Journal of clinical endocrinology and metabolism 90, 5234–5240 [DOI] [PubMed] [Google Scholar]
- *65.Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Curro N, Boschi A, Bernard M, von Arx G, and European Group on Graves, O. (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. The Journal of clinical endocrinology and metabolism 97, 4454–4463 [DOI] [PubMed] [Google Scholar]
- 66.Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, and Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17, 357–362 [DOI] [PubMed] [Google Scholar]
- 67.Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campenni A, Morelli S, Puxeddu E, Sisti E, Trimarchi F, Vermiglio F, and Marino M (2016) Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy. Endocrine 54, 259–268 [DOI] [PubMed] [Google Scholar]
- 68.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM, and European Group on Graves, O. (2016) The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J 5, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott DL, Wolfe F, and Huizinga TW (2010) Rheumatoid arthritis. Lancet (London, England) 376, 1094–1108 [DOI] [PubMed] [Google Scholar]
- 70.Eisenberg R, and Albert D (2006) B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol 2, 20–27 [DOI] [PubMed] [Google Scholar]
- 71.Hasselbalch HC (2003) B-cell depletion with rituximab-a targeted therapy for Graves’ disease and autoimmune thyroiditis. Immunol Lett 88, 85–86 [DOI] [PubMed] [Google Scholar]
- 72.Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, Guastella C, Avignone S, Pirola G, Ratiglia R, and Beck-Peccoz P (2006) Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. European journal of endocrinology 154, 511–517 [DOI] [PubMed] [Google Scholar]
- 73.El Fassi D, Nielsen CH, Hasselbalch HC, and Hegedus L (2006) The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. European journal of endocrinology 154, 623–632 [DOI] [PubMed] [Google Scholar]
- 74.El Fassi D, Nielsen CH, Hasselbalch HC, and Hegedus L (2006) Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid 16, 709–710 [DOI] [PubMed] [Google Scholar]
- 75.Salvi M, Vannucchi G, Curro N, Campi I, Covelli D, Dazzi D, Simonetta S, Guastella C, Pignataro L, Avignone S, and Beck-Peccoz P (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. The Journal of clinical endocrinology and metabolism 100, 422–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, and Bahn RS (2015) Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. The Journal of clinical endocrinology and metabolism 100, 432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kahaly GJ, Riedl M, Konig J, Pitz S, Ponto K, Diana T, Kampmann E, Kolbe E, Eckstein A, Moeller LC, Fuhrer D, Salvi M, Curro N, Campi I, Covelli D, Leo M, Marino M, Menconi F, Marcocci C, Bartalena L, Perros P, and Wiersinga WM (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6, 287–298 [DOI] [PubMed] [Google Scholar]
- 78.Yamamura T, Kleiter I, Fujihara K, Palace J, Greenberg B, Zakrzewska-Pniewska B, Patti F, Tsai CP, Saiz A, Yamazaki H, Kawata Y, Wright P, and De Seze J (2019) Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. The New England journal of medicine 381, 2114–2124 [DOI] [PubMed] [Google Scholar]
- *79.Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodriguez Alvarez FM, Castillo Laguarta JM, Del Estad Cabello A, Gessa Sorroche M, Espana Gregori E, and Sales-Sanz M (2018) Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol 195, 181–190 [DOI] [PubMed] [Google Scholar]
- 80.Perez-Moreiras JV, Alvarez-Lopez A, and Gomez EC (2014) Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic plastic and reconstructive surgery 30, 162–167 [DOI] [PubMed] [Google Scholar]
- 81.Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, and Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, and Gershengorn MC (2006) A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem 281, 9841–9844 [DOI] [PubMed] [Google Scholar]
- 83.Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, and Gershengorn MC (2009) Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A 106, 12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumann S, Eliseeva E, McCoy JG, Napolitano G, Giuliani C, Monaco F, Huang W, and Gershengorn MC (2011) A new small-molecule antagonist inhibits Graves’ disease antibody activation of the TSH receptor. The Journal of clinical endocrinology and metabolism 96, 548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neumann S, Nir EA, Eliseeva E, Huang W, Marugan J, Xiao J, Dulcey AE, and Gershengorn MC (2014) A selective TSH receptor antagonist inhibits stimulation of thyroid function in female mice. Endocrinology 155, 310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, and Gershengorn MC (2010) A small molecule inverse agonist for the human thyroid-stimulating hormone receptor. Endocrinology 151, 3454–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Nunez Miguel R, Blundell TL, Furmaniak J, and Rees Smith B (2004) Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14, 560–570 [DOI] [PubMed] [Google Scholar]
- 88.Sanders J, Allen F, Jeffreys J, Bolton J, Richards T, Depraetere H, Nakatake N, Evans M, Kiddie A, Premawardhana LD, Chirgadze DY, Miguel RN, Blundell TL, Furmaniak J, and Smith BR (2005) Characteristics of a monoclonal antibody to the thyrotropin receptor that acts as a powerful thyroid-stimulating autoantibody antagonist. Thyroid 15, 672–682 [DOI] [PubMed] [Google Scholar]
- 89.Chen CR, McLachlan SM, and Rapoport B (2009) A monoclonal antibody with thyrotropin (TSH) receptor inverse agonist and TSH antagonist activities binds to the receptor hinge region as well as to the leucine-rich domain. Endocrinology 150, 3401–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanders JM,RN; Furmaniak J; Smith BR. (2010) TSH Receptor Monoclonal Antibodies With Agonist, Antagonist, And Agonist, And Inverse Agonist Activities/. in Constitutive Activity in Receptors and Other Proteins (Simon MI ed.), Elsevier Science. pp [DOI] [PubMed] [Google Scholar]
- 91.Sanders P, Young S, Sanders J, Kabelis K, Baker S, Sullivan A, Evans M, Clark J, Wilmot J, Hu X, Roberts E, Powell M, Nunez Miguel R, Furmaniak J, and Rees Smith B (2011) Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol 46, 81–99 [DOI] [PubMed] [Google Scholar]
- *92.Jansson L, Vrolix K, Jahraus A, Martin KF, and Wraith DC (2018) Immunotherapy With Apitopes Blocks the Immune Response to TSH Receptor in HLA-DR Transgenic Mice. Endocrinology 159, 3446–3457 [DOI] [PubMed] [Google Scholar]
- 93.Pearce SHS, Dayan C, Wraith DC, Barrell K, Olive N, Jansson L, Walker-Smith T, Carnegie C, Martin KF, Boelaert K, Gilbert J, Higham CE, Muller I, Murray RD, Perros P, Razvi S, Vaidya B, Wernig F, and Kahaly GJ (2019) Antigen-Specific Immunotherapy with Thyrotropin Receptor Peptides in Graves’ Hyperthyroidism: A Phase I Study. Thyroid [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pritchard J, Tsui S, Horst N, Cruikshank WW, and Smith TJ (2004) Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves’ disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. Journal of immunology (Baltimore, Md. : 1950) 173, 3564–3569 [DOI] [PubMed] [Google Scholar]
- *95.Smith TJ (2010) Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacological reviews 62, 199–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gong Y, Yao E, Shen R, Goel A, Arcila M, Teruya-Feldstein J, Zakowski MF, Frankel S, Peifer M, Thomas RK, Ladanyi M, and Pao W (2009) High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507). PLoS One 4, e7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, Toner GC, Maki RG, Meyers PA, Chugh R, Ganjoo KN, Schuetze SM, Juergens H, Leahy MG, Geoerger B, Benjamin RS, Helman LJ, and Baker LH (2011) R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29, 4541–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma H, Zhang T, Shen H, Cao H, and Du J (2014) The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy. British journal of clinical pharmacology 77, 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *99.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, and Douglas RS (2017) Teprotumumab for Thyroid-Associated Ophthalmopathy. The New England journal of medicine 376, 1748–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Terwee CB, Dekker FW, Mourits MP, Gerding MN, Baldeschi L, Kalmann R, Prummel MF, and Wiersinga WM (2001) Interpretation and validity of changes in scores on the Graves’ ophthalmopathy quality of life questionnaire (GO-QOL) after different treatments. Clinical endocrinology 54, 391–398 [DOI] [PubMed] [Google Scholar]
- 101.Douglas RS (2019) Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis. Eye (Lond) 33, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Douglas RS, Francis-Sedlak M, and Holt R (2018) Teprotumumab in TED: Diplopia Outcome Analysis (abstract) in American Society of Ophthalmic Plastic & Recontructive Surgery (ASOPRS) Annual Fall Meeting, Chicago, IL [Google Scholar]
- 103.Kahaly G, douglas R, Holt R, Perdok R, Ball J, and Smith T (2018) 48-week follow-up of a multicenter, randomized, double-masked, placebo-controlled treatment trial of teprotumumab in thyroid-associated ophthalmopathy (abstract) in American Thyroid Association (ATA) 88th Annual Meeting, Washington DC [Google Scholar]


